Abstract
Objectives:
The signaling pathways upstream of glycogen synthase kinase-3β (GSK-3β) get reduced during ischemic preconditioning (IPC) in hyperlipidemic rat heart. Pioglitazone, an insulin sensitizer, exerts cardioprotection through GSK-3β. The objective of the study is to investigate the role of pioglitazone on the attenuated cardioprotective effect of IPC in hyperlipidemic rat heart.
Materials and Methods:
The rats were administered high-fat diet for 8 weeks to induce experimental hyperlipidemia (HL). After mounting on a Langendorff apparatus, isolated perfused hearts were given four cycles of IPC; each consists of 5 min of both ischemia and reperfusion followed by 30 min of ischemia and 120 min of reperfusion. Insulin (50 mU/ml) was perfused alone and in combination with pioglitazone (2 μM), while in other groups, this combination was repeated with wortmannin (100 nM), a selective PI3K inhibitor and rapamycin (1 nM), a selective mammalian target of rapamycin (mTOR) inhibitor, separately, and in combination. Myocardial injury was assessed by measuring infarct size and the levels of creatinine kinase-myocardial band (CK-MB) and lactate dehydrogenase (LDH) in the coronary effluent.
Results:
IPC significantly decreased the infarct size and levels of LDH and CK-MB in normal but not in HL rat heart. Perfusion of insulin along with pioglitazone significantly reduced the infarct size and release of CK-MB and LDH in IPC-treated HL rat hearts. Perfusion of wortmannin or rapamycin alone significantly and in combination almost completely abolished the pioglitazone-induced restored cardioprotection (P < 0.05).
Conclusion:
Cardioprotective effect of IPC gets lost in hyperlipidemic rat heart. The results suggest that perfusion of pioglitazone restored the cardioprotective effect of IPC in hyperlipidemic rat heart, an effect that may be via PI3K and mTOR.
KEY WORDS: Creatinine kinase, glycogen synthase kinase-3β, ischemia-reperfusion, lactate dehydrogenase, myocardial injury, pioglitazone, rapamycin, wortmannin
Introduction
Hyperlipidemia (HL) is a well-known risk for the cardiovascular complications including coronary artery disease.[1] It has been reported that ischemic preconditioning (IPC), i.e., short intermittent cycles of sublethal ischemia followed by reperfusion before the subsequent prolonged ischemic insult, produces cardioprotection against ischemia-reperfusion (I/R) induced injury.[2,3] The absence of the cardioprotective effect of IPC during the HL is reported.[4] Also, the decrease of the insulin sensitivity is a major risk of HL.[5]
Pioglitazone is a ligand for peroxisome proliferator activated receptor-gamma thereby produces the cardioprotection against I/R induced injury by activation of the signals upstream to glycogen synthase kinase-3μ (GSK-3μ).[6,7] IPC produces cardioprotection by the activation of PI3K and mammalian target of rapamycin (mTOR).[8,9,10] Both the pathways were reported to be diminished during the HL.[11,12] We have reported that impairment of upstream pathways of GSK-3μ is responsible for the attenuation of IPC-mediated cardioprotection in hyperlipidemic rat heart while administration of GSK-3μ inhibitor restored it.[13]
Therefore, the present study is designed to investigate whether or not pioglitazone, an insulin sensitizer, restores the attenuated cardioprotective effect of IPC in hyperlipidemic rat heart and to determine the possible signaling pathway involved in this effect.
Materials and Methods
The experimental protocol used in the present study was approved by Institutional Animal Ethical Committee (Reg. no. 816/04/c/CPCSEA). All the experiments were carried out according to the guidelines of Indian National Science Academy for care and use of animals in scientific research. Wistar rats (180–250 g) of either sex, divided into nine groups of six animals each, were employed in the present study. They were acclimatized in animal house and were exposed to normal cycle of light and darkness.
Drugs and Chemicals
Insulin (50 mU/ml) (Torrent Pharmaceutical Ltd., India) was dissolved in Krebs–Henseleit (K–H) sol. Pioglitazone (2 μM) (Enzo Life Sciences, AG), wortmannin (100 nM), and rapamycin (1 nM) (Cayman Chemical Co., USA) were dissolved in dimethyl sulfoxide. The 1% w/v solution of triphenyltetrazolium chloride (TTC) stain (CDH Pvt. Ltd., New Delhi), prepared in Tris-chloride buffer (CDH Pvt. Ltd., New Delhi), was used to measure infarct size. The lactate dehydrogenase (LDH) and creatinine kinase-myocardial band (CK-MB) enzymatic estimation kits were purchased from coral clinical systems, Goa, India. All other reagents used in this study were of analytical grade and always freshly prepared before use.
Induction of Experimental Hyperlipidemia
The rats were administered high-fat diet (containing corn starch 44.74 g, casein 14 g, sucrose 10 g, butter 20 g, fiber 5 g, mineral mix 3.5 g, vitamin mix 1 g, choline 0.25 g, tert-butylhydroquinone 0.0008 g, cholesterol 1 g, and cholic acid 0.5 g) for 8 weeks to induce experimental HL.[14] HL was documented by estimating the serum level of total cholesterol (TC) and triglycerides (TG) using commercially available kits (Vital Diagnostics [P] Ltd., Mumbai, India).
Isolated Rat Heart Preparation
Hearts from heparinized rats (500 IU/i.p.) were rapidly excised and immediately mounted on Langendorff's apparatus (Digital Langendorff system, Radnoti LLC, Monrovia, USA). The heart was enclosed by a double-walled jacket with circulating water maintained at 37.8°C. The heart was retrogradely perfused at constant pressure (By using peristaltic pump) and flow rate (7–9 ml/min) with freshly prepared K–H buffer solution (NaCl 118 mM; KCl 4.7 mM; CaCl2 2.5 mM; MgSO4.7H2O 1.2 mM; NaHCO3 25 mM; KH2PO4 1.2 mM; C6H12O6 11 mM), pH 7.4, bubbled with 95% O2 and 5% CO2. IPC was produced by four I/R episodes in which closing the inflow of K–H solution for 5 min followed by 5 min of reperfusion. Global ischemia was produced for 30 min followed by reperfusion for 120 min.[15] Coronary effluent was collected before ischemia, immediately, 5 min and 30 min after reperfusion for the estimation of LDH and CK-MB.[16]
Assessment of Myocardial Injury
Estimation of LDH and CK-MB in the coronary effluent was done to assess the myocardial injury. The assessment of myocardial infarct size was done using TTC staining method. Values of LDH and CK-MB were expressed in international units per liter (IU/L).
Myocardial Infarct Size
At the end of reperfusion, hearts were removed from the Langendorff's apparatus, ventricles were kept overnight at − 4°C after excising both the atria and root of aorta, and sliced into uniform sections of 1–2 mm thickness. The slices were incubated in 1% TTC at 37°C in 0.2 M Tris buffer pH 7.4 for 30 min.[17] The normal myocardium was stained brick red whereas the infarcted portion remained unstained. Infarct size was measured by the volume method.[18]
Experimental Protocol
A diagrammatic representation of experimental protocol is shown in Figure 1. In all groups, the isolated rat heart was perfused with K–H solution and allowed for 10 min of stabilization. Group 1, sham control n = 6; isolated rat heart was perfused continuously for 190 min after the stabilization, without subjecting them to global ischemia. Group 2, I/R control; n = 6; after stabilization heart was perfused for 40 min followed by 30 min of global ischemia and 120 min of reperfusion. Group 3, IPC control n = 6; heart was subjected to four cycles of IPC. Each cycle comprises 5 min ischemia and 5 min of reperfusion, followed by 30 min of global ischemia and 120 min of reperfusion. Group 4, IPC in hyperlipidemic heart; n = 6; isolated heart from hyperlipidemic rat was subjected to IPC as described in group 3. Group 5, IPC in insulin (50 mU/ml) perfused hyperlipidemic heart, n = 6; isolated heart from hyperlipidemic rat was perfused with K–H solution containing insulin (50 mU/ml) for 10 min and in each cycle of reperfusion during IPC followed by 30 min of ischemia and 120 min of reperfusion. Group 6, IPC in insulin and pioglitazone perfused hyperlipidemic heart, n = 6; isolated heart from hyperlipidemic rat was perfused by solution containing insulin (50 mU/ml) and pioglitazone (2 μM) during stabilization and 5 min in each cycle of IPC followed by 30 min of ischemia and 120 min of reperfusion. Group 7, IPC in insulin and pioglitazone in the presence of wortmannin (PI3K inhibitor) (100 nM) perfused in hyperlipidemic heart, n = 6; isolated hyperlipidemic rat was perfused for 10 min during stabilization and 5 min each cycle of IPC with K–H solution containing insulin (50 mU/ml), pioglitazone (2 μM) with wortmannin (100 nM) followed by 30 min of ischemia and 120 min of reperfusion. Group 8, IPC in insulin (50 mU/ml) and pioglitazone (2 μM) in the presence of rapamycin (mTOR inhibitor) (1 nM) in hyperlipidemic heart, n = 6; isolated heart from hyperlipidemic rat was perfused for 10 min during stabilization and 5 min in each cycle of IPC with K–H solution containing insulin (50 mU/ml), pioglitazone (2 μM) with rapamycin (1 nM) followed by 30 min of ischemia and 120 min of reperfusion. Group 9, IPC in insulin (50 mU/ml) and pioglitazone (2 μM) in the presence of wortmannin (100 nM) and rapamycin (1 nM) in hyperlipidemic heart, n = 6; isolated heart from hyperlipidemic rat was perfused for 10 min during stabilization and 5 min in each episode of IPC with K–H solution containing insulin (50 mU/ml), pioglitazone (2 μM) with wortmannin (100 nM) and rapamycin (1 nM) followed by 30 min of ischemia and 120 min of reperfusion.
Figure 1.
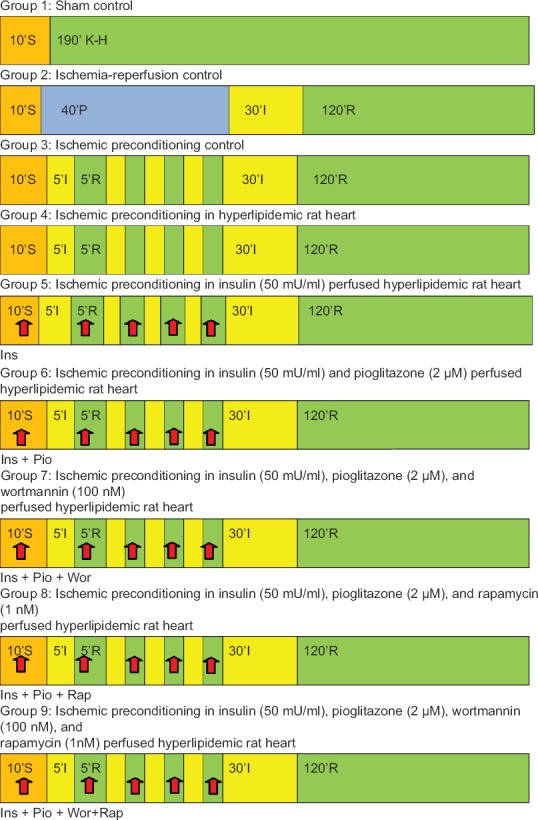
Diagrammatic representation of experimental protocol. S, P, I, R, Ins, Pio, Wor, Rap denote stabilization, perfusion, ischemia, reperfusion, insulin, pioglitazone, wortmannin, and rapamycin, respectively
Statistical Analysis
All values were expressed as mean ± standard error of mean. The data obtained from various groups were statistically analyzed using one-way ANOVA followed by Tukey's multiple comparisons test. P < 0.05 was considered to be statistically significant.
Results
Effect of High Fat Diet on Serum Total Cholesterol and Triglycerides
The administration of high fat diet for 8 weeks significantly increased the serum TC and TG levels as compared to basal value.
Effect of Ischemic Preconditioning on Myocardial Injury in Normal and Hyperlipidemic Rat Heart
Global ischemia for 30 min, followed by 120 min of reperfusion markedly increased the infarct size and the release of LDH and CK-MB, as compared to sham group. IPC significantly (P < 0.05) reduced the I/R-induced myocardial infarct size and release of LDH and CK-MB of hearts obtained from normal rats. However, in case of hyperlipidemic rat hearts, IPC failed to decrease the I/R-induced myocardial infarct size and the release of LDH and CK-MB [Figures 2–4].
Figure 2.
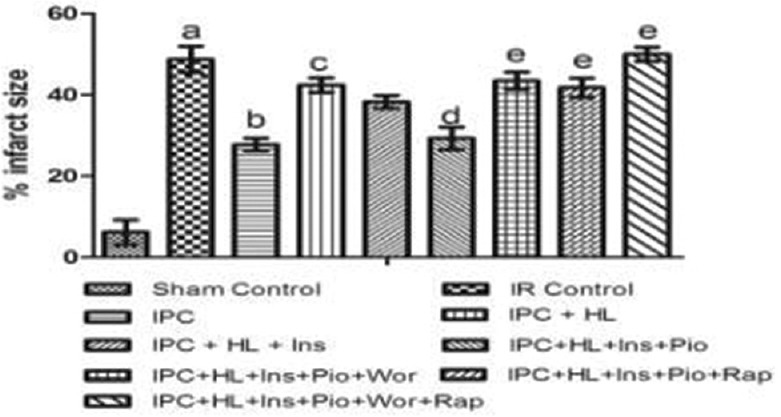
Effect of pharmacological interventions on myocardial infarct size. I R denotes ischemia reperfusion; IPC denotes ischemic preconditioning; HL denotes hyperlipidemia; Ins denotes insulin; Pio denotes pioglitazone; Wor denotes wortmannin; Rap denotes rapamycin. Values are expressed as mean ± standard deviation, a = P < 0.05 versus Sham Control and basal value; b = P < 0.05 versus ischemia-reperfusion control; c = P < 0.05 versus ischemic preconditioning control; d = P < 0.05 versus ischemic preconditioning + hyperlipidemia; e = P < 0.05 versus ischemic preconditioning + hyperlipidemia + Ins + Pio
Figure 4.
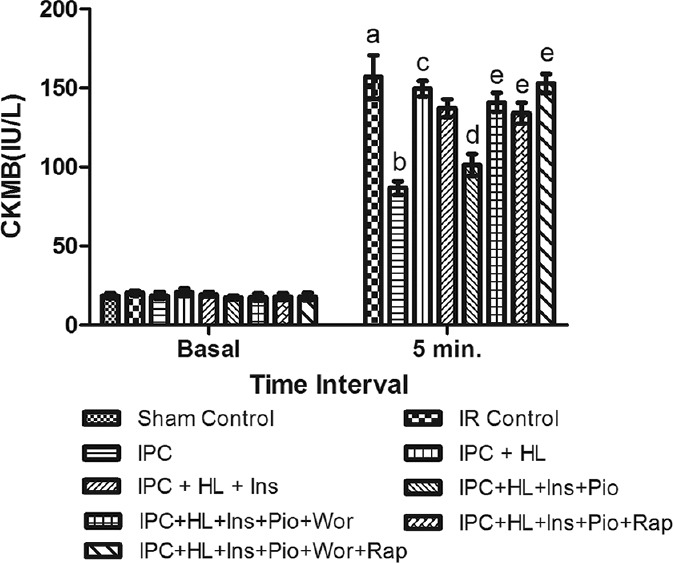
Effect of pharmacological interventions on myocardial release of creatinine kinase-myocardial band. I R denotes ischemia reperfusion; IPC denotes ischemic preconditioning; HL denotes hyperlipidemia; ins denotes insulin; Pio denotes pioglitazone; Wor denotes wortmannin; Rap denotes rapamycin. Values are expressed as mean ± standard deviation, a = P < 0.05 versus Sham control and basal value; b = P < 0.05 versus ischemia-reperfusion control; c = P < 0.05 versus ischemic preconditioning control; d = P < 0.05 versus ischemic preconditioning + hyperlipidemia; e = P < 0.05 versus ischemic preconditioning + hyperlipidemia + Ins + Pio
Figure 3.
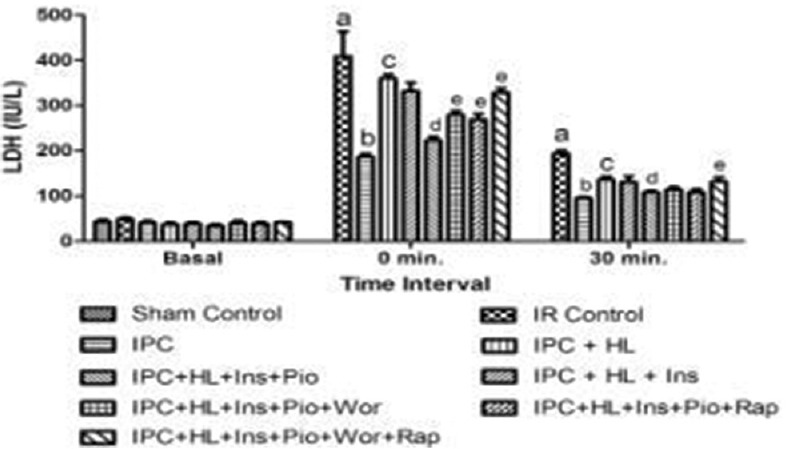
Effect of pharmacological interventions on myocardial release of lactate dehydrogenase. I R denotes ischemia reperfusion; IPC denotes ischemic preconditioning; HL denotes hyperlipidemia; Ins denotes insulin; Pio denotes pioglitazone; Wor denotes wortmannin; Rap denotes rapamycin. Values are expressed as mean ± standard deviation, a = P < 0.05 versus Sham control and basal value; b = P < 0.05 versus ischemia-reperfusion control; c = P < 0.05 versus ischemic preconditioning control; d = P < 0.05 versus ischemic preconditioning + hyperlipidemia; e = P < 0.05 versus ischemic preconditioning + hyperlipidemia + Ins + Pio
Effect of Ischemic Preconditioning in Insulin and Pioglitazone Perfused Hyperlipidemic Rat Heart
Perfusion of insulin (50 mU/ml) alone did not demonstrate any cardioprotective effect of IPC in hyperlipidemic rat heart whereas perfusion of pioglitazone (2 μM) with insulin (50 mU/ml) significantly (P < 0.05) restored the IPC-induced cardioprotection as shown by decrease in myocardial infarct size and release of LDH and CK-MB from hyperlipidemic rat heart [Figures 2–4].
Effect of Wortmannin or Rapamycin in the Restoration of Ischemic Preconditioning Mediated Cardioprotection in Insulin and Pioglitazone Perfused Hyperlipidemic Rat Heart
Perfusion of wortmannin (100 nM) along with pioglitazone (2 μM) and insulin (50 mU/ml) increases the myocardial infarct size and the release of LDH and CK-MB in coronary effluent obtained from hyperlipidemic rat heart. Perfusion of rapamycin also significantly (P < 0.05) attenuated the IPC-mediated cardioprotection in insulin (50 mU/ml) and pioglitazone (2 μM) perfused hyperlipidemic rat heart noted in terms of increase in myocardial infarct size and the release of LDH and CK-MB in coronary effluent. Perfusion of combination of both wortmannin (100 nM) and rapamycin (1 nM) almost completely (P < 0.05) attenuated the IPC-induced cardioprotection in insulin and pioglitazone-perfused hyperlipidemic rat heart [Figures 2–4].
Discussion
CK-MB is a specific marker of myocardial injury. The peak release of CK-MB at 5 min after reperfusion and LDH immediately after reperfusion has been reported.[19] In this study, global ischemia of 30 min followed by 120 min of reperfusion increased the level of LDH and CK-MB in coronary effluent measured at 0 min and 5 min of reperfusion, respectively, and increased the myocardial infarct size which is in accordance with earlier studies.[20] Hyperlipidemia is one of the major risk factors for coronary artery disease and it has been shown to abolish the cardioprotective effect of IPC.[21,22,23] In the present study, the administration of high fat diet for 8 weeks significantly increased the levels of serum TC and TG. IPC in normal rat heart significantly produced the cardioprotection; however, in hyperlipidemic rat hearts, this cardioprotection is abolished in terms of increase in infarct size and release of LDH and CK-MB in coronary effluent which is in agreement with our earlier studies.[24]
Infusion of insulin before the ischemia produces protection against I/R induced injury in normal heart.[25] In our study, perfusion of insulin (50 mU/ml) was unable to exert any cardioprotection in hyperlipidemic rat heart. Our result is in accordance of the previous reported work, i.e., insulin is unable to exert cardioprotection during the pathological conditions such as diabetes mellitus and HL.[26] IPC in insulin and pioglitazone-perfused hyperlipidemic rat heart, significantly restored the attenuated cardioprotection as compared to insulin-perfused hyperlipidemic rat heart which reflects that the attenuated cardioprotection in hyperlipidemic rat heart may be a part by impaired insulin mediated signaling.
In our study, perfusion of pioglitazone, an insulin sensitizer,[7,27] significantly restored the cardioprotection in hyperlipidemic rat heart. In our study, administration of rapamycin (mTOR inhibitor) in hyperlipidemic rat heart significantly attenuated the observed cardioprotection of IPC in insulin and pioglitazone perfusion. This finding is supported by other laboratories which also states that insulin exert cardioprotection through mTOR pathway.[28] In previous studies, pioglitazone is reported to produce cardioprotection by the activation of PI3K/Akt pathway.[6] In our study, administration of wortmannin (a specific inhibitor of PI3K) significantly attenuated the observed cardioprotection in insulin- and pioglitazone-perfused hyperlipidemic rat heart which strengthens the earlier finding. Moreover, administration of wortmannin and rapamycin in a combination followed by perfusion of insulin and pioglitazone completely blocked the observed cardioprotection in insulin- and pioglitazone-perfused hyperlipidemic rat heart. These results indicate that pioglitazone produces cardioprotection by the activation of both PI3K and mTOR pathways.
The mechanism of IPC-mediated signaling depends on both stimuli, i.e., PI3K and mTOR.[29] Both PI3K and mTOR have also been implicated as an upstream of GSK 3μ,[29] thereby its activation results in the inhibition of GSK 3μ. We have earlier reported that failure of IPC-mediated cardioprotection in hyperlipidemic rat heart is due to impaired signaling pathway, upstream of GSK 3μ.[13,24]
The results show that the attenuated cardioprotective effect of IPC in hyperlipidemic rat heart is may be due to the impaired signaling of PI3K and mTOR. Also, attenuation of the cardioprotective effect by combined administration of wortmannin and rapamycin was greater than that observed when these drugs were used alone. This clearly suggests that pioglitazone in the presence of insulin produces cardioprotection through PI3K/Akt and mTOR at a same time during HL.
Conclusion
Perfusion of pioglitazone restored the IPC-induced cardioprotection by the activation of both pathways, i.e. PI3K/Akt and mTOR in hyperlipidemic rat heart, which was completely attenuated in the presence of rapamycin and wortmannin.
Financial Support and Sponsorship
Institutional funding.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss GB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Am Heart Assoc. 1995;92:3172–7. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Tomai F, Crea F, Chiariello L, Gioffrè PA. Ischemic preconditioning in humans: Models, mediators, and clinical relevance. Circulation. 1999;100:559–63. doi: 10.1161/01.cir.100.5.559. [DOI] [PubMed] [Google Scholar]
- 4.Gagandeep K, Ramnik S. Possible mechanisms involved in attenuated cardioprotection in high fat diet induced hyperlipidemic rat hearts. J Pharm Res. 2011;4:1108. [Google Scholar]
- 5.Rohatgi N, Aly H, Marshall CA, McDonald WG, Kletzien RF, Colca JR, et al. Novel insulin sensitizer modulates nutrient sensing pathways and maintains ß-cell phenotype in human islets. PLoS One. 2013;8:e62012. doi: 10.1371/journal.pone.0062012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda S, Kobayashi H, Iwasa M, Kawamura I, Sumi S, Narentuoya B, et al. Antidiabetic drug pioglitazone protects the heart via activation of PPAR-gamma receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1558–65. doi: 10.1152/ajpheart.00712.2008. [DOI] [PubMed] [Google Scholar]
- 7.Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: A role for the prosurvival kinases PI3K and P42/44MAPK. J Cardiovasc Pharmacol. 2005;46:817–22. doi: 10.1097/01.fjc.0000188365.07635.57. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114(Pt 16):2903–10. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 9.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–70. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 10.Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2006;12(3 Pt 1):679–89. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Sobkiw CL, Hirshman MF, Logsdon MN, Li TQ, Goodyear LJ, et al. Loss of class IA PI3K signaling in muscle leads to impaired muscle growth, insulin response, and hyperlipidemia. Cell Metab. 2006;3:355–66. doi: 10.1016/j.cmet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 13.Yadav HN, Singh M, Sharma PL. Involvement of GSK-3ß in attenuation of the cardioprotective effect of ischemic preconditioning in diabetic rat heart. Mol Cell Biochem. 2010;343:75–81. doi: 10.1007/s11010-010-0500-z. [DOI] [PubMed] [Google Scholar]
- 14.Lorkowska B, Bartus M, Franczyk M, Kostogrys RB, Jawein J, Pisulewski PM, et al. Hypercholesterolemia does not alter endothelial function in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2006:1019–26. doi: 10.1124/jpet.105.098798. [DOI] [PubMed] [Google Scholar]
- 15.Das DK, Maulik N, Sato M, Ray PS. Reactive oxygen species function as second messenger during ischemic preconditioning of heart. Mol Cell Biochem. 1999;196:59–67. [PubMed] [Google Scholar]
- 16.Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff-still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113–26. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, et al. Early phase acute myocardial infarct size quantification: Validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 18.Chopra K, Singh M, Kaul N, Andrabi KI, Ganguly NK. Decrease of myocardial infarct size with desferrioxamine: Possible role of oxygen free radicals in its ameliorative effect. Mol Cell Biochem. 1992;113:71–6. doi: 10.1007/BF00230887. [DOI] [PubMed] [Google Scholar]
- 19.Parikh V, Singh M. Resident cardiac mast cells and the cardioprotective effect of ischemic preconditioning in isolated rat heart. J Cardiovasc Pharmacol. 1997;30:149–56. doi: 10.1097/00005344-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Parikh V, Singh M. Possible role of adrenergic component and cardiac mast cell degranulation in preconditioning-induced cardioprotection. Pharmacol Res. 1999;40:129–37. doi: 10.1006/phrs.1999.0501. [DOI] [PubMed] [Google Scholar]
- 21.Ferdinandy P. Myocardial ischaemia/reperfusion injury and preconditioning: Effects of hypercholesterolaemia/hyperlipidaemia. Br J Pharmacol. 2003;138:283–5. doi: 10.1038/sj.bjp.0705097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, et al. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation. 2005;112:2149–56. doi: 10.1161/CIRCULATIONAHA.105.566190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giricz Z, Lalu MM, Csonka C, Bencsik P, Schulz R, Ferdinandy P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: Role of matrix metalloproteinase-2 inhibition. J Pharmacol Exp Ther. 2006;316:154–61. doi: 10.1124/jpet.105.091140. [DOI] [PubMed] [Google Scholar]
- 24.Yadav HN, Singh M, Sharma PL. Modulation of the cardioprotective effect of ischemic preconditioning in hyperlipidaemic rat heart. Eur J Pharmacol. 2010;643:78–83. doi: 10.1016/j.ejphar.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Fuglesteg B, Tiron C, Jonassen AK, Mjos OD, Ytrehus K. Insulin and ischemic preconditioning induce cardioprotective GSK 3β blockade via STAT3 signaling in the isolated rat heart. Acta Physiol. 2006;187:659. [Google Scholar]
- 26.Fullmer TM, Pei S, Zhu Y, Sloan C, Manzanares R, Henrie B, et al. Insulin suppresses ischemic preconditioning-mediated cardioprotection through Akt-dependent mechanisms. J Mol Cell Cardiol. 2013;64:20–9. doi: 10.1016/j.yjmcc.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki H, Ogawa K, Shimizu M, Mori C, Takatsuka H, Okazaki F, et al. The insulin sensitizer pioglitazone improves the deterioration of ischemic preconditioning in type 2 diabetes mellitus rats. Int Heart J. 2007;48:623–35. doi: 10.1536/ihj.48.623. [DOI] [PubMed] [Google Scholar]
- 28.Fuglesteg BN, Tiron C, Jonassen AK, Mjøs OD, Ytrehus K. Pretreatment with insulin before ischaemia reduces infarct size in Langendorff-perfused rat hearts. Acta Physiol (Oxf) 2009;195:273–82. doi: 10.1111/j.1748-1716.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 29.Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, et al. GSK-3ß at the crossroads in the signalling of heart preconditioning: Implication of mTOR and Wnt pathways. Cardiovasc Res. 2011;90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]


