Abstract
Fixed drug eruption (FDE) is a common type of drug eruption seen in skin clinics. It is characterized by solitary or multiple, round to oval erythematous patches with dusky red centers, some of which may progress to bulla formation. Bullous FDE may be caused by a number of drugs. We hereby describe a case of azithromycin-induced bullous FDE; to the best of our knowledge, this is the first such case being reported.
KEY WORDS: Azithromycin, bullous, fixed drug eruption
Introduction
fixed drug eruption (FDE) characteristically recurs in the same site or sites each time the drug is administered; however, the number of involved sites may increase with each exposure. Acute lesions usually develop within 30 min to 8 h as sharply marginated, round to oval erythematous, and edematous plaques, which become dusky violaceous or brown and sometimes vesicular or bullous after re-administration of incriminating drug. Various morphological types of FDEs include morbilliform, scarlatiniform, erythema multiforme-like, Stevens–Johnson syndrome-like, eczematous, urticaria, nodular, vesicular, bullous (may become toxic epidermal necrolysis-like in severe cases), nonpigmenting, and diffuse hypomelanosis.[1,2] Diagnosis can be confirmed by provocation test (patch and/or oral). A hitherto unreported bullous FDE to azithromycin is being reported here.
Case Report
A 35-year-old male presented to clinic with a bullous lesion over left ankle and erosions over genitalia. He had been prescribed azithromycin for Grade II acne vulgaris (tablet azithromycin 500 mg for three consecutive days a week for 4 weeks). The patient had taken a single tablet of azithromycin, the day before presenting to clinic. He developed burning sensation and itching in genitalia at midnight and multiple nonbullous lesions on lower leg and a single bulla over left ankle next morning. Patient was not receiving any concomitant drug. Patient had a history of similar lesions over the genitalia and left ankle in May 2014 following use of azithromycin for throat infection. This time, he developed additional lesions over the lower legs.
Cutaneous examination revealed multiple 2–4 mm erythematous patches over the legs [Figure 1], erosive lesions over genitalia [Figure 2], and a bullous lesion over left ankle [Figure 3]. Patient was afebrile. Systemic examination did not reveal any abnormality. Patient did not give consent for skin biopsy. Routine blood parameters were found to be normal.
Figure 1.
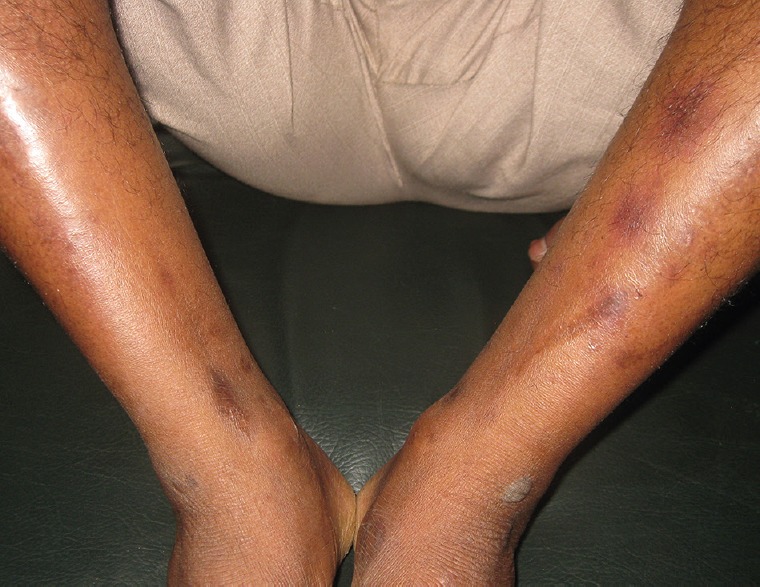
Multiple hyperpigmented patches over extensor aspect of lower limbs
Figure 2.
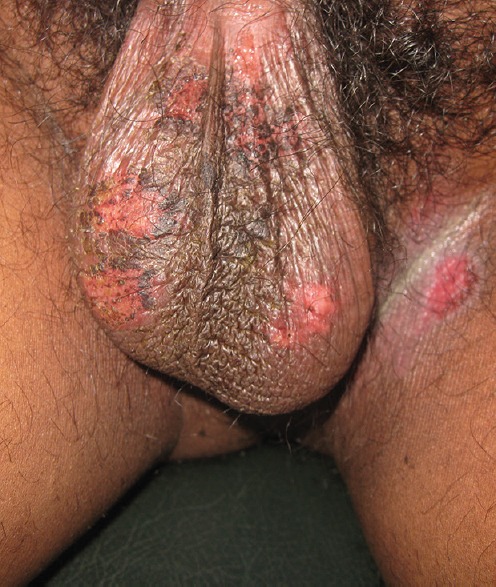
Multiple erosions in genitalia suggestive of preexisting bulla
Figure 3.
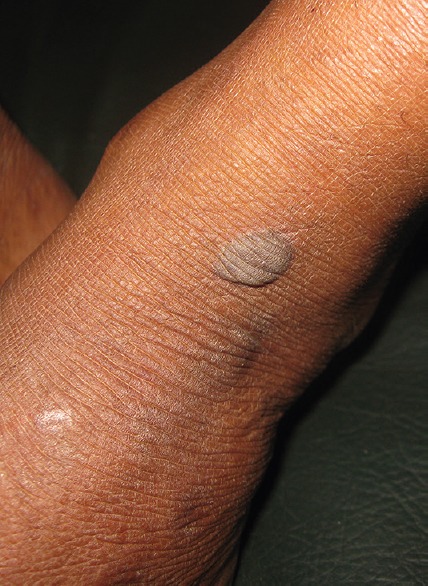
Bullous lesion over left ankle
A diagnosis of bullous FDE was made on the basis of clinical evidence. Oral provocation test and patch test with azithromycin were not performed. Azithromycin was stopped, and the patient was treated for FDE with oral prednisolone (30 mg/day for 5 days) and antihistaminics (levocetirizine 5 mg once daily for 7 days). On the first follow-up visit (after 7 days), the bulla was found to have ruptured leaving a pigmentation mark; the irritation over the lesions had decreased, and all the drugs were stopped. He was under periodic follow-up for treatment of the postinflammatory hyperpigmentation, but it was refractory to treatment. He has been counseled regarding the condition.
Causality assessment by Naranjo score and WHO-Uppsala Monitoring Centre (UMC) scale categorized the reaction as “probable” (Naranjo's score = 6) adverse effect induced by azithromycin. Severity assessment using Modified Hartwig and Siegel ADR Severity Assessment Scale labeled the reaction as “moderate” (Level 3).
Discussion
FDE is a specific cutaneous adverse reaction induced by an ever-expanding list of drugs. Bullous FDE has been reported with several drugs including phenylbutazone, acetaminophen, fluconazole, nimesulide, ciprofloxacin, and metronidazole.[3,4,5]
Brocq first introduced the term FDE in 1894. Pathogenesis of FDE is complex. Interleukin (IL)-mediated survival of memory T cells is considered the most probable mechanism for development of FDE. CD8+ cells, liberating interferon-gamma, are found in large numbers in FDE lesions and IL-20 is thought to be responsible for site-specificity of lesions. Generally, lesions of FDE heal with residual pigmentation, thus being of cosmetic concern to patient. Treatment of FDE is symptomatic with systemic antihistamines and topical corticosteroids. Time interval between ingestion of offending drug and appearance of symptoms varies from 30 min to 8 h with a mean duration of about 2 h. In the present case, lesions developed 3 h following ingestion of suspect drug. Severe cases of generalized bullous FDE (GBFDE) warrant the attention and aggressive management of SJS or TEN.[2] Furthermore, patients need to be counseled to avoid common offending drugs for FDE in future.
Some workers suggest use of oral provocation tests and patch tests to confirm the diagnosis of FDE. However, evidence of efficacy of these tests is still lacking.[6] Tests were not performed in the present case due to severity of the episode. Temporal association with azithromycin and previous history of a milder reaction with same drug at same sites, with residual hyperpigmentation, suggested the diagnosis of bullous FDE due to azithromycin. Azithromycin was labeled as “probable” cause of bullous FDE on the basis of Naranjo scale and WHO-UMC scale.[7,8] Besides, severity was graded as “moderate” based on the Modified Hartwig and Siegel ADR Severity Assessment Scale.[9]
Azithromycin is widely used and is generally considered a safe medicine. Acne vulgaris, an extremely common dermatosis, responds well to pulse azithromycin therapy, with an added advantage of better patient compliance.[10] Gastrointestinal symptoms and reversible hearing loss are common. Potentially serious ADRs include angioedema and cholestatic jaundice in >1% of patients. Azithromycin can cause severe skin reaction-fever, sore throat, swelling in face or tongue, burning in eyes, skin pain, followed by a red or purple skin rash that spreads, especially in the face or upper body and causing blistering and peeling.
An extensive literature search on PubMed using the keywords “azithromycin” and “bullous fixed drug rash” did not reveal any results as on November 02, 2015. Inducing awareness among physicians about this hitherto unreported side effect of azithromycin is important since more such cases may be reported with extensive usage of the drug in future.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Sehgal VN, Srivastava G. Fixed drug eruption (FDE): Changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897–908. doi: 10.1111/j.1365-4632.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- 2.Lipowicz S, Sekula P, Ingen-Housz-Oro S, Liss Y, Sassolas B, Dunant A, et al. Prognosis of generalized bullous fixed drug eruption: Comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726–32. doi: 10.1111/bjd.12133. [DOI] [PubMed] [Google Scholar]
- 3.Nath AK, Adityan B, Thappa DM. Multifocal bullous fixed drug eruption due to fluconazole. Indian J Dermatol. 2008;53:156–7. doi: 10.4103/0019-5154.43212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumaran S, Sandhu K, Saikia UN, Handa S. Nimesulide induced bullous fixed drug eruption of the labial mucosa. Indian J Dermatol Venereol Leprol. 2004;70:44–5. [PubMed] [Google Scholar]
- 5.Ada S, Yilmaz S. Ciprofloxacin-induced generalized bullous fixed drug eruption. Indian J Dermatol Venereol Leprol. 2008;74:511–2. doi: 10.4103/0378-6323.44324. [DOI] [PubMed] [Google Scholar]
- 6.Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: General considerations. Allergy. 2003;58:854–63. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 8.The Use of the WHO-UMC System for Standardized Case Causality Assessment. World Health Organization (WHO) – Uppsala Monitoring Centre. [Last accessed on 2015 Sep 12]. Available from: http://www.who-umc.org/Graphics/24734.pdf .
- 9.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 10.Singhi MK, Ghiya BC, Dhabhai RK. Comparison of oral azithromycin pulse with daily doxycycline in the treatment of acne vulgaris. Indian J Dermatol Venereol Leprol. 2003;69:274–6. [PubMed] [Google Scholar]


