Abstract
Anthraquinones are natural phenolic compounds, which are reported to act as anti-aging, anti-inflammatory, antioxidant, anti-cancer, laxative and antitumor agents. They are abudant in plants like candle bush, aloes, cascara bark and rhubarb. The present work was to observe the effect of different forced degradation conditions by high-performance thin layer chromatography on potential markers i.e. aloe emodin and emodin. Both aloe emodin and emodin were subjected to various forced degradation studies such as oxidation, acid and alkaline hydrolysis, photolysis, hydrolytic and thermal degradation. Aloe emodin, was more susceptible to acid hydrolysis and degradation was found to a lesser extent under thermal degradation whereas significant degradation was observed under acid hydrolysis, lesser extent was observed under alkali hydrolysis for emodin. Forced degradation studies on aloe emodin and emodin gives information about its storage and intrinsic stability conditions considering the advanced pharmaceutical aspects of formulation.
Keywords: Anthraquinones, aloe emodin, emodin, forced degradation, high-performance thin layer chromatography
Aloe emodin 1,8-Dihydroxy-3-(hydroxymethyl)-9,10-anthracenedione] and emodin [6-methyl-1,3,8-trihydroxyanthraquinone] are anthraquinones naturally present in rhubarb (Rheum emodi), candle bush (Cassia alata), aloes (Aloe barbadensis dried leaf juice) and cascara (Cascara sagrada)[1]. They act as antiinflammatory[2], laxative[3], antiaging, anticancer[4], antioxidant[5] and antitumor agents. Aloe emodin and emodin show cell cycle arrest, cytotoxic to cells and leads to apoptosis in human tongue and breast cancer cells thus acting as antiproliferative agents. There are many herbal marketed formulations, which have aloe emodin and emodin as major constituents such as Amlycure D.S. capsules, N-Zime syrup, Divya Udarkalp churna, and LIV-SR syrup. Considerable interest on herbal drugs has been aroused because of their beneficial effects on humans. The aim of the present work was to carry out forced degradation studies and to provide the information on stability inherent conditions of the potential markers i.e. aloe emodin and emodin and to ascertain the degradation pathways. The force degradation studies of drug substances should be carried out in various conditions such as effect of light, humidity, elevated temperature, oxidizing agents, acid and base degradation (across a range of pH values) according to stability guidelines by ICH Q1AR (International Conference on Harmonisation). The effect of various forced degradation conditions was observed using high-performance thin layer chromatography (HPTLC) analysis[6]. There is no method reported for analysis of forced degradation studies of aloe emodin and emodin.
Analytical grade reagents: ethyl acetate, formic acid, toulene and methanol were obtained from S.D. Fine-Chem Limited, Mumbai, India. Aloe emodin and emodin standards were procured from Total Herb Solutions, Mumbai. Aloe emodin and emodin stock solutions (1000 µg/ml) were prepared by dissolving accurately weighed 10 mg of each standard in 10 ml methanol. TLC plates pre-coated with silica gel 60 F254 was used as stationary phase. Standard and sample solutions were applied as a band to the 10×10 cm plate (6.0 mm wide) by using Camag Linomat V sample applicator (100 µl Hamilton syringe). Development of plate was done ascending to a distance of 80 mm with mobile phase at room temperature (24±2°) in Camag glass twin-trough chamber. The chamber was saturated for 30 min previously and the plates were scanned at 263 nm after development with Camag TLC Scanner- 3 using the deuterium lamp.
In situ HPTLC spectral overlaying of aloe emodin and emodin was taken and isoabsorptive point i.e 263 nm was selected as scanning wavelength. Accurate, precise, robust HPTLC method was developed using toluene: ethyl acetate: Formic acid, 10:2:1 (v/v/v) as mobile phase. Good resolution for aloe emodin and emodin was observed with Rf values at 0.37±0.03 and 0.55±0.03, respectively fig. 1. Linear relationship was found to be in the concentration range of 300-800 ng/spot (y=9.4274x+906.14 with r2=0.9993) for aloe emodin and 150-400 ng/spot (y=13.031x-115.96 with r2=0.9996) for emodin.
Fig. 1.
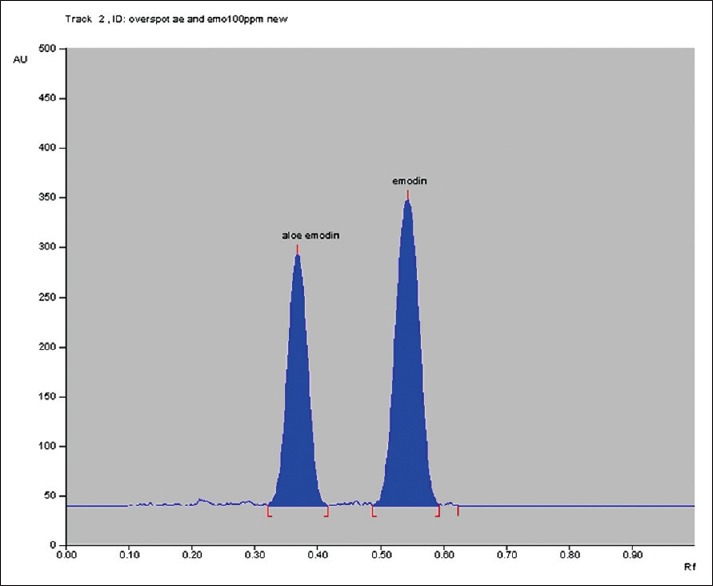
HPTLC chromatogram of standard aloe emodin and emodin.
Chromatogram of aloe emodin and emodin at Rf 0.37 and 0.55, respectively by TLC.
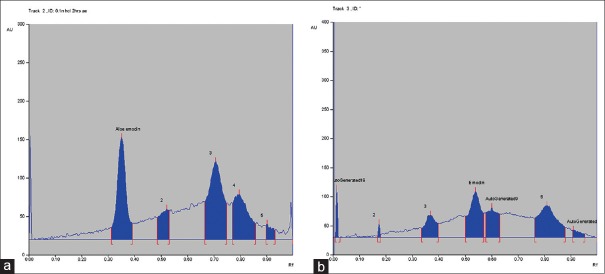
On treating the solutions with 0.1 N HCl for 2 h, the peak areas corresponding to aloe emodin and emodin decreased and additional peaks was observed at Rf 0.52, 0.71 for aloe emodin and 0.01, 0.36, 0.58 for emodin. This shows that aloe emodin and emodin undergoes degradation under acidic conditions (fig. 2a and b). On treating the solutions with 0.1 N NaOH for 2 h, aloe emodin and emodin was found less susceptible to base degradation. Additional peaks were observed at Rf0.01, 0.64 for aloe emodin and 0.01, 0.39, 0.69 for emodin. Oxidative degradation was observed in presence of 6% v/v of hydrogen peroxide when exposed for 3 h and the HPTLC chromatogram shows additional peaks at Rf0.56, 0.68 for aloe emodin and 0.63 for emodin. Aloe emodin and emodin shows moderate degradation when exposed to dry heat at 105° for 8 h. Additional peaks were observed at Rf0.02, 0.65 for aloe emodin and 0.01, 0.08, 0.65 for emodin. Aloe emodin undergoes hydrolytic degradation to a large extent at 80° for 8 h and shows additional peak at 0.62 Rf whereas emodin shows moderate degradation and additional peak was observed at 0.31. Aloe emodin and emodin shows less degradation when exposed to sunlight or UV-254 nm for 8 h. Degradant peaks were observed at Rf 0.45, 0.65 for aloe emodin and 0.70 for emodin. The concentration of unaffected aloe emodin and emodin was calculated under several forced degradation conditions based on the area under curve (Table 1).
Fig. 2.
(a) HPTLC chromatogram of standard aloe emodin subjected to acid degradation. Subjected to acid degradation and Rf of degradants=0.52, 0.71. (b) HPTLC chromatogram of standard emodin subjected to acid degradation. Subjected to acid degradation and Rf of degradants=0.01 0.36 0.58.
TABLE 1.
FORCED DEGRADATION DATA OF ALOE EMODIN AND EMODIN
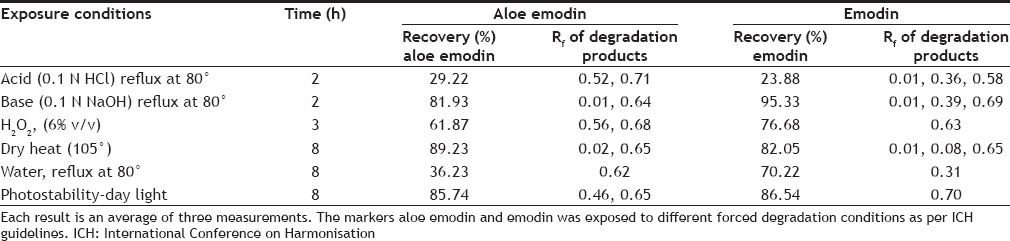
Forced degradation studies on aloe emodin and emodin were carried out and it was found that aloe emodin was more susceptible to acid (29.22%) and water degradation (36.23%), moderate to oxidation induced degradation (61.87%) and to lesser extent to day light (85.74%) and dry heat (89.23%) induced degradation. Emodin was found to be more susceptible to acid (23.88%) degradation. Moderate degradation was observed in water (70.22%), oxidation (76.68%) and dry heat (82.05%) induced degradation and to lesser extent to day light (86.54%) and base (95.332%) induced degradation. The HPTLC technique developed for both drugs estimation resolves the degradation products under all forced degradation conditions thus providing information on intrinsic stability of aloe emodin and emodin. Care should be taken when stored under acidic conditions as both drugs undergo rapid degradation. These studies and observations regarding stability of aloe emodin and emodin may help during storage and in its modern and traditional formulation aspects in pharmacy. The forced degradation studies on aloe emodin and emodin was performed and it can be concluded that these findings provide an insight and information about the storage and intrinsic stability conditions of aloe emodin and emodin with respect to the advanced formulation aspects.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgements:
Authors are thankful to the Anchrom Laboratory, Mumbai for giving training on HPTLC instrument.
Footnotes
Narayanan, et al.: Degradation Studies of Aloe Emodin and Emodin
REFERENCES
- 1.Evans WC. 15th ed. London (UK): Elsevier Publishers; 2004. Trease and Evans Pharmacognosy. [Google Scholar]
- 2.Park MY, Kwon HJ, Sung MK. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci Biotechnol Biochem. 2009;73:828–32. doi: 10.1271/bbb.80714. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HQ, Zhou CH, Wu YQ. Effect of emodin on small intestinal peristalsis of mice and relevant mechanism. World J Gastroenterol. 2005;11:3147–50. doi: 10.3748/wjg.v11.i20.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60:2800–4. [PubMed] [Google Scholar]
- 5.Vargas F, Díaz Y, Carbonel K. Antioxidant and scavenging activity of emodin, aloe-emodin, and rhein on free-radical and reactive oxygen species. Pharm Biol. 2004;42:342–8. [Google Scholar]
- 6.Reich E, Schibli A. 1st ed. Switzerland: Thieme Medical Publishers; 2006. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants. [Google Scholar]