Abstract
Introduction
Biomarkers are urgently needed for the critical yet understudied preclinical stage of Alzheimer's disease (AD).
Methods
Cerebrospinal fluid (CSF) collection, [C-11]Pittsburgh compound B (PiB) amyloid imaging, and magnetic resonance imaging were acquired in 104 cognitively healthy adults enriched with risk for sporadic AD. Image-derived cerebral β-amyloid (Aβ) burden, measured concurrently and longitudinally, was regressed on CSF measures of Aβ, neural injury, and inflammation, as well as ratios with Aβ42. Linear mixed-effects regression was used to model the effect of the CSF measures that predicted longitudinal brain amyloid accumulation on longitudinal cognitive decline, measured by memory test scores.
Results
At baseline, Aβ42/Aβ40 and all CSF ratios to Aβ42 were associated with PiB binding in AD-vulnerable regions. Longitudinally, Aβ42/Aβ40 and ratios of total tau (t-tau), phosphorylated-tau (p-tau), neurofilament light protein, and monocyte chemoattractant protein-1 to Aβ42 were associated with increased Aβ deposition over 2 years, predominantly in lateral parietal and temporal cortex. However, these CSF ratios were not significantly associated with cognitive decline, and the effect seems to be largely driven by Aβ42 in the denominator.
Discussion
These results corroborate previous findings that t-tau/Aβ42 and p-tau/Aβ42 are the strongest candidate biomarkers during the preclinical time frame. They support a framework in which neural injury and amyloid deposition are likely occurring simultaneously. It may be that neurodegenerative processes influence progressive amyloid accumulation, even in the preclinical time frame. CSF biomarkers for nonspecific axonal injury and inflammation may provide more information at more advanced stages of the preclinical time course.
Keywords: Amyloid, PiB, PET, Cerebrospinal fluid, Biomarkers, Preclinical AD, Alzheimer's disease, Biological parametric mapping, Aβ42, Tau, NFL, MCP-1, YKL-40, Linear mixed-effects
1. Introduction
Although it is widely accepted that Alzheimer's disease (AD) pathology, including β-amyloid (Aβ) deposition in plaques and microvessels and tau pathology in the form of neurofibrillary tangle formation, begins decades before symptom onset [1], [2], [3], [4], there is a paucity of longitudinal research in the late mid-life time frame [1] necessary to establish this effect empirically. Greater understanding of biomarkers associated with these hallmark features of AD, including tau and Aβ proteins in cerebrospinal fluid (CSF) and in-vivo neuroimaging measures of Aβ burden, during preclinical stages is important for early detection and future treatment and prevention efforts [1], [2], [5].
The most widely accepted model of AD etiology proposes that amyloid deposition in the brain is an early and critical step in driving the pathophysiological processes of AD that in turn initiate neurodegeneration, in the form of synaptic failure and neuronal death, and eventual symptom manifestation [1], [6], [7]. Increasing evidence suggests that amyloid may be necessary—although even this is contested by some [8]—but not sufficient for developing AD [6], [8], [9], [10], [11]. Aβ may be neither the primary nor the only neurotoxin that causes AD; but it is likely the key initiator of many complex, often tau-dependent, pathologic changes in the brain culminating in neurodegeneration years later [12]. In accordance with this theory, recent in vitro and in vivo work demonstrates a dynamic positive feed-forward mechanism whereby Aβ drives the disease pathway through tau, and tau further increases Aβ levels [13]. Aβ imaging by itself is not sufficient for a clinical prognosis in the preclinical time frame, and it may be that the most promising AD biomarkers will encompass multiple aspects of the disease, including amyloid and tau-mediated neural injury.
Because of the molecular exchange of metabolites between the brain and CSF, CSF analytes reflect biochemical and pathologic processes in the brain [5], [14], [15], thereby providing means for examining multiple indicators of disease processes occurring in the central nervous system. CSF Aβ and tau have high diagnostic accuracy for AD [16], but their reliability for the detection of possible preclinical AD is still unclear.
To examine features associated with spatial amyloid binding and longitudinal accumulation in the preclinical time frame, we conducted a multimodal study with Pittsburgh compound B (PiB) positron emission tomography (PET) imaging and CSF biomarkers in a late middle-aged, cognitively healthy sample enriched with the risk factors of apolipoprotein E ε4 (APOE ε4) genotype and parental history of sporadic AD from the Wisconsin Registry for Alzheimer's Prevention (WRAP; [17]). Our study had three aims as follows: (1) investigate relationships between amyloid pathology in the brain and concurrent CSF biomarkers associated with amyloid deposition, neuronal injury, and inflammation; (2) determine whether CSF biomarker levels at baseline lumbar puncture (LP) predict changes in PiB amyloid deposition over a subsequent 2-year period; and (3) investigate relationships between CSF predictors of brain Aβ and longitudinal cognitive decline.
We hypothesized that CSF biomarkers of AD pathology including amyloid burden (lower Aβ42), tangle pathology (elevated phosphorylated-tau [p-tau]), axonal injury (elevated total-tau [t-tau] and neurofilament light protein [NFL]), and microglial activation/inflammation (elevated monocyte chemoattractant protein 1 [MCP-1] and chitinase-3–like protein [YKL-40]) would be associated with greater amyloid deposition at baseline and greater longitudinal amyloid accumulation over 2 years. We further hypothesized that Aβ42 ratios (e.g., t-tau/Aβ42) would most likely be associated with cognitive decline, as ratios reflect multiple AD-related pathologies simultaneously, suggesting greater risk for imminent disease.
2. Material and methods
Extended materials and methods are included as Supplementary Material.
2.1. Participants
Participants in this study were recruited from WRAP [17] if they had participated in biomarkers substudies. For this analysis, subjects were included if they had one or more amyloid and magnetic resonance imaging (MRI) scans, and CSF collected at the time of the first amyloid scan, resulting in a sample of 104. The University of Wisconsin Institutional Review Board approved all study procedures, and each subject provided signed informed consent before participation.
2.2. Biomarker collection
All participants underwent baseline MRI, [C-11]PiB PET, and LP as described previously [18], [19]. A total of 78 additionally underwent a second PiB scan approximately 2 years later. The first visit at which amyloid scans, MRI scans, and CSF were obtained is referred to as baseline or visit 1; the second imaging visit is referred to as follow-up or visit 2. PiB distribution volume ratio (DVR) maps were used as the amyloid variable for all voxel-wise analyses. A composite measurement of global amyloid derived from eight bilateral regions of interest (ROIs) was calculated for visit 1 and visit 2 as described previously [20] and is henceforth referred to as PiB burden.
CSF measures were selected based on their ability to detect known pathology in AD including amyloid deposition (Aβ42), neurofibrillary tangles (p-tau), neuronal damage (t-tau and NFL), and inflammation (MCP-1 and YKL-40). We also examined the most commonly described CSF ratios of p-tau/Aβ42, t-tau/Aβ42, and Aβ42/Aβ40 as well as more novel ratios of NFL, MCP-1, and YKL-40 to Aβ42. To ensure that these ratios truly reflect cumulative pathology, we additionally examined the inverse of Aβ42 (1/Aβ42) to mimic the effect of CSF Aβ42 in the denominator without a marker of pathology in the numerator.
2.3. Cognitive data measures and collection
At each WRAP visit, participants completed a comprehensive neuropsychological battery. To investigate the relationship between CSF biomarkers and episodic memory performance, we selected the delayed memory scores from the Rey auditory verbal learning test (RAVLT) and the Wechsler memory scale-revised (WMS-R) [21], [22], [23], [24]. For RAVLT, which was initiated at the first wave of WRAP neuropsychology data collection, one participant had two time points, 25 had three time points, 52 had four time points, and 25 had five time points. For WMS-R, which was initiated at the second wave, 25 participants had two time points, 52 had three time points, and 25 had four time points.
2.4. Statistical analyses
Separate models were run for each CSF measure unless otherwise stated. Covariates always include age at LP, sex, parental family history (FH), and APOE ε4.
For models run in Statistical Package for the Social Sciences (SPSS) 22, significance is inferred at P < .05, adjusted for multiple comparisons with false discovery rate (FDR) correction [25]. Regressions were hierarchical such that the model's first step includes only covariates and the second step adds the individual CSF measure. R2 change is calculated as the difference between the R2 of the first and second steps of the model. Effect sizes were calculated using Cohen's f2 for hierarchical multiple regression; effect sizes of 0.02, 0.15, and 0.35 are interpreted as small, medium, and large, respectively. Results are visualized using partial regression plots, which are scatter plots of residuals from regressing PiB burden and the CSF variable on all other predictors.
2.4.1. Concurrent CSF measures and amyloid
2.4.1.1. PiB burden
PiB burden at the initial scan was entered as the dependent variable in a multiple regression model in SPSS 22, and individual CSF measures were entered as the independent variable of interest, along with covariates.
To minimize effects of multicollinearity among CSF measures, a separate model was run for each CSF analyte or ratio, adjusting for covariates. However, AD is a multifaceted disease and ratios are only able to capture two pathologic features. Therefore, we also examined a comprehensive regression model with all CSF biomarkers with the exception of p-tau because it was highly correlated with t-tau (Spearman's ρ = .881). Using baseline PiB burden as the outcome, we performed hierarchical regression with z-scores of CSF t-tau, MCP-1, YKL-40, NFL, and Aβ42 in addition to the standard covariates to determine the additional predictive power of each biomarker to the overall model. For each of these CSF measures, a model would include all covariates and four of the five CSF variables as the first step and then the fifth CSF measure would be added as the second step. Standardized rather than unstandardized β-coefficients are reported for easier comparison of contributions of each variable to the model.
2.4.1.2. Regional Aβ
A multiple regression framework in statistical parametric mapping (SPM) 12 (http://www.fil.ion.ucl.ac.uk/spm/) was used to assess relationships between CSF markers associated with AD pathology and amyloid in the brain. Baseline PiB DVR images were entered as the dependent variable, and individual CSF measures were input as the independent variable of interest in addition to the standard covariates. Analyses were restricted to the cerebral gray matter using a template-based mask. Significance was inferred at the voxel peak-level when α <0.05 with multiple comparisons by family-wise error (FWE) correction and a cluster extent >100 voxels.
2.4.2. Baseline CSF measures and longitudinal amyloid
Although there are many ways to approach longitudinal data analysis with two time points, we chose a standard approach of regressing a follow-up variable on the baseline variable and covariates [26], [27]. This method statistically controls for variance in the baseline state, enabling the interpretation of variables affecting follow-up state independent of baseline.
2.4.2.1. PiB burden
PiB burden at visit 2 was entered as the dependent variable in a multiple regression model in SPSS 22. In addition to the standard set of covariates, we also controlled for PiB burden at visit 1 and then looked at the effect of the CSF analyte. By controlling for amyloid load at visit 1 and looking at amyloid load at visit 2 as the outcome, the coefficient on an individual CSF measure is interpreted as the effect of that CSF measure on longitudinal amyloid load, controlling for baseline amyloid load. Amyloid scans occurred about 2 years apart (mean = 25.47 months; standard deviation = 2.37 months; range = 21–33 months) because the study was designed to keep this interval uniform, and variation was relatively symmetrical, it was not included as a covariate in the regression models.
We repeated the hierarchical regression model, which included the five CSF measures (t-tau, MCP-1, YKL-40, NFL, and Aβ42) simultaneously, for longitudinal PiB burden. Each model included the standard covariates as well as baseline PiB burden, and four of the five CSF variables as the first step; then the fifth CSF measure was added as the second step. Standardized rather than unstandardized β-coefficients are reported for easier comparison of contributions of each variable to the model.
2.4.2.2. Regional Aβ
To gain spatial resolution on findings from the mentioned analyses, regressions using biological parametric mapping, an SPM5 toolbox for multimodal image analysis based on a voxel-wise use of the general linear model [28], were performed on CSF measures that were associated with longitudinal PiB burden. The PiB DVR scan from the second visit was the dependent variable and the PiB DVR scan from the first visit was used as an imaging covariate in addition to five nonimaging covariates: age, sex, APOE ε4, FH, and CSF biomarker level. Because visit 1 amyloid burden was such a strong predictor of visit 2 amyloid burden, we chose a more moderate threshold for the resultant longitudinal statistical maps of α <0.001 (uncorrected) together with a cluster extent >250 voxels; however, our primary inference was still at the peak voxel where significance was again evaluated at α(FWE) <0.05.
2.4.3. CSF measures and cognitive decline
Linear mixed-effects regression was used to model the effect of the CSF biomarkers that predicted longitudinal Aβ accumulation on longitudinal cognitive decline measured by tests of delayed recall. First, unconditional means models adjusting for random effects were examined using unstructured covariance structure. Next, conditional models were run which included significant random effects plus fixed effects of sex, APOE ε4, FH, interval between first cognitive evaluation and LP (months), literacy (time 1 Wide Range Achievement Test III reading scores), CSF biomarker level, time (age at each visit), and the interaction of time × CSF measure (slope).
3. Results
3.1. Sample characteristics
Sample characteristics are summarized in Table 1.
Table 1.
Sample characteristics
| Demographics | Range | Mean (SD) or % |
|---|---|---|
| Age at LP (years) | 49.04–70.96 | 61.15 (5.58) |
| Sex (% female) | 66% | |
| APOE ε4∗ (% positive) | 38% | |
| FH (% positive) | 77% | |
| Education (years) | 12–21 | 16.63 (2.47) |
| PiB burden | Range | n | Mean (SD) |
|---|---|---|---|
| PiB visit 1 | 0.995–1.68 | 104 | 1.17 (0.15) |
| PiB visit 2 | 0.991–1.83 | 78 | 1.17 (0.17) |
| PiB burden change from visit 1 to visit 2† | −0.12 to 0.20 | 78 | 0.0047 (0.059) |
| CSF measures (ng/L) | n PiB visit 1, n PiB visit 2 | Mean (SD)‡ |
|---|---|---|
| Amyloid | ||
| Aβ42 | 103, 78 | 735.91 (205.06) |
| Aβ42/Aβ40 | 104, 78 | 0.096 (0.018) |
| 1/Aβ42 | 101, 76 | 0.0014 (0.0004) |
| Neural injury | ||
| T-tau | 103, 77 | 317.50 (112.53) |
| P-tau | 102, 76 | 43.09 (13.77) |
| NFL | 102, 77 | 597.21 (185.06) |
| T-tau/Aβ42 | 100, 75 | 0.440 (0.191) |
| P-tau/Aβ42 | 99, 75 | 0.060 (0.024) |
| NFL/Aβ42 | 99, 76 | 0.844 (0.317) |
| Inflammation | ||
| MCP-1 | 104, 78 | 558.27 (131.56) |
| YKL-40 | 102, 76 | 139,470.69 (41,248.72) |
| MCP-1/Aβ42 | 101, 76 | 0.810 (0.317) |
| YKL-40/Aβ42 |
99, 74 |
194.11 (60.91) |
| Intervals |
Range |
Mean (SD) |
| PiB visit 1 to visit 2 (months) | 21–33 | 25.47 (2.37) |
| PiB visit 1 to LP (days) | −57 to 68 | −1.35 (18.32) |
| PIB visit 1 to NP testing wave 1 (months) | 0–107 | 70.24 (19.88) |
| First RAVLT testing (wave 1) to LP (months) | 36–109 | 70.29 (19.88) |
| First WMS-R testing (wave 2) to LP (months) | −11 to 58 | 20.67 (17.04) |
Abbreviations: SD, standard deviation; LP, lumbar puncture; APOE ε4, possession of apolipoprotein E ε4 allele; FH, parental family history; PiB, Pittsburgh compound B amyloid imaging; CSF, cerebrospinal fluid; Aβ, beta-amyloid; t-tau, total tau; p-tau, phosphorylated-tau; NFL, neurofilament light protein; MCP-1, monocyte chemoattractant protein 1; YKL-40, chitinase-3–like protein; NP, neuropsychology; RAVLT, Rey auditory verbal learning test; WMS-R, Wechsler memory scale-revised.
APOE ε4 allele breakdown: 38% APOE ε4; n = 13 ε2/ε3, n = 52 ε3/ε3, n = 3 ε2/ε4, n = 35 ε3/ε4, and n = 1 ε4/ε4.
PiB burden change is displayed for descriptive purposes only, it was not used in any statistical analyses.
CSF was only collected once, mean and SD values are for all subjects at PiB visit 1.
3.2. Concurrent CSF measures and amyloid
3.2.1. PiB burden
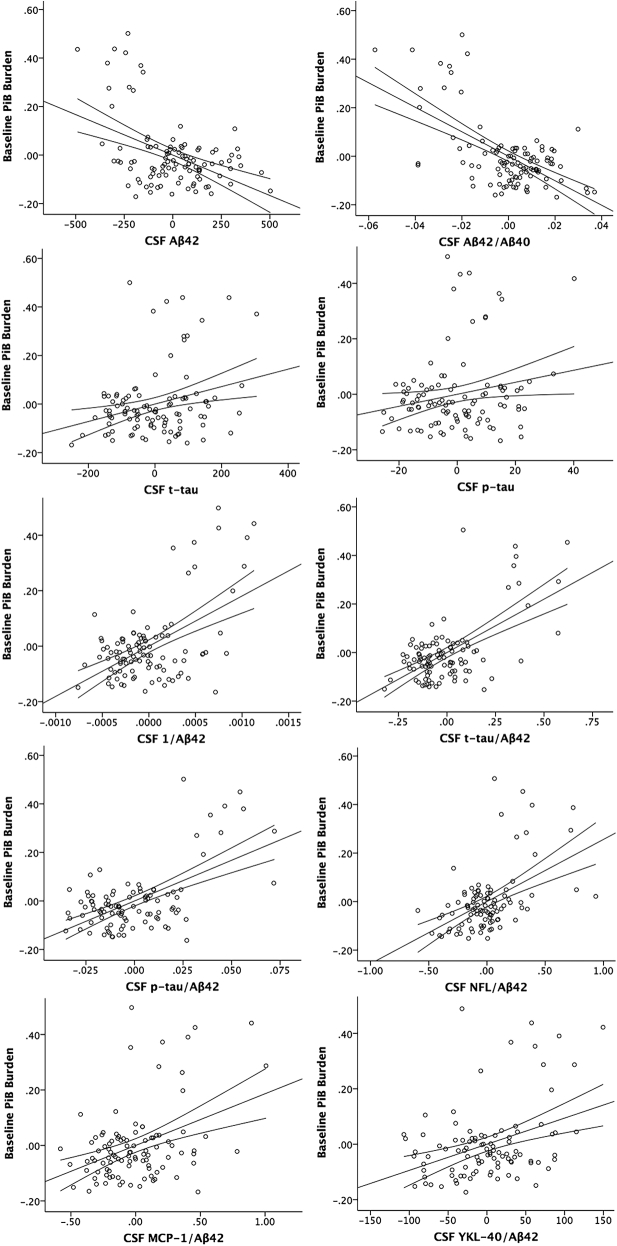
CSF t-tau, Aβ42, Aβ42/Aβ40, 1/Aβ42, t-tau/Aβ42, p-tau/Aβ42, NFL/Aβ42, MCP-1/Aβ42, and YKL-40/Aβ42 all significantly predicted baseline PiB burden at P(FDR) <.05 (Supplementary Table 2, Fig. 1). Additionally, p-tau (P = .04) was significant at an uncorrected threshold of P < .05. Aβ42/Aβ40, 1/Aβ42, t-tau/Aβ42, and p-tau/Aβ42 had large effect sizes; CSF Aβ42, NFL/Aβ42, MCP-1/Aβ42, and YKL-40/Aβ42 had medium effect sizes; and t-tau and p-tau had small effect sizes.
Fig. 1.
Partial regression plots of CSF measures and baseline PiB burden. Y-axis: DVR of baseline PiB burden adjusted for all other predictors in the model (age, sex, APOE ε4, and FH). X-axis: CSF measure adjusted for all other predictors in the model. 95% confidence intervals for the regression line are displayed. Abbreviations: PiB, Pittsburgh compound B; CSF, cerebrospinal fluid; DVR, distribution volume ratio; Aβ, beta-amyloid; NFL, neurofilament light protein; t-tau, total tau; p-tau, phosphorylated-tau; MCP-1, monocyte chemoattractant protein 1; YKL-40, chitinase-3–like protein; FH, parental family history.
When CSF measures of amyloid, neural injury, and inflammation were simultaneously included as predictors in the model, only t-tau and Aβ42 significantly accounted for variance in baseline PiB burden. Inclusion of CSF Aβ42 (R2 change = 0.229; f2 = 0.495) and t-tau (R2 change = 0.104; f2 = 0.225) significantly improved the model. Aβ42 (standard β = −0.582, P < .001) and t-tau (standard β = 0.385, P < .001) were the strongest predictors of baseline brain amyloid burden.
3.2.2. Regional Aβ
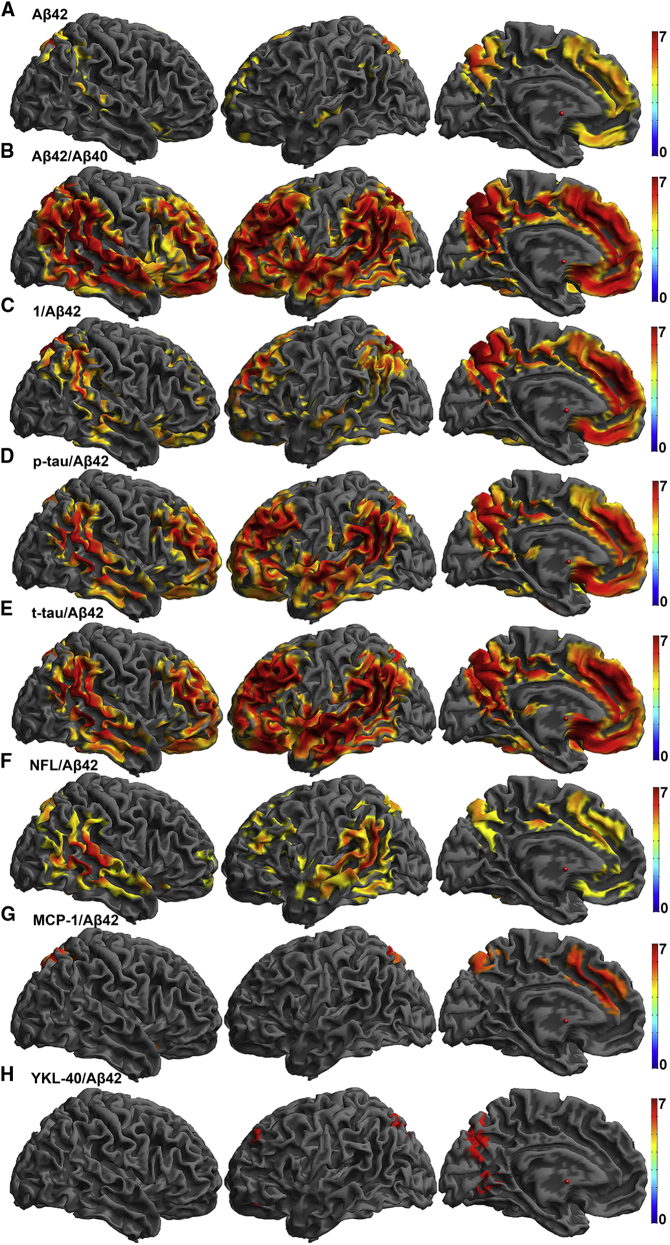
CSF Aβ42, Aβ42/Aβ40, and all ratios of individual CSF markers to CSF Aβ42 were consistently related to a spatial pattern of amyloid in the brain in regions commonly affected in AD including posterior cingulate, lateral parietal cortex, precuneus, and medial prefrontal cortex (Fig. 2). Supplementary Table 3 provides statistical details and anatomic locations for the peaks that survived P(FWE) <.05. Although tau alone did not survive FWE at the voxel-level, tau/Aβ42 showed a significant positive relationship with amyloid burden. These data support a model where amyloid burden is more severe in the presence of neurofibrillary pathology. The statistical map of the inverse of Aβ42 (1/Aβ42) shows a significant relationship that is consistent but not identical with the overall pattern of other CSF measures.
Fig. 2.
Heat maps of T-statistics for voxel-wise regressions of baseline CSF measures on baseline PiB images rendered onto a template brain for (A) Aβ42, (B) Aβ42/Aβ40, (C) 1/Aβ42, (D) p-tau/Aβ42, (E) t-tau/Aβ42, (F) NFL/Aβ42, (G) MCP-1/Aβ42, and (H) YKL-40/Aβ42. The negative contrast is displayed for Aβ42 and Aβ42/Aβ40, and the positive contrast is displayed for all other ratios. Color bars representing t-statistics from 0 to 7 are on the right. All voxels are significant at P(FWE) <.05. Abbreviations: CSF, cerebrospinal fluid; Aβ, beta-amyloid; PiB, Pittsburgh compound B; p-tau, phosphorylated-tau; t-tau, total tau; NFL, neurofilament light protein; MCP-1, monocyte chemoattractant protein 1; FWE, family-wise error; YKL-40, chitinase-3–like protein.
3.3. Baseline CSF measures and longitudinal amyloid
3.3.1. PiB burden
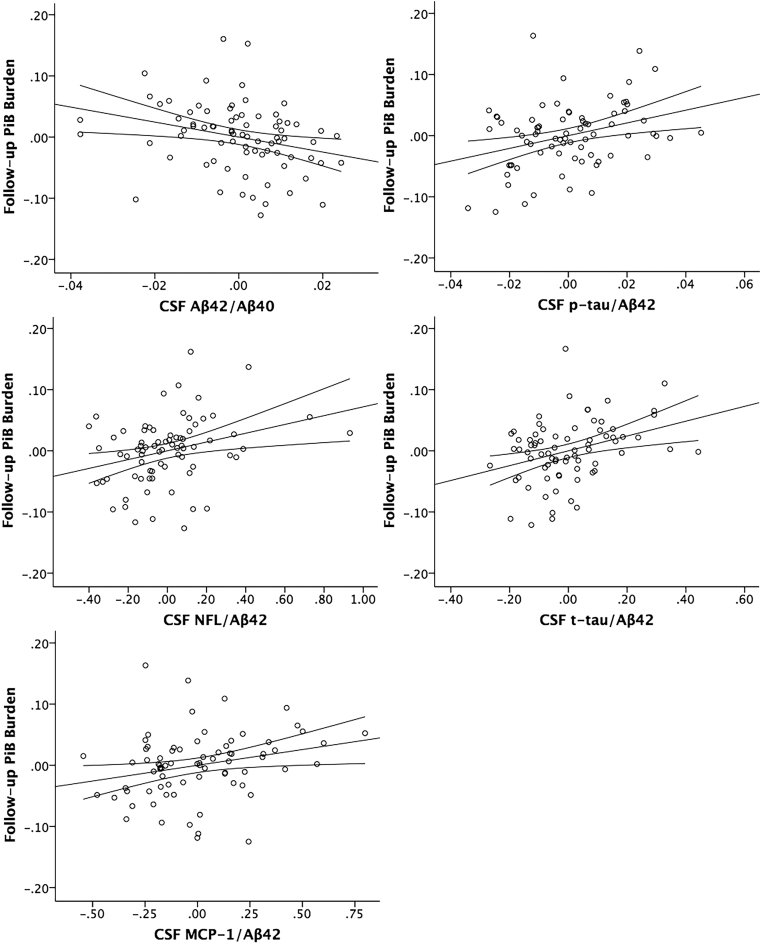
T-tau/Aβ42, p-tau/Aβ42, and NFL/Aβ42 were significantly associated with PiB burden at visit 2 at P(FDR) <.05 (Supplementary Table 4, Fig. 3). Aβ42/Aβ40 (P = .017) and MCP-1/Aβ42 (P = .03) were additionally associated with PiB burden at visit 2 at P(uncorrected) <.05. All results were in the expected direction (CSF/Aβ42 were positive, whereas Aβ42 and Aβ42/Aβ40 were negative). These results suggest that a composite CSF measurement, here in the form of a ratio, which is sensitive to both amyloid (Aβ42) and neural injury (tau, p-tau, or NFL) or inflammation (MCP-1), predicts amyloid deposition over time. All f2 effect sizes for CSF markers were small because visit 1 PiB burden is a very strong predictor of visit 2 PiB burden. T-tau/Aβ42 and p-tau/Aβ42 had the largest effect sizes (f2 >0.12) of the CSF biomarkers.
Fig. 3.
Partial regression plots of CSF measures and follow-up PiB burden. Y-axis: DVR of follow-up PiB burden adjusted for all other predictors in the model (age, sex, APOE ε4, FH, and baseline PiB burden). X-axis: CSF measure adjusted for all other predictors in the model. 95% confidence intervals for the regression line are displayed. Abbreviations: CSF, cerebrospinal fluid; PiB, Pittsburgh compound B; DVR, distribution volume ratio; Aβ, beta-amyloid; p-tau, phosphorylated-tau; t-tau, total tau; NFL, neurofilament light protein; MCP-1, monocyte chemoattractant protein 1; FH, parental family history.
When CSF measures of amyloid, neural injury, and inflammation were simultaneously included as predictors in the longitudinal model, only t-tau significantly accounted for variance in amyloid accumulation (R2 change = 0.007; f2 = 0.08). Besides amyloid load at visit 1 (standard β = 0.794, P < .001), Aβ42 (standard β = −0.107, P = .053) and t-tau (standard β = 0.113, P = .034) were the strongest predictors of longitudinal amyloid accumulation.
3.3.2. Regional Aβ
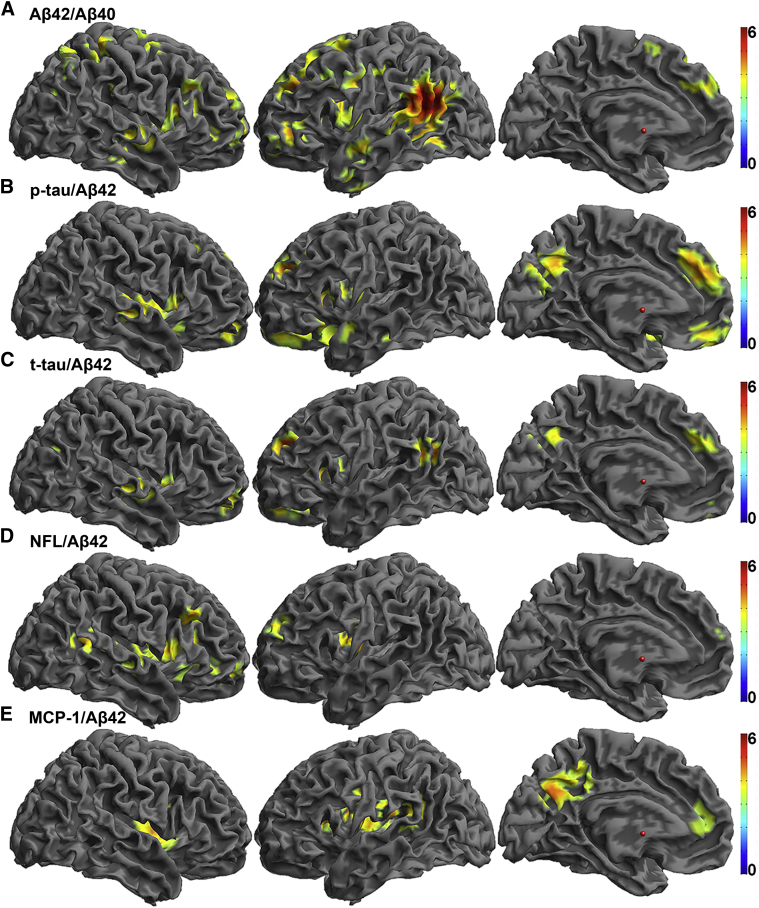
T-tau/Aβ42, p-tau/Aβ42, NFL/Aβ42, and MCP-1/Aβ42 were positively associated and Aβ42/Aβ40 was negatively associated with longitudinal amyloid deposition in the eight ROIs (Fig. 4). Supplementary Table 5 provides statistical details and anatomic locations for the peaks that survived P(FWE) <.05. Ratios were primarily associated with amyloid increases in the frontal and lateral parietal and temporal lobes, and p-tau/Aβ42 and MCP-1/Aβ42 were additionally associated with amyloid increases in the precuneus.
Fig. 4.
Heat maps of T-statistics for voxel-wise BPM regressions of CSF measures on longitudinal PiB rendered onto a template brain for (A) Aβ42/Aβ40, (B) p-tau/Aβ42, (C) t-tau/Aβ42, (D) NFL/Aβ42, and (E) MCP-1/Aβ42. The negative contrast is displayed for Aβ42 and Aβ42/Aβ40, and the positive contrasts are displayed for all other ratios. BPM statistical maps are thresholded at P < .001 with cluster extent >250 voxels. All clusters are significant at P(FWE) <.05. Color bars representing t-values from 0 to 6 are on the right. Abbreviations: BPM, biological parametric mapping; CSF, cerebrospinal fluid; PiB, Pittsburgh compound B; Aβ, beta-amyloid; FWE, family-wise error; p-tau, phosphorylated-tau; t-tau, total tau; NFL, neurofilament light protein; MCP-1, monocyte chemoattractant protein 1.
3.4. CSF measures and cognitive decline
A significant relationship between the CSF ratios which predicted longitudinal Aβ accumulation (CSF Aβ42/Aβ40, t-tau/Aβ42, p-tau/Aβ42, NFL/Aβ42, and MCP-1/Aβ42) and slope of cognitive decline was not detected.
4. Discussion
The etiology of AD involves multiple pathologic processes occurring decades before disease onset, with the two primary forms of pathology consisting of amyloid plaques and neurofibrillary tangles. AD is also associated with inflammation [29] and changes in white matter structural integrity [30]. The aim of the present study was to better characterize early coincident preclinical changes by examining the relationship between CSF biomarkers covering different aspects of AD neuropathology and PET-PiB amyloid deposition in a relatively younger cognitively healthy adult sample. Our overarching hypothesis, that CSF markers of neural injury would predict both baseline and longitudinal Aβ burden, was supported. CSF ratios to Aβ42 were spatially related to amyloid deposition in regions commonly affected in AD at baseline, and CSF ratios of tau to Aβ42 were related to an increase in global Aβ load and to a spatial pattern of Aβ in the brain in AD-sensitive ROIs longitudinally assessed over a 2-year time span. Although an array of CSF biomarkers have been shown to be related to amyloid longitudinally in a similar cohort [31], this is the first known study to provide spatial specificity with voxel-wise analyses, and to investigate novel ratios reflecting simultaneous gliosis and Aβ deposition.
Voxel-wise analyses showed CSF markers had a highly conserved relationship to amyloid within regions commonly labeled as the default mode network (DMN) including lateral parietal cortex, precuneus, and medial prefrontal cortex. It has been suggested that susceptibility to amyloid deposition in these regions may be due to high activity or metabolism [32], [33], [34] and relatively greater extent of neuroplasticity over the life span [35], [36]. Buckner et al. [37] (2005) found convergence of five different in vivo imaging methods on the DMN in young adults and older adults with AD, postulating that lifetime cerebral metabolism in the DMN predisposes these cortical regions to AD-related changes including amyloid deposition, metabolic disruption, and atrophy.
CSF ratios with Aβ42 were consistently significantly associated with PiB amyloid burden in the brain at baseline and 2-year follow-up. Correspondingly, previous literature has demonstrated that Aβ42/Aβ40 is decreased in AD [5], [38] and improves accuracy of distinguishing prodromal AD and dementia compared with Aβ42 alone [39]. The ratio has been suggested to normalize the Aβ42 concentration to a measure of overall amyloidogenic processing by amyloid precursor protein, making it possible to detect low Aβ42 in high Aβ producers and vice versa [40]. Similarly, studies have shown that combinations of tau and Aβ42 improve sensitivity and specificity [5], [41], [42], predict subjective cognitive decline [43], and predict conversion from a clinical dementia rating of 0 (cognitively normal) to 1 (mild dementia; [44]) and from mild cognitive impairment to AD [45]. This is likely because they encompass not only strong amyloid effects but also more subtle disease-relevant tau changes, with simultaneous pathology more indicative of disease. This is particularly interesting in light of recent and compelling evidence showing that tau increases Aβ production [13].
The NFL/Aβ42 findings further support a theory of coincident axonal degeneration and amyloid pathology. Interestingly, the ratio of the inflammatory marker of MCP-1 to Aβ42 was also related to both baseline and longitudinal amyloid, but YKL-40/Aβ42 only predicted baseline amyloid. MCP-1 has been found near amyloid plaques [46], is upregulated in CSF of AD patients [47], and predicts future conversion to AD and the rate of cognitive decline [48]. Although these findings and ours suggest a role for coincident Aβ and microglial/inflammatory processes in AD pathogenesis, other studies have shown mixed results for the diagnostic value of these CSF markers [49]. More studies are needed to validate these CSF analytes, and their ratios to Aβ42, as AD biomarkers.
Our results indicated that 1/Aβ42 was a strong predictor of baseline Aβ in a consistent but not identical pattern to other CSF measures. This suggests not only that Aβ42 in the denominator is not solely driving results but also that 1/Aβ42 may even be more sensitive to amyloid in the brain than Aβ42 alone, possibly because the inverse transformation corrects for positive skewness. However, 1/Aβ42 did not survive significance thresholds in the longitudinal frameworks, suggesting that ratios with markers of neural injury truly reflect cumulative pathology rather than a mathematical phenomenon. Effect sizes further support the theory that ratios provide more information than 1/Aβ42 alone. For baseline analyses, t-tau/Aβ42 and p-tau/Aβ42 had larger effect sizes than 1/Aβ42 (Supplementary Table 2). Coincident markers seem to be even more relevant for predicting longitudinal amyloid accumulation, which showed larger effect sizes for all ratios with Aβ42 except YKL-40/Aβ42 compared with 1/Aβ42, which was not significant (Supplementary Table 4). Therefore, at a single time point, CSF Aβ42 (or 1/Aβ42) may be sufficient to detect brain Aβ, but CSF ratios indicative of simultaneous pathology predict progressive accumulation over time, which is a stronger indicator of disease progression than stable brain amyloid levels. However, a supplemental analysis suggests that although t-tau/Aβ42 and p-tau/Aβ42 account for unique variance above and beyond 1/Aβ42, the story is less clear for MCP-1/Aβ42 and NFL/Aβ42 (Supplementary Table 6). These remain important biomarkers to investigate, and may prove to be better predictors of other AD-relevant outcomes, or more informative at more advanced ages and stages of the preclinical AD time course. This will be ascertained with a future study.
The majority of our baseline and longitudinal analyses indicate that CSF measures of amyloid alone but not tau alone corresponded with amyloid load in the brain, consistent with previous findings [50]. This is likely because our sample is relatively young and, thus, may show considerable amyloid pathology while only beginning to show changes in tau. Consistent with this theory, Buchhave et al. [51] (2009) found that among AD patients, Aβ42 levels did not change over time but tau increased 16% over 2 years, with higher levels associated with faster conversion. This suggests that Aβ42 levels increase earlier in the disease stage and then plateau, whereas tau biomarkers may increase gradually closer to symptom onset [45]. Similarly, findings from Kanai et al. [52] suggest that although CSF Aβ42 and Aβ42/Aβ40 may make the most significant changes at early AD stages, CSF tau likely increases with clinical progression of dementia. The data here suggest that at the earliest stages of increasing tau pathology—likely at an intermediary stage of amyloid accumulation—tau may interact to accelerate brain amyloid accumulation. The comprehensive model with all CSF biomarkers highlights the important contribution of tau to preclinical amyloid accumulation. This finding that tau significantly contributes to a model when Aβ42 is also included in the model in addition to findings that tau/Aβ42 but not tau alone was significant in voxel-wise models further corroborates our theory that tau's impact on AD pathogenesis is dependent on the presence of amyloid.
Although there are likely subtle cognitive changes occurring during the preclinical time period [1], [2], [53], we did not find a significant association between CSF biomarkers and slopes of episodic memory. However, because of the relatively young age and cognitive health of our sample, associations between CSF biomarkers and cognitive decline are likely to be subtle. These findings do not preclude the likely possibility that CSF ratios are related to preclinical cognitive decline. Furthermore, others have found that change in CSF levels may be more closely related to cognition [31], [54]. Longitudinal CSF collection and ongoing neuropsychological testing is underway in this cohort and will be critical to understanding the relationship of CSF changes over time to disease progression, cognitive decline, and an interacting cascade between tau and amyloid.
There are limitations to our study that deserve mentioning. First, CSF from LP is only an indirect measure of pathology; however, it is a common clinical and research technique that is currently much less expensive and more widely available than PET imaging. Second, although this cohort is enriched for risk factors for AD, our sample's relatively younger age and lack of significant clinical symptoms restrict our clinical interpretation of the results. Third, the correlational design of this study prevents us from describing causal mechanisms between CSF analytes and AD pathology or symptoms. Fourth, serial CSF was not available, further preventing this study from ascertaining whether amyloid and tau are interacting in a reciprocal positive feedback loop, as recent work suggests [13]. Fifth, CSF analytes and ratios were correlated, making interpretation of a model with multiple CSF analytes less interpretable, even though neural injury, inflammation, and amyloid likely interact to impact amyloid accumulation. Although they are interpreted with this caution, the findings from the cumulative model suggest that tau and Aβ42 are the strongest CSF predictors and statistically improve modeling of amyloid accumulation, and normal tolerance and variance inflation factors suggest that each variable was contributing unique variance.
This study adds important breadth to the investigation of biomarkers for AD by first, examining a spread of biomarkers spanning neural injury, inflammation, and gliosis in addition to the traditional measures of tau and Aβ42; second, recruiting participants who are enriched for AD risk factors but are still in late-middle age and are cognitively normal; and third, longitudinally assessing both brain amyloid and cognitive decline. Follow-up longitudinal studies investigating clinical outcomes are necessary to determine the diagnostic accuracy of these biomarkers.
Research in context.
-
1.
Systematic review: The authors reviewed and appropriately cited the literature using traditional online sources and data presented at relevant conferences. There is estimable analysis on Alzheimer's disease (AD) fluid and imaging biomarkers in older adults who are normal or cognitively impaired, but considerably less information is available regarding the important preclinical late-midlife time frame.
-
2.
Interpretation: These findings support a theory whereby presence of β-amyloid (Aβ) in addition to other pathologic features of AD including neural injury, neurofibrillary tangles, and gliosis predict amyloid accumulation in the preclinical time frame.
-
3.
Future directions: Longitudinal analysis of biomarkers is critical for understanding their utility, especially when collection and analysis begins in the preclinical time frame which by definition and design implies a lack of clinical outcomes initially. This work, therefore, sets the groundwork for baseline and earliest change, and future research will investigate clinical end points, longitudinally measured cerebrospinal fluid biomarkers, and longitudinal multimodal imaging.
Acknowledgments
This research was supported by NIH grants AG021155 (S.C.J.), AG000213 (S.A.), AG027161 (S.C.J.), AG037639 (B.B.B.), and P50 AG033514 (S.A.); by P30 HD003352, by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison; by NSF Award SES 0849122, and by the Swedish Research Council, the Swedish Brain Foundation and Torsten Söderberg's Foundation to the University of Gothenburg. Portions of this research were supported by the Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation, and from the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The authors gratefully acknowledge Sharon Lu, Nancy Davenport-Sis, Amy Hawley, Sandra Harding, Jennifer Bond, Chuck Illingworth, Erika Starks, and the support of researchers and staff at the Waisman Center, University of Wisconsin-Madison for their assistance in recruitment, data collection, and data analysis. Above all, the authors thank their dedicated volunteers for their participation in this research.
Footnotes
H.A.R. has a pending patent for an magnetic resonance imaging pulse sequence. K.B. reports personal fees associated with Advisory Boards from IBL International, Eli Lilly, and Roche Diagnostics. S.A. reports grants during the conduct of the study from NIH/NIA, which support the Alzheimer's Disease Research Center, and grants from Merck Pharmaceutical and ADCS/Toyoma Phramaceutical for activities outside the submitted work. All other authors have no conflicts of interest to disclose.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.11.006.
Supplementary data
References
- 1.Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K., Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2009;18:413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: An appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 7.Hardy J.A., Higgins G.A. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 8.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 9.Chetelat G. Alzheimer disease: Abeta-independent processes-rethinking preclinical AD. Nat Rev Neurol. 2013;9:123–124. doi: 10.1038/nrneurol.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis D.G., Schmitt F.A., Wekstein D.R., Markesbery W.R. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Nievas B.G., Stein T.D., Tai H.C., Dols-Icardo O., Scotton T.C., Barroeta-Espar I. Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology. Brain. 2013;136:2510–2526. doi: 10.1093/brain/awt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bright J., Hussain S., Dang V., Wright S., Cooper B., Byun T. Human secreted tau increases amyloid-beta production. Neurobiol Aging. 2015;36:693–709. doi: 10.1016/j.neurobiolaging.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Lista S., Garaci F.G., Ewers M., Teipel S., Zetterberg H., Blennow K. CSF Abeta1-42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer's disease. Alzheimers Dement. 2014;10:381–392. doi: 10.1016/j.jalz.2013.04.506. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 17.Sager M.A., Hermann B., La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 18.Johnson S.C., Christian B.T., Okonkwo O.C., Oh J.M., Harding S., Xu G. Amyloid burden and neural function in people at risk for Alzheimer's disease. Neurobiol Aging. 2014;35:576–584. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendlin B.B., Carlsson C.M., Johnson S.C., Zetterberg H., Blennow K., Willette A.A. CSF T-Tau/Abeta42 predicts white matter microstructure in healthy adults at risk for Alzheimer's disease. PLoS One. 2012;7:e37720. doi: 10.1371/journal.pone.0037720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprecher K.E., Bendlin B.B., Racine A.M., Okonkwo O.C., Christian B.T., Koscik R.L. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lezak M.D. 4th ed. Oxford University press; New York, New York: 2004. Neuropsychological assessment. [Google Scholar]
- 22.Spreen O., Strauss E. Oxford University Press; USA: 1998. A compendium of neuropsychological tests: Administration, norms, and commentary. [Google Scholar]
- 23.Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey auditory verbal learning test: A handbook. [Google Scholar]
- 24.Wechsler . The Psychological Corporation, Harcourt Brace Jovanovich, inc for Psychological Corp; New York: 1987. Wechsler memory scale-revised. [Google Scholar]
- 25.Curran-Everett D. Multiple comparisons: Philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 26.Locascio J.J., Atri A. An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Dis Extra. 2011;1:330–357. doi: 10.1159/000330228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senn S., Stevens L., Chaturvedi N. Repeated measures in clinical trials: Simple strategies for analysis using summary measures. Stat Med. 2000;19:861–877. doi: 10.1002/(sici)1097-0258(20000330)19:6<861::aid-sim407>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Casanova R., Srikanth R., Baer A., Laurienti P.J., Burdette J.H., Hayasaka S. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–143. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S., Pu F., Shi F., Xie S., Wang Y., Jiang T. Regional white matter decreases in Alzheimer's disease using optimized voxel-based morphometry. Acta Radiol. 2008;49:84–90. doi: 10.1080/02841850701627181. [DOI] [PubMed] [Google Scholar]
- 31.Sutphen C.L., Jasielec M.S., Shah A.R., Macy E.M., Xiong C., Vlassenko A.G. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72:1029–1042. doi: 10.1001/jamaneurol.2015.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simic G., Babic M., Borovecki F., Hof P.R. Early failure of the default-mode network and the pathogenesis of Alzheimer's disease. CNS Neurosci Ther. 2014;20:692–698. doi: 10.1111/cns.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirrito J.R., Yamada K.A., Finn M.B., Sloviter R.S., Bales K.R., May P.C. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Neill D. Should Alzheimer's disease be equated with human brain ageing? A maladaptive interaction between brain evolution and senescence. Ageing Res Rev. 2012;11:104–122. doi: 10.1016/j.arr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Fjell A.M., McEvoy L., Holland D., Dale A.M., Walhovd K.B. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner R.L., Snyder A.Z., Shannon B.J., LaRossa G., Sachs R., Fotenos A.F. Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skoog I., Davidsson P., Aevarsson O., Vanderstichele H., Vanmechelen E., Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- 39.Molinuevo J.L., Blennow K., Dubois B., Engelborghs S., Lewczuk P., Perret-Liaudet A. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: A consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Lewczuk P., Lelental N., Spitzer P., Maler J.M., Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer's disease: Validation of two novel assays. J Alzheimers Dis. 2015;43:183–191. doi: 10.3233/JAD-140771. [DOI] [PubMed] [Google Scholar]
- 41.De Meyer G., Shapiro F., Vanderstichele H., Vanmechelen E., Engelborghs S., De Deyn P.P. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: Easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Stomrud E., Hansson O., Blennow K., Minthon L., Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 44.Fagan A.M., Roe C.M., Xiong C., Mintun M.A., Morris J.C., Holtzman D.M. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 45.Buchhave P., Minthon L., Zetterberg H., Wallin A.K., Blennow K., Hansson O. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 47.Galimberti D., Schoonenboom N., Scarpini E., Scheltens P. Chemokines in serum and cerebrospinal fluid of Alzheimer's disease patients. Ann Neurol. 2003;53:547–548. doi: 10.1002/ana.10531. [DOI] [PubMed] [Google Scholar]
- 48.Westin K., Buchhave P., Nielsen H., Minthon L., Janciauskiene S., Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer's disease. PLoS One. 2012;7:e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lautner R., Mattsson N., Scholl M., Augutis K., Blennow K., Olsson B. Biomarkers for microglial activation in Alzheimer's disease. Int J Alzheimers Dis. 2011;2011:939426. doi: 10.4061/2011/939426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagan A.M., Mintun M.A., Mach R.H., Lee S.Y., Dence C.S., Shah A.R. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 51.Buchhave P., Blennow K., Zetterberg H., Stomrud E., Londos E., Andreasen N. Longitudinal study of CSF biomarkers in patients with Alzheimer's disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanai M., Matsubara E., Isoe K., Urakami K., Nakashima K., Arai H. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer's disease: A study in Japan. Ann Neurol. 1998;44:17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- 53.Okonkwo O.C., Oh J.M., Koscik R., Jonaitis E., Cleary C.A., Dowling N.M. Amyloid burden, neuronal function, and cognitive decline in middle-aged adults at risk for Alzheimer's disease. J Int Neuropsychol Soc. 2014;20:422–433. doi: 10.1017/S1355617714000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stomrud E., Hansson O., Zetterberg H., Blennow K., Minthon L., Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.