Abstract
We generated 18F-labeled antibody fragments for positron emission tomography (PET) imaging using a sortase-mediated reaction to install a trans-cyclooctene-functionalized short peptide onto proteins of interest, followed by reaction with a tetrazine-labeled-18F-2-deoxyfluoroglucose (FDG). The method is rapid, robust, and site-specific (radiochemical yields > 25%, not decay corrected). The availability of 18F-2-deoxyfluoroglucose avoids the need for more complicated chemistries used to generate carbon–fluorine bonds. We demonstrate the utility of the method by detecting heterotopic pancreatic tumors in mice by PET, using anti-Class II MHC single domain antibodies. We correlate macroscopic PET images with microscopic two-photon visualization of the tumor. Our approach provides easy access to 18F-labeled antibodies and their fragments at a level of molecular specificity that complements conventional 18F-FDG imaging.
Short abstract
18F-2-Deoxyfluoroglucose combined with sortase is used to site-specifically label antibody fragments. A new mouse Class II MHC specific 18F-labeled VHH not only decorated lymphoid organs but also detected a heterotopic pancreatic tumor (∼1.5 mm) due to the presence of immune cells. 1, 2, 3: lymph nodes, 4: thymus, 5: tumor.
Introduction
Imaging of medically relevant specimens by positron emission tomography (PET) using 18F-labeled biomolecules is increasingly important for both clinical diagnosis and in biomedical research.1−7 By exploiting differences in the rate of glucose uptake and its metabolism,8−11 2-deoxy-2-18F-fluoroglucose (18F-FDG)-PET imaging can distinguish many tumors with increased metabolic activity from surrounding normal tissue. 18F-Labeled ligands can also be used to track expression of the receptors to which they bind.2,12,13 While 18F-FDG is readily available in most radiopharmacies, the generation of other 18F-labeled bioactive molecules of interest can require elaborate synthetic strategies.12−14 A further challenge is the short half-life of 18F (t1/2 = 110 min), which requires use within hours of production. In terms of radiation exposure, the use of 18F-fluorine has advantages over longer-lived isotopes such as 89Zr (t1/2 3.27 days)15 and 124I (t1/2 4.18 days).16 Use of 18F-FDG is potentially more practical in a clinical setting than are methods using elemental 18F.17
Although antibodies are endowed with exquisite specificity and are of considerable therapeutic value, the use of 18F-labeled antibody fragments has yet to see widespread application for imaging purposes.18,19 Our approach enables the use of 18F-FDG to achieve efficient labeling of proteins and does so in a manner that is reproducible and site-specific, leaving intact the antibody fragment’s antigen binding site. The method also could be applicable to other suitably modified biologicals, such as cytokines and chemokines.20 The ability to determine the biodistribution of therapeutically useful antibodies or their fragments and a comparison of these measurements with clinical outcomes can thus expand the repertoire of diagnostic tools.
Results and Discussion
Our strategy relies on a two-step process for labeling proteins equipped with a sortase recognition motif.21,22 Sortases are bacterial transpeptidases that are finding increasing use as tools for protein engineering. Sortases stand out for their ease of production, high degree of specificity, fast and efficient conversion of the appropriately modified protein substrate, and ready access to a wide variety of nucleophiles in the transacylation reaction.23−25
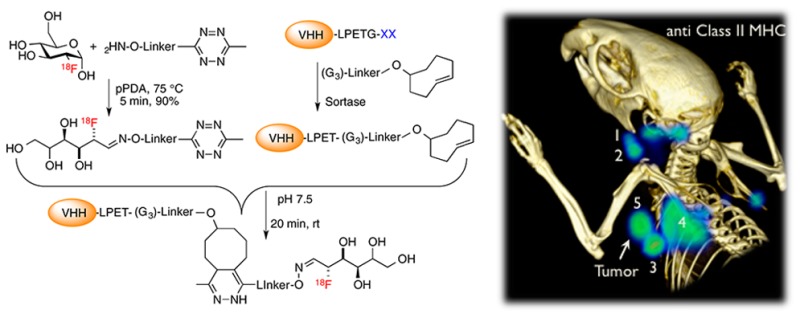
As a first step, we generated a short synthetic peptide, (Gly)3-R, where R contains a trans-cyclooctene (TCO) functionality that enables a TCO-tetrazine ligation reaction with a 18F-tetrazine. The TCO-tetrazine reaction is fast, with an estimated second order rate constant of 210–26000 M–1 s–1.26−28 We established a method for 18F labeling using commercially available 18F-FDG, the principal source of 18F in clinical use. The dynamic equilibrium between an aldohexose in its linear aldehyde form (the reactive molecular species) and its cyclical hemiacetal derivative permits the installation of 18F-FDG on an aminooxy-functionalized molecule.29
In view of the t1/2 of 18F ≈ 110 min, any synthetic process using 18F as a substrate and the necessary downstream purification steps must be rapid. Thus, we first optimized reaction conditions using nonradioactive FDG and characterized the reaction products by liquid chromatography–mass spectrometry (LC–MS) (Supporting Information). Several different catalysts have been reported for the oxime ligation reaction, of which the phenylenediamines are among the most efficient. While m-phenylenediamine is a more efficient catalyst than p-phenylenediamine (pPDA), its Schiff base is more stable and can block oxime formation if its concentration relative to the aminooxy or aldehyde is high.30,38 In our case, the concentration of aldehyde (18F-FDG) is extremely low (<nM). We therefore used pPDA as the catalyst at ∼0.4–0.6 M and tetrazine-aminooxy in the ∼0.2–0.3 M range. We incubated the aminooxy-tetrazine with fluorodeoxyglucose in the presence of the catalyst, pPDA, with constant agitation at 75 °C for ∼5–10 min. High-performance liquid chromatography (HPLC) of the reaction mixture showed (near)-complete consumption of FDG (Supporting Information). To produce the radioactive aminooxy-tetrazine derivative, we performed the incubation with 18F-FDG in the presence of the catalyst, pPDA, with constant agitation at 75 °C for ∼5–10 min. Radio-HPLC showed that the coupling reaction with 18F-FDG proceeded rapidly, yielding >90% oxime 18F-FDG-tetrazine in ∼5–10 min (Figure 1). We separated the 18F-oxime product by HPLC, followed by capture of the product via a Sep-pak C18 column. A solution containing the TCO-labeled protein of interest, prepared previously using sortase, was then added to the purified oxime 18F-FDG-tetrazine. The reaction was allowed to proceed for ∼15–20 min at 25 °C with constant agitation. The 18F-labeled protein was purified by size exclusion in phosphate buffer, providing the final 18F-labeled protein ready for injection.
Figure 1.
(A–C) Site-specific 18F-labeling of proteins using 18F-FDG and sortase. (A) A tetrazine-aminooxy and 18F-FDG were combined in the presence of p-phenylenediamine to produce 18F-tetrazine. Dynamic equilibrium between hemiacetal and linear forms of the aldohexose allows capture of the FDG into a tetrazine molecule via an oxime ligation; the 18F-tetrazine product is purified via HPLC. (B) A single domain antibody fragment (VHH) equipped at its C-terminus with the LPXTG sortase-recognition motif is site-specifically modified with a (Gly)3-trans-cyclooctene (TCO), as confirmed by LC-MS (Supporting Information). (C) 18F-Tetrazine was added to the TCO-modified VHH, and after ∼20 min the labeled VHH was retrieved by rapid size exclusion chromatography. (D–F) 18F-VHH7 (anti-mouse class II MHC) detects secondary lymphoid organs. (D) PET images of a representative C57BL/6 mouse 2 h postinjection of 18F-VHH7; numbers indicate (i) lymph nodes: 1, 2, 3, 4, 7, 8, 9; (ii) thymus: 5; (iii) spleen: 6. (E) PET-CT images of C57BL/6 mouse imaged with 18F-VHH7 from two different viewpoints (top and bottom panels); clearly lymph nodes and thymus are visible. See movie 01 in Supporting Information for a 3D visualization of lymphoid organs. (F) PET signals in vivo in different organs. Experiments are representative of three mice with similar results.
We previously used a 18F-TCO-tetrazine to label proteins with 18F to image lymphoid organs using an anti-Class II MHC single domain antibody, VHH7.31 We evaluated the present labeling method to confirm that the binding site of the nanobody remained intact. 18F-VHH7, produced as described above, detected secondary lymphoid organs exactly as reported31 (Figure 1 and movie 01 in the Supporting Information).
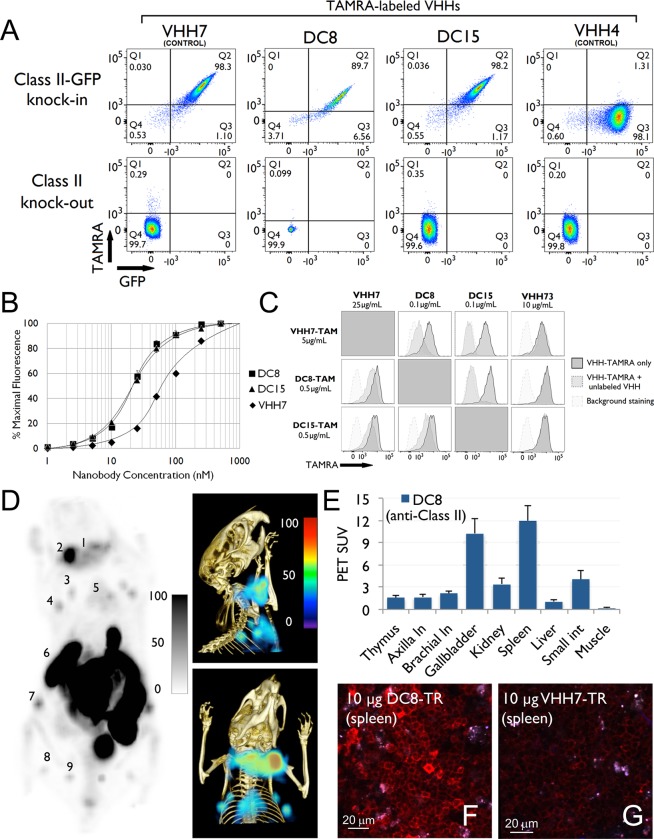
The half-maximal binding of VHH7 for Class II MHC+ cells on splenocytes is in the ∼55–60 nM range (Figure 2). Possible in vivo applications might benefit from single domain antibodies (VHHs) with improved affinities for their targets. To that end, we identified higher affinity anti-Class II VHHs in a phage display library generated from an alpaca immunized with murine splenocytes. Specificity of the anti Class II VHHs was ascertained by the absence of staining of splenocytes from class II MHC knockout mice, and perfect costaining with GFP-positive cells from class II MHC-GFP knock-in mice32 using fluorescently labeled VHH derivatives. The affinity of newly identified class II MHC-specific VHHs was compared to that of VHH7. VHHDC8 and VHHDC15 bind ∼3–4 fold better to Class II MHC molecules (Figure 2) than does VHH7. In competition experiments both VHHDC8 and VHHDC15 interfered with each other’s ability to bind spleen cells and inhibited binding of VHH7; similarly, an excess of VHH7 inhibited binding of VHHDC8 and VHHDC15 (Figure 2). These findings imply that these different Class II MHC-specific VHHs recognize a closely related epitope.
Figure 2.
(A) DC8 and DC15 specifically recognize the mouse Class II MHC complex: 106 splenocytes isolated from C57BL/6 Class II-GFP knock-in and Class II knockout mice were stained with labeled VHHs as indicated. Plots are gated on live, CD19+ cells. VHH7 has been previously demonstrated to recognize murine Class II MHC. DC8 and DC15 are novel VHHs isolated through staining of dendritic cells. VHH4 is specific for human Class II MHC and does not recognize the murine homologue. (B) DC8 and DC15 are able to stain murine B cells at concentrations too low for VHH7 staining: 106 splenocytes isolated from WT C57BL/6 mice were stained with the indicated concentrations of Alexa647-labeled VHHs. Populations were gated on live, CD19+ cells, and the mean Alexa647 fluorescence of each population is plotted. (C) DC8 and DC15 outcompete VHH7 for an overlapping epitope: 106 splenocytes isolated from WT C57/BL6 mice were costained with TAMRA-labeled VHH and a variable concentration of unlabeled VHH. The costained splenocytes (dark gray peak) were compared to splenocytes stained only with the TAMRA-labeled nanobody (light gray peak). VHH73 does not bind to class II MHC molecules and is used as a control. (D) 18F-DC8 (anti-mouse class II MHC), produced using 18F-FDG and sortagging, detects secondary lymphoid organs. PET (left) and PET-CT (right-top and bottom) images of a representative C57BL/6 mouse 2 h postinjection of 18F-DC8; clearly lymph nodes, spleen, and thymus are visible. Numbers indicate (i) lymph nodes: 1, 2, 3, 4, 7, 8, 9; (ii) thymus: 5; (iii) spleen: 6. See movie 02 in Supporting Information for a 3D visualization of lymphoid organs. (E) PET signals in vivo in all organs. (F, G) DC8 and VHH7 (both anti-mouse class II MHC) stain secondary lymphoid organs with different affinities. Images were acquired using two-photon microscopy. VHHs were site-specifically labeled with Texas Red via sortagging. F and G are images of spleen of C57BL/6 mice injected with 10 μg of DC8-Texas Red (F) or VHH7-Texas Red (G) 90 min prior to imaging. Clearly DC8-Texas Red stains Class II positive cells with higher affinities compared to VHH7. Experiments are representative of three mice with similar results.
For in vivo analysis, we prepared Texas Red-conjugated VHH7 for comparison with similarly labeled VHHDC8. We injected mice with 10 μg of the Texas Red-conjugated VHHs. Ninety minutes postinjection we excised spleen and lymph nodes for analysis by two-photon microscopy. The signal obtained from VHHDC8-stained lymphoid organs was substantially stronger than that seen for VHH7, indicating that higher affinity for the target improved image intensity (Figure 2). Having established the utility of the new anti-Class II MHC VHH for in vivo staining, we used it for PET imaging. 18F-VHHDC8 prepared as described above detected secondary lymphoid organs (Figure 2 and movie 02 in the Supporting Information) in a manner comparable to 18F-VHH7 (Figure 1). Compared to VHH7, we observed stronger binding of VHHDC8 to spleen relative to lymph nodes (compare SUVs in Figures 1 and 2). The higher affinity of VHHDC8 and its short circulatory half-life, typical of a VHH, might lead to its more efficient capture upon passage through the spleen, leaving comparatively less available for exit from the bloodstream and staining of lymph nodes.
Pancreatic tumors are often poorly infiltrated with immune cells and develop a dense stroma, implicated in the resistance to standard chemotherapy and immunomodulatory antitumor treatments.33 We used the pancreatic cancer cell line Panc02 as a model for pancreatic cancer and explored the possibility of imaging its presence by tracking the arrival of Class II MHC-positive cells (activated host macrophages, dendritic cells) using 18F-VHHDC8. Panc02 itself does not express Class II MHC products. Mice injected subcutaneously with 1 × 106 Panc02 cancer cells were imaged with 18F-VHHDC8 2 weeks after injection of the tumor. Although the tumors were not palpable at the time of imaging (tumor size estimated at ∼1.2 mm in diameter), PET images clearly showed their presence (Figure 3 and movie 03, Supporting Information). PET imaging using 18F-FDG failed to detect the tumor, likely due to its small size and/or low metabolic activity (Figure 3 and movie 04 in the Supporting Information). To correlate the results obtained by PET with microscopy, we injected tumor-bearing mice with 20 μg of Texas Red-VHHDC8. Two hours postinjection, the tumor was excised and imaged by two-photon microscopy. The tumor was infiltrated with or surrounded by Class II MHC+ cells, consistent with the PET imaging result (Figure 3; see image 01 in the Supporting Information for high resolution visualization).
Figure 3.
18F-DC8 (anti mouse Class II MHC) detects infiltration of Class II+ immune cells in/around a tumor. Tumor-associated class II MHC+ cells were visualized using 18F-VHHDC8. A C57BL/6 mouse was inoculated subcutaneously on the back of the left shoulder with 106 murine panc02 cancer cells and imaged 2 weeks post injection. (A–C) PET (A) and PET-CT (B, C) images. In A–C, different sets of lymph nodes (1, 2, 3, 4, 8, 9, 10 and their symmetrical counterparts), thymus (5), tumor (6), and spleen (7) are visible. In A–C, as pointed by the arrow, tumor-associated Class II MHC positive cells are visible, attributable to influx of host-derived Class II MHC positive cells. See movie 03 in Supporting Information for a 3D visualization of lymph nodes and tumor-associated Class II MHC positive cells. (D–F) 18F-FDG fails to detect the tumor. A C57BL/6 mouse was inoculated subcutaneously on the back of the left shoulder with 106 murine panc02 cancer cells and imaged 2 weeks post injection. 18F-FDG, routinely used in clinic, was used to image tumor-bearing mice. Only highly active tissues (heart, brown fat, mouth muscles) were visible due to their high metabolic activity. The tumor was not visible, probably due to its very small size (∼1.5 mm in diameter) and low metabolic activity. See movie 04 in Supporting Information for a 3D visualization. (G) PET signals in vivo in different organs. (H) 2-photon microscopy image of an explanted tumor with MHC class II positive (VHHDC8 stained) infiltrating immune cells. VHHDC8 was site-specifically labeled with Texas Red via sortagging. A C57BL/6 mouse was inoculated subcutaneously on the back of the left shoulder with 106 murine panc02 cancer cells. 2 weeks post panc02 cancer cell injection, 20 μg of VHHDC8-Texas Red was injected IV 90 minutes prior to explant imaging of the panc02-tumor. See image 01 in the Supporting Information for high-resolution visualization. Experiments are representative of three mice with similar results.
The short half-life of VHHs (∼10–20 min) likely requires compensation in terms of affinity of the VHH for its target to ensure retention by the tumor. An important limitation of the use of VHHs for immuno-PET is their accumulation in the kidneys and intestine. The use of a longer-lived isotope such as 64Cu or 89Zr might permit an observation window that allows adequate clearance from kidneys and intestine without compromising imaging quality, but this remains to be explored experimentally.
In conclusion, we have site-specifically labeled biomolecules with 18F, starting from a widely available precursor, 18F-FDG. The method avoids the far more demanding generation of carbon–18F bonds and thus facilitates access to 18F-labeled biomolecules, provided these tolerate the presence of a sortase recognition motif, for example, as shown for 4-helix bundle cytokines.20 We successfully applied immuno-PET to the detection of small heterotopic pancreatic tumor transplants, using high affinity anti-Class II MHC VHHs to decorate the tumor-surrounding immune cells.
The VHH-PET method provides information on the tumor immune microenvironment, while the use of 18F-FDG-PET can identify tumors based on their increased metabolic activity compared to surrounding normal tissue. Both approaches can be applied to the same specimen repeatedly to obtain information on tumor growth and regression, for example, in response to therapy. Immunogenicity of VHHs remains an issue of concern in the case of repeated administration, but approaches for humanization of camelid-derived VHHs have been described34 to address this issue. The small size of VHHs and their ease of enzymatic modification relative to other formats commonly applied to antibody fragments present a powerful addition to the radiodiagnostic toolbox.35−37
Acknowledgments
M.R. is a Cancer Research Institute Irvington Fellow supported by the Cancer Research Institute. This work was funded by NIH R01-AI087879-06 (to H.L.P.), DP1-GM106409-03 (an NIH Pioneer Award to H.L.P.), and R01-GM100518-04 (to H.L.P.) and by the Lustgarten Foundation (H.L.P.). We acknowledge the Whitehead Flow Cytometry Core Facility for support. RW and NK are supported in part by P50CA086355, 5R01EB010011, P01-CA117969, and the Lustgarten Foundation.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.5b00121.
The authors declare no competing financial interest.
Supplementary Material
References
- Quigley H.; Colloby S. J.; O’Brien J. T. PET imaging of brain amyloid in dementia: a review. Int. J. Geriatr. Psychiatry 2011, 2610991–999. [DOI] [PubMed] [Google Scholar]
- Clark C. M.; et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA, J. Am. Med. Assoc 2011, 3053275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers E. C.; et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 875586–592. [DOI] [PubMed] [Google Scholar]
- Pichler B. J.; Kolb A.; Nägele T.; Schlemmer H.-P. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J. Nucl. Med. 2010, 513333–336. [DOI] [PubMed] [Google Scholar]
- Schlemmer H. P.; Pichler B. J.; Schmand M.; Burbar Z.; Michel C.; Ladebeck R.; Jattke K.; Townsend D.; Nahmias C.; Jacob P. K.; Heiss W. D.; Claussen C. D. Simultaneous MR/PET Imaging of the Human Brain: Feasibility Study. Radiology 2008, 24831028–1035. [DOI] [PubMed] [Google Scholar]
- Boellaard R.; et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2010, 371181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R. Molecular imaging in cancer. Science 2006, 31257771168–1171. [DOI] [PubMed] [Google Scholar]
- Groheux D.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 383426–435. [DOI] [PubMed] [Google Scholar]
- Higashi K.; Clavo A. C.; Wahl R. L. Does FDG uptake measure proliferative activity of human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J. Nucl. Med. 1993, 343414–419. [PubMed] [Google Scholar]
- Huebner R. H.; et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J. Nucl. Med. 2000, 4171177–1189. [PubMed] [Google Scholar]
- Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J. Nucl. Med. 2011, 52181–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester H. J.; et al. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur. J. Nucl. Med. Mol. Imaging 2003, 301117–122. [DOI] [PubMed] [Google Scholar]
- Liu Q.; et al. 18F-Labeled magnetic-upconversion nanophosphors via rare-Earth cation-assisted ligand assembly. ACS Nano 2011, 543146–3157. [DOI] [PubMed] [Google Scholar]
- Liu S.; et al. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol. Imaging Biol. 2010, 125530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosjan M. J.; et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 54739–743. [DOI] [PubMed] [Google Scholar]
- Knowles S. M.; et al. Quantitative ImmunoPET of Prostate Cancer Xenografts with 89Zr- and 124I-Labeled Anti-PSCA A11 minibody. J. Nucl. Med. 2014, 553452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keliher E. J.; Reiner T.; Turetsky A.; Hilderbrand S. A.; Weissleder R. High-Yielding, Two-Step 18F Labeling Strategy for 18F-PARP1 Inhibitors. ChemMedChem 2011, 63424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan G.; Bigner D. D.; Zalutsky M. R. Fluorine-18-labeled monoclonal antibody fragments: a potential approach for combining radioimmunoscintigraphy and positron emission tomography. J. Nucl. Med. 1992, 3381535–1541. [PubMed] [Google Scholar]
- Cai W.; et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J. Nucl. Med. 2007, 482304–310. [PubMed] [Google Scholar]
- Popp M. W.; Dougan S. K.; Chuang T.-Y.; Spooner E.; Ploegh H. L. Sortase-catalyzed transformations that improve the properties of cytokines. Proc. Natl. Acad. Sci. 2011, 10883169–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S. K.; Liu G.; Ton-That H.; Schneewind O. Staphylococcus aureus Sortase, an Enzyme that Anchors Surface Proteins to the Cell Wall. Science 1999, 2855428760–763. [DOI] [PubMed] [Google Scholar]
- Guimaraes C. P.; et al. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 2013, 891787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian M.; Dozier J. K.; Distefano M. D. Enzymatic labeling of proteins: techniques and approaches. Bioconjugate Chem. 2013, 2481277–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijbis K.; Spooner E.; Ploegh H. L. Protein Ligation in Living Cells Using Sortase. Traffic 2012, 136780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiji S.; Nagamune T. Sortase-Mediated Ligation: A Gift from Gram-Positive Bacteria to Protein Engineering. ChemBioChem 2009, 105787–798. [DOI] [PubMed] [Google Scholar]
- Blackman M. L.; Royzen M.; Fox J. M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels–Alder Reactivity. J. Am. Chem. Soc. 2008, 1304113518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karver M. R.; Weissleder R.; Hilderbrand S. A. Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconjugate Chem. 2011, 22112263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karver M. R.; Weissleder R.; Hilderbrand S. A. Bioorthogonal reaction pairs enable simultaneous, selective, multi-target imaging. Angew. Chem., Int. Ed. Engl. 2012, 514920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavari M.; et al. A novel method for direct site-specific radiolabeling of peptides using [18F]FDG. Bioconjugate Chem. 2009, 203432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian M.; et al. A highly efficient catalyst for oxime ligation and hydrazone-oxime exchange suitable for bioconjugation. Bioconjugate Chem. 2013, 243333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler M.; Grinberg L.; Wang X.; Dawson P. E.; Baca M. Enhanced catalysis of oxime-based bioconjugations by substituted anilines. Bioconjugate Chem. 2014, 25193–101 10.1021/bc400380f. [DOI] [PubMed] [Google Scholar]
- Rashidian M.; et al. Non-invasive Imaging of Immune Responses. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M.; et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 2002, 4186901983–988. [DOI] [PubMed] [Google Scholar]
- Feig C.; et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012, 18164266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincke C.; et al. General Strategy to Humanize a Camelid Single-domain Antibody and Identification of a Universal Humanized Nanobody Scaffold. J. Biol. Chem. 2009, 28453273–3284. [DOI] [PubMed] [Google Scholar]
- Chakravarty R.; Goel S.; Cai W. Nanobody: the “magic bullet” for molecular imaging?. Theranostics 2014, 44386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Huyvetter M.; et al. Targeted Radionuclide Therapy with A (177)Lu-labeled Anti-HER2 Nanobody. Theranostics 2014, 47708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer T.; Muyldermans S.; Depicker A. Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 2014, 325263–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.