Abstract
It is now well established that mitochondria are organelles that, far from being static, are subject to a constant process of change. This process, which has been called mitochondrial dynamics, includes processes of both fusion and fission. Loss of Pink1 (PTEN-induced putative kinase 1) function is associated with early onset recessive Parkinson’s disease and it has been proposed that mitochondrial dynamics might be affected by loss of the mitochondrial kinase. Here, we report the effects of silencing Pink1 on mitochondrial fusion and fission events in dopaminergic neuron cell lines. Cells lacking Pink1 were more sensitive to cell death induced by C2-Ceramide, which inhibits proliferation and induces apoptosis. In the same cell lines, mitochondrial morphology was fragmented and this was enhanced by application of forskolin, which stimulates the cAMP pathway that phosphorylates Drp1 and thereby inactivates it. Cells lacking Pink1 had lower Drp1 and Mfn2 expression. Based on these data, we propose that Pink1 may exert a neuroprotective role in part by limiting mitochondrial fission.
Keywords: Pink1, Ceramide, Parkinson, Rotenone, Mitochondrial dynamics
1. Introduction
Parkinson disease (PD) is an age-related neurological disorder characterized by the loss of dopaminergic neurons and the accumulation of intracellular inclusions known as Lewy bodies (Forno, 1996). PD causes slowed movements, rigidity and tremor and is slowly progressive. Although most PD cases are sporadic, about 10% are inherited. Of the known genes associated with familial forms of PD, several are involved in the regulation of mitochondrial function, including Parkin, DJ1 and Pink1 (Gasser, 2007).
Pink1 (PTEN-induced kinase 1) is a 581 amino acid protein with a serine–threonine kinase domain and a mitochondrial localization sequence (Unoki and Nakamura, 2001). Although the physiological roles of Pink1 have not been completely determined, several pieces of evidence suggest that Pink1 has important functions in mitochondrial physiology. Pink1 stabilizes mitochondrial membrane potential (ΔΨm) and protects cells from apoptosis when they are exposed to various neurotoxins (Valente et al., 2004). Pink1 is necessary for HtrA2 phosphorylation (a mitochondrial protein involved in quality control) (Plun-Favreau et al., 2007) and regulates mitochondrial calcium flux through the Na+/Ca2+ exchanger (Gandhi et al., 2009). Pink1 deficiency causes mitochondrial calcium overload (Marongiu et al., 2009), which is associated with increased production of ROS through NADPH oxidase (Gandhi et al., 2012).
One additional function of Pink1 is in the control of mitophagy (Youle and Narendra, 2011). Specifically, kinase active Pink1 is required for the recruitment of parkin to depolarized mitochondria in mammalian cells. Loss of Pink1/parkin also causes changes in mitochondrial morphology (Santos and Cardoso, 2012) but the effects of deficiency appear to be different in different model systems. In Drosophila, Pink1 promotes mitochondrial fission (Deng et al., 2008; Poole et al., 2008), but in human cells Pink1 seems to promote mitochondrial fusion (Lutz et al, 2009; Sandebring et al., 2009). Potentially resolving this discrepancy, mitochondrial fragmentation is an early effect of Pink1 downregulation in Drosophila S2 cells (Lutz et al., 2009). Furthermore, data from large scale screens of parkin substrates under conditions where mitophagy is triggered have shown that both fusion and fission proteins on the outer mitochondrial membrane are targeted for removal (Chan et al., 2011; Sarraf et al., 2013). Recessive genes involved in PD have been associated with effects on mitochondrial morphology, but α-synuclein may also participate in this process. Surprisingly, the mitochondrial phenotype caused by expression of α-synuclein rescued by co-expression of Pink1, Parkin and DJ1 (Kamp et al., 2010).
Genetic studies have revealed the importance of mitochondrial fusion and fission in the normal function of cells and have also described key molecular components of each. Mitochondrial fusion requires Mitofusin-1 (Mfn1) and Mitofusin-2 (Mfn2), two highly conserved GTPases located in the outer mitochondrial membrane (Chen et al., 2003). Another protein involved in mitochondrial fusion is Opa1, which was initially identified as a gene mutation in autosomal dominant optic atrophy (Delettre et al., 2000). Opa1 down regulation leads to aberrations in morphology of the mitochondrial cristae and generates mitochondrial fragmentation (Chen and Chan, 2005). Two additional proteins, Fis1 and Drp1 are important components of mitochondrial fission machinery. Although Drp1 is located in the cytosol, a subpopulation is located at specific sites of mitochondrial tubules that mark the places where fission occurs (Chan, 2006). Drp1 contains dynein-like GTPase domains that are important in the constriction of mitochondrial membranes. Mitochondrial MIEF1 factor, also known as MiD51, induces extensive mitochondrial fusion when overexpressed but depletion leads to mitochondrial fragmentation (Zhao et al., 2011).
There are still many unanswered questions regarding the control of mitochondrial fusion and fission. It is not known how different proteins linked to these processes interact, but healthy mitochondria tend to merge while fission can be a mechanism by which cells get rid of damaged mitochondria through lysosomal degradation (Itoh et al., 2013). Here, we demonstrate that downregulation of Pink1 alters the balance of mitochondrial fusion and fission and sensitizes cells to neuronal death induced by rotenone and C2-ceramide.
2. Experimental procedure
2.1. Cell culture
CAD cells, originally obtained from a mouse mesencephalic tumor (Horton et al., 2001; Qi et al., 1997), were grown in DMEM-F12 (Sigma–Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified 5% CO2 incubator. They were seeded at a density of 2 × 105 per well on 6 well plates. After overnight attachment, they were switched to serum free, transferrin 1X and sodium selenite (50 ng/ml) to achieve neuronal like differentiation (48 h). CAD cells were treated with C2-ceramide (25 μM; Sigma–Aldrich, St. Louis, MO, USA) for 6h and cells were collected. The dose had been previously determined to cause apoptotic cell death (Arboleda et al., 2009).
BE(2)-M17 cells (ATCC designation CRL-2267) are human neuroblastoma cells that express dopamine synthesis enzymes such as tyrosine hydroxylase and dopamine-β-hydroxylase (Thiele, 1991). M17 cells were seeded in OPTIMEM I supplemented with 10% FBS and differentiated by treatment with retinoic acid 1 μM and 2% FBS.
2.2. Transduction of CAD and M17 cells
We used lentiviral plasmids to knockdown Pink1. For CAD cells we used commercial Pink1 shRNA plasmid for mouse (sc-44599-SH, SantaCruz Biotechnology, Dallas, TX, USA) and a control shRNA plasmid A (sc-108060, SantaCruz Biotechnology, Dallas, TX, USA) with resistance to puromycin (Sigma–Aldrich, St. Louis, MO, USA). M17 cells were transfected with the following construct against Pink1: 5′-GCTGGAGGAGTATCTGATAGG-3′; and a control shRNA 5′-CCTAGACGCGATAGTATGGAC-3′ and stable clones were established by selection with blasticidin (Invitrogen, Carlsbad, CA, USA). The doses used for selection were 6 μg/ml for CAD cells and 5 μg/ml for M17 cells and both were administered for 3 days. Transduction of Pink1 from CAD cells used 1 μg of shRNA plasmids with Hifect (Lonza; Cologne, Germany) for 24 h while transduction of Pink1 from the M17 cell line used X-treme Gene 9 (Roche Molecular Biochemicals; Mannheim, Germany). Positive clones were isolated after approximately 15 days.
2.3. Quantitative PCR (qPCR)
Total RNA was isolated from cells with Trizol according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). The isolated RNA was then treated with DNase I (Invitrogen, Carlsbad, CA, USA). Five micrograms of total RNA was reverse transcribed with SuperScriptIII Reverse Transcriptase (Invitrogen, Carlsbad, CA USA) using OligodT’s. cDNA templates were diluted ten-fold before performing qPCR. The primers used for Pink1 in CAD cells were 5′-GTGGAACATCTCGGCAGGTT-3′ (forward) and 5′-CCTCTCTTGGATTTTCTGTAAGTGAC-3′ (reverse), while the primers for β-actin were 5′-CTTGGGTATGGAATCCTGTGG-3′ (forward) and 5′-TCAGGAGGAGCAATGATCTTG-3′ (reverse). The primers used for Pink1 in M17 cells were: 5′-AGACGCTTGCAGGGCTTTC-3′ (forward) and 5′-GGCAATGTAGGCATGGTGG-3′ (reverse) and β-actin were 5′-AGAAAATCTGGCACCACACC-3′ (forward) and 5′-AGAGGCGTACAGGGATAGCA-3′ (reverse); qPCR was performed on a LightCycler (Roche Molecular Biochemicals; Mannheim, Germany). Results were analyzed using the (ΔΔCt) method (Kubista et al, 2006; Livak and Schmittgen, 2001).
2.4. MTT and LDH release assays
Cellular metabolic activity was evaluated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay) (Invitrogen, Carlsbad, CA, USA) in CAD cells. They were seeded at a density of 7 × 103 in 200 μl of medium per well in a 96 well plate. The cells were treated with 25 μM C2-ceramide (Aldrich, St. Louis, MO, USA) for 6 h. After stirring, 50 μl of medium, 25 μl of the stock solution of MTT (5 mg/ml in PBS) was added to each well. This was followed by incubation for 2 h at 37 °C. The medium was then removed and the cells were lysed. The purple formazan product was solubilized by adding 100 μl of lysis buffer (20% SDS, 50% dimethylformamide, pH 4.7) per well. The plates were incubated at 37 °C for 120 min and absorbance read at 590 nm. The percentage of cell survival was calculated relative to untreated cells.
LDH release was measured in CAD cells (control and shPink1) treated with or without C2-ceramide as previously described with CytoTox96® Assay (Promega, Madison, WI, USA) following manufacturer’s instructions. 50 μl of the supernatant were transferred from each well of the assay plate to a new plate, and 50 μl of the reconstituted substrate mix was added to each well. The plates were covered and protected from light and incubated 30 min at 37 °C. Then 50 μl of the stop solution was added and absorbance read at 490 nm. Total cell lysis was used as a positive control.
2.5. Western blotting
Control shPink1 and shPink1 M17 cells were treated with a dose of 100 nM of rotenone (known as an inhibitor of complex I of mitochondrial electron transport chain) during 24 h, 25 μM of forskolin (FK, Sigma–Aldrich, St. Louis, MO, USA) during 3 h, and 10 μM of CCCP (Sigma–Aldrich, St. Louis, MO, USA) during 3 h. FK was used because it is a positive control for mitochondrial fusion. It stimulates cAMP signaling pathway that phosphorylates Drp1. Phosphorylated Drp1 cannot execute fission. CCCP as a mitochondrial-uncoupling reagent generates rounded and fragmented mitochondria.
The cells were scraped, pelleted and lysed at 4–8 °C for 3 min using RIPA lysis buffer. Lysates were centrifuged at 13,000 rpm for 10 min at 4 °C. The protein concentration of the supernatant was determined using the BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) using bovine serum albumin (BSA) as the standard. Protein samples (40 μg) were separated using poly-acrylamide gels at 100 V. After electrophoresis, proteins were transferred to nitrocellulose membranes (Hybond-C GE Healthcare, Piscataway, NJ, USA) and incubated in blocking buffer (5% powdered milk in TBS-T) for 1 h. The membrane was then incubated overnight at 4 °C with 3 ml of an antibody mixture containing anti-total Drp1 (1:500 BD Biosciences, San Jose, California, USA), anti-Mfn2 (1:500 Sigma–Aldrich, St. Louis, MO, USA), anti-Fis1 (1:500 Biovision, Milpitas, CA, USA), anti-Opa1 (1:500 BD Biosciences, San Jose, CA, USA) and anti-β-actin (1:5000 Sigma–Aldrich, St. Louis, MO, USA). The following day, the membranes were washed three times with TBS-T for 5 min. This was followed by incubation with appropriate secondary antibodies for 1 h (1:2000) and then washed three times with TBS-T. Bound antibodies were detected using the ECL system (Thermo Scientific, Rockford, IL, USA), and images were taken using Storm Phosphor-Imager 840 (Molecular Dynamics, Sunnyvale, CA, USA). Densitometry was performed with Image J software.
2.6. Mitochondria morphology analysis
Control shPink1 and shPink1 M17 cells were treated as shown in Section 2.5 and transiently transfected with 1 μg of mito-YFP plasmid using X-treme Gene 9 (Roche Molecular Biochemicals; Mannheim, Germany), fixed with 4% paraformaldehyde and placed on slides with Prolong Gold Antifade Reagent (Roche Molecular Biochemicals; Mannheim, Germany). Images were acquired on a Zeiss LSM 500 Confocal Microscope (Carl Zeiss, Jena, Germany). Mito-YFP was image with a 63×/1.4 Oil PlanApochromat DIC objective under illumination with a HeNe laser at 488 nm. Pictures of mitochondria were obtained by using z-stacks of 20 images separated by 1 μm along the z axis. The images obtained by confocal microscopy were contrast optimized and converted to 8-bit images. Using Image J 1.47 software (National Institutes of Health, USA) each mitochondrion was marked to analyze morphological characteristics such as area, perimeter and major and minor axes. Based on these parameters, the aspect ratio (AR; relationship between major and minor axes of the ellipse) of a mitochondrion and its form factor (F/F; perimeter 2/4π × area) were calculated (Mitra and Lippincott-Schwartz, 2010). A numeric cut off was applied to divide mitochondria into two groups: fragmented/unbranched and associated/branched.
2.7. Immunofluorescence
Mitochondria from control shPink1 and control shPink1 CAD cells were stained with 200 nM of MitoTracker® Red CM-H2XRos (Invitrogen, Carlsbad, CA USA) for 30 min and washed twice with DMEM-F12 which was preheated to 37 °C. The medium was removed, and cells were fixed with 4% paraformaldehyde for 20 min then washed three times with PBS1X for 5 min and permeabilized with Triton X-100 0.1% PBS1X for 10 min. Blocking buffer (l% BSA and TritonX-100 in PBS 1X0.05%) was added for 1 h. Then cells were incubated overnight with anti Fis1 (1:50 sc-98900 Santa Cruz Biotechnology, Dallas, TX, USA), anti p-Drp1 (Ser-637) (Cell Signaling, 1:50) and anti Mfn1 (1:50 sc-50330 Santa Cruz Biotechnology, Dallas, TX, USA). The primary antibody was washed with PBS1X 3 times for 5 min. The secondary fluorescent antibody (Alexa Fluor 568 goat anti-rabbit IgG, 1:200, A-11079 Invitrogen, Carlsbad, CA, USA) was added for 2 h. Then the mitochondria were washed again and stained with Hoechst (1:5000, 33258 Sigma–Aldrich, St. Louis, MO, USA). Cell images were visualized in a confocal microscope (C2-Confocal, Nikon, Melville, NY, USA).
2.8. Statistical analyses
All data are presented as mean ± SEM from at least three independent experiments. Statistical analysis was performed using one-way ANOVA, followed by the Tukey-Kramer multiple comparison test (Prism 5, GRaphPad Software, La Jolla, CA, USA).
3. Results
3.1. Downregulation of Pink1 reduces mitochondrial activity and increases vulnerability to damage
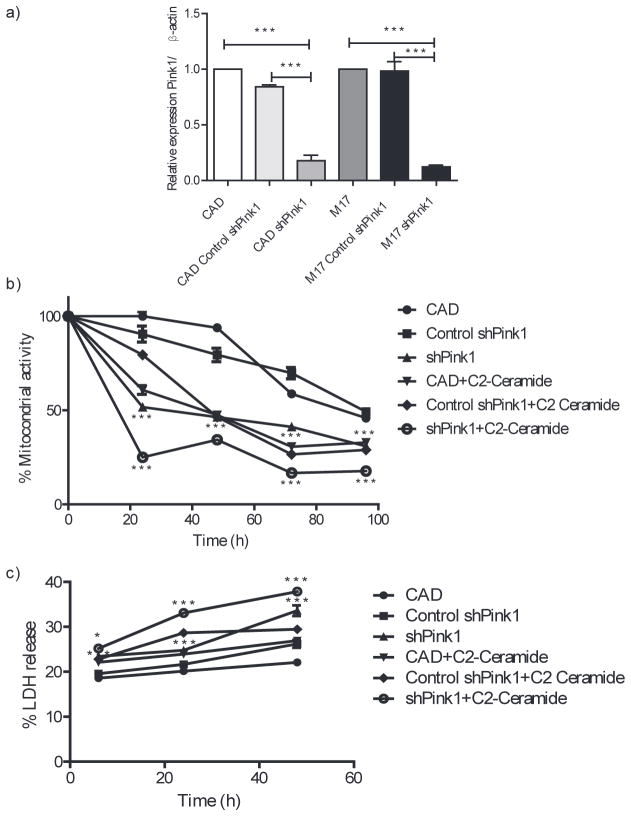
CAD and M17 cells were analyzed for expression of Pink1 using qRT-PCR. shRNA decreased Pink1 expression by 83% ±0.08 for CAD cells and 88% ±0.02 for M17 cells (Fig. 1a). After transfection, shPink1 cells had lower MTT activity and higher LDH release than basal and control cells at all times (Fig. 1b and c). ShPink1 cells were also more sensitive to C2-ceramide treatment at the different times analyzed (Fig. 1b and c).
Fig. 1.
Pink1 is necessary for a proper mitochondrial activity; (a) The mean relative Pink1 expression estimated using quantitative RT-PCR, normalized to β-actin expression; (b) Mitochondrial activity measured by MTT assay in shPink1, control shPink1 in CAD cells treated with and without C2-ceramide (25 μM); (c) LDH release was examined for control shPink1 in CAD cells treated with and without C2-ceramide (25 μM) at different times. ***p < 0.0001 compared to control cells, n = 3, 16 data were taken per cell line/treatment.
3.2. Downregulation of Pink1 induces changes in mitochondrial distribution and morphology
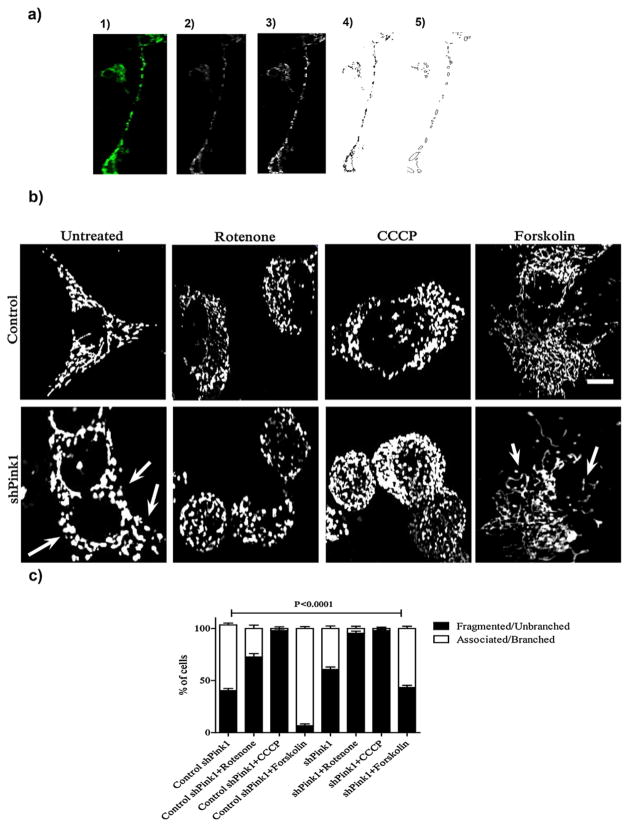
Mitochondrial morphology was analyzed by confocal microscopy in M17 cells (Fig. 2a). ShPink1 cells showed a larger percentage of fragmented mitochondria (61% ±4.0) than did control cells (40% ± 3.5) (Fig. 2b and c). In addition, rotenone caused an increase in the level of mitochondrial fragmentation which was further augmented in shPink1 cells. Treatment with FK decreases mitochondrial fragmentation in control and in shPink1 cells (Fig. 2a and b). FK treatment generates elongated mitochondria and extensive networks throughout the cells in control and shPink1 cells (Fig. 2b, bottom panel). ShPink1 cells treated with FK show 57% ± 3.5 of cells with branched or associated mitochondria whereas control cells have 93% ±3.0 of cells with the same morphology. In control cells, mitochondria are distributed throughout most of the cell body. In contrast, in shPink1 cells and in cells treated with rotenone and CCCP mitochondria tend to concentrate around the nuclei (Fig. 2b).
Fig. 2.
Absence of Pink1 causes fragmentation of mitochondria. (a) Mitochondrial morphology analysis. (1) Shows original image (superposition of each of the 20 images that compose the stack); (2) 3D projection of the image obtained in (1); (3) Contrast adjust and application of spatial filters of the image obtained in (2); (4) Conversion of the image to binary (8 bits) for further analysis of particles and shape descriptors, as shown in (5). Analysis was done by using Image J software. (b) Representative images of the mitochondria of the M17 cells. Cells were transfected with mito-YFP and treated with rotenone, FK and CCCP. The arrows point fragmented mitochondria. Scale bar: 5 μm; (c) Quantification of mitochondrial morphology in M17 cells. 280–400 cell per group/treatment were evaluated, n = 3. The values are presented as the percent of the total number of counted cells. Statistical studies were made with two-way ANOVA.
3.3. Mitochondrial fusion/fission proteins are affected by downregulation of Pink1
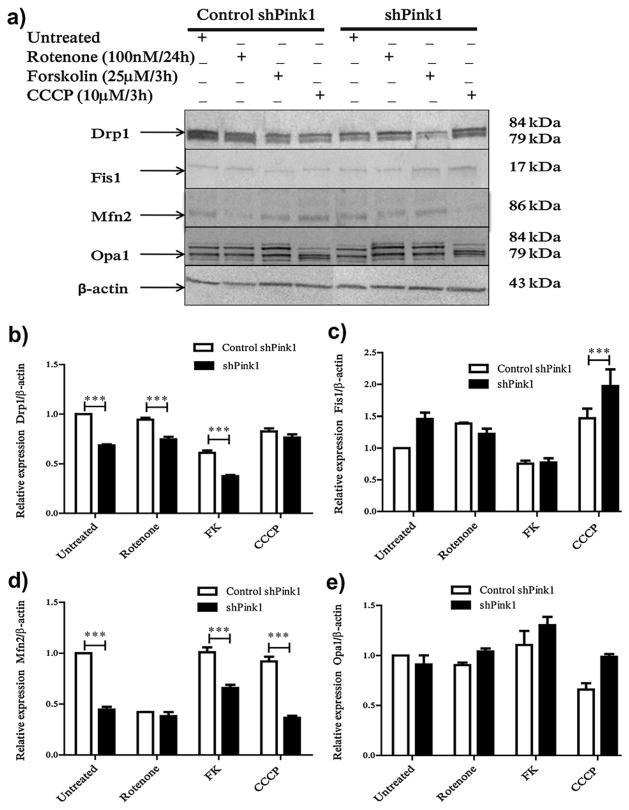
We assessed whether the differences in mitochondrial morphology were related to the expression of some of the proteins involved in fusion (Mfn2 and Opa1) and fission (Drp1 and Fis1) in M17 cells. Total Drp1 was significantly lower in shPink1 cells compared to control cell lines. Nevertheless, rotenone does not affect Drp1 in control and shPink1 cells and treatment with FK decrease its levels in both types of cells (Fig. 3a and b). Quantities of Fis1 significantly different from those in control cells were only observed in shPink1 cells treated with CCCP (Fig. 3a and c). In control cells, rotenone exposure was associated with a decrease in the expression of Mfn2 while neither exposure to FK or CCCP did not change Mfn2 levels (Fig. 3a and d). In shPink1 cells, the expression level of Mfn2 was significantly less than in control cells and neither rotenone nor CCCP change this pattern (Fig. 3a and d). No significant changes in Opa1 were observed in any of the treatments used (Fig. 3a and e).
Fig. 3.
Pink1 modifies the expression of some proteins involved in mitochondrial fusion and fission. Western blot of some of the proteins involved in fusion/fission processes; (a) Representative images of 3 independent experiments. (b) Bars show the relative levels of Drp1; (b) Fis1; (c) Mfn2, and (d) Opa1 by densitometry. ***p < 0.0001 compared to control cells, n = 3.
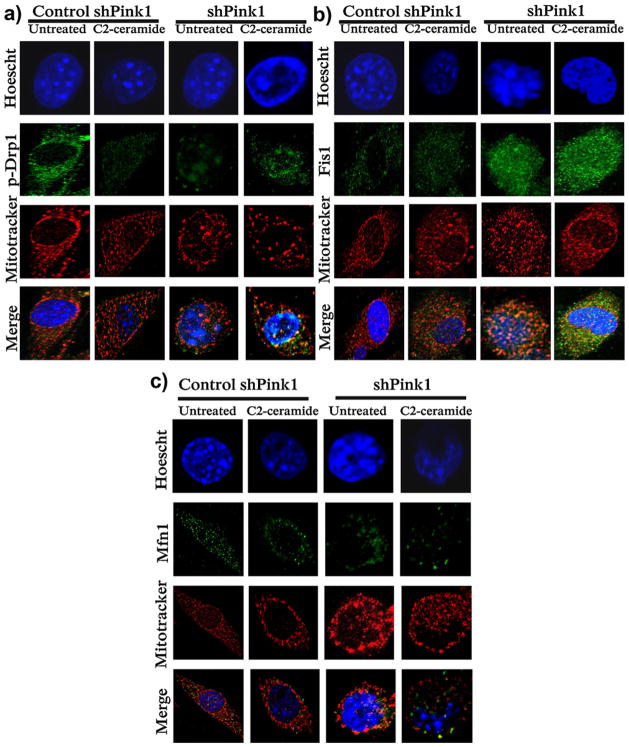
Immunofluorescence was used to detect Fis1, p-Drp1 and Mfn1 in CAD cells. The intensity of fluorescence increased over control cells levels in the presence of Fis1 in shPink1 cells (Fig. 4b), whereas the opposite was found for p-Drp1 and Mfn1 (Fig. 4a and c). No changes in this pattern were observed resulting from C2-ceramide treatment (Fig. 4a–c). Similar changes in the intensity of fluorescence when Fis1, Drp1 and Mfn1 were present in shPink1 cells were also observed in controls treated with C2-ceramide (Fig. 4a–c). Mitotracker staining further confirmed the perinuclear distribution of fragmented mitochondria in shPink1 cells (Fig. 4a–c).
Fig. 4.
Pink1 deficiency affects the fluorescence intensity of some fusion/fission proteins; (a) Mfn1; (b) Fis1 and (c) p-Drp1 in CAD cells. Representative pictures of shPink1 and control cells in the presence and absence of C2-ceramide. There is a greater green intensity corresponding to Fis1 in shPink1 cells (b), whereas the fluorescence intensity of p-Drp1 and Mfn1 is stronger in control cells (a and c). Mitochondria stained with Mitotracker have mainly a perinuclear localization in shPink1 cells and in cells treated with C2-ceramide. Scale bar: 3.2 μm.
4. Discussion
Although important advances about the possible biological role exerted by Pink1 have recently been made, the biochemistry, functioning and signaling pathways that Pink1 regulates are still largely unknown. In this study we demonstrate that downregulation of Pink1 has compromised fusion–fission machinery which alters the normal response to diverse stimuli and displays a default fission phenotype, associated with increased expression of Fis1 and decreased expression of Drp1 and Mfh2, and toward a perinuclear distribution of mitochondria. The absence of Pink1 also sensitizes cells to neurotoxic insults such as neuronal death induced by C2-ceramide (Fig. 5).
Fig. 5.
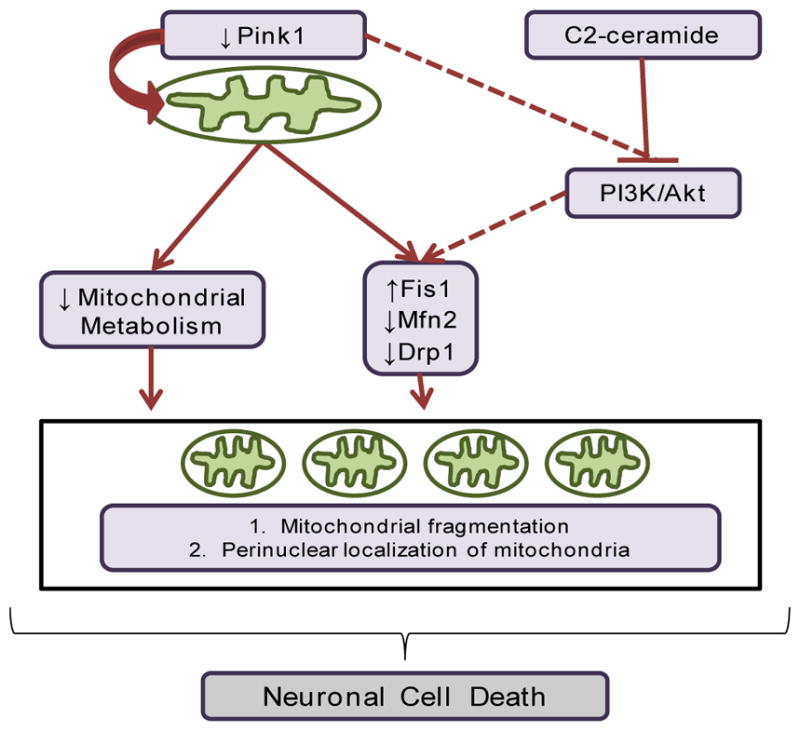
Model of the effects of the downregulation of Pink1 in CAD and M17 cells at mitochondria level. Pink1 absence produces a lower mitochondrial metabolism, leading to cell death when cells are exposed to the toxic effects of C2-ceramide. It is also proposed that the inhibition of the PI3K/Akt pathway by C2-ceramide could regulate the fusion/fission machinery, but this has to be addressed in neurons. Likewise, there is a decrease in the expression of Drp1 and Mfn2 and an increase of Fis1, related to mitochondrial fragmentation.
Several previous studies have suggested that there is a relationship between mitochondrial dynamics and apoptosis, but the molecular basis of these proteins roles remains unknown (Suen et al., 2008). Furthermore, there is controversy about the role of Pink1 in the balance between mitochondrial fusion and fission. The differences found could be attributed to the phase of the cell cycle at which the models used are (Yu et al., 2011), because it has been demonstrated that mitochondrial dynamics may influence the level of proteins involved in the regulation of the cell cycle machinery (Mitra, 2013). Here, we found that when Pink1 is downregulated, the number of cells containing fragmented mitochondria significantly increases (from 40% ±3.5 to 61% ± 4.0). As expected, treatment with rotenone or CCCP increases the amount of mitochondrial fission while treatment with FK decreases the level of fission. The response to FK is ameliorated in shPink1 cells are less obvious than the response in control cells, suggesting that Pink1 normally contributes to the response of mitochondrial dynamics to FK. Because FK controls phosphorylation and diminishes the ability of Drp1 to promote fusion (Cribbs and Strack, 2007, 2009), Pink1 could control the functioning or the expression levels of Drp1. However, shPink1 cells have significantly lower expression levels of total Drp1 and a greater proportion of fragmented mitochondria. Lower levels do not imply less activity. Another explanation is the fact that there are alternative fission mechanisms other than Drp1, which can independently cause mitochondrial fragmentation (Ishihara et al., 2009). Although Drp1 undergoes complex post-translational modifications, their functional significance is still unknown (Arduino et al., 2011; Reddy et al., 2011; Santel and Frank, 2008). Proteins such as Bax and Bak have also been reported to regulate the mitochondrial fission process (Martinou and Youle, 2011).
Increased mitochondrial fragmentation in response to further mitochondrial damage caused by rotenone in shPink1 cells seems not to be associated with the control of fusion–fission proteins, at least at the steady state level, but most probably is related to the generation of ROS (Reddy, 2008), NOS, DNA damage and elevated glucose levels (Knott and Bossy-Wetzel, 2008). The effect of CCCP on mitochondrial fission seems to be associated with increased expression of Fis1. It has been demonstrated that Fis1 expression leads to increased mitochondrial calcium accumulation and to outer mitochondrial membrane depolarization (Iwasawa et al., 2011).
Increased levels of fission in shPink1 cells are also associated with decreases in the expression level of Mfn2. Although the relationship between these proteins is not clear, it has been suggested that Mfn2 mediates Parkin recruitment to damaged mitochondria in a manner dependent on Pink1 (Chen and Dorn, 2013). Some studies have shown that Opa1 and mitofusins act at different steps of the fusion process since Opa1 knockout MEFs maintain fusion of the outer membrane but their inner membranes do not fuse (Chan, 2012).
There is clear evidence showing that damaged mitochondria are removed by a process known as mitophagy (Jin and Youle, 2012; Vives-Bauza and Przedborski, 2011). This process is closely regulated by the interaction between Pink1 and Parkin (Vives-Bauza et al., 2010) so that, in the absence of Pink1, all this machinery is compromised and becomes associated with neuronal cell death (Dagda et al., 2009). Previous studies have shown that wild-type Pink1 protects against rotenone-induced mitochondrial fragmentation while Pink1 deficient cells present fewer mitochondrial connections (Sandebring et al., 2009). The interaction between mitophagy and mitochondrial dynamics is not completely understood but some studies have suggested that fission might be essential for mitochondrial autophagy (Twig et al., 2008) and that Pink1 is central in this process (Chu, 2010).
Previously, we have demonstrated that C2-ceramide is able to inhibit the PI3K/AKT pathway leading to cell death, but this effect can be counteracted by overexpression of Pink1 (Sanchez-Mora et al., 2012). Interestingly, shPink1 cells exhibit increased sensitivity to C2-ceramide, a neurotoxin that induces similar changes in mitochondrial dynamics and distribution as downregulation of Pink1. In this context, the inhibition of the PI3/AKT pathway by the downregulation of Pink1 (Qi et al., 2011; Stiles, 2009; Timmons et al., 2009) maybe an important central regulator of proteins involved in mitochondrial fusion and fission. Some studies have found that Akt may have a role in the regulation of mitochondrial fusion in a cardiac cell model (Ong and Hausenloy, 2010) and mitofusins could be intermediates in the Akt signaling pathway (Perumalsamy et al., 2010). This evidence could potentially explain the present results (Fig. 5).
We also observed highly repetitive differences in the distribution of mitochondria, which are characterized by perinuclear accumulation upon downregulation of Pink1 or treatment with a C2-ceramide, rotenone and CCCP. This organization may have important functional implications because perinuclear mitochondria play a role in the spatial regulation of Ca2+ waves (Hashitani et al., 2010).
5. Conclusions
Pink1 is important for mitochondrial activity and this effect is related to the balance between fusion and fission. The absence of Pink1 in this model tips the balance toward fission, an effect which is not related to higher levels of Drp1 but which is related to significant decreases in Mfn2. In this context, Pink1 could promote neuroprotection by regulating mitochondrial fusion regulated by Mfn2. Possibly other mechanisms are involved in this process and further studies are needed to understand the nature of this regulation.
Acknowledgments
CAD cells were a kind gift from Dr. Dona M. Chikaraishi, Department of Neurobiology, Duke University Medical Center, Durham, NC, USA. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and by COLCIENCIAS (202010016543) and the Facultad de Medicina, Universidad Nacional de Colombia (201010018824).
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009;59:333–46. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Arduino DM, Esteves AR, Cardoso SM. Mitochondrial fusion/fission, transport and autophagy in Parkinson’s disease: when mitochondria get nasty. Parkinsons Dis. 2011;2011:767230. doi: 10.4061/2011/767230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–87. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–37. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(Spec No. 2):R283–9. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet. 2010;19:R28–37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Functional characterization of phosphorylation sites in dynamin-related protein 1. Methods Enzymol. 2009;457:231–53. doi: 10.1016/S0076-6879(09)05013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Cherra SJ, III, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–55. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–10. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Vaarmann A, Yao Z, Duchen MR, Wood NW, Abramov AY. Dopamine induced neurodegeneration in a PINK1 model of Parkinson’s disease. PLoS ONE. 2012;7:e37564. doi: 10.1371/journal.pone.0037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Update on the genetics of Parkinson’s disease. Mov Disord. 2007;22(Suppl 17):S343–50. doi: 10.1002/mds.21676. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Lang RJ, Suzuki H. Role of perinuclear mitochondria in the spatiotemporal dynamics of spontaneous Ca2+ waves in interstitial cells of Cajal-like cells of the rabbit urethra. Br J Pharmacol. 2010;161:680–94. doi: 10.1111/j.1476-5381.2010.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton CD, Qi Y, Chikaraishi D, Wang JK. Neurotrophin-3 mediates the autocrine survival of the catecholaminergic CAD CNS neuronal cell line. J Neurochem. 2001;76:201–9. doi: 10.1046/j.1471-4159.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–66. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S. Fis1 and Bap31 bridge the mitochondria–ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556–68. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Youle RJ. J Cell Sci. 2012;125:795–9. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, et al. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–89. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann NY Acad Sci. 2008;1147:283–92. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonak J, Lind K, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–51. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, et al. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem. 2009;108:1561–74. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra K. Mitochondrial fission–fusion as an emerging key regulator of cell proliferation and differentiation. Bioessays. 2013;35:955–64. doi: 10.1002/bies.201300011. [DOI] [PubMed] [Google Scholar]
- Mitra K, Lippincott-Schwartz J. Analysis of mitochondrial dynamics and functions using imaging approaches. Curr Protoc Cell Biol. 2010;chapter 4(Unit-21) doi: 10.1002/0471143030.cb0425s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010;88:16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci USA. 2010;107:6882–7. doi: 10.1073/pnas.0910060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, et al. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–52. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–25. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Yang W, Liu Y, Cui T, Gao H, Duan C, et al. Loss of PINK1 function decreases PP2A activity and promotes autophagy in dopaminergic cells and a murine model. Neurochem Int. 2011;59:572–81. doi: 10.1016/j.neuint.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromol Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67:103–18. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mora RM, Arboleda H, Arboleda G. PINK1 overexpression protects against C2-ceramide-induced CAD cell death through the PI3K/AKT pathway. J Mol Neurosci. 2012;47:582–94. doi: 10.1007/s12031-011-9687-z. [DOI] [PubMed] [Google Scholar]
- Sandebring A, Thomas KJ, Beilina A, van der Brug M, Cleland MM, Ahmad R, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60:448–55. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- Santos D, Cardoso SM. Mitochondrial dynamics and neuronal fate in Parkinson’s disease. Mitochondrion. 2012;12:428–37. doi: 10.1016/j.mito.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–6. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles BL. PI-3-K and AKT: onto the mitochondria. Adv Drug Deliv Rev. 2009;61:1276–82. doi: 10.1016/j.addr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele CJ. Patterns of regulation of nuclear proto-oncogenes MYCN and MYB in retinoic acid treated neuroblastoma cells. Prog Clin Biol Res. 1991;366:151–6. [PubMed] [Google Scholar]
- Timmons S, Coakley MF, Moloney AM, O’Neill C. Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett. 2009;467:30–5. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001;20:4457–65. doi: 10.1038/sj.onc.1204608. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Przedborski S. Mitophagy: the latest problem for Parkinson’s disease. Trends Mol Med. 2011;17:158–65. doi: 10.1016/j.molmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2010;107:378–83. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sun Y, Guo S, Lu B. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet. 2011;20:3227–40. doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin S, Wang X, Qu M, Uhlen P, et al. Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 2011;30:2762–78. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]