Abstract
The endothelium lines the internal surfaces of blood and lymphatic vessels and has a critical role in maintaining homeostasis. Endothelial dysfunction is involved in the pathology of many diseases and conditions, including disorders such as diabetes, cardiovascular diseases, and cancer. Given this common etiology in a range of diseases, medicines targeting an impaired endothelium can strengthen the arsenal of therapeutics. Nanomedicine – the application of nanotechnology to healthcare – presents novel opportunities and potential for the treatment of diseases associated with an impaired endothelium. This review discusses therapies currently available for the treatment of these disorders and highlights the application of nanomedicine for the therapy of these major disease complications.
Keywords: endothelium, nanomedicine, endothelial disorder, diabetes, atherosclerosis, cancer, permeability
Graphical Abstract
Endothelial function and dysfunction
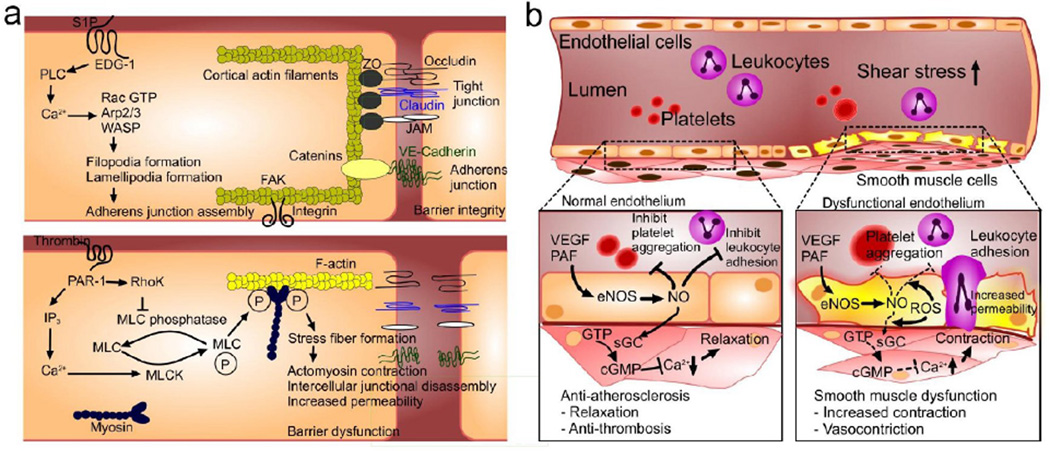
The endothelium is a semi-selective barrier that lines vessels, controls their degree of permeability towards biologically active molecules via membrane-bound receptors, and regulates blood clotting (thrombosis and fibrinolysis), inflammation, blood pressure (vasoconstriction and vasodilation), and leukocyte trafficking [1]. The endothelium is anchored to the extracellular matrix through focal adhesions that are controlled by transmembrane integrins (e.g. αvβ3, α2β1, α5β1) [2] and to cytoskeleton-linking proteins (e.g. cadherin, claudin, occludin) [3] that act jointly with intercellular connections, such as adherens and tight junctions. This intertwined structure maintains the integrity of the endothelial barrier and low basal permeability (Figure 1a). The permeability of a vascular endothelial layer is regulated through cytoskeletal connections [4] and by signaling cascades [3] including myosin light chain kinase (MLCK) [5], PKC isoforms (PKC-β, -δ, and -θ) [6, 7], Rhokinase [8], focal adhesion kinase (FAK) [9], src kinase [10], small GTPases [11], and soluble mediators, such as anaphylatoxin [12] and bradykinin [13].
Figure 1. Mechanism and mediators of endothelial function and permeability.
(a) Endothelial cells maintain the tight cell-cell connections and the underlying matrix for increased barrier integrity. Sphingosine-1-phosphate (S1P) binds to its EDG-1 receptor, which ultimately strengthens EC barrier function through lamellipodia formation and subsequent AJ assembly. In dysfunctional barriers, thrombin binds to the PAR-1 receptor, which induces inositol trisphosphate (IP3) production and a subsequent increase in intracellular Ca2+. Increased Ca2+ activates the myosin light chain kinase (MLCK) to phosphorylate MLCs, leading to increased actomyosin contractility. Furthermore, thrombin inhibits MLC phosphatase activity through Rho/Rho kinase (RhoK), which increases MLC phosphorylation. The resulting actomyosin contraction contributes to increased permeability of the EC layer. (b) In normal ECs, activated eNOS promotes nitric oxide (NO) production, inhibiting platelet aggregation, leukocyte adhesion, and smooth muscle cell proliferation. Reduced availability of NO leads to endothelial disorder and compromised production of NO by oxidative stress. This inhibits eNOS-derived NO production and results in platelet aggregation and leukocyte adhesion as well as increased contractions of smooth muscle cells.
Endothelial disorder (EnD) arises from disruptions in the regulation of the endothelial barrier function due to hemodynamic alteration, cytotoxicity, physical injury, and immune-mediated responses (Figure 1b). When an injury, such as a hemorrhage, occurs at a location within the vascular system, leukocytes are released as part of the immune system response. They produce gelatinase, a matrix metalloproteinase that causes the degradation of tight junction proteins; this destroys the endothelial barrier [14]. This results from the degradation of type IV collagen in the extracellular matrix [15] and leads to the leaking of blood through the degraded tight junction, which can lead to further complications. For example, it can cause the rupture of plaques in an atherosclerotic artery. The disruption of the plaque results in the release of coagulants from platelets, causing thrombi to form in the blood stream and leading to more serious problems such as stroke and ischemia. This can occur not only with atherosclerotic tissues but with any disturbance that causes excess and/or unwanted coagulation of blood proteins [16].
EnD is a crucial hallmark of many diseases, including diabetes mellitus [17], tumorigenesis [18], hypertension [19], hypercholesterolemia [20], ischemia/reperfusion injury [21], respiratory disorders [22], chronic renal failure [23], and autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematous, and Wegener’s granolumatosis [24, 25]. EnD therapy could therefore serve as a potential target for the prevention and treatment of these disorders, including cardiovascular diseases (CVDs) [26].
Current treatments for EnD-associated diseases often involve the improvement of nitric oxide (NO) bioavailability through mediators (e.g. NO synthase) and their pathways (e.g. PI3K-AKT) [27], restoring endothelial function with reduced oxidative stresses. At present, available treatments mostly target CVD and related complications. For example, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (known as statins) have anti-inflammatory and anti-hypertensive effects as well as cholesterol-lowering roles, but have been linked to increased diabetes risk [28]. Renin angiotensin system inhibitors (e.g. angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB)) are used for the treatments of chronic kidney failure, diabetic nephropathy, and hypertension [29, 30], which could also induce hyperkalemia (a condition characterized by high blood levels of potassium). Endothelin receptor antagonists (ERA) [31] and oral hypoglycemic drugs [32] are used to treat hypertension and kidney disease in diabetic patients, but these drugs also have adverse effects, including fluid retention and edema [33]. With the ability to deliver a wide range of therapeutics specifically to disease locations and in a sustained manner, nanomedicines are emerging as new treatments with the potential to minimize the adverse effects of current EnD therapies.
In this review, we highlight the key features of EnD-associated diseases and current representative nanomedicine platforms for their treatment and diagnosis. Focusing on diabetes mellitus, atherosclerosis, and cancer, we discuss their main characteristics, limitations of current therapies, and how nanomedicines can improve the outlook of these endothelial complications. Lastly, we draw attention to steps required in order to transform current nanomedicines into enabling technologies to treat EnD-associated diseases.
Nanomedicine for endothelial disorder-associated diseases
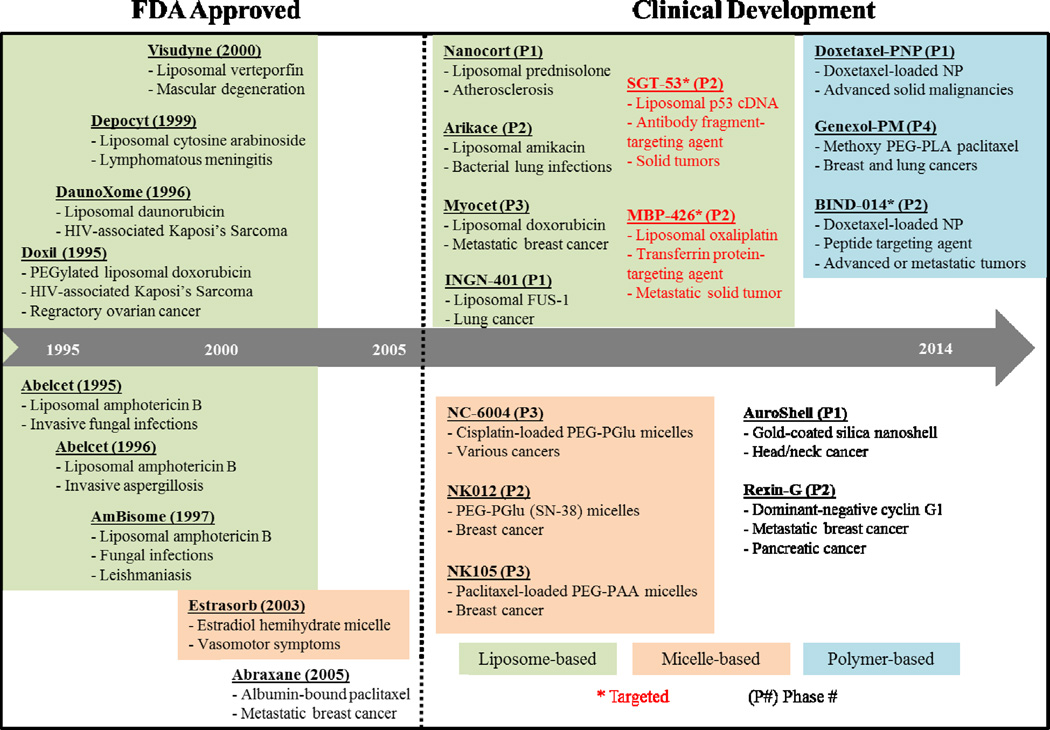
Nanomedicine – the application of nanotechnology to medical diagnostics and therapies – encompasses the rapidly expanding field of drug delivery using nanoparticles (NPs) [34]. A variety of materials have been used to formulate nanomedicines for drug delivery and imaging applications to date. These range from lipids (micelles [35] and liposomes [36]) to polymers [37, 38] and lipid-polymer hybrids [39, 40], as well as organic precursors (dendrimers) [41], carbon (carbon nanotubes and pipes) [42], metal oxides (metal organic frameworks) [43], and inorganic molecules (gold [44], iron oxide [45], quantum dots [46]), and biological components (proteins) [47]. Liposomes and polymeric NPs comprise the majority of NPs in clinical trials (Figure 2). The approval and commercial success of Doxil, a PEGylated NP platform for the treatment of cancer, in 1995 paved the way for the development and FDA-approval of current NPs in clinical development.
Figure 2. Timeline of selected clinical stage nanomedicines (FDA-approved and in clinical development).
PEG = polyethylene glycol; siRNA = small interfering RNA; GAH TNF = tumor necrosis factor; Bik = Bcl-2 interacting killer; PEG–PGlu = polyethylene glycol-poly(glutamate); and PEG–PLA = polyethylene glycol-polylactic acid. Phase trial is as of June 2015, in the United States. Source: www.clinicaltrials.gov.
Specific to drug delivery applications, NPs can provide the following advantages [48–50]: 1) the ability to encapsulate and deliver poorly water-soluble drugs, 2) the enhanced circulation of NPs due to PEGylation, resulting in prolonged drug circulation times [51], 3) the reduction of systemic toxicities observed with the use of free drugs, 4) the incorporation of targeting elements that allow highly localized release of drugs [52, 53], 5) the co-delivery of two or more types of drugs to sites of action for combination therapies [54], 6) the simultaneous visualization of drug delivery and therapeutic response [55, 56], and 7) the intracellular delivery of plasma sensitive nucleic acids, such as siRNA [57, 58]. These advantages could be used to provide better therapeutic solutions to disorders arising from EnD, particularly by targeting the specific endothelial tissues and malfunctions that lead to the observed symptoms and diseases. Nevertheless, the overall number of FDA-approved NPs is small. Since the early 2000s, FDA approval of NP systems has slowed notably despite the large number of NPs currently in clinical trials. This may be in part due to the rising cost of clinical trials, as well as the rise in the understanding of the complex pathologies of disease progression. In the next section, we highlight disease pathologies and the complex role that the endothelium plays in their progression, as well as examples of nanomedicines currently being explored for these diseases.
Endothelial disorder in major pathologies and the nanomedicine research
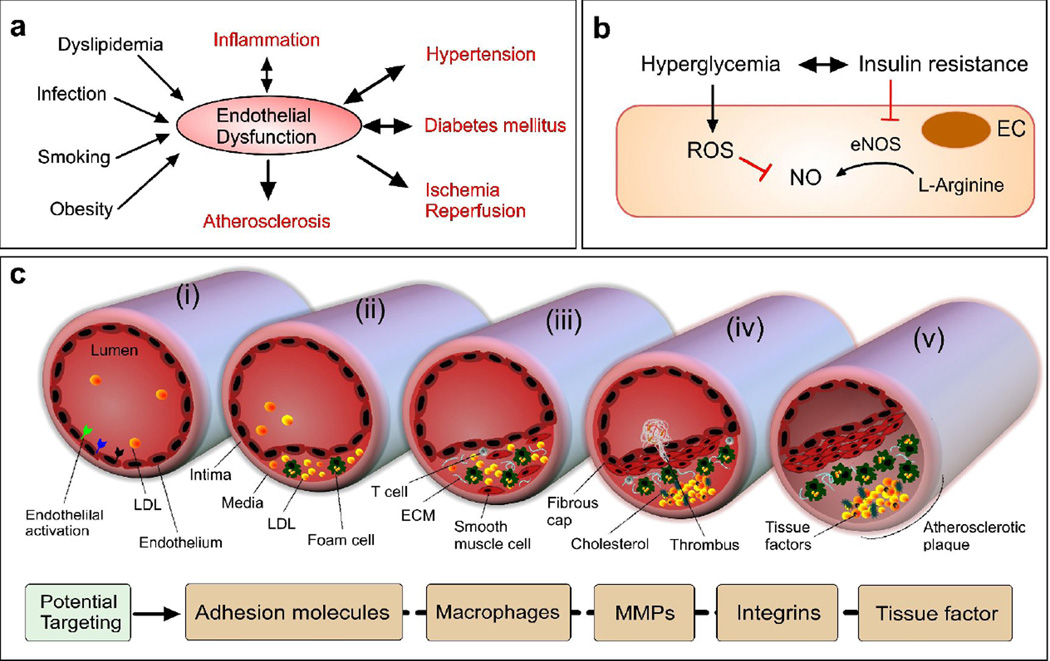
A malfunctioning endothelium has critical implications; it is closely involved with the pathogenesis of many diseases and conditions. We highlight the features of EnD-associated diseases, along with selected samples of corresponding nanomedicine therapies being studied (Table 1). Many EnD-associated diseases including diabetes, atherosclerosis, and cancer have common inducers (Figure 3a). These diseases have common endothelial pathologies, such as disordered cell junctions within endothelial cell layers. Nevertheless, there exist different ligands and proteins that are better targets for each condition.
Table 1.
Selected complications of endothelial disorders and related nanomedicine research
| Disease pathology |
Endothelial Disorder Relation | Nanomedicine therapy |
|---|---|---|
| Acute lung injury (ALI) |
ALI results from disrupted capillary- endothelial interfaces. P38 mitogen- activated protein kinase, which can be activated by inflammatory cytokines (e.g. TNF-α) and lipopolysaccharides, has been implicated in the disruption of normal endothelial permeability in ALI [59]. |
Human glucagon-like peptide 1 (GLP-1) is an immunomodulatory, anti-inflammatory, and anti-apoptotic peptide. Researchers encapsulated GLP-1 with PEGylated phospholipid micelles, allowing them to mitigate side effects, including immunogenicity, pancreatitis, and renal failure. S.c. delivery of NPs suppressed neutrophil accumulation and lung activation was observed, offering effective protection against the inflammatory effects of LPS-induced ALI in murine models [60]. An inhalable liposomal formulation of amikacin, or Arikace, has been undergoing phase 2 clinical trials for bronchiectasis, cystic fibrosis, and bacterial lung infections [61]. |
| Alzheimer disease (AD) |
Normal interactions of endothelial, neuronal, and glial cells are disrupted in AD. Amyloid-β (Aβ), deposited in cerebrovascular walls in AD patients, is cytotoxic to ECs and affects NO production, mitochondrial function, and induction of apoptosis [62]. |
Research has shown that NPs conjugated with transition metal chelators (polystyrene and the iron chelator MAEHP) can protect human corticol neurons against Aβ-related cytotoxicity by preventing Aβ aggregation [63]. The conjugated NPs, which showed a higher efficacy for crossing the blood-brain barrier, were used to reverse Aβ deposition These NPs may provide an alternate method for the treatment of neurodegenerative diseases associated with excess transition metals. |
| Asthma | Airways in asthma patients are distinguished by elevated endothelial permeability, partly due to sensory neuropeptides that can create intercellular gaps and cause interstitial edema [64]. |
Silver is a known antimicrobial agent. Researchers used nebulized silver NPs to attenuate inflammatory responses in murine models of inflamed airways. By inhibiting ROS and NF-κB expression, the NPs suppressed the expression and activation of pro-inflammatory cytokines (e.g. IL-4, IL-5, TNF-α), chemokines, and adhesion molecules. The exact location and mechanism of action of the silver NPs at the cellular level, however, remains unclear [65]. |
| Burn injury | Oxidants damage endothelial membranes through peroxidation of cell lipids, disruption of interstitial matrix components (e.g. collagen degradation), and inflammation (e.g. recruitment of lymphocytes and macrophages) [66]. These lead to increased vascular permeability and edema formation. |
Topical application of antimicrobial nanoemulsions (NB-201), composed of water, surfactants, alcohol, and vegetable oil, reduced burn wound infection and dermal inflammatory responses [67]. In rat models, NB-201 treatment decreased bacterial and pro-inflammatory IL-1β and CINC-3 levels, while mitigating capillary leakage and edema; this was associated with reduced dermal inflammation. In this way, topical nanoemulsions may provide a method for protecting thermally injured skin from inflammatory and immunosuppressive effects. |
| Glaucoma | EnD in glaucoma patients arises from altered production and function of NO and endothelin-1 (ET-1), a vasoconstrictive peptide. Decreased NO and increased ET-1 levels lead to vasospasms and decreased blood flow and vasodilator responses, leading to ocular hypertension and ischemia [68]. |
Egg-phosphaticylcholine (EggPC) liposomes were loaded with latanoprost, a prostaglandin analogue used to increase ocular outflow of fluids. Encapsulation of latanoprost allowed slow and sustained release of the drug. Compared to daily topical applications of free latanoprost, the liposomes were more effective at reducing intraocular pressure in rabbit eyes for up to 90 days after a single subconjunctival injection [69]. |
| Heart failure | Congestive heart failure (CHF) can lead to pulmonary hypertension and eventual ventricular failure [70]. Impaired homeostasis and signaling of endothelial Ca2+ in CHF is related to increased formation of β-actin and F-actin, resulting in the cytoskeletal remodeling of pulmonary vessels and disrupted endothelial functions. |
Nanofibers, composed of self-assembling peptides, were injected into mice. These fibers self-assembled into microenvironments in the myocardium for ECs [71]. Previously, it was shown that ECs survive well within RAD16-II peptide microenvironments in vitro. Working as scaffold, the injectable version of the peptides also promoted vascular cell recruitment and aided endothelial regeneration, as observed by the presence of endothelial markers. This presents an injectable tissue regeneration strategy. |
| Hepatitis | Constant liver inflammation in Hepatitis C patients leads to elevated levels of soluble intercellular adhesion molecule- 1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1), which indicate endothelial damage [72]. |
PEGylated IFN-α-2a nanostructures have been used in clinical trials to enhance antiviral activity, pharmacokinetic properties, and tolerability of drugs in hepatitis C patients [73]. Pegylation extends a drug’s half-life and activity, lessening immunogenicity and enhancing plasma residence times and concentrations compared to standard IFN-α-2a. |
| Hypertension | EnD has been shown to be an early marker of cardiovascular diseases such as hypertension [74]. In patients with hypertension, a dysfunctional endothelium with oxidative stresses and thus reduced NO availability may contribute to increased peripheral resistance, vascular and organ damage, and cardiovascular disorders [75, 76]. |
Many liposomes and polymeric NPs, loaded with peptides, genes, and small molecules, have been studied to treat pulmonary hypertension [77] and normalize mean arterial pressure [78]. These NPs are popular due to their enhanced drug protective abilities, as well as modifiable surface moieties and sustained-release capabilities. Combinations of drugs, such as verapamil and trandolapril, have also shown potential to stabilize hypertension-associated vascular endothelial dysfunction and damage [79]. |
| Inflammatory bowel disease (IBD) |
Altered expression of inducible NO synthase and eNOS in ECs decrease NO- and acetylcholine-mediated dilation of vessels, resulting in compromised vasodilatory responses to ischemia. This accounts for the decreased blood flow observed in patients with IBD [80]. |
Orally administered TNF-α siRNA/polyethyleneimine nanocomplexes decreased TNF-α levels in mice colons [81]. The nanocomplex is able to protect the siRNA from enzymatic degradation. Furthermore, compared to free siRNA, the nanocomplexes contain more siRNA, have decreased cytocoxicity, and are more efficiently taken up by macrophages. Drug- loaded nanocarriers have also been explored, including tacrolimus-loaded PLGA NPs for the treatment of colitis in murine models [82]. See a recent review [83]. |
| Ischemia- Reperfusion (I/R) injury |
Reperfusion injury arises due to the return of blood flow to previously ischemic tissues, and the re-established oxygen causes free radical formation, with NADPH oxidase being the source of ROS during I/R injury [84]. |
ATP-loaded liposomes have shown positive results in both in vitro and in vivo treatment of myocardial ischemia [85]. In rabbit models, for example, ATP- liposomes had significantly lower levels of ventricular damage compared to free ATP. In its free form, ATP has a very short half- life in blood and cannot freely enter cells. By using liposomes, researchers were able to not only protect ATP and its crease its half-life but also to protect human ECs from energy failure. |
| Myocardial infarction (MI) |
In acute MI, inflammatory cytokines (i.e. IL-1β, IL-6, TNF-α) are activated, and these act in conjunction with ROS, also generated during MI. They disrupt Ca2+ intracellular homeostasis, increasing cell permeability, eventually leading to cell necrosis and EnD [86]. |
NPs loaded with drugs and siRNA were shown to mitigate MI symptoms [87]. Polyketal NPs loaded with p38 inhibitor SB239063 decreased in apoptotic events and infarct sizes in murine models of MI [88]. Furthermore, by coating the NPs with sugar N-acetylglucosamine, researchers were able to significantly enhance uptake of the NPs by cardiomyocytes compared to plain particles. |
Figure 3. Endothelial disorder in metabolic and cardiovascular diseases.
(a) Key EnD inducers and EnD-associated diseases. (b) A key EnD mechanism in diabetes. NO is formed from L-arginine by eNOS. In diabetes characterized by insulin resistance and hyperglycemia, EnD results from reduced production of NO. This arises through decreased activation of eNOS due to insulin resistance and increased breakdown of NO by ROS, promoted by hyperglycemia. (c) Initiation and progression of atherosclerosis with an activated endothelium (adapted from [95]). Atherogenic lipoproteins enter the intima and aggregate within the extracellular intimal space (i). Unregulated uptake of these atherogenic lipoproteins by macrophages leads to the generation of foam cells (ii). In addition to monocytes, other types of leukocyte, particularly T cells, are recruited to atherosclerotic lesions and cause chronic inflammation. The growth of plaque induces tissue remodeling (iii). The foam cells release cellular debris and crystalline cholesterol. Smooth muscle cells form a fibrous cap beneath the endothelium, contributing to the formation of a necrotic core within the plaque. The resulting non-obstructive plaque may rupture, resulting in the formation of a thrombus in the lumen (iv), which can lead to tissue infarction. Ultimately, if the plaque does not rupture and the lesion continues to grow, the lesion can encroach on the lumen and result in clinically obstructive disease (v). Potential NP therapies in atherosclerosis could benefit from the increased microvessel permeability, which is caused by hypoxia-induced neovascularization of the vasa vasorum and would allow the delivery of NPs to plaques within vascular vessel walls.
Diabetes
Diabetes mellitus is characterized by increasing and sustained blood glucose concentration, which can be subdivided into type 1 (due to insulin deficiency arising from dysfunction or loss of insulin-secreting β cells in the pancreas) and type 2 (due to defects in insulin action within tissues or insulin resistance) diabetes. Conventional therapeutics, including insulin sensitizers, secretagogues, and analogs, can improve hyperglycemia and endothelial function in patients with diabetes [89]. The general recommended medication for type 2 diabetes, for example, is metformin; metformin is a biguanide compound insulin sensitizer that decreases hepatic glucose levels and improves endothelial function [90]. Insulin secretagogues, or incretin hormones (e.g. glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP)), are used to trigger insulin release by inhibiting the KATP channel of the pancreatic beta cells and by restricting activity of enzyme dipeptidyl peptidase-4 (DDP-4) [91]. Insulin analogs, also referred to as insulin receptor ligands, can act like human insulin in terms of glycemic control (e.g., insulin detemir, a long-acting peptide that binds to circulating albumin). Despite their therapeutic effects, these drugs have been shown to cause adverse effects such as increased incidence of lactic acidosis in metformin-treated patients. These adverse effects can be mitigated by targeted drug delivery to the site of action. Metformin has been successfully incorporated into O-carboxymethyl chitosan NPs and shown to preferentially target pancreatic cells [92]. GLP-1 conjugate NPs have been synthesized for gene therapy, increasing the bioavailability of GLP-1 [93] and allowing not only more effective gene therapy but also decreasing the possibility of off-target effects. Furthermore, there has been increased interest in the delivery of insulin orally. Insulin NPs for oral delivery have been developed by using chitosan to encapsulate insulin. These NPs reach circulation through intestinal absorption by disrupting endothelial tight junctions, and they have been shown to decrease glycemia in diabetic rats [94]. Researchers continue to develop NP platforms that are able to not only target specific endothelial functions (such as tight junctions formed by gastrointestinal endothelial cells in the case of orally delivered NPs) but also to mitigate adverse toxic effects that might arise.
In type 2 diabetes, EnD is illustrated through activation of PKC, formation of advanced glycation end-products (AGEs), impaired vasodilation and vasoconstriction (due to decreased NO), and inflammatory signaling (Figure 3b) [17, 96]. Chronic imbalance of oxygen-related chemical reaction (ox/redox) in ECs, caused by increased ROS and decreased antioxidant capacity, promotes EnD and insulin resistance. For example, insulin-mediated NO production decreases due to insulin resistance with impaired phosphoinositide 3-kinase (PI3K) effects. Increased superoxide anion production induced by hyperglycemia causes increased activity of alternative pathways, including PKC and AGE pathways [97]. Overexpression of PKC isoforms can directly induce insulin resistance. Current therapies being studied for EnD and vascular complications in type 2 diabetes center on increasing NO bioavailability, reducing oxidative stress, and inhibiting PKC and AGE activity. As an example, ruboxistaurin [98] inhibits PKC-β mediation of the insulin receptor phosphorylation and PI3K-AKT signaling [99], and Benfotiamine (a highly bioavailable thiamine derivative) reduces AGE levels and markers of EnD in patients with type 2 diabetes [100].
Islet cell regeneration and encapsulation provide additional options with therapeutic potential for achieving insulin independence in human trials without the use of immunosuppressant drugs [101]. For instance, polymeric nanoparticles containing couramin and composed of poly(lactide-co-glycolide)-poly(ethylene glycol) (PLGA-PEG) polymer with the CHBLWSTRC (Pep I) peptide have been synthesized. These NPs have been shown to target pancreatic islet microvessels and were recently reported to target pancreatic islet endothelial cells (ECs) for immunodulatory therapy of autoimmune type 1 diabetes. These Pep I NPs showed up to a 3-fold increase in islet capillary EC binding compared to controls, which contained peptides of the same amino length but without binding preferences [102]. Genetic approaches for correcting malfunctioning islet ECs is promising, and the delivery of plasma-sensitive nucleic acids in NPs is advantageous. However, the levels of insulin secretion achieved from these methods still need to be regulated due to the limited numbers of vectors delivered. As an example, polyethylenimine dendrimers incorporating the exendin-4 expression vector (PEI25k/pbeta-SP-Ex-4 complex) were developed to protect isolated β cells from apoptosis during islet transplantation [103]. Exendin-4 shows similar effects to those of GLP-1, in that it inhibits pancreatic islet cell death; however, exendin-4 has a longer half-life in serum. This amplifies the survival rate and extent of cells, which in turn would ultimately produce insulin after transplantation for the treatment of type 1 diabetes. Although further investigation is needed, approaches to diabetes using genetic methods as well as islet cell generation and encapsulation therapies are at early stages of preclinical development and offer alternatives to existing therapies for controlling glucose levels. Nevertheless, their safety and efficacy profiles remain to be elucidated.
Atherosclerosis
Atherosclerosis refers to the buildup of plaques within arteries. Increased endothelial permeability, both at the luminal and adventitial sides, is a hallmark of the atherosclerotic process, with plaque progression enhancing inflammatory responses in blood vessels and thus leading to further EnD [104]. The activated and inflamed endothelium plays a major role in atherosclerotic disease from initial lesion formation to disease progression and final thrombotic complications (Figure 3c) [105], as well as in aggravating atherosclerotic plaques due to myocardial infarction [106]. Besides surgical interventions, including the use of stents to physically widen the arterial lumen in advanced plaques, the current standard of care for at-risk patients includes lowering plasma lipid levels through lifestyle changes, by dieting and exercising, and in particular the prescription of statins [107]. Statins are orally administered drugs that help lower blood cholesterol levels by competitively inhibiting cholesterol synthesis in the liver [108]. Nevertheless, significant residual risk remains for patients treated with these conventional preventative and therapeutic options. For example, more than half of patients hospitalized after myocardial infarctions (MIs) with high recurrence rates show normal or low LDL levels [109]. This indicates that current treatments, primarily aimed at lowering blood cholesterol levels, do not effectively treat atherosclerosis.
Research has highlighted the critical role that many immune cells play in the development and later complications of atherosclerosis; EnD plays a critical role in atherosclerotic pathology, given that the normal function of the endothelium in blood vessels are disturbed and disrupted as atherosclerosis progresses. As shown in Figure 3c, lipids and lipid byproducts can activate the endothelium. This triggers the recruitment of leukocytes into plaques, subsequently activating the inflammatory cascade [86]. Given the major role that immune cells play in atherosclerosis, their direct treatment with anti-inflammatory agents can lead to significant reduction in plaque accumulation in animal models [110]. To this end, nanomedicine provides an enhanced way of treating atherosclerosis. Encapsulating the therapeutic agents in NPs enhances their bioavailability and aids their delivery to immune cells in plaques, ensuring more effective treatments.
Early nanomedicine studies in atherosclerosis primarily focused on diagnosis, some of which used noninvasive magnetic resonance imaging (MRI) of atherosclerotic plaques and their macrophages using ultrasmall superparamagnetic iron oxide particles (USPIOs) [111, 112]. USPIO-enhanced MRI has been shown to enable the quantification of atherosclerotic plaque macrophage burden and the effects of atorvastatin lipid-lowering therapy in patients [113]. Similarly, target-specific imaging of adhesion molecules (e.g. VCAM-1) has been used to monitor the efficacy of statin therapy in mice using cross-linked iron oxide (CLIO) [114]. VCAM-1-expressing cells have successfully been used as targets for nanoparticles to detect inflammation within plaques [115]. Atherosclerosis causes the release of cytokines that activate endothelial cells, which in turn leads to the expression of VCAM-1 on endothelial cells [116]. Radiolabeled NPs have also been used to quantify macrophage inflammation noninvasively in experimental atherosclerosis models through PET/computed tomography (CT) and PET/MRI [117, 118].
During atherosclerosis development, microvessels sprout from the vasa vasorum and into the plaques. Due to their inflammatory state, the new microvessels have enhanced permeability. This allows NPs to gather within plaques via non-specific accumulation [119], through a mechanism of action believed to be similar to the enhanced permeability and retention (EPR) effect observed in tumor tissues. Using fluorine MRI and magnetic resonance spectroscopy (MRS) of NPs, researchers have quantified the progressive deterioration of vascular endothelial barriers in later-stage plaques, implicating the disrupted endothelium is a potential contributor to plaque rupture susceptibility [120]. Theranostic liposomes, containing both imaging labels and the anti-inflammatory prednisolone phosphate (PLP), were developed to treat atherosclerotic inflammation. The NPs accumulated in plaques due to the increased permeability of vessels through a passive mechanism. Unlike animals treated with free PLP, significant reduction in macrophage burden was observed in rabbit models treated with the NPs [56].
Besides exploiting the vascular permeability associated with EnD, the activated endothelium in atherosclerotic plaques can be directly targeted with surface-functionalized NPs. For example, Chan et al. developed lipid-polymer NPs functionalized with collagen IV-targeting peptides and loaded with paclitaxel. These NPs were successfully used to target injured vasculature and suppress stenosis [121]. Similarly, functionalization of NPs with VCAM-1-targeted peptides has enabled quantification of NP accumulation in targeted tissues of mice [122]. Researchers have also used αVβ3 integrin-targeted perfluorocarbon NPs loaded with both fumagillin (antiangiogenic drug) and Gd-DTPA (MRI signal enhancer) for imaging guided drug delivery in atherosclerotic rabbits [123]. In this study, a reduction in angiogenesis was observed through the resultant MRI enhancement data, which showed a decrease in angiogenesis when the drug was present as compared to the control.
Failure to resolve the maladaptive inflammatory response to excessive subendothelial lipoproteins leads to enhanced inflammatory cell recruitment, macrophage death, and defective clearance of apoptotic cells [124]. The imbalance between the pro- and anti-inflammatory responses within blood vessels, with the former dominating the later, is a hallmark of atherosclerosis. Significant attention has therefore focused on not only using nanomedicine to prevent inflammation but also to enhance natural endothelial anti-inflammatory mechanisms [125]. Researchers recently developed collagen IV-targeted NPs containing the anti-inflammatory peptide Ac2–26, with the goal of enhancing inflammation resolution by effectively limiting recruitment of neutrophils and decreasing IR injury-induced tissue damage in chronic inflammatory disease models [126]. By encapsulating Ac2–26, researchers were able to enhance the peptide’s bioavailability once injected. In murine models, the NPs significantly decreased polymononuclear neutrophils recruitment (56% vs. 30% for peptide-only) and improved the prevention of I/R-induced tissue damage.
High-density lipoprotein (HDL) is a natural compound that transports cholesterol from atherosclerotic plaques to the liver, exhibiting athero-protective properties [127–130]. HDL also can restore endothelial function [131, 132] and reduce risk of coronary artery disease [133]. Direct infusions of HDL NPs have been shown to control fatty acid metabolism in patients with type 2 diabetes [134], to promote cholesterol efflux in humans [135], and to reduce human atherosclerotic plaques [136]. HDL’s inherent interaction with plaque macrophages renders it an attractive NP platform for targeted delivery of diagnostic and/or therapeutic agents. HDL-derived nanomaterials have been reconstituted to encapsulate inorganic nanocrystals for medical imaging [137, 138] as well as to deliver therapeutic molecules and silencing RNAs [139, 140]. Traditional methods of synthesizing these HDL NPs involve lengthy procedures, often difficult to scale up. By contrast, a recent method for the reconstitution of HDL-based nanomaterials uses microfluidics technology and has shown potential for the scale-up production and effective optimization of these multifunctional HDL NPs [141]. This platform allows the synthesis of reproducible and homogeneous HDL NPs in a single-step process, significantly decreasing the complexity and time needed for NP formulation. Furthermore, this technology has been used to successfully encapsulate hydrophobic molecules, as well as imaging agents such as gold, iron oxide, quantum dot nanocrystals, and fluorophores for CT, MRI, and fluorescence microscopy, respectively.
Although significant progress has been made in using nanomedicine for atherosclerosis in a preclinical context, clinical studies have primarily been concentrated on the diagnosis of inflammation, with a focus on the use of iron oxide NPs [142, 143]. Two clinical trials with nanomedicine therapies for atherosclerosis are being conducted for the investigation of NP plaque targeting and silencing inflammatory activity [144, 145]. The trial uses PEGylated liposomal NPs loaded with prednisolone sodium phosphate, an anti-inflammatory drug; investigators hope to intravenously deliver the NPs to plaques. Their aim is to minimize the effects of immunosuppression by targeting macrophages located within plaques directly. Furthermore, the investigators hope that the NPs will prolong the efficacy of the drug, thereby enhancing its anti-inflammatory benefits.
Ultimately, the success of atherosclerotic treatments will depend not only on the development of novel anti-inflammatory nanomedicines but also on the appropriate identification of patients and corresponding clinical endpoints. Despite a deeper understanding of the critical role that EnD and subsequent inflammation plays, as well as advances in the treatment of atherosclerotic plaques through anti-inflammatory nanomedicine, current standards of diagnosis and care for atherosclerotic patients focus on blood lipid and cholesterol levels. Some investigators have suggested other biomarkers to diagnose atherosclerosis, such as high-sensitivity C-reactive protein [146] and plasma osteoprotegerin [147]; both of these markers have been correlated with traditional risk factors of atherosclerosis. Until this issue is appropriately addressed, it will be difficult to not only correctly identify and diagnose patients at risk of atherosclerotic events but to treat patients to an appropriate clinical endpoint.
Cancer
Tumors larger than 1mm3 in volume require blood vessels to grow via angiogenesis. While EC monolayers in normal blood vessels form tight junctions between each other with no overlap, tumor ECs grow and branch excessively and uncontrollably. This results in chaotic structures of enlarged inter-endothelial gaps, with associated break-down of tight junctions between ECs and disrupted basement membranes [150]. EnD is also observed in the pathogenesis of metastasis, wherein tumor cells invade the endothelium, enter the circulatory system, migrate to new niche locations, and subsequently colonize distant sites [151].
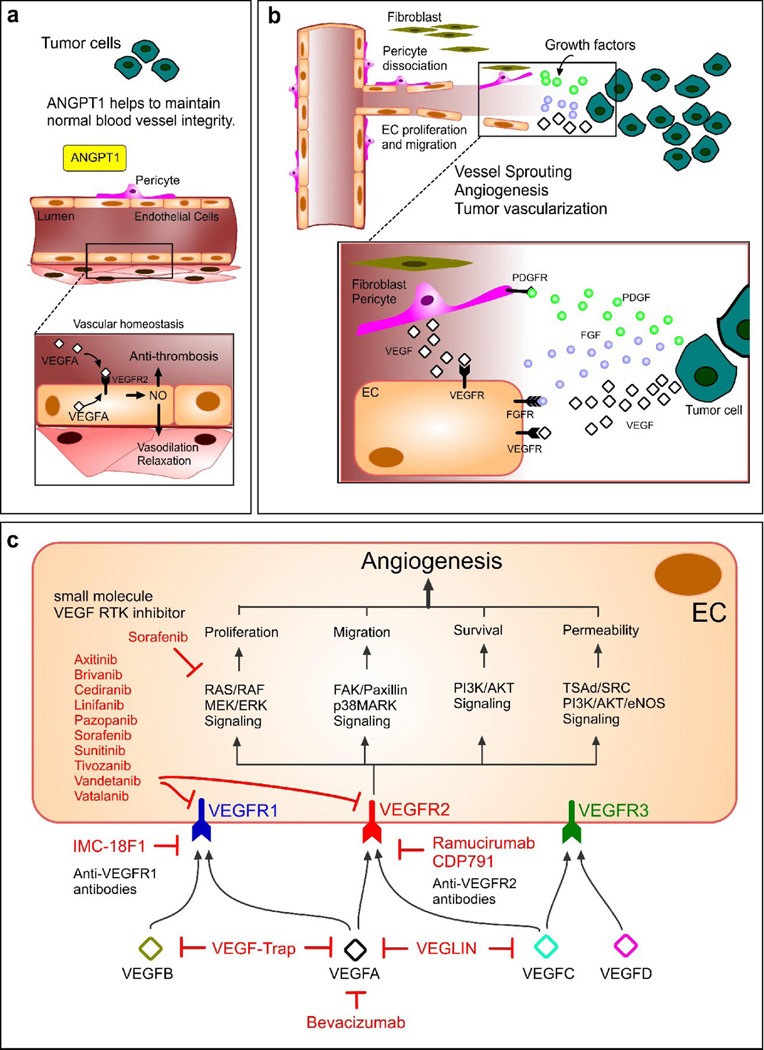
One of vascular endothelial growth factor (VEGF)’s main functions is to support EC proliferation and migration (Figure 4a), stimulating angiogenesis and vasculogenesis. However, chronic stimulation or overexpression of VEGF in tumors causes abnormal fluid leakage across the EC monolayer and leads to the deposition of signaling molecules in the interstitium. For example, VEGF receptors and their complexes with NRP-1,-2 enhance endothelial proliferation and survival [152, 153], but cells expressing VEGF receptor 1 (VEGFR1) establish cluster formation to upregulate fibronectin for metastasized cancer cell adhesion [154]. Tumor angiogenesis is therefore facilitated in tumors (Figure 4b) [155]. Solid tumors often produce large concentrations of vascular permeability factors as a result of rapidly growing tumor cells, which require an increased supply of nutrients and oxygen. As a result, the definitive vascular biology features of tumors arise, including an imbalance between angiogenic inhibitors (e.g. thrompospondin-1) and angiogenic stimulators (e.g., VEGF) [156] and overexpression and activation of various integrins [157].
Figure 4. The role of VEGF in tumor growth and inhibition of VEGF as a cancer therapy.
(a) Angiopoietin 1 (ANGPT1) and VEGF play important roles through the actions of circulating VEGF and intracrine actions of endothelial-derived VEGF. (b) Tumors express several pro-angiogenic factors (e.g. VEGF, bFGF, PDGF). Interstitial fibroblasts and dissociated microvascular pericytes, stimulated by PDGF from tumors, release VEGF and contribute to EC proliferation and migration, thereby exerting paracrine EC protective effects during angiogenesis [148]. Blockage of these mechanisms is expected to improve the efficacy of cancer therapy as well as inhibition of pro-angiogenic factors. (c) Strategies to inhibit VEGF signaling include monoclonal antibodies targeting VEGFA, such as Bevacizumab, and VEGF receptors such as IMC-18F1 and Ramucirumab. Also, soluble VEGF receptors, such as VEGF-Trap or VEGLIN, have been used to inhibit VEGF signaling. In ECs, many small-molecule VEGF RTK inhibitors have been tested to prevent ligand-dependent receptor phosphorylation of VEGFR1 and VEGFR2, which would otherwise trigger various signaling pathways, eventually activating angiogenesis [149].
The investigation of anti-VEGF therapies for the regression of tumor growth has been studied for over three decades, beginning with the pioneering work of Folkman in the early 1970 [158]. For instance, VEGF inhibitors (e.g. bevacizumab (Avastin®)) bind VEGFA to inhibit cell proliferation and block signaling through VEGFR1 and VEGFR2 (Figure 4c) [159]. However, abrupt VEGF inhibition can disrupt vascular homeostasis, resulting in vascular contraction, hypertension, regression of blood vessels, increased vascular tone, and proteinuria [160]. Through nanomedicine, the sustained release of bevacizumab can be facilitated; its rate of release can be tuned using varying compositions and ratios of polymeric PLGA and PLA-PEG NPs [161]. Furthermore, single agent VEGF inhibitory therapy is minimally effective [162], whereas combination therapy of low-dose antiangiogenic drugs with chemotherapy drugs has higher efficacy than either drug alone [163]. Bevacizumab with paclitaxel, for example, is dramatically better at fighting tumors in in vivo studies than either of the two alone [164]. Chemotherapy with simultaneous administration of anti-angiogenic therapy has been shown to have synergistic effects [165, 166]. Anti-angiogenic polymeric nanoparticles loaded with paclitaxel, which exhibits anti-angiogenic effects at low doses and bear RGDfK integrin-targeting ligands, were shown to inhibit the growth of proliferating αvβ3-expressing ECs in several cancers [167]. Targeted nanoparticle-mediated nucleic acid and drug delivery can be effectively used for tumor anti-angiogenic therapies [168–172]. Recently nano-graphene was developed as a vascular marker for tumor angiogenesis - whereby 27nm PEGylated nano-graphene oxide NPs were successfully directed to tumor neovasculature in vivo by targeting CD105 (endoglin) [173]. The efficacy of this system was investigated in vitro, in vivo and ex vivo by PET.
One area of intense research in drug delivery concerns the development of NPs that can penetrate further within tumors and remain within the tumor or bound to cancer cells. The large gaps between tumor ECs facilitate the extravasation of particulate material from the surrounding vessels into tumors [174], and they are a major contributor to the EPR effect [175]. Recently, there has been a growing awareness in understanding the prevalence and degree of EPR in humans, given that the various processes involved in the EPR effect are heterogeneous across patient populations [176]. It may be beneficial to be able to predict the degree of vessel permeability in tumors via imaging using theranostics or companion diagnostics, and nanomedicine can play a major role here [176–181]. In addition to abnormal architecture, tumor blood vessels also have impaired receptors for angiotensin II, which controls vessel constriction [182].
There are a number of vascular mediators which facilitate the EPR effect and these include bradykinin, nitric oxide (NO), peroxynitrite (ONOO), prostaglandins, angiotensin-converting enzyme (ACE) inhibitors, VEGF, and numerous other cytokines [183]. Methods of elevating blood pressure or introducing NO-secreting compounds have been investigated by means of administering adjuvants in addition to NP injections [183, 184]. For example, VEGF was shown to increase vascular permeability and enhance the extravasation of NPs across tumor vasculature when co-administered with liposome NPs [185]. In addition to bradykinin, NO and prostaglandins are factors involved in the regulation of vascular permeability, and the administration of a number of kinase inhibitors has led to an enhanced EPR effect [186]. The co-administration of a transforming growth factor beta (TGFβ) receptor inhibitor led to an enhancement of EPR-mediated accumulation of both liposomal and micelle NPs, a direct result of reduced pericyte coverage on tumor neovasculature [186]. Enhancing vascular permeability and lowering the pressure difference can increase the overall “leakiness” of tumor vessels and therefore ‘passive’ accumulation of NPs. Nanomedicines, such as Doxil and Abraxane, have shown improvements in drug toxicity and response rates in cancer therapy [50]. On the other hand, ‘active’ targeting is a term used to describe the mode of action of NPs with surfaces bearing affinity ligands that specifically target cell populations [50]. The majority of FDA-approved nanomedicines are non-targeted, whilst targeted NPs in clinical trials are mostly limited to a few receptors such as transferrin that is overexpressed on the surface of various proliferating cancer cells [50]. Nonetheless, the successful clinical translation of these nanomedicines has led to the investigation of more advanced NP formulations, including targeted nanomedicines such as BIND-014 [187, 188]. BIND-014 is a prostate specific membrane antigen (PSMA)-targeted docetaxel (Dtxl)-encapsulated polymeric NP, and it is currently undergoing clinical development22. PSMA is a transmembrane protein overexpressed on the surface of prostate cancer cells and tumor-associated neovasculature of virtually all solid tumors, making PSMA an ideal cancer target [189, 190]. Other examples of nanomedicines relevant to EnD include doxorubicin- and MRI contrast agent-loaded polymeric micelles with a cRGD ligand that can target α v β 3 integrins on tumor ECs [191] and cisplatin-loaded poly(acrylamide) NPs with a F3 peptide targeting moiety that can treat ovarian tumor ECin vivo [192].
Concepts in the field of anti-angiogenesis research are rapidly changing as tumor endothelium, and thus vessels, are highly complex and heterogeneous. Inhibiting VEGF is not without complications (i.e., upregulation of compensatory angiogenic pathways, heterogeneity in tumor types, etc.) [193], and in many cases, sustained release of potent anti-angiogenic agents is required. Therefore, novel nanomedicines that can improve bioactivity and prolong the bioavailability of drugs targeting the vascular and neovasculature of tumors may offer new and more effective treatment modalities. For a more detailed cancer-focused review, including the latest advances in tumor nanotherapy, please refer to the article by Xu and colleagues [194].
Concluding remarks and future perspectives
Understanding EnD is critical for the advancement of translational nanomedicine in the treatment of many EnD-associated diseases. Targeting the dysfunctional endothelium, specifically the common pathogenic factors that cause its dysfunction, may provide a method for treating multiple disorders simultaneously. Notably, NPs may be used to aid the treatment of EnD that involves life-threatening conditions such as stroke, ALI, and ischemia. For instance, successful targeting of the VEGF pathway for controlled EC activation and proliferation (i.e. regulatory microvascular permeability) could provide a common therapy to treat EnD associated diseases including diabetes, ARDS/ALI, I/R injury, cancer, and CVDs.
Conventional drug delivery, without the use of nanocarriers, leads to the distribution of drugs and therapeutic agents throughout the body. Nanomedicine using surface engineering approaches for active targeting bestows high specificity to the NPs and therefore facilitates their selective accumulation at the site of disease, in addition to increasing the effectiveness of treatments. For example, peptide-coated NPs have enabled more effective ligand-specific gene delivery to human ECs with enhanced efficacy, specificity, biodegradability, and low cytotoxicity [195]. Another study using liposome-DNA complexes coated with human serum albumin showed that these NPs enhanced transfection of lymphocytes and macrophages compared to non-coated NPs [196].
Commercially available nanomedicine therapies today are mostly administered intravenously. Due to the invasive and often painful nature, patient compliance is an issue. Consequently, the drive for the development of orally administered nanomedicine has gained traction, with emphasis on the treatment of diabetes, since diabetes tends to require constant monitoring and systemic administration of treatment. Standard care for diabetes using open-loop insulin administration requires periodic and invasive insulin injections and tends to lead to poor glucose control [197]. A major advantage of diabetes nanomedicine geared towards oral delivery is the enhanced bioavailability of these drugs, whereas direct delivery of free peptides and protein drugs through the gastrointestinal track is accompanied by low bioavailability due to the gastro-intestinal barriers [198–201]. For example, the use of polymeric materials has also shown potential for improved oral insulin delivery, particularly owing to their excellent biocompatibility (e.g. polycaprolatone [202])) and mucoadhesion (e.g. chitosan [203, 204]), which can enhance epithelial permeability in the gut (e.g. by disrupting TJs [205]). Polymeric NPs loaded with CoQ10 were shown to reduce blood pressure to normal levels when orally administered to hypertensive rats [206]. The process was as effective as the commercial formation of CoQ10 that is currently administered, highlighting the effectiveness of noninvasive drug administration through the use of a NP platform. To effectively translate to the clinic, these NP platforms need to demonstrate long-term stability, efficacy, and superiority over existing common injectable insulin treatments, including prolonged regulation of glucose levels in patients at least on par with those shown in animal models [207].
In order to develop effective nanotherapies, the following outstanding questions for effective clinical translation of nanomedicine for EnD should be considered: 1) How do nanocarriers interact with and pass through vascular endothelial barriers that are dynamically regulated for homeostasis?; 2) How does the dysfunctional endothelium influence NP behavior compared to a regularly function endothelium?; 2) Is passive transport of nanocarriers via the EPR effect a promising mechanism for targeted delivery of therapeutic agents into the endothelium?; and 4) Does the NP penetrate the endothelium by changing the endothelium’s chemical and biological properties or by direct mechanical effects? To answer these questions, the biophysicochemical properties of NPs need to be investigated with consideration of how these particles will interact with biological systems [208]. The following challenges first need to be taken into consideration for optimal nanomedicine design: 1) the building blocks of NPs need to be non-toxic, biodegradable, and bioeliminable; 2) the synthetic components and formulation procedures should be simple, scalable, and cost-effective with high productivity and reproducibility; 3) the NPs need to be stable with a suitable shelf-life period; and 4) a clear therapeutic advantage over the free form of the drug (and possibly existing therapies) should be demonstrated.
In addition to these intrinsic challenges associated with designing and developing NPs, there exist broader challenges. In particular, the challenges related to NP regulation and manufacturing need to be kept in mind. Any new NP therapy must not only comply with existing regulatory guidelines but also pass the Food and Drug Administration’s three-phase human clinical trials. Furthermore, the challenges associated with scaling up the production of NPs may impede their penetration and assimilation as standard therapeutic agents.
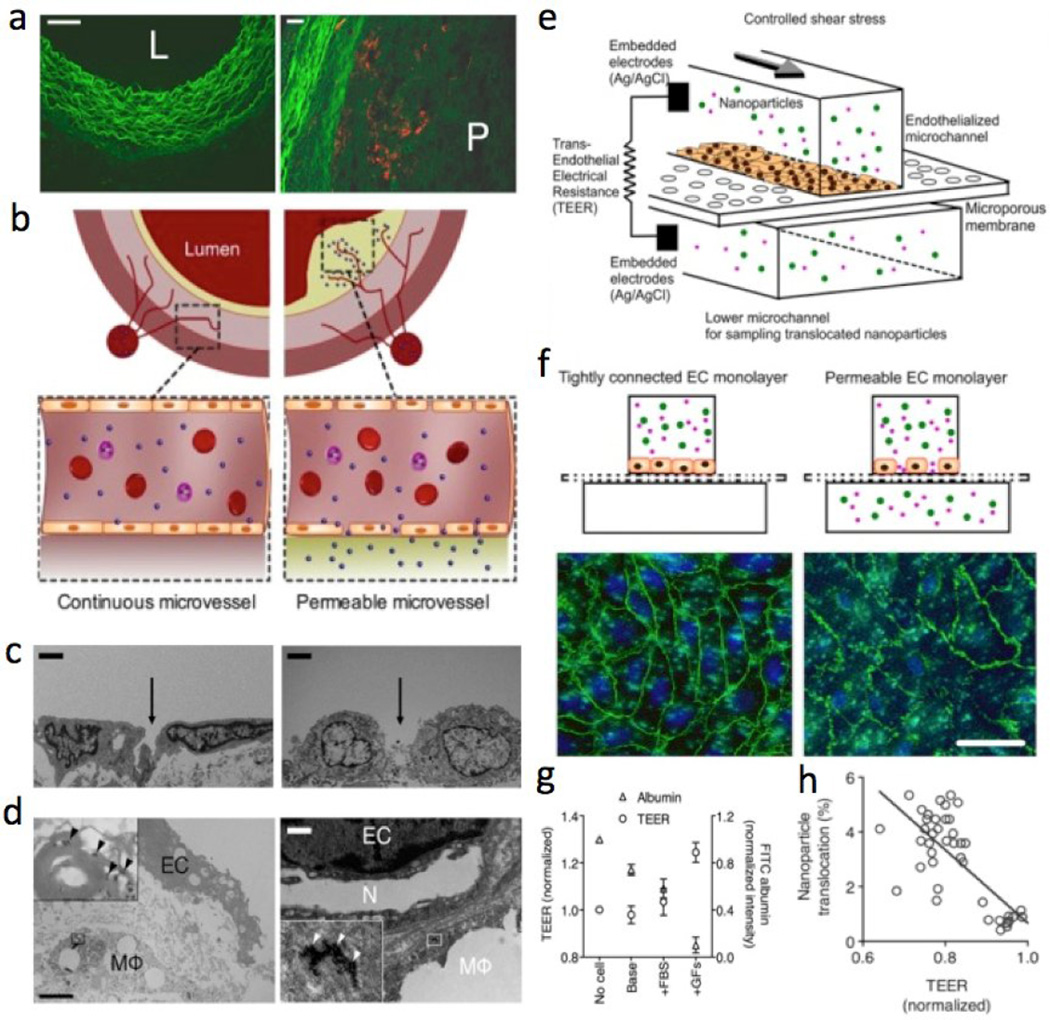
Fortunately, novel microfluidic approaches, not only related to the development of NPs [141, 210–213] but also as diagnostic techniques [214–217], have gained huge traction over the last years [218–221]. Indeed, the evolution of microfluidics has seen the incorporation of in vitro cellular approaches and integration on chips [222], which will provide a better understanding of EC function and dysfunction [223, 224]. Our recent study demonstrated that an endothelialized microfluidic model can serve as a complementary in vitro platform to examine NP delivery to dysfunctional ECs and/or mural cells for the screening of NPs in pathophysiological conditions, including in vivo validation of NP translocation across the atherosclerotic endothelium (Figure 5) [209]. This integrative approach can be applied to optimize nanoparticles and study their potentials in a range of other diseases, including cancer, diabetes, and inflammation. In the future, the ability to create physiologically realistic microsystems that can mimic in vitro microvessels [225, 226], and manipulate in vivo small organisms [227] or ex vivo embryonic tissue excised from live embryos [228–231], will allow the identification and prediction of the potential of nanomedicines and pave the path for their rapid clinical translation.
Figure 5. Probing nanoparticle translocation across the permeable endothelium using an in vitro microfluidic model with in vivo validation.
(a) Fluorescence microscopy images of typical cross-sections of the healthy wall (L, lumen) and the atherosclerotic vessel with plaque (P). (b) Cross-section schematics of continuous normal and permeable capillaries that penetrate into the atherosclerotic plaque from the vasa vasorum. (c) TEM of the endothelial lining of a normal/healthy vessel wall (left) compared to a permeable endothelial layer with a large gap (right). (Scale bar, 2µm). (d) High resolution TEM showing ECs with a gap between them and a macrophage (MΦ) behind it. At a higher magnification, multiple individual nanoparticles (arrowheads) can be found. (Scale bar, 2µm.) The neovessel (N) within the plaque is bordered with a lipid-loaded MΦ, which itself has taken up nanoparticles as well. (Scale bar, 1µm.) (e) Diagram of an endothelialized microfluidic device enabling TEER measurement across the EC layer. (f) Permeable EC layer with disrupted adherens junctions between ECs, as evidenced by patchy expression of VE-cadherin (green) in the image on the right compared to the left. Nuclei (DAPI). (Scale bar, 20µm). (g) ECs in different culture media show different permeability (shown by TEER and FITC-albumin translocation). (h) Nanoparticle translocation and TEER from these experiments are inversely correlated (r2 = 0.54, P < 0.0001). Reprinted with permission from PNAS [209].
Highlights.
We review the key features of a functioning and a malfunctioning endothelium.
We highlight endothelial pathologies in asthma, burns, heart failure, and more.
We discuss overlapping pathologies between diabetes, atherosclerosis, and cancer.
The VEGF pathway is a possible target for treating multiple endothelial disorders.
Nanotechnology can provide safer treatments and better in vivo study validations.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) and the National Institutes of Health (NIH), as a Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C (Z.A.F. and R.L.), the National Cancer Institute Grant CA151884 (R.L. and O.C.F.), the David H. Koch–Prostate Cancer Foundation Award in Nanotherapeutics (R.L. and O.C.F.), ACTSI Emory/GA Tech Regenerative Engineering and Medicine (REM) Seed Grant (Y.K.), the Center for Pediatric Nanomedicine (CPN) of Children’s Healthcare of Atlanta Seed Grant (Y.K.), American Heart Association Scientist Development Grant 15SDG25080314 (Y.K.), and the National Institute Of Neurological Disorders and Stroke of the National Institutes of Health R21NS091682 (Y.K.), and Dr. Zhen Gu and Dr. May Tun Saung for insightful comments.
Biographies
Bomy Lee Chung

Bomy Lee Chung received her B.S. in chemical and biomolecular engineering from the Georgia Institute of Technology in 2010. She then joined the chemical engineering department at the Massachusetts Institute of Technology, where she is a PhD candidate in the laboratory of Dr. Robert Langer. Her research focuses on the design and development of nanoparticles for theranostic (therapeutic + diagnostic) uses.
Michael J. Toth

Michael Toth is a PhD student in the Laboratory of Multiscale Biosystems and Multifunctional Nanomaterials at Georgia Institute of Technology. He received his B.S. from the Department of Biomedical Engineering at Georgia Institute of Technology. Michael has focused on the development of microfluidic technologies through the applications system dynamics and control theory. These technologies are used to better understand the synthesis of nanoparticles and to optimize the process for a variety of applications including nanomedicines and imaging agents.
Nazila Kamaly

Nazila Kamaly, PhD is an Instructor in the Laboratory of Nanomedicine and Biomaterials at Harvard Medical School (HMS) and Brigham and Women’s Hospital (BWH). She received her combined bachelors and masters MSci degree in Medicinal Chemistry from University College London and completed her Ph.D. on the development of diagnostic and theranostic nanoparticles in the Department of Chemistry at Imperial College London in 2008. She has worked as a postdoctoral fellow at HMS/BWH and Imperial College London. Her research is focused on the development of multifunctional nanoparticles for therapeutic and imaging applications in a number of diseases.
Yoshitaka J. Sei

Yoshitaka Sei is a PhD student in the Laboratory of Multiscale Biosystems and Multifunctional Nanomaterials at Georgia Institute of Technology. He received his B.S. from the Department of Mechanical Engineering at The Johns Hopkins University. Yoshitaka has since shifted his focus to developing nanomedicines using microfluidic-based approaches and exploring their viability within biomimetic microenvironments, commonly known as “organ-on-a-chip” devices.
Jacob Becraft

Jacob is a PhD student at MIT in the mammalian synthetic biology laboratory of Dr. Ron Weiss in the Biological Engineering department. His primary research focus is the development of smart vaccines and therapeutics. His PhD thesis focuses on the development of synthetic translational control mechanisms to merge the fields of RNA therapeutics and synthetic biology to create next generation therapies. Before attending MIT, Jacob studied at the University of Illinois at Urbana-Champaign, where he studied Chemical and Biomolecular Engineering and worked on polymer-based gene delivery vector targeting via advanced synthesis methods under Dr. Daniel Pack.
Willem J.M. Mulder

Willem Mulder obtained a PhD in Biomedical Engineering from the Eindhoven University of Technology in 2006 after which he founded the Nanomedicine Laboratory at the Icahn School of Medicine at Mount Sinai. In 2013 he was also appointed Professor of Cardiovascular Nanomedicine at the Academic Medical Center of the University of Amsterdam. His research is funded by several National Institute of Health (NIH) R01 grants as well as a Vidi grant from the Netherlands Organisation for Scientific Research (NWO). His group focuses on the development of nanomedicinal approaches for targeted diagnosis and treatment of cardiovascular disease and cancer.
Zahi A. Fayad

Zahi A. Fayad, PhD is the Mount Sinai Endowed Professor in Medical Imaging and Bioengineering at the Icahn School of Medicine at Mount Sinai in New York City. He is Director the Translational and Molecular Imaging Institute and Vice Chair of Research in the Department of Radiology. His appointments as Professor is in the Departments of Radiology and Medicine (Cardiology). Dr. Fayad completed his graduate trainings at the Johns Hopkins University (MS, Biomedical Engineering ‘91) and at the University of Pennsylvania (PhD, Bioengineering ‘96). His interests are in Cardiovascular Imaging and Nanomedicine.
Omid C. Farokhzad

Omid Farokhzad is an Associate Professor at Harvard Medical School (HMS) and physician- scientist in the Department of Anesthesiology at Brigham and Women’s Hospital (BWH), where he is the director of the Laboratory of Nanomedicine and Biomaterials. His research focuses on developing therapeutic nanoparticle technologies. He has authored 120 papers, holds over 140 issued/pending US and International patents. He completed postgraduate clinical and research trainings, respectively, at the BWH/HMS and MIT. He received his M.D. and M.A. from Boston University School of Medicine and his M.B.A. from the Sloan School of Management at MIT.
YongTae Kim

YongTae Kim is an Assistant Professor at the George W. Woodruff School of Mechanical Engineering at Georgia Institute of Technology, where he is the director of the Laboratory of Multiscale Biosystems and Multifunctional Nanomaterials. His research focuses on the development of nanomedicines using microsystems that reconstitute organ-level functions on a chip. He was a Postdoctoral Associate in the Koch Institute at MIT. He received a Ph.D. in Mechanical Engineering from Carnegie Mellon University after he had worked at R&D Divisions of Hyundai-Kia Motors and Samsung Electronics for 6 years. He received Bachelor’s and Master’s degrees from Seoul National University.
Robert Langer

Robert Langer is the Koch Institute Professor at MIT. He has written more than 1,250 articles and has 1,050 issued and pending patents worldwide. His awards include the US National Medal of Science, the US National Medal of Technology and Innovation, the Charles Stark Draper Prize, Albany Medical Center Prize, the Wolf Prize for Chemistry, the 2014 Kyoto Prize and the Lemelson-MIT prize, for being “one of history’s most prolific inventors in medicine.” Langer is one of the very few individuals ever elected to the National Academy of Medicine, the National Academy of Engineering and the National Academy of Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
R.L. and O.C.F. have financial interests in BIND Therapeutics, Selecta Biosciences, and Blend Therapeutics, which are developing nanoparticle technologies for medical applications. These companies did not support the aforementioned research and currently have no rights to any technology or intellectual property developed as part of this research. All other authors declare no conflicts.
References
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Luo BH, Carman CV, Springer TA. Annual review of immunology. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta D, Malik AB. Physiological reviews. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bogatcheva NV, Verin AD. Microvascular research. 2008;76:202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Cardiovascular research. 2010;87:272–280. doi: 10.1093/cvr/cvq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Idris I, Gray S, Donnelly R. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 7.Rigor RR, Beard RS, Jr, Litovka OP, Yuan SY. American journal of physiology. Cell physiology. 2012;302:C1513–C1522. doi: 10.1152/ajpcell.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh K, Fukumoto Y, Shimokawa H. American journal of physiology. Heart and circulatory physiology. 2011;301:H287–H296. doi: 10.1152/ajpheart.00327.2011. [DOI] [PubMed] [Google Scholar]
- 9.Mehta D. Microvascular research. 2012;83:1–2. doi: 10.1016/j.mvr.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Hu G, Minshall RD. Microvascular research. 2009;77:21–25. doi: 10.1016/j.mvr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Birukov KG. Microvascular research. 2009;77:46–52. doi: 10.1016/j.mvr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeger M, Bengtson A, Karlsson K, Heideman M. Obstetrics and gynecology. 1989;73:551–556. [PubMed] [Google Scholar]
- 13.Liu LB, Xue YX, Liu YH, Wang YB. Journal of neuroscience research. 2008;86:1153–1168. doi: 10.1002/jnr.21558. [DOI] [PubMed] [Google Scholar]
- 14.Brown H, Chau T, Mai N, Day N, Sinh D, White N, Hien T, Farrar J, Turner G. Neurology. 2000;55:104–111. doi: 10.1212/wnl.55.1.104. [DOI] [PubMed] [Google Scholar]
- 15.Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Ridker PM, Hansson GK. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 17.Tabit CE, Chung WB, Hamburg NM, Vita JA. Reviews in endocrine & metabolic disorders. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaisman BL, Andrews KL, Khong SM, Wood KC, Moore XL, Fu Y, Kepka-Lenhart DM, Morris SM, Jr, Remaley AT, Chin-Dusting JP. PloS one. 2012;7:e39487. doi: 10.1371/journal.pone.0039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S, Singh M, Sharma PL. Vascular pharmacology. 2011;54:80–87. doi: 10.1016/j.vph.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Lefer AM. Journal of thrombosis and thrombolysis. 1997;4:63–65. doi: 10.1023/a:1017542201505. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis NA, Orfanos SE. Current opinion in critical care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 23.Annuk M, Zilmer M, Fellstrom B. Kidney international. 2003;63:50–53. doi: 10.1046/j.1523-1755.63.s84.2.x. [DOI] [PubMed] [Google Scholar]
- 24.Murdaca G, Colombo BM, Cagnati P, Gulli R, Spano F, Puppo F. Atherosclerosis. 2012;224:309–317. doi: 10.1016/j.atherosclerosis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Bijl M. The Netherlands journal of medicine. 2003;61:273–277. [PubMed] [Google Scholar]
- 26.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Diabetes care. 2009;32(Suppl 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banquet S, Delannoy E, Agouni A, Dessy C, Lacomme S, Hubert F, Richard V, Muller B, Leblais V. Cellular signalling. 2011;23:1136–1143. doi: 10.1016/j.cellsig.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Golomb BA, Evans MA. American journal of cardiovascular drugs: drugs, devices, and other interventions. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafiq MM, Menon DV, Victor RG. The American journal of medicine. 2008;121:265–271. doi: 10.1016/j.amjmed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persson F, Rossing P, Parving HH. British journal of clinical pharmacology. 2013;76:580–586. doi: 10.1111/bcp.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers KE, Sethna C. Pediatric nephrology. 2013;28:711–720. doi: 10.1007/s00467-012-2316-4. [DOI] [PubMed] [Google Scholar]
- 32.Hamnvik OP, McMahon GT. The Mount Sinai journal of medicine, New York. 2009;76:234–243. doi: 10.1002/msj.20116. [DOI] [PubMed] [Google Scholar]
- 33.Baltatu OC, Iliescu R, Zaugg CE, Reckelhoff JF, Louie P, Schumacher C, Campos LA. Frontiers in physiology. 2012;3:103. doi: 10.3389/fphys.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghimi SM, Hunter AC, Murray JC. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 35.Kataoka K, Harada A, Nagasaki Y. Advanced drug delivery reviews. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 36.Torchilin VP. Nature reviews. Drug discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 37.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Journal of controlled release : official journal of the Controlled Release Society. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 38.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Biomaterials. 2009;30:2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. Angewandte chemie. 2011;50:7027–7031. doi: 10.1002/anie.201101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosman AW, Janssen HM, Meijer EW. Chemical reviews. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 42.Bianco A, Kostarelos K, Prato M. Current opinion in chemical biology. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Ferey G. Angewandte chemie. 2006;45:5974–5978. doi: 10.1002/anie.200601878. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Journal of the American Chemical Society. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Gupta M. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Mulder WJ, Koole R, Brandwijk RJ, Storm G, Chin PT, Strijkers GJ, de Mello Donega C, Nicolay K, Griffioen AW. Nano letters. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 47.Kratz F. Journal of controlled release : official journal of the Controlled Release Society. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Farokhzad OC, Langer R. ACS nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 49.Shi JJ, Xiao ZY, Kamaly N, Farokhzad OC. Accounts of chemical research. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 50.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chemical Society reviews. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao W, Langer R, Farokhzad OC. Angewandte chemie. 2010;49:6567–6571. doi: 10.1002/anie.201001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao Z, Levy-Nissenbaum E, Alexis F, Luptak A, Teply BA, Chan JM, Shi J, Digga E, Cheng J, Langer R, Farokhzad OC. ACS nano. 2012;6:696–704. doi: 10.1021/nn204165v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, Farokhzad OC. Nanomedicine. 2012;8:687–698. doi: 10.2217/nnm.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gianella A, Jarzyna PA, Mani V, Ramachandran S, Calcagno C, Tang J, Kann B, Dijk WJ, Thijssen VL, Griffioen AW, Storm G, Fayad ZA, Mulder WJ. ACS nano. 2011;5:4422–4433. doi: 10.1021/nn103336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, Dickson SD, Nicolay K, Banciu M, Schiffelers RM, Metselaar JM, van Bloois L, Wu HS, Fallon JT, Rudd JH, Fuster V, Fisher EA, Storm G, Mulder WJ. Molecular pharmaceutics. 2010;7:2020–2029. doi: 10.1021/mp100309y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao W, Xiao Z, Radovic-Moreno A, Shi J, Langer R, Farokhzad OC. Methods in molecular biology (Clifton, N.J) 2010;629:53–67. doi: 10.1007/978-1-60761-657-3_4. [DOI] [PubMed] [Google Scholar]
- 58.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Nature biotechnology. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Z, Yang J, Xia YF, Huang WZ, Wang ZT, Dai Y. Journal of biochemical and molecular toxicology. 2012;26:282–290. doi: 10.1002/jbt.21420. [DOI] [PubMed] [Google Scholar]
- 60.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. Pharmaceutical research. 2011;28:662–672. doi: 10.1007/s11095-010-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Zhang Y, Wurtz W, Lee JK, Malinin VS, Durwas-Krishnan S, Meers P, Perkins WR. Journal of aerosol medicine and pulmonary drug delivery. 2008;21:245–254. doi: 10.1089/jamp.2008.0686. [DOI] [PubMed] [Google Scholar]
- 62.Salmina AB, Inzhutova AI, Malinovskaya NA, Petrova MM. J Alzheimers Dis. 2010;22:17–36. doi: 10.3233/JAD-2010-091690. [DOI] [PubMed] [Google Scholar]
- 63.Liu G, Men P, Kudo W, Perry G, Smith MA. Neuroscience letters. 2009;455:187–190. doi: 10.1016/j.neulet.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wanner A, Mendes ES. American journal of respiratory and critical care medicine. 2010;182:1344–1351. doi: 10.1164/rccm.201001-0038PP. [DOI] [PubMed] [Google Scholar]
- 65.Park HS, Kim KH, Jang S, Park JW, Cha HR, Lee JE, Kim JO, Kim SY, Lee CS, Kim JP, Jung SS. International journal of nanomedicine. 2010;5:505–515. doi: 10.2147/ijn.s11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demling RH. The Journal of burn care & rehabilitation. 2005;26:207–227. [PubMed] [Google Scholar]
- 67.Hemmila MR, Mattar A, Taddonio MA, Arbabi S, Hamouda T, Ward PA, Wang SC, Baker JR., Jr Surgery. 2010;148:499–509. doi: 10.1016/j.surg.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Acta ophthalmologica. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 69.Natarajan JV, Ang M, Darwitan A, Chattopadhyay S, Wong TT, Venkatraman SS. International journal of nanomedicine. 2012;7:123–131. doi: 10.2147/IJN.S25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerem A, Yin J, Kaestle SM, Hoffmann J, Schoene AM, Singh B, Kuppe H, Borst MM, Kuebler WM. Circulation research. 2010;106:1103–1116. doi: 10.1161/CIRCRESAHA.109.210542. [DOI] [PubMed] [Google Scholar]
- 71.Lukyanenko V. Nanomedicine. 2007;2:831–846. doi: 10.2217/17435889.2.6.831. [DOI] [PubMed] [Google Scholar]
- 72.de Castro IF, Micheloud D, Berenguer J, Guzman-Fulgencio M, Catalan P, Miralles P, Alvarez E, Lopez JC, Cosin J, Lorente R, Munoz-Fernandez MA, Resino S. AIDS (London, England) 2010;24:2059–2067. doi: 10.1097/QAD.0b013e32833ce54d. [DOI] [PubMed] [Google Scholar]
- 73.Thomas T, Foster G. International journal of nanomedicine. 2007;2:19–24. doi: 10.2147/nano.2007.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghiadoni L, Taddei S, Virdis A. Current vascular pharmacology. 2012;10:42–60. doi: 10.2174/157016112798829823. [DOI] [PubMed] [Google Scholar]
- 75.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. British journal of pharmacology. 2009;157:527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caballero AE. Obesity Research. 2012;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 77.Mansour HM, Rhee YS, Wu X. International journal of nanomedicine. 2009;4:299–319. doi: 10.2147/ijn.s4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubinstein I, Ikezaki H, Onyuksel H. International journal of pharmaceutics. 2006;316:144–147. doi: 10.1016/j.ijpharm.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 79.Versari D, Virdis A, Ghiadoni L, Daghini E, Duranti E, Masi S, Magagna A, Taddei S. Atherosclerosis. 2009;205:214–220. doi: 10.1016/j.atherosclerosis.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 80.Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, Rostom A, Anderson TJ, Beck PL. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:175–182. doi: 10.1016/j.cgh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 81.Laroui H, Theiss AL, Yan Y, Dalmasso G, Nguyen HT, Sitaraman SV, Merlin D. Biomaterials. 2011;32:1218–1228. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamprecht A, Yamamoto H, Takeuchi H, Kawashima Y. Journal of pharmacology and experimental therapeutics. 2005;315:196–202. doi: 10.1124/jpet.105.088146. [DOI] [PubMed] [Google Scholar]
- 83.Pichai MV, Ferguson LR. World journal of gastroenterology : WJG. 2012;18:2895–2901. doi: 10.3748/wjg.v18.i23.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, Deanfield JE. Circulation. 2010;121:2310–2316. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 85.Hartner WC, Verma DD, Levchenko TS, Bernstein EA, Torchilin VP. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2009;1:530–539. doi: 10.1002/wnan.46. [DOI] [PubMed] [Google Scholar]
- 86.Hori M, Nishida K. Cardiovascular research. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 87.Buxton DB. Nanomedicine. 2012;7:173–175. doi: 10.2217/nnm.11.184. [DOI] [PubMed] [Google Scholar]
- 88.Gray WD, Che P, Brown M, Ning X, Murthy N, Davis ME. Journal of cardiovascular translational research. 2011;4:631–643. doi: 10.1007/s12265-011-9292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verspohl EJ. Pharmacological reviews. 2012;64:188–237. doi: 10.1124/pr.110.003319. [DOI] [PubMed] [Google Scholar]
- 90.Zhang TX, Xu JX, Peng F, Chai DJ, Lin JX. Blood pressure. 2013;22:106–113. doi: 10.3109/08037051.2012.732761. [DOI] [PubMed] [Google Scholar]
- 91.Hocher B, Reichetzeder C, Alter ML. Kidney & blood pressure research. 2012;36:65–84. doi: 10.1159/000339028. [DOI] [PubMed] [Google Scholar]
- 92.Snima K, Jayakumar R, Unnikrishnan A, Nair SV, Lakshmanan V-K. Carbohydrate polymers. 2012;89:1003–1007. doi: 10.1016/j.carbpol.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 93.Jean M, Alameh M, De Jesus D, Thibault M, Lavertu M, Darras V, Nelea M, Buschmann MD, Merzouki A. European Journal of Pharmaceutical Sciences. 2012;45:138–149. doi: 10.1016/j.ejps.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Ma Z, Lim TM, Lim L-Y. International journal of pharmaceutics. 2005;293:271–280. doi: 10.1016/j.ijpharm.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 95.Rader DJ, Daugherty A. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 96.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Cell and tissue research. 2009;335:165–189. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 97.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Current medicinal chemistry. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 98.Mehta NN, Sheetz M, Price K, Comiskey L, Amrutia S, Iqbal N, Mohler ER, Reilly MP. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2009;23:17–24. doi: 10.1007/s10557-008-6144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura S, Chikaraishi Y, Tsuruma K, Shimazawa M, Hara H. Experimental eye research. 2010;90:137–145. doi: 10.1016/j.exer.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 100.Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Diabetes care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 101.O'Sullivan ES, Vegas A, Anderson DG, Weir GC. Endocrine reviews. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh K, Kanapathipillai M, Korin N, McCarthy JR, Ingber DE. Nano letters. 2012;12:203–208. doi: 10.1021/nl203334c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ah Kim H, Lee S, Park JH, Lee S, Lee BW, Ihm SH, Kim TI, Kim SW, Ko KS, Lee M. Journal of drug targeting. 2009;17:242–248. doi: 10.1080/10611860902718664. [DOI] [PubMed] [Google Scholar]
- 104.Wallaschofski H, Orda C, Fuhrer D, Holzapfel HP, Krohn K, Miehle K, Neumann S, Georgi P, Paschke R. Thyroid : official journal of the American Thyroid Association. 2001;11:710–711. doi: 10.1089/105072501750362817. [DOI] [PubMed] [Google Scholar]
- 105.Libby P. The American journal of clinical nutrition. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 106.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruckert E, Rosenbaum D. Current opinion in lipidology. 2011;22:43–48. doi: 10.1097/MOL.0b013e328340b8e7. [DOI] [PubMed] [Google Scholar]
- 108.Descamps OS, De Sutter J, Guillaume M, Missault L. Atherosclerosis. 2011;217:308–321. doi: 10.1016/j.atherosclerosis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 109.Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr, Dai D, Hernandez A, Fonarow GC. American heart journal. 2009;157:111–117. e112. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 110.Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM. Nature communications. 2014;5 doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 112.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 113.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JM UK-I, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, Hayes PD, Varty K, Boyle JR, Gaunt ME, Zalewski A, Gillard JH. Journal of the American College of Cardiology. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 114.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 115.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P. Nano letters. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tedgui A, Mallat Z. Physiological reviews. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 117.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Circulation research. 2013;112:755–761. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lobatto ME, Fuster V, Fayad ZA, Mulder WJ. Nature reviews. Drug discovery. 2011;10:835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang H, Zhang L, Myerson J, Bibee K, Scott M, Allen J, Sicard G, Lanza G, Wickline S. PloS one. 2011;6:e26385. doi: 10.1371/journal.pone.0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chan JM, Rhee JW, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan H, Myerson JW, Hu L, Marsh JN, Hou K, Scott MJ, Allen JS, Hu G, San Roman S, Lanza GM, Schreiber RD, Schlesinger PH, Wickline SA. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:255–264. doi: 10.1096/fj.12-218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 124.Tabas I. Nature reviews. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klingenberg R, Hansson GK. European heart journal. 2009;30:2838–2844. doi: 10.1093/eurheartj/ehp477. [DOI] [PubMed] [Google Scholar]
- 126.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luthi AJ, Zhang H, Kim D, Giljohann DA, Mirkin CA, Thaxton CS. ACS nano. 2012;6:276–285. doi: 10.1021/nn2035457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heeren J, Beisiegel U, Grewal T. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:442–448. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 129.Glickson JD, Lund-Katz S, Zhou R, Choi H, Chen IW, Li H, Corbin I, Popov AV, Cao W, Song L, Qi C, Marotta D, Nelson DS, Chen J, Chance B, Zheng G. Molecular imaging. 2008;7:101–110. [PubMed] [Google Scholar]
- 130.Nicholls SJ, Uno K, Tuzcu EM, Nissen SE. Current atherosclerosis reports. 2010;12:301–307. doi: 10.1007/s11883-010-0125-4. [DOI] [PubMed] [Google Scholar]
- 131.Bisoendial RJ, Hovingh GK, Levels JH, Lerch PG, Andresen I, Hayden MR, Kastelein JJ, Stroes ES. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 132.Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C, Corti R, Ruschitzka F, Luscher TF, Noll G. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 133.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. JAMA : the journal of the American Medical Association. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 134.Drew BG, Carey AL, Natoli AK, Formosa MF, Vizi D, Reddy-Luthmoodoo M, Weir JM, Barlow CK, van Hall G, Meikle PJ, Duffy SJ, Kingwell BA. Journal of lipid research. 2011;52:572–581. doi: 10.1194/jlr.P012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoang A, Drew BG, Low H, Remaley AT, Nestel P, Kingwell BA, Sviridov D. European heart journal. 2012;33:657–665. doi: 10.1093/eurheartj/ehr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Circulation research. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 137.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJ. Nano letters. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Skajaa T, Cormode DP, Jarzyna PA, Delshad A, Blachford C, Barazza A, Fisher EA, Gordon RE, Fayad ZA, Mulder WJ. Biomaterials. 2011;32:206–213. doi: 10.1016/j.biomaterials.2010.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang S, Damiano MG, Zhang H, Tripathy S, Luthi AJ, Rink JS, Ugolkov AV, A TKS, Dave SS, Gordon LI, Thaxton CS. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2511–2516. doi: 10.1073/pnas.1213657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McMahon KM, Mutharasan RK, Tripathy S, Veliceasa D, Bobeica M, Shumaker DK, Luthi AJ, Helfand BT, Ardehali H, Mirkin CA, Volpert O, Thaxton CS. Nano letters. 2011;11:1208–1214. doi: 10.1021/nl1041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim Y, Fay F, Cormode DP, Sanchez-Gaytan BL, Tang J, Hennessy EJ, Ma M, Moore K, Farokhzad OC, Fisher EA, Mulder WJ, Langer R, Fayad ZA. ACS nano. 2013;7:9975–9983. doi: 10.1021/nn4039063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Degnan AJ, Patterson AJ, Tang TY, Howarth SP, Gillard JH. Cerebrovascular diseases. 2012;34:169–173. doi: 10.1159/000339984. [DOI] [PubMed] [Google Scholar]
- 143.Tang TY, Muller KH, Graves MJ, Li ZY, Walsh SR, Young V, Sadat U, Howarth SP, Gillard JH. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 144. [accessed 2014/09/30]; http://clinicaltrials.gov/ct2/show/NCT01647685.
- 145. [accessed 2014/09/30]; http://clinicaltrials.gov/ct2/show/NCT01601106.
- 146.Bian F, Yang X, Zhou F, Wu PH, Xing S, Xu G, Li W, Chi J, Ouyang C, Zhang Y. British journal of pharmacology. 2014;171:2671–2684. doi: 10.1111/bph.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mogelvang R, Pedersen SH, Flyvbjerg A, Bjerre M, Iversen AZ, Galatius S, Frystyk J, Jensen JS. The American journal of cardiology. 2012;109:515–520. doi: 10.1016/j.amjcard.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 148.Folkman J. Nature reviews. Drug discovery. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 149.Huang H, Bhat A, Woodnutt G, Lappe R. Nature reviews. Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 150.Carmeliet P, Jain RK. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen DX, Bos PD, Massague J. Nature reviews. Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 152.Zachary I, Gliki G. Cardiovascular research. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 153.Ferrara N, Gerber HP, LeCouter J. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 154.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hicklin DJ, Ellis LM. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 156.Jain RK. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 157.Desgrosellier JS, Cheresh DA. Nature reviews. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Folkman J. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 159.Wang Y, Fei D, Vanderlaan M, Song A. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 160.Kamba T, McDonald DM. British journal of cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li F, Hurley B, Liu Y, Leonard B, Griffith M. The open ophthalmology journal. 2012;6:54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, Francis P, Cheiken R, Elting J, McNabola A, Wilkie D, Petrenciuc O, Chen EX. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 163.Bocci G, Nicolaou KC, Kerbel RS. Cancer research. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 164.Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y, Nishimura G, Matsuda H, Tsukuda M. Oncology reports. 2007;18:47–51. [PubMed] [Google Scholar]
- 165.Duda DG, Jain RK, Willett CG. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4033–4042. doi: 10.1200/JCO.2007.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jain RK. Nature reviews. Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 167.Eldar-Boock A, Miller K, Sanchis J, Lupu R, Vicent MJ, Satchi-Fainaro R. Biomaterials. 2011;32:3862–3874. doi: 10.1016/j.biomaterials.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim WJ, Chang CW, Lee M, Kim SW. Journal of controlled release : official journal of the Controlled Release Society. 2007;118:357–363. doi: 10.1016/j.jconrel.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 169.Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC. Nucleic acids research. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ravindran J, Nair HB, Sung B, Prasad S, Tekmal RR, Aggarwal BB. Biochem Pharmacol. 2010;79:1640–1647. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 172.Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Biomaterials. 2010;31:2278–2292. doi: 10.1016/j.biomaterials.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 173.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, Nayak TR, Goel S, Bean J, Theuer CP, Barnhart TE, Liu Z, Cai W. ACS nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Danquah MK, Zhang XA, Mahato RI. Advanced drug delivery reviews. 2011;63:623–639. doi: 10.1016/j.addr.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 175.Greish K. Methods in molecular biology (Clifton, N.J. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 176.Prabhakar U, Blakey DC, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Cancer research. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, Lowik CW, Kaijzel EL, Que I, Storm G, Strijkers GJ, Griffioen AW, Nicolay K. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- 178.Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS. Nano letters. 2008;8:2599–2606. doi: 10.1021/nl080141f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mulder WJ, Strijkers GJ, Habets JW, Bleeker EJ, van Schaftder Schaft DW, Storm G, Koning GA, Griffioen AW, Nicolay K. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2008–2010. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- 180.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, Lacy EK, Zhang H, Robertson JD, Wickline SA, Lanza GM. Cancer research. 2003;63:5838–5843. [PubMed] [Google Scholar]
- 181.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Nature medicine. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 182.Suzuki M, Hori K, Abe I, Saito S, Sato H. J Natl Cancer Inst. 1981;67:663–669. [PubMed] [Google Scholar]
- 183.Fang J, Nakamura H, Maeda H. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 184.Nagamitsu A, Greish K, Maeda H. Jpn J Clin Oncol. 2009;39:756–766. doi: 10.1093/jjco/hyp074. [DOI] [PubMed] [Google Scholar]
- 185.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 186.Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K. Proc Natl Acad Sci U S A. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Science translational medicine. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 188.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Accounts of chemical research. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 189.Elsasser-Beile U, Buhler P, Wolf P. Curr Drug Targets. 2009;10:118–125. doi: 10.2174/138945009787354601. [DOI] [PubMed] [Google Scholar]
- 190.Slovin SF. Expert Opin Ther Targets. 2005;9:561–570. doi: 10.1517/14728222.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Nano letters. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 192.Winer I, Wang S, Lee YE, Fan W, Gong Y, Burgos-Ojeda D, Spahlinger G, Kopelman R, Buckanovich RJ. Cancer research. 2010;70:8674–8683. doi: 10.1158/0008-5472.CAN-10-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dudley AC. Cold Spring Harbor perspectives in medicine. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Trends in molecular medicine. 2015;21:223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Green JJ, Chiu E, Leshchiner ES, Shi J, Langer R, Anderson DG. Nano letters. 2007;7:874–879. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]
- 196.Simões S, Slepushkin V, Pires P, Gaspar R, Pedroso de Lima MC, Düzgüneş N. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2000;1463:459–469. doi: 10.1016/s0005-2736(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 197.Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Advanced healthcare materials. 2012;1:267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rekha MR, Sharma CP. International journal of pharmaceutics. 2013;440:48–62. doi: 10.1016/j.ijpharm.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 199.Hasan AA, Madkor H, Wageh S. Drug delivery. 2013;20:120–126. doi: 10.3109/10717544.2013.779332. [DOI] [PubMed] [Google Scholar]
- 200.Jean M, Alameh M, Buschmann MD, Merzouki A. Gene therapy. 2011;18:807–816. doi: 10.1038/gt.2011.25. [DOI] [PubMed] [Google Scholar]
- 201.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2792–2797. doi: 10.1073/pnas.1214518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Damge C, Maincent P, Ubrich N. Journal of controlled release : official journal of the Controlled Release Society. 2007;117:163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 203.Lin YH, Mi FL, Chen CT, Chang WC, Peng SF, Liang HF, Sung HW. Biomacromolecules. 2007;8:146–152. doi: 10.1021/bm0607776. [DOI] [PubMed] [Google Scholar]
- 204.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Biomaterials. 2010;31:3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 205.Yeh TH, Hsu LW, Tseng MT, Lee PL, Sonjae K, Ho YC, Sung HW. Biomaterials. 2011;32:6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 206.Ankola D, Viswanad B, Bhardwaj V, Ramarao P, Kumar M. European Journal of Pharmaceutics and Biopharmaceutics. 2007;67:361–369. doi: 10.1016/j.ejpb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 207.Sonaje K, Lin KJ, Wey SP, Lin CK, Yeh TH, Nguyen HN, Hsu CW, Yen TC, Juang JH, Sung HW. Biomaterials. 2010;31:6849–6858. doi: 10.1016/j.biomaterials.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 208.Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC. Advanced drug delivery reviews. 2012;64:1363–1384. doi: 10.1016/j.addr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim Y, Lobatto ME, Kawahara T, Lee Chung B, Mieszawska AJ, Sanchez-Gaytan BL, Fay F, Senders ML, Calcagno C, Becraft J, Tun Saung M, Gordon RE, Stroes ES, Ma M, Farokhzad OC, Fayad ZA, Mulder WJ, Langer R. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1078–1083. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mieszawska AJ, Kim Y, Gianella A, van Rooy I, Priem B, Labarre MP, Ozcan C, Cormode DP, Petrov A, Langer R, Farokhzad OC, Fayad ZA, Mulder WJ. Bioconjugate chemistry. 2013;24:1429–1434. doi: 10.1021/bc400166j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sanchez-Gaytan BL, Fay F, Lobatto ME, Tang J, Ouimet M, Kim Y, van der Staay SE, van Rijs SM, Priem B, Zhang L, Fisher EA, Moore KJ, Langer R, Fayad ZA, Mulder WJ. Bioconjugate chemistry. 2015;26:443–451. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim Y, Lee Chung B, Ma M, Mulder WJ, Fayad ZA, Farokhzad OC, Langer R. Nano letters. 2012;12:3587–3591. doi: 10.1021/nl301253v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rhee M, Valencia PM, Rodriguez MI, Langer R, Farokhzad OC, Karnik R. Advanced Materials. 2011;23:H79–H83. doi: 10.1002/adma.201004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Neeves KB, Onasoga AA, Wufsus AR. Current opinion in hematology. 2013;20:417–423. doi: 10.1097/MOH.0b013e3283642186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Warren AD, Kwong GA, Wood DK, Lin KY, Bhatia SN. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sharma S, Zapatero-Rodriguez J, Estrela P, O'Kennedy R. Biosensors. 2015;5:577–601. doi: 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Niu L, Zhang N, Liu H, Zhou X, Knoll W. Biomicrofluidics. 2015;9:052611. doi: 10.1063/1.4929579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kim Y, Langer R. Microfluidics in nanomedicine. In: Meyers RA, editor. Reviews in Cell Biology and Molecular Medicine. Wiley-VCH Verlag GmbH & Co. KGaA; 2015. pp. 127–152. [Google Scholar]
- 219.Kim Y, Pekkan K, Messner WC, Leduc PR. Journal of the American Chemical Society. 2010;132:1339–1347. doi: 10.1021/ja9079572. [DOI] [PubMed] [Google Scholar]
- 220.Kim Y, Messner WC, LeDuc P. Disruptive Science and Technology. 2012;1:41–53. [Google Scholar]
- 221.Valencia PM, Farokhzad OC, Karnik R, Langer R. Nature nanotechnology. 2012;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sei Y, Justus K, LeDuc P, Kim Y. Microfluidics and Nanofluidics. 2014;16:907–920. [Google Scholar]
- 223.Song JW, Munn LL. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hovell CM, Sei YJ, Kim Y. Journal of laboratory automation. 2015;20:251–258. doi: 10.1177/2211068214560767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chronis N, Zimmer M, Bargmann CI. Nature methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- 228.Kim Y, Joshi SD, Messner WC, LeDuc PR, Davidson LA. PloS one. 2011;6:e14624. doi: 10.1371/journal.pone.0014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim Y, Hazar M, Vijayraghavan DS, Song J, Jackson TR, Joshi SD, Messner WC, Davidson LA, LeDuc PR. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14366–14371. doi: 10.1073/pnas.1405209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Song J, Shawky JH, Kim Y, Hazar M, LeDuc PR, Sitti M, Davidson LA. Biomaterials. 2015;58:1–9. doi: 10.1016/j.biomaterials.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hazar M, Kim Y, Song J, LeDuc PR, Davidson LA, Messner WC. Lab on a chip. 2015;15:3293–3299. doi: 10.1039/c5lc00530b. [DOI] [PMC free article] [PubMed] [Google Scholar]