Abstract
OBJECTIVE
The American College of Radiology (ACR) Appropriateness Criteria panel has recommended that patients with prostate cancer who have received treatment undergo imaging only after suspected cancer recurrence. We examined whether local physicians followed this recommendation and what types of imaging examinations were ordered in a cohort of patients with local prostate cancer.
MATERIALS AND METHODS
The Rochester Epidemiology Project, a research consortium that collects, links, and stores medical record information of Olmsted County, Minnesota, residents, was used to capture the complete medical history of treated patients with prostate cancer from 2000 through 2011. Clinical information and imaging examinations performed were retrieved by chart review. Suspected recurrence was defined as treatment-specific prostate-specific antigen level elevations, bone pain, or abnormal digital rectal examination findings.
RESULTS
Of the 670 treated patients with prostate cancer who were included in the final analysis, 129 (19%) underwent posttreatment imaging. After excluding imaging related to retreatment or another cancer, 13 patients (i.e., 2% of the entire cohort and 10% of imaged patients) underwent imaging in the absence of suspected recurrence. A total of 90 patients (70% of imaged patients) underwent imaging after suspected recurrence. Of these 90 patients, 62 (69%) underwent a bone scan as their first imaging modality either alone or in combination with other imaging modalities. Of the providers who ordered a bone scan first, 27% were urologists, 23% were radiation oncologists, and 24% were primary care physicians.
CONCLUSION
Most patients in this study did not undergo imaging in the absence of suspected recurrence. Various types of imaging examinations were ordered for patients with suspected recurrence.
Keywords: American College of Radiology appropriateness criteria, bone scan, imaging guidelines, imaging utilization, prostate cancer
Prostate cancer is the most common noncutaneous cancer in men in the United States. An estimated 220,800 men will receive a diagnosis of prostate cancer in the United States in 2015, and an estimated one in seven men will develop prostate cancer during their lifetime [1]. Although many patients are considered to be at low risk and undergo active surveillance, it is recommended that high-risk patients with aggressive cancer undergo treatment. Radical prostatectomy, radiation therapy, and hormone therapy (i.e., androgen deprivation therapy) are the three major treatments for prostate cancer. The choice of treatment depends on the type of cancer, as defined by tumor pathologic Gleason grade and stage, on physician and patient preference, and on expected survival [2].
Patient monitoring after treatment is critical, because an estimated 20–50% of men will have recurrence of their cancer in the 5 years after treatment [3–5]. Patients are typically followed at periodic intervals with serum prostate-specific antigen (PSA) level measurements and digital rectal examinations (DREs). Elevations in PSA level over a posttreatment baseline level are suggestive of cancer recurrence or progression. Additional imaging, including radionuclide bone scan, MRI, CT, and transrectal ultrasound, is often then performed to determine whether the cancer has recurred or metastasized [2].
However, there are several options for follow-up imaging with varying levels of evidence to confirm clinical effectiveness at detecting local tumor recurrence and metastasis. This range of choices may contribute to physician uncertainty on which examination to order, likely leading to variation in clinical practice that may not benefit, and potentially even negatively affect, patient outcome, anxiety, burden, and cost. To reduce this uncertainty, the American College of Radiology (ACR) Appropriateness Criteria panel has published guidelines detailing which types of imaging examinations are considered appropriate as backed by clinical evidence and which examinations are considered inappropriate or of inconclusive benefit [6–9]. These guidelines, published and updated in 2000, 2005, 2007, and 2011, divide patients into radical prostatectomy, radiation therapy, and androgen deprivation therapy treatment groups. The panel notes that imaging examinations are typically not appropriate unless the patient has clinical indications of cancer recurrence, including an elevation in PSA level, abnormal DRE findings, or bone pain. In patients with suspected recurrence, the panel rated a list of examinations for each treatment group on a scale from 1 (usually not appropriate) to 9 (usually appropriate). Radionuclide bone scans are the most highly recommended modality (8 rating for all treatment groups) in patients with suspected cancer recurrence, but CT and MRI examinations of the abdomen and pelvis are also highly recommended (7 rating for all treatment groups). Bone scan is readily available and standardized. The documentation of skeletal metastasis indicates a poor prognosis despite treatment [10].
Although these ACR appropriateness criteria have existed for more than a decade, little information exists on whether and how practice patterns follow or deviate from practice guidelines. The level of adherence according to the physician’s clinical specialty (e.g., general practitioner, internal medicine, oncology, urology, and so forth) has also not been studied. This information would be valuable in determining whether the guidelines have been appropriately disseminated to physicians. In addition, areas of discrepancy, such as a group of patients for whom imaging examinations are inappropriately ordered, can be identified and examined in more detail. Discrepancies between practice patterns and practice guidelines caused by a lack of knowledge could be targeted with education. Discrepancies caused by legitimate clinical circumstances where nonadherence is best for the patient could be identified and studied.
The purpose of this study was to obtain an accurate and detailed assessment of how local physicians use radiologic imaging examinations for the clinical care of patients with prostate cancer after treatment, and whether this utilization follows the recommendation of the ACR Appropriateness Criteria panel that patients undergo imaging only after there is suspicion of cancer recurrence. The specific imaging modalities first ordered after suspected recurrence were also examined in this cohort.
Materials and Methods
Study Population
The protocol for this study was HIPAA compliant and was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. The population for this study was derived from patients in Olmsted County, Minnesota, followed by the Rochester Epidemiology Project (REP). The REP is a compilation of medical records of all health care providers in Olmsted County, enabling the longitudinal analysis of a patient’s complete medical history [11–13]. Residency status is estimated for all patients on the basis of mailing addresses to confirm whether a patient was a resident of Olmsted County during a particular time. All patient data were retrieved from the archived medical records of Mayo Clinic Rochester, Olmsted Medical Center, and the Rochester Family Medicine Clinic.
Patients were included in this study if they had a documented biopsy-confirmed diagnosis of prostate cancer during 2000–2011; underwent radical prostatectomy, radiation therapy, or androgen deprivation therapy for prostate cancer during 2000–2011; were residents of Olmsted County for at least 5 years after prostate cancer treatment; and had authorized the use of their medical record for research purposes. Patients were excluded if a diagnosis of prostate cancer was suspected but not confirmed by biopsy, if they did not receive treatment for their cancer, or if they received treatment before or after the 2000–2011 study period. Because the ACR guidelines do not have recommendations for imaging of patients with metastatic cancer, patients with metastatic cancer that was diagnosed before their treatment were also excluded.
Patients were classified by whether they received prostatectomy, radiation therapy, or androgen deprivation therapy. Patients who received multiple different treatment types were classified as follows: patients who received prostatectomy treatment in combination with salvage radiation therapy or androgen deprivation therapy or both were classified in the prostatectomy category, and patients who received radiation therapy in combination with androgen deprivation therapy were classified in the radiation therapy category.
Data Retrieval
Patients with a diagnosis of prostate cancer were identified from the REP using the International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic code 185 and confirmed via manual chart review. Patient date of birth and place of residence were retrieved from the REP. Detailed cancer information, including date of diagnosis, Gleason score, treatment type and date, whether the cancer metastasized and location of metastasis, whether the cancer recurred and date of recurrence, and whether the patient had any other type of cancer, were retrieved from the Mayo Clinic Cancer Registry, the Mayo Prostate Cancer Database, and manual chart review. Dates and results of serum PSA tests were retrieved from a combination of automated retrieval and manual chart review.
All radiology examinations of included patients in the 5 years after their initial prostate cancer treatment were identified by a combination of automated retrieval of relevant Current Procedural Terminology codes and manual chart review. Imaging examinations of patients with previously documented metastatic prostate cancer were excluded, because the ACR guidelines do not have specific recommendations for metastatic imaging. Imaging examinations specifically noted in the ACR guidelines, including 99mTc-methylene diphosphonate bone scan, CT scan, MRI scan, transrectal ultrasound (TRUS), TRUS-guided prostate biopsy, 111In-capromab pendetide (ProstaScint, Cytogen) scan, and 18F-FDG PET scan, were included for further review. Chart review was performed to determine the indication for the examination. Examinations performed for reasons other than prostate cancer, including those specifically listed for another cancer type and not for prostate cancer, were excluded. Examinations with no listed indication were included. The specialty of the ordering physician for each examination was determined by chart review.
Defining Adherence to American College of Radiology Guidelines
The ACR guidelines published in 2000 and updated in 2005 and 2007 were used to encompass this study period [6, 8, 9]. These guidelines, classified into postradical prostatectomy, postradiation therapy, and post–androgen deprivation therapy recommendations, rate various imaging examinations by a modified Delphi method on a scale from 1 to 9, with 9 considered the most appropriate. Recommendations for imaging examinations remained consistent in the 2000, 2005, and 2007 publications, enabling the use of one set of recommendations for this study. We reviewed the ACR panel recommendations of only performing imaging in patients with suspected cancer recurrence and not performing chest x-ray examinations “because prostatic lung metastasis is only found in late stage disease after other metastatic sites are well established [8,9].” Medical records were reviewed to determine how frequently imaging examinations were ordered for patients without suspected recurrence, which was defined as biochemical recurrence (for patients undergoing prostatectomy, any detectable posttreatment PSA test result; for patients undergoing radiation therapy, a ≥ 2.0 ng/mL increase in PSA level above the posttreatment nadir; and for patients undergoing androgen deprivation therapy, any increase in PSA level above the posttreatment nadir), abnormal DRE findings, or reported bone pain. Records were also reviewed to determine which imaging examinations were first ordered in patients with suspected recurrence. Imaging examinations performed within 3 days of each other were considered as being performed on the same day to account for examinations that were ordered at the same time but performed on different days.
Statistical Analysis
The purpose of this analysis was to provide a descriptive summary of how well patient care aligned with ACR guidelines. As such, descriptive statistics were used instead of inferential statistics. Data were analyzed using JMP (SAS Institute). Continuous variables are reported as median and interquartile range to account for nonparametric data distributions. Categoric variables are reported as percentages.
Results
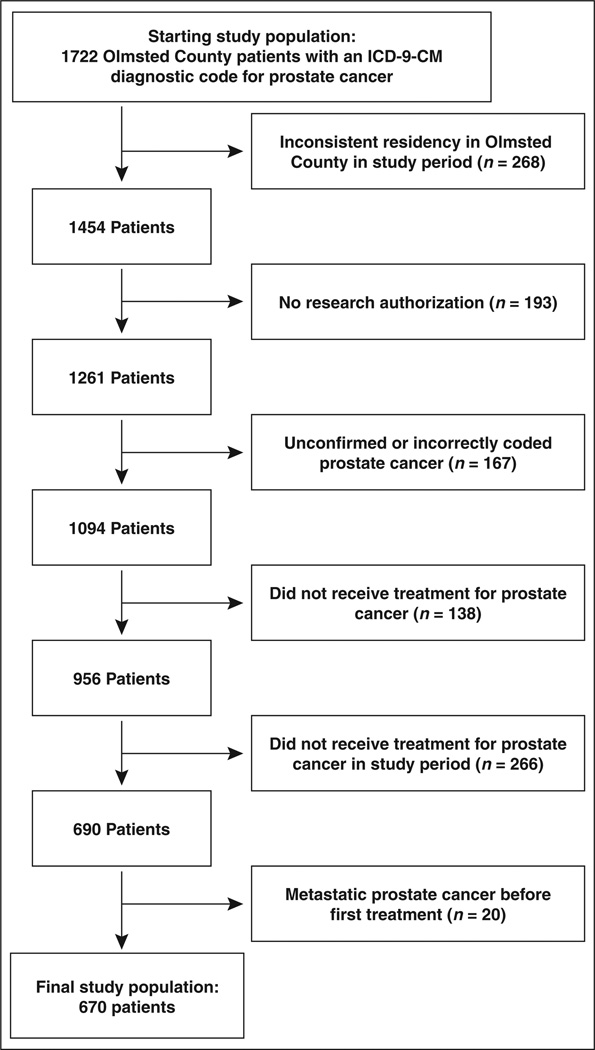
A total of 1722 patients with a diagnosis of prostate cancer from 2000 through 2011 were initially identified in the REP. Inclusion and exclusion of these patients on the basis of criteria defined in the Materials and Methods section is shown in Figure 1. Demographics of the final included study population of 670 treated patients are shown in Table 1. After all exclusions, the study cohort consisted of 670 patients, with 399 (60%) undergoing prostatectomy, 205 (31%) receiving radiation therapy, and 66 (10%) receiving androgen deprivation therapy (percentages do not total 100% because of rounding). The median Gleason score at the time of diagnosis was 6. Of these patients, 146 (22%) had documented suspicion of cancer recurrence, whether because of elevated PSA results, abnormal DRE findings, or bone pain, with 39 of those patients eventually receiving a diagnosis of metastatic disease. A total of 192 (29%) patients received a diagnosis of another type of cancer before or during the study period, with skin cancer being the most common type (n = 92 [48%]) of second primary cancers.
Fig. 1.
Study population flowchart.
TABLE 1.
Demographic Characteristics of 670 Study Participants
| Characteristic | Value |
|---|---|
| Age at first treatment (y), median (IQR) | 66 (59–72) |
| Gleason score, median (IQR) | 6 (6–7) |
| Gleason score subgroups | |
| ≤ 6 | 395 (59) |
| 7 | 202 (30) |
| 8 | 37 (5.5) |
| 9–10 | 33 (4.9) |
| Unknown | 3 (0) |
| PSA level at diagnosis (ng/mL), median (IQR) | 5.7 (3.9–8.6) |
| Treatment type | |
| Prostatectomy | 399 (60) |
| Radiation therapy | 205 (31) |
| Androgen deprivation therapy | 66 (10) |
| Documented outcome of cancer | |
| No suspicion of recurrence | 524 (78) |
| Suspected recurrence | 146 (22) |
| Documented recurrent or metastatic disease (n = 146) | |
| Local recurrence | 107 (73) |
| Confirmed metastatic disease | 39 (27) |
| Bone | 25 (64) |
| Distant lymph node | 7 (17) |
| Other | 10 (26) |
| Diagnosed with second primary cancer | |
| No | 478 (71) |
| Yes | 192 (29) |
| Skin | 92 (47) |
| Bladder | 18 (9) |
| Lung | 14 (7) |
| Melanoma | 13 (6) |
| Other | 58 (30) |
Note—Except where noted otherwise, data are number (%) of patients. Not all percentages total 100% because of rounding. IQR = interquartile range, PSA = prostate-specific antigen.
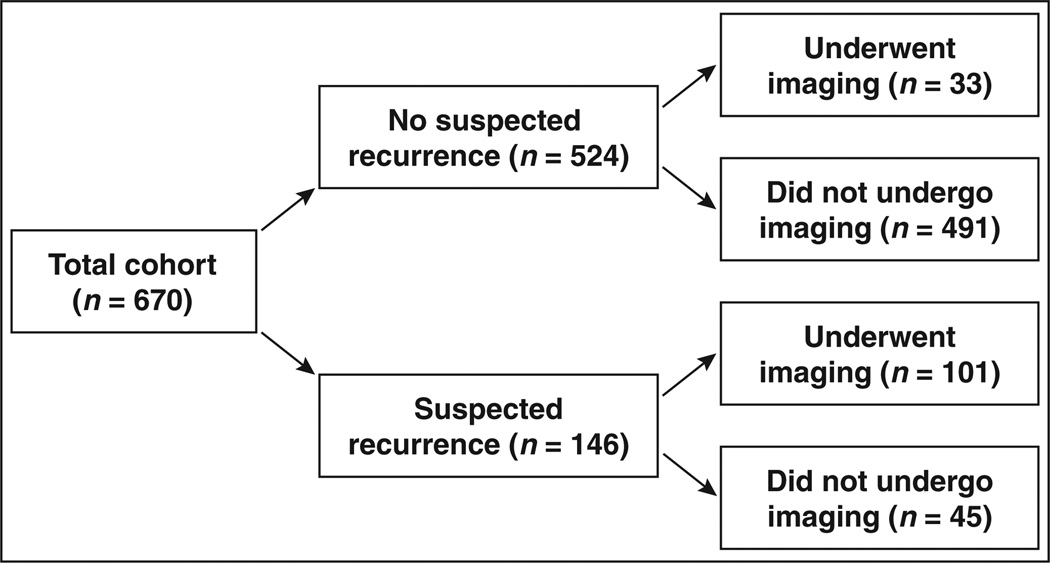
A total of 129 patients (19% of cohort) underwent 360 imaging examinations ordered by 104 unique providers in relation to their prostate cancer (Table 2). Of all the patients who underwent imaging, more than half underwent imaging in the absence of PSA elevations suggestive of cancer recurrence (no sign of recurrence, 37/129 [29%]; before signs of recurrence, 48/129 [37%]). The medical records of these patients were examined to determine whether there were PSA-independent clinical signs of cancer recurrence (Table S1, supplemental data, which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org). Bone pain was reported in 29 patients (40 examinations), and biochemical recurrence from outside PSA results or abnormal DRE findings was reported in five patients (10 examinations). After recategorizing these patients as undergoing imaging under suspicion of cancer recurrence, 61 patients underwent 114 examinations ordered by 43 unique providers in the absence of clinical evidence of cancer recurrence (Table 2). After recategorization, few patients without documented evidence of cancer recurrence underwent imaging (Fig. 2; 33/524 [6%]). Two thirds of patients with suspected cancer recurrence underwent at least one imaging examination either before or after clinical signs of recurrence (101/146 [69%]).
TABLE 2.
Imaging Examinations Performed on Study Population
| Reason for Imaging Examination | No. of Patients (n = 129)a |
No. of Imaging Examinations (n = 360) |
|---|---|---|
| Cancer recurrence as defined by only treatment-specific PSA level elevations | ||
| No signs of recurrence | 37 (29) | 79 (22) |
| Before signs of recurrence | 48 (37) | 85 (24) |
| After signs of recurrence | 68 (53) | 191 (53) |
| Unknown recurrence | 3 (2) | 5 (1) |
| Cancer recurrence as defined by both treatment-specific PSA level elevations and chart review | ||
| No signs of recurrence | 33 (25) | 71 (20) |
| Before signs of recurrence | 28 (22) | 43 (12) |
| After signs of recurrence | 90 (70) | 241 (67) |
| Unknown recurrence | 3 (2) | 5 (1) |
Note—Data are number (%) of patients or examinations. PSA = prostate-specific antigen.
Some patients underwent imaging both before and after signs of recurrence. Thus, percentages do not total 100%.
Fig. 2.
Imaging performed in study cohort in relation to suspected recurrence. ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Thorough chart review of the 61 patients who underwent imaging in the absence of suspected cancer recurrence determined that 39 examinations were actually performed for reasons other than prostate cancer, even though prostate cancer was listed as the examination indication, with 16 examinations performed for other clinical reasons (e.g., wellness checkup or pneumonia) in 10 patients and 23 examinations performed for monitoring another cancer in seven patients (Table S1). Another 35 examinations of 20 patients were performed for treatment-related reasons, including restaging before receiving another treatment type (20 examinations of 10 patients) and checking for interstitial brachytherapy seed migration (13 examinations of eight patients). Four examinations were performed as follow-up for prior imaging unrelated to prostate cancer that suggested metastasis. Nine patients had examinations performed for other reasons, including 11 examinations performed of two patients who had high tumor grade prostate cancer at the time of diagnosis but no clinical evidence of cancer recurrence and eight examinations performed for a variety of clinical reasons that could suggest cancer recurrence, including testicular mass, hematuria, and abdominal fullness. A total of 17 examinations of 13 patients had no specific reason for ordering listed in the medical records. Cumulatively, this suggests that at most 22 patients (nine patients with other reasons for examination and 13 patients with no reason given, representing 2% of the entire cohort and 10% of imaged patients) had imaging potentially performed in the absence of PSA elevations, abnormal DRE findings, or bone pain suggestive of cancer recurrence.
Ninety patients (13% of cohort) underwent 241 imaging examinations ordered by 84 unique providers after suspected recurrence. The specific types of imaging examinations, subdivided by whether the patients received a diagnosis of another type of cancer in addition to prostate cancer, are listed in Table S2, supplemental data, which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org. The most common modalities ordered were CT of the abdomen or pelvis, endorectal coil MRI, spinal x-ray, and bone scan. Spinal x-ray was ordered more frequently in patients with additional cancers, whereas endorectal coil MRI was ordered more frequently in patients with only prostate cancer. Chest x-ray was ordered for 14 patients.
The types of imaging examinations first ordered in patients with suspected recurrence are shown in Table 3. Of these 90 patients, 62 (69%) underwent a bone scan as their first imaging modality, either alone (n = 37 [41%]) or in combination with other imaging modalities (n = 25 [28%]). The most common examinations performed in combination with bone scan were CT of the abdomen or pelvis (14 patients) and endorectal coil MRI (nine patients). The remaining 28 patients (31%) underwent another imaging modality examination first beside bone scan. Endorectal coil MRI (10 patients), spinal x-ray (six patients), and TRUS (five patients) were the most common types of imaging examinations ordered. Chest x-ray was ordered for seven patients (five patients in combination with bone scan and two patients without bone scan).
TABLE 3.
First Imaging Modalities Ordered for Patients With Suspected Cancer Recurrence
| Imaging Modality | No. of Patients | No. of Examinations |
|---|---|---|
| Bone scan alone | 37 | 37 |
| Bone scan with other modalities | 25 | 64 |
| Bone scan | 25 | 25 |
| CT | ||
| Abdomen or pelvis | 14 | 14 |
| Pelvis | 1 | 1 |
| Chest | 2 | 2 |
| MRI | ||
| Abdomen | 1 | 1 |
| Abdomen or pelvis | 1 | 1 |
| Pelvis (endorectal) | 9 | 9 |
| Spine | 1 | 1 |
| X-ray | ||
| Pelvis | 1 | 1 |
| Spine | 2 | 2 |
| Chest | 5 | 5 |
| Transrectal ultrasound | 2 | 2 |
| Other modalities alone | 28 | 33 |
| CT | ||
| Abdomen or pelvis | 3 | 3 |
| Pelvis | 1 | 1 |
| MRI | ||
| Pelvis (endorectal) | 10 | 10 |
| Spine | 1 | 1 |
| X-ray | ||
| Pelvis | 4 | 4 |
| Spine | 6 | 6 |
| Chest | 2 | 2 |
| Transrectal ultrasound | 5 | 5 |
| ProstaScint (Cytogen) | 1 | 1 |
Patient demographics and the specialty of the physician who ordered the imaging examinations were compared between patients who underwent only a bone scan, a bone scan with other imaging modalities, and only other modalities first (Table 4). Patient age at the time of first postrecurrence imaging examination and treatment type were similar among the three groups. Patients who underwent a bone scan in combination with other modalities had higher PSA levels both at the time of their initial cancer diagnosis and at the time of their postrecurrence imaging examination than did patients who only underwent a bone scan or another imaging modality. Almost all patients with a Gleason score of 9 or higher at diagnosis (12/13 [92%]) and all patients with a PSA level greater than or equal to 10 ng/mL at the time of the examination (8/8) underwent a bone scan alone or in combination with other modalities. Most of the orders for bone scan alone were made by primary care (41% [15/37]), urology (19% [7/37]), and other specialty (19% [7/37]) providers, whereas most of the orders for bone scan with other examinations were made by urology (40% [10/25]) and radiation oncology (36% [9/25]) providers. Most of the orders for other modalities were made by urology (43% [12/28]) and primary care (32% [9/28]) providers. The percentage of patients who received a bone scan first, either alone or in combination with other imaging examinations, ranged from 44% (17/39) of patients seen by urologists to 86% (6/7) of patients seen by medical oncologists and 90% (9/10) of patients seen by other specialty providers.
TABLE 4.
Characteristics of Patients With Suspected Recurrence by Type of Imaging Modality Ordered First
| Characteristic | Bone Scan Alone |
Bone Scan and Other Modalities |
Other Modalities Alone |
Percentage of Patients for Whom Bone Scan Was Ordered Firsta (95% CI) |
|---|---|---|---|---|
| No. of patients | 37 | 25 | 28 | |
| Total no. of examinations | 37 | 64 | 33 | |
| Patient demographics | ||||
| Age at first postrecurrence imaging examination (y), median (IQR) | 73 (65–81) | 73 (68–78) | 71 (65–80) | |
| Gleason score subgroups | ||||
| ≤ 6 | 14 (38) | 8 (32) | 10 (36) | 69 (51–82) |
| 7 | 16 (43) | 11 (44) | 14 (50) | 66 (51–78) |
| 8 | 1 (3) | 0 | 3 (11) | 25 (5–70) |
| 9–10 | 6 (16) | 6 (24) | 1 (3.6) | 92 (67–99) |
| PSA level at time of diagnosis (ng/mL), median (IQR) | 6.7 (4.6–14) | 10.3 (6.2–21) | 7.3 (5.3–14) | |
| PSA level at time of first postrecurrence imaging examination (ng/mL), median (IQR) | 0.4 (0.1–3.2) | 3.5 (0.6–8.3) | 0.7 (0.3–1.1) | |
| PSA level ≥ 10 ng/mL at time of examination | 3 (8.1) | 5 (20) | 0 | 100 (68–100) |
| Treatment type | ||||
| Radical prostatectomy | 18 (49) | 11 (44) | 12 (43) | 71 (56–82) |
| Definitive radiation therapy | 12 (32) | 10 (40) | 10 (36) | 69 (51–82) |
| Androgen deprivation therapy | 7 (19) | 4 (16) | 6 (21) | 65 (41–83) |
| Ordering physician’s specialty | ||||
| Urology | 7 (19) | 10 (40) | 12 (43) | 41 (26–59) |
| Radiation oncology | 5 (14) | 9 (36) | 5 (18) | 74 (51–88) |
| Medical oncology | 2 (5.4) | 4 (16) | 1 (3.6) | 86 (49–97) |
| Primary careb | 15 (41) | 0 | 9 (32) | 63 (43–79) |
| Otherc | 7 (19) | 2 (8) | 1 (3.6) | 90 (60–98) |
| Unknown | 1 (3) | 0 | 0 | 100 (21–100) |
Note—Except where noted otherwise, data are number (%) of patients. Not all percentages total 100% because of rounding. IQR = interquartile range, PSA = prostate-specific antigen.
Bone scan ordered alone or with other modalities.
Defined as internal medicine and family practice specialties.
Other specialties include physical medicine, geriatric medicine, gastroenterology, cardiology, anesthesiology, and emergency medicine.
Discussion
In this cohort of 670 treated patients with prostate cancer, at most only 22 patients (3%) underwent imaging in the absence of clinical symptoms of cancer recurrence. Several different imaging modalities were performed on patients with suspected cancer recurrence, with 69% (62/90) undergoing a bone scan first, either alone or in combination with other imaging modalities. Chest x-ray examinations were performed for 16% (14/90) of patients with suspected recurrence. These findings show that there is substantial variability in which imaging examinations are chosen by providers for monitoring prostate cancer.
Urologists were less likely than oncologists to order a bone scan as the first imaging modality. One explanation may be that oncologists are more likely to see patients who are more at risk of developing metastases, whereas urologists are more likely to see patients who are more at risk of local recurrence. Furthermore, urologists may be more familiar with patients with low- or intermediate-grade tumors and slow PSA doubling times, which are more indicative of local recurrence. Endorectal coil MRI, spinal x-ray, and TRUS examinations were performed most often in lieu of a bone scan. The decision to not perform a bone scan first may reflect the lower diagnostic accuracy of bone scan in certain patients with prostate cancer. Bone scan is reported to have a low sensitivity and specificity, especially in patients without high elevations in PSA level (< 20 ng/mL or < 10 ng/mL in some urology guidelines) or high PSA velocity. In our study, all the patients with recurrent PSA level greater than 10 ng/mL underwent a bone scan, either alone or in combination with other imaging modalities. The prevalence of endorectal coil MRI as an alternative modality choice may reflect a shift toward the use of that modality over bone scan. Endorectal coil MRI has been shown to be useful in depicting local recurrent tumor in the prostatectomy bed even with low PSA values [14]. This information may be of importance when the patient is a candidate for localized salvage therapy.
To our knowledge, this is the first population-based observational study to examine imaging utilization for the clinical care of posttreatment patients with prostate cancer and the first to compare ACR Appropriateness Criteria recommendations in these patients. Several prior studies have found significant national variability in imaging utilization and overuse of imaging in patients with prostate cancer before treatment [15–19]. National-level analyses of imaging utilization in patients with prostate cancer after treatment would likely also show such variation and overuse. Other studies examining physician adherence to ACR recommendations have also shown wide variations [20, 21].
Studies examining adherence to imaging appropriateness criteria and efforts to improve adherence are especially timely and important. The Protecting Access to Medicare Act of 2014 [22] has mandated that starting in 2017, providers must use physician-derived appropriateness criteria when ordering advanced imaging (CT, MRI, nuclear medicine, and PET) for Medicare patients. To receive reimbursement, claims for these examinations must confirm that the relevant appropriateness criteria were consulted and detail what clinical decision support tool was used for the consultation and whether the ordered examination adhered to the criteria. The use of appropriateness criteria for ordering imaging examinations should be encouraged through both educational means (i.e., dissemination of the criteria at national and institutional meetings) and technologic means (i.e., clinical decision support). However, we think that deviation from the criteria should not necessarily be punitive, because such deviation may be warranted depending on the clinical situation. Instances of deviation should be captured and examined to determine whether such deviations are occurring routinely enough to warrant review and revision of the appropriateness criteria.
Some of the variability in imaging utilization can be attributed to the heterogeneity of the population of patients with prostate cancer and the fact that providers are often ordering imaging examinations on the basis of the likelihood of local versus metastatic recurrence. For example, patients with low-grade prostate cancer, low risk of recurrence, and a long PSA doubling time are more likely to have local recurrence, whereas patients with high-grade cancer, high risk of recurrence, and a short PSA doubling time are more likely to have metastatic recurrence. With the former group, CT, endorectal coil MRI, or TRUS would be considered an appropriate first step, whereas with the latter group, bone scan would be considered appropriate. We observed these practice patterns in our study, where patients with a higher Gleason score and higher PSA concentrations at recurrence were more likely to have a bone scan ordered compared with patients with lower Gleason scores and PSA concentrations. Although the ACR Appropriateness Criteria do discuss the effectiveness of certain imaging modalities in identifying local or metastatic recurrence, more explicit recommendations and tables for appropriate imaging to perform in the event of local versus metastatic recurrence could help reduce imaging utilization variability.
The ACR Appropriateness Criteria are one of several guidelines for the management of patients with prostate cancer. It is likely that some ordering providers base their decisions on patient management on recommendations from other medical societies instead of the ACR. The American Urologic Association and National Comprehensive Cancer Network both have guidelines that include managing treated patients with prostate cancer; however, neither is as detailed in its recommendations for imaging as the ACR Appropriateness Criteria [2, 23]. Neither the American Urologic Association nor the National Comprehensive Cancer Network guidelines explicitly state that imaging should not be performed in the absence of clinical suspicion of recurrence. The American Urologic Association guidelines also do not explicitly state which types of imaging modalities to perform in the presence of potential recurrence, whereas the National Comprehensive Cancer Network guidelines list a variety of possible modalities, including CT or MRI TRUS, bone scan, abdominal or pelvic CT or MRI, endorectal ultrasound, and PET choline, without ranking them. One way to increase awareness of the ACR Appropriateness Criteria, and subsequently decrease variability in imaging utilization, would be for the ACR Appropriateness Criteria panel to work directly with these other medical societies to provide more detailed imaging recommendations in their guidelines.
The results of our study reflect the providers, practice patterns, and patients seen at a small number of medical centers in one geographic location. National-level analyses would provide a better picture of imaging variation across a wider range of providers and patients. However, our use of a comprehensive collection of regional medical records led to a much more accurate study than one possible at the national level for several reasons. First, by confirming patient addresses throughout the study period we could identify a subset of patients who received all of their medical care at the included centers. Not all national-level patient databases contain comprehensive longitudinal patient records, leading to a higher risk of missing treatments, examinations, and other medical care. Second, chart review enabled us to identify patients with a confirmed diagnosis of prostate cancer instead of relying on less-specific International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes. Third, we were able to include only imaging examinations that were performed in relation to the patient’s prostate cancer. Our chart review identified numerous examinations performed in patients without suspected cancer recurrence that were actually performed for unrelated reasons or reasons related to prostate cancer retreatment. If these examinations had been included in our analysis, then the frequency of imaging in these patients would have been overestimated. Finally, we were able to identify patients with suspected cancer recurrence through retrieval of all PSA results and clinical notes. This allowed us to differentiate between patients with suspected cancer recurrence, for whom imaging and additional follow-up are appropriate, and patients with stable PSA levels, for whom imaging is largely seen as inappropriate. Our analysis of clinical notes, imaging examination indications, and laboratory test results gave a more detailed and accurate clinical picture of the patients in this study than would have been possible with larger but less detailed administrative databases such as the Medicare database.
Several study limitations exist. First, our findings represent local patients and providers and may therefore not be generalizable to other practices. Second, our study only examined clinical practice patterns through 2011 and did not include utilization of newer imaging technologies, such as 11C-choline PET/CT [24–26]. Third, although we used the REP to identify local patients who would most likely seek care only at the included medical centers, patients may have received care, including PSA testing, imaging, and other prostate cancer management, at outside centers that would have not been captured in our analysis. Fourth, indications given for the imaging examinations can be inaccurate, so even though we made efforts to include only examinations performed in relation to prostate cancer, examinations actually performed for other reasons may have falsely elevated the number of examinations. Fifth, imaging examinations ordered by primary care providers may have actually been recommended by specialist providers when the patient was referred to a specialty service. Finally, because this is a retrospective study, we were unable to determine why physicians ordered an imaging examination. We therefore cannot conclude whether incidences of nonadherence were attributable to lack of knowledge about the ACR guidelines or intentional deviation from the guidelines for legitimate clinical reasons. Prospective studies that directly survey providers to determine the reasons for ordering imaging examinations would provide a more accurate picture of imaging utilization.
In conclusion, our analysis of a local cohort of posttreatment patients with prostate cancer determined that imaging examinations are not frequently ordered in the absence of suspected cancer recurrence. However, a wide variety of imaging examinations was ordered in patients with suspected recurrence. This variation suggests that additional provider education and reanalysis of recommendations by the ACR Appropriateness Criteria panel may be needed. Additional studies are necessary to determine whether this variation affects patient outcomes.
Supplementary Material
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (NIH) under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Supplemental Data
Available online at www.ajronline.org.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. [Accessed July 8, 2015];SEER website. seer.cancer.gov/archive/csr/1975_2011/. Published April 2014. Updated December 17, 2014.
- 2.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 4.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 5.Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology. 2006;68:593–598. doi: 10.1016/j.urology.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 6.Amis ES, Jr, Bigongiari LR, Bluth EI, et al. Post-treatment follow-up of prostate cancer: American College of Radiology—ACR Appropriateness Criteria. Radiology. 2000;215(suppl):773–778. [PubMed] [Google Scholar]
- 7.Casalino DD, Remer EM, Arellano RS, et al. ACR Appropriateness Criteria® posttreatment follow-up of prostate cancer. J Am Coll Radiol. 2011;8:863–871. doi: 10.1016/j.jacr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Kawashima A, Choyke PL, Bluth EI, et al. Post-treatment follow-up of prostate cancer. Reston, VA: American College of Radiology; 2005. [PubMed] [Google Scholar]
- 9.Kawashima A, Francis IR, Baumgarten DA, et al. Post-treatment follow-up of prostate cancer. Reston, VA: American College of Radiology; 2007. [Google Scholar]
- 10.Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linder BJ, Kawashima A, Woodrum DA, et al. Early localization of recurrent prostate cancer after prostatectomy by endorectal coil magnetic resonance imaging. Can J Urol. 2014;21:7283–7289. [PubMed] [Google Scholar]
- 15.Choi WW, Williams SB, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Overuse of imaging for staging low risk prostate cancer. J Urol. 2011;185:1645–1649. doi: 10.1016/j.juro.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Lavery HJ, Brajtbord JS, Levinson AW, Nabizada-Pace F, Pollard ME, Samadi DB. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77:274–278. doi: 10.1016/j.urology.2010.07.491. [DOI] [PubMed] [Google Scholar]
- 17.Makarov DV, Desai R, Yu JB, et al. Appropriate and inappropriate imaging rates for prostate cancer go hand in hand by region, as if set by thermostat. Health Aff (Millwood) 2012;31:730–740. doi: 10.1377/hlthaff.2011.0336. [DOI] [PubMed] [Google Scholar]
- 18.Prasad SM, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer. 2012;118:1260–1267. doi: 10.1002/cncr.26416. [DOI] [PubMed] [Google Scholar]
- 19.Saigal CS, Pashos CL, Henning JM, Litwin MS. Variations in use of imaging in a national sample of men with early-stage prostate cancer. Urology. 2002;59:400–404. doi: 10.1016/s0090-4295(01)01543-6. [DOI] [PubMed] [Google Scholar]
- 20.Broder JS, Hamedani AG, Liu SW, Emerman CL. Emergency department contrast practices for abdominal/pelvic computed tomography-a national survey and comparison with the American College of Radiology Appropriateness Criteria®. J Emerg Med. 2013;44:423–433. doi: 10.1016/j.jemermed.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Raichlin E, Williamson EE, et al. Evaluation of coronary CTA Appropriateness Criteria in an academic medical center. J Am Coll Radiol. 2010;7:125–131. doi: 10.1016/j.jacr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 22.The Protecting Access to Medicare Act of 2014, HR 4302, Pub L 113-93. 2014 [Google Scholar]
- 23.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of 11C-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308–1313. doi: 10.1016/j.juro.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 25.Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 26.Kitajima K, Murphy RC, Nathan MA, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med. 2014;55:223–232. doi: 10.2967/jnumed.113.123018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.