Abstract
Prevailing theory holds that abnormally large increases in renal salt retention and cardiac output are early pathophysiologic events mediating initiation of most instances of salt-induced hypertension. This theory has come under increasing scrutiny because it is based on studies that lack measurements of sodium balance and cardiac output obtained during initiation of salt-loading in proper normal controls, i.e., salt-resistant subjects with normal blood pressure. Here we make the case for a “vasodysfunction” theory for initiation of salt-induced hypertension: In response to an increase in salt intake, a subnormal decrease in total peripheral resistance that involves a subnormal decrease in renal vascular resistance, in the absence of abnormally large increases in sodium retention and cardiac output, is the hemodynamic abnormality that usually mediates initiation of salt-induced increases in blood pressure (BP). It is the failure to normally decrease vascular resistance in response to salt loading that enables a normal increase of cardiac output to initiate the salt-induced increase in blood pressure. This theory is based on the results of properly controlled studies which consistently demonstrate that in salt-sensitive subjects, salt-loading initiates increased BP through a hemodynamic mechanism that: 1) does not usually involve early increases in sodium retention and cardiac output greater than those which occur with salt-loading in normal controls, and 2) usually involves an early failure to decrease vascular resistance to the same extent as that observed during salt-loading in normal controls. Multiple mechanisms including disturbances in nitric oxide and sympathetic nervous system activity likely underlie this subnormal vasodilatory response to salt that usually precedes and initiates salt-induced hypertension.
Keywords: salt sensitivity hypertension, salt-sensitive, hypertension, kidney, vasculature
Introduction
It has long been recognized that in some people substantially increasing dietary intake of salt (NaCl) increases blood pressure whereas in others, “salt loading” has little or no effect on blood pressure.1 Blood pressure so affected by salt has been termed “salt-sensitive” and “salt-resistant,” respectively. While the blood pressure response to salt is a continuous variable and the trait of salt-sensitivity like that of hypertension is arbitrarily defined,2 it has been estimated that 30%-50% of hypertensive humans are salt-sensitive and about 25% of normotensive humans are salt-sensitive.3, 4 Salt-sensitivity confers an increased risk for the occurrence of hypertension, and for cardiovascular disease.5–7 Further, pathophysiologic mechanisms mediating salt-sensitivity may contribute to risk for cardiovascular disease beyond their effects on blood pressure.5 Accordingly, the mechanisms of salt-sensitivity continue to be intensively studied with the hope that better understanding of those mechanisms could lead to improved approaches to the prevention and treatment of salt-induced increases in blood pressure and cardiovascular disease.
Prevailing Theory of Initiation of Salt-Sensitivity and Salt-Induced Hypertension
Prevailing theory holds that an abnormally large increase in renal retention of salt8–16 is an early pathophysiologic event in the causation of salt-sensitivity and salt-induced hypertension. In accord with this theory, it is held that a substantial increase in dietary salt does not induce a pressor effect in salt-resistant subjects because they excrete a salt load more rapidly and retain less sodium than salt-sensitive individuals.12, 17 In the current analysis, we challenge this conventional view of salt-sensitivity/salt-resistance and make the case for a “vasodysfunction” theory for initiation of salt-induced hypertension: An abnormal vascular resistance response to increases in salt intake, in the absence of an abnormally large increase in renal retention of sodium, usually mediates the initiation of salt-induced increases in blood pressure.
We focus this analysis on the initiation of salt-induced increases in blood pressure because we are interested in: 1) how increases in salt intake cause increased blood pressure from the outset and 2) why individuals vary in their blood pressure responses to initiation of a high salt diet. Further, it is possible that the mechanisms mediating abnormalities in vascular resistance during initiation of salt-induced hypertension may also contribute to the abnormally increased systemic vascular resistance that characterizes sustained hypertension.
According to prevailing theory, salt-sensitive subjects have an impaired renal ability to excrete a salt load that usually causes them to retain more sodium than normal salt-resistant subjects.8, 10, 12–16 The retention of abnormally large amounts of salt and water is held to cause abnormally large increases in sodium balance, blood volume, and a transient, abnormally large increase in cardiac output that contributes importantly to the hemodynamic initiation of increased blood pressure.8, 11, 12 Many investigators also note a role for abnormalities in the control of systemic vascular resistance in the hemodynamic initiation of salt-induced increases in blood pressure, i.e., they do not contend that only an abnormally large increase in cardiac output induced by abnormally large increases in renal retention of sodium and water is responsible for the hemodynamic initiation of salt-induced increases in blood pressure.15, 18–24
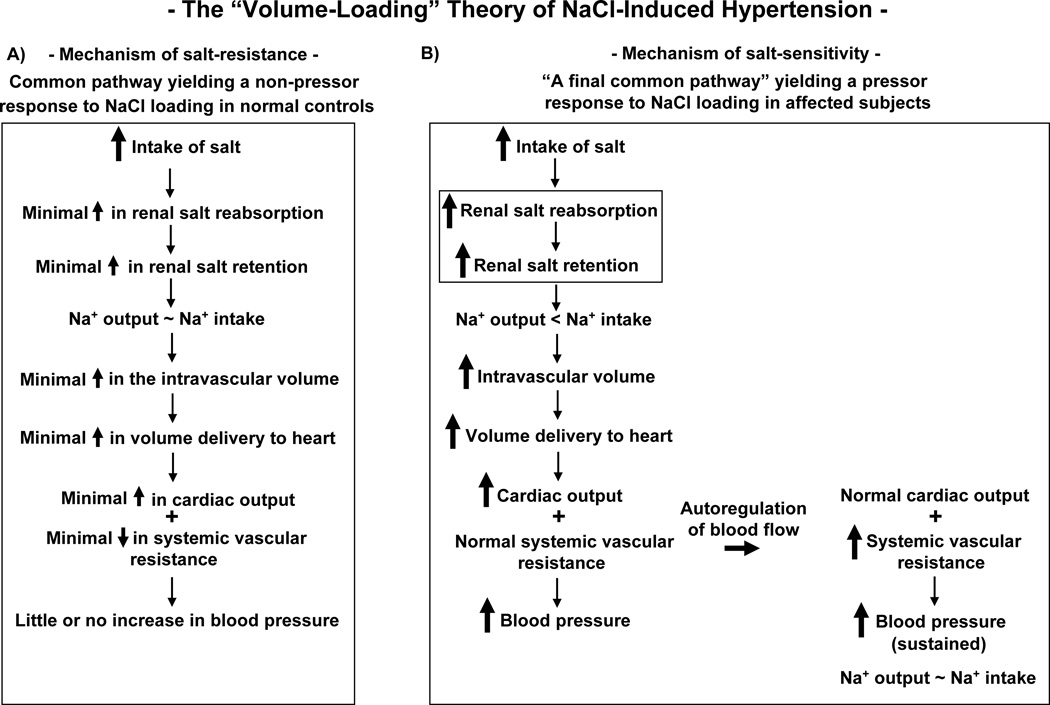
It should be noted that views holding that abnormal levels of both cardiac output and systemic vascular resistance contribute to the hemodynamic initiation of salt-induced hypertension differ from the view championed by Guyton, Lifton, and others.8, 11, 12, 25–27 This view shown in Figure 1 holds that during initiation of salt-induced hypertension, systemic vascular resistance is “normal” and pathogenically uninvolved in salt’s initial pressor effect. Abnormal systemic vascular resistance is held to be only secondary to increases in cardiac output and blood pressure caused by abnormal increases in renal retention of salt and water (Figure 1). This “volume-loading” theory (Figure 1), sometimes referred to as the “autoregulation” theory,28 also describes the sequence of events through which the phenomenon of “abnormal pressure natriuresis” is said to initiate and sustain salt-induced hypertension.29–32 Most theories of salt-induced hypertension share two core tenets: 1) salt-sensitive subjects have a subnormal ability to excrete a salt load that causes them to retain more sodium than normal salt-resistant subjects, and 2) such an abnormally increased renal retention of sodium causes an abnormally large increase in cardiac output and thereby contributes importantly to the hemodynamic initiation of salt-induced increases in blood pressure. Here we challenge the validity of both these tenets.
Figure 1.
The “volume-loading” theory of the pathogenesis of NaCl-induced hypertension. A) The mechanism held to mediate a normal, i.e., non-pressor, response to increases in salt intake (common pathway for salt-resistance). According to the current Guyton and Hall Textbook of Medical Physiology, “raising salt intake in the absence of impaired kidney function or excessive formation of antinatriuretic hormones usually does not increase arterial pressure much because the kidneys rapidly eliminate the excess salt and blood volume is hardly altered.”12 B) The mechanism held to mediate an abnormal, i.e., pressor, response to increases in salt intake (mechanism of salt-sensitivity) as originally developed by Borst et al,25 Coleman and Guyton,28 and others, and adapted here from the diagram by Lifton et al.8 Note that in this theory, the level of systemic vascular resistance becomes abnormal only after blood pressure has increased and only after the increase in cardiac output has initiated the phenomenon of autoregulation. According to the theory, an abnormal increase in the amount of renal salt reabsorption/retention is usually an early, critical abnormality that enables increased salt intake to initiate hypertension (as emboxed within the Figure).
Pro Position: Vasodysfunction Theory for Initiation of Salt-Induced Hypertension
We first define key terms as they are used in this analysis. We use the term “vasodysfunction” to indicate a subnormal decrease in systemic vascular resistance in response to increases in salt intake. For all variables, we define “normal” responses to increases in salt intake as the responses to increases in salt intake observed in normal subjects, i.e., salt-resistant subjects with normal blood pressure. We define “substantial” increases in salt intake as increases equivalent to approximately 200 mmol per day or more occurring within the dietary range of salt intake reported in humans (a range of approximately 10 mmol/day to 350 mmol/day).33 The terms “systemic vascular resistance” and “total peripheral resistance” are used interchangeably.
We take the position that “vasodysfunction,” i.e., a subnormal decrease in systemic vascular resistance in response to increases in salt intake, is the hemodynamic abnormality that mediates initiation of most instances of salt-induced hypertension. Further, in contrast to most theories of salt-induced hypertension, the “vasodysfunction” theory holds that the initiation of increased blood pressure by increased salt intake does not usually involve: 1) abnormally large increases in renal sodium retention, i.e., does not usually involve retention of sodium in an amount greater than that retained during salt loading in normal controls and 2) abnormally large increases in cardiac output, i.e., does not usually involve an increase in cardiac output greater than that occurring during salt loading in normal controls.
The Normal Natriuretic and Hemodynamic Responses to Increases in Salt Intake
To determine which responses of salt-sensitive subjects to salt loading are normal or abnormal, it is first necessary to establish the responses of normal subjects to salt-loading. In normal salt-resistant humans, substantial increases in salt intake usually induce substantial renal sodium retention34–41 and substantial increases in blood volume,40, 42 plasma volume,39, 43 stroke volume,37, 38, 44 and cardiac output.37, 38, 44 In response to increases in salt intake, it takes considerable time (days) for normal humans to increase sodium excretion enough to achieve sodium balance (sodium excretion = sodium intake)35–41 and thus, normal humans retain substantial amounts of sodium in response to substantial increases in salt intake.34–41
In normal humans, substantial increases in salt intake, renal sodium retention, fluid volumes, and cardiac output induce little or no increase in blood pressure because normal humans rapidly undergo substantial decreases in systemic vascular resistance in response to such increases in salt intake.37, 38 In normal humans, the decreases in systemic vascular resistance induced by salt are usually great enough to offset the pressor effects that would otherwise be expected to result from the substantial salt-induced increases in cardiac output.37, 38, 44 The normal decrease in SVR upon NaCl loading appears to involve an early decrease in renal vascular resistance.(RVR).45, 46
In normal animals (salt-resistant animals with normal blood pressure), increases in salt intake also usually induce increases in renal sodium retention,47–51 blood volume/plasma volume,52–54 and cardiac output47, 53, 55, 56 but induce little or no increase in blood pressure, because normal animals also vasodilate and substantially reduce systemic vascular resistance in response to such increases in salt intake.47, 53, 55, 56 These observations in normal humans and animals contradict the contention that normal subjects resist the pressor effects of increased salt intake because they excrete sodium chloride “almost as rapidly as it can be infused or drunk”17 and their “blood volume is hardly altered.”12
The Lack of Appropriate Normal Controls in Studies of Salt-Induced Increases in Blood Pressure
We know of no publications which show that during the period when salt loading initiates increases in blood pressure in salt-sensitive subjects, they both retain more sodium and have greater levels of cardiac output than do salt-loaded normal controls. The “volume-loading” theory (Figure 1) that: 1) salt-induced hypertension is initiated by abnormally large increases in sodium retention and cardiac output, and 2) systemic vascular resistance becomes abnormal only when it increases above baseline, is based on publications28–30, 57–66 that lack reference to appropriate normal controls. Specifically, the studies cited in support of the “volume-loading” theory28–30, 57–66 do not compare salt-sensitive subjects to normal controls with respect to the observed changes in sodium balance, cardiac output, and systemic vascular resistance that occur during salt loading. Without such comparisons to salt-loaded normal controls, one cannot judge whether the changes observed to occur in salt-sensitive subjects during salt loading are normal or abnormal.
Salt-sensitive hypertensive subjects have been reported to have greater salt-induced increases in sodium balance and cardiac output than salt-resistant hypertensive subjects.60, 61 However, as cautioned by Campese et al.,67 such increases cannot be judged to be abnormal without comparing them to results of salt loading studies in proper normal controls, i.e., salt-resistant subjects with normal blood pressure (salt-resistant normotensive controls). Indeed, in a salt loading study of Ishii et al, salt-sensitive hypertensive subjects did not have greater salt-induced increases in sodium balance than those observed in salt-resistant normotensive controls.35 It remains to be determined whether the mechanisms that contribute to salt-resistance in normal subjects also contribute to salt-resistance in hypertensive subjects.
The Natriuretic and Hemodynamic Responses of Salt-Sensitive Subjects to Increases in Salt Intake
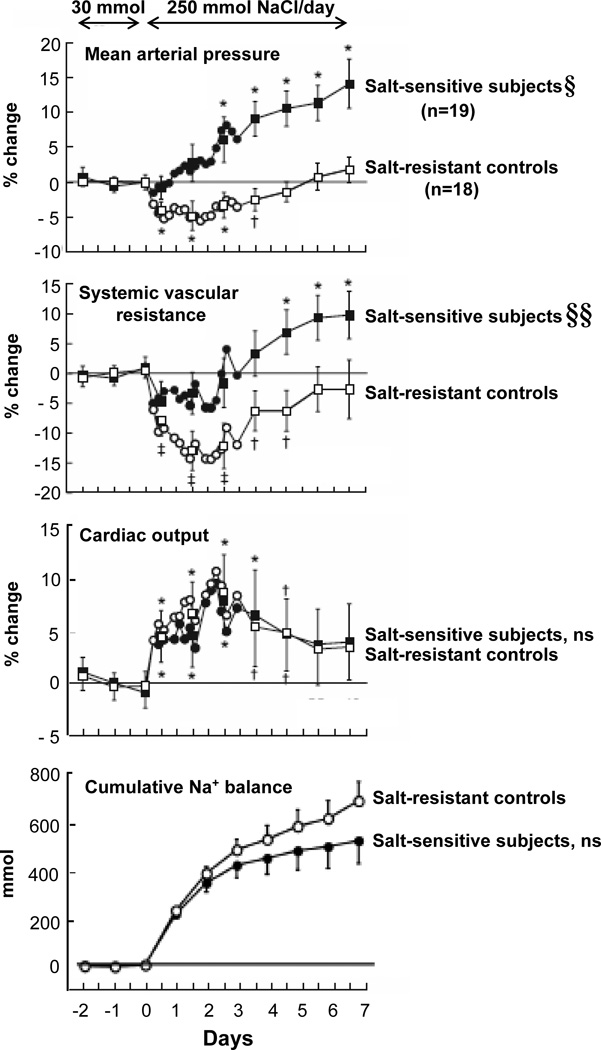
Figure 2 shows the results of studies from our group demonstrating that at no time during initiation of salt-induced increases in blood pressure in normotensive salt-sensitive humans, do salt-sensitive subjects usually retain more sodium or have greater increases in cardiac output than do salt-loaded normal control subjects.37, 38 The results of these and all other relevant, properly controlled studies demonstrate that in normal subjects, salt-loading usually induces increases in sodium retention 35–38, 48–51 and cardiac output37, 38, 44, 53, 55, 56 that are just as great as those observed with salt-loading in salt-sensitive subjects (Figure 2). Thus, the finding that blood pressure during salt loading is substantially greater in salt-sensitive subjects than in normal controls cannot be attributed to abnormal (greater) increases in sodium retention and cardiac output in the salt-sensitive subjects.
Figure 2.
Time course of NaCl-induced changes in cumulative sodium balance and hemodynamic variables in salt-sensitive subjects and normal, salt-resistant controls. Results of studies in normotensive African Americans in which Schmidlin et al continually monitored blood pressure, cardiac output, systemic vascular resistance, and cumulative sodium retention before and after increasing dietary intake of NaCl from 30 mmol/day to 250 mmol/day (adapted from Schmidlin et al38 with permission from the publisher). Hemodynamic values are shown as percentage of change from the period of low NaCl intake (average of values during the last 3 days of the one week period in which the low NaCl diet was consumed). During the last 3 days of the low NaCl diet, the salt-sensitive subjects (n=19) did not differ from the salt-resistant subjects (n=18) with respect to absolute levels of mean arterial pressure, systemic vascular resistance, cardiac output, or cumulative sodium balance. The changes in cardiac output are secondary to changes in stroke volume, not in heart rate. § denotes significant difference between salt-sensitive subjects versus salt-resistant controls for salt-induced changes in blood pressure on day 1 of high NaCl intake (P<.05) and on days 2 to 7 of high NaCl intake (P<.001). §§ denotes significant difference between salt-sensitive subjects versus salt-resistant controls on day 2 to day 7 of high NaCl intake (P<.003). ns, no significant differences between salt-sensitive subjects and salt-resistant controls. Significant difference compared to period of low NaCl intake denoted by †, P <.05; *, P<.01; ‡, P<.001. Changes in hemodynamic values are shown at 4 hour intervals for the first 72 hours and then at 24 hour intervals thereafter. Results are displayed as means and 95% confidence intervals.
Note, we do not dispute that sodium retention and increases in cardiac output occur during salt-loading in salt-sensitive subjects. Rather, we emphasize that the increases in cumulative sodium retention35–38, 48–51 and in cardiac output37, 38, 44, 53, 55, 56 that usually occur in salt-sensitive subjects when salt-loading increases blood pressure are not abnormally large, i.e., not greater than those that occur during salt-loading in normal controls (Figure 2).
Systemic Vascular Resistance is Abnormal Throughout Initiation of Salt-Induced Increases in Blood Pressure
With respect to changes in systemic vascular resistance induced by increases in salt intake, Figure 2 shows results from our laboratory demonstrating that during initiation of salt-loading, normal salt-resistant humans vasodilate and reduce systemic vascular resistance, whereas salt-sensitive humans fail to vasodilate and reduce systemic vascular resistance normally.37, 38 Specifically, the salt-sensitive subjects fail to vasodilate and reduce systemic vascular resistance to the same extent as that observed during initiation of salt loading in normal controls. In studies in salt-sensitive humans and in animals that have included salt-loaded normal controls, it has been consistently observed that salt-sensitive subjects fail to decrease systemic vascular resistance to a normal extent in response to increases in salt intake.37, 38, 44, 53, 55, 56 In salt-sensitive subjects, this failure of systemic vascular resistance to robustly decrease during the initiation of salt-loading is clearly abnormal.
Given that in normal salt-resistant humans, a robust decrease in systemic vascular resistance begins within 12 – 24 hours of initiating salt loading (Figure 2), the failure of systemic vascular resistance to decrease to the same extent in the salt-sensitive subjects as in the normal controls, indicates the occurrence of a very early salt-induced abnormality in vascular resistance. This “vasodysfunction,” i.e., this subnormal decrease in systemic vascular resistance in response to salt loading, is a critical pathogenic event in the initiation of the salt-induced increase in blood pressure: Without this vasodysfunction, the normal increase of cardiac output induced by salt in the salt-sensitive subjects would not have elicited a pressor effect, just as it did not in the salt-resistant normal controls (Figure 2). Furthermore, in salt-sensitive subjects, the failure of systemic vascular resistance to normally decrease in response to increases in salt intake occurs before blood pressure increases above baseline. Thus, the abnormal vascular resistance response to salt loading cannot be a consequence of the salt-induced increases in blood pressure (Figure 2).
The “Vasodysfunction” Theory for Initiation of Salt-Induced Hypertension
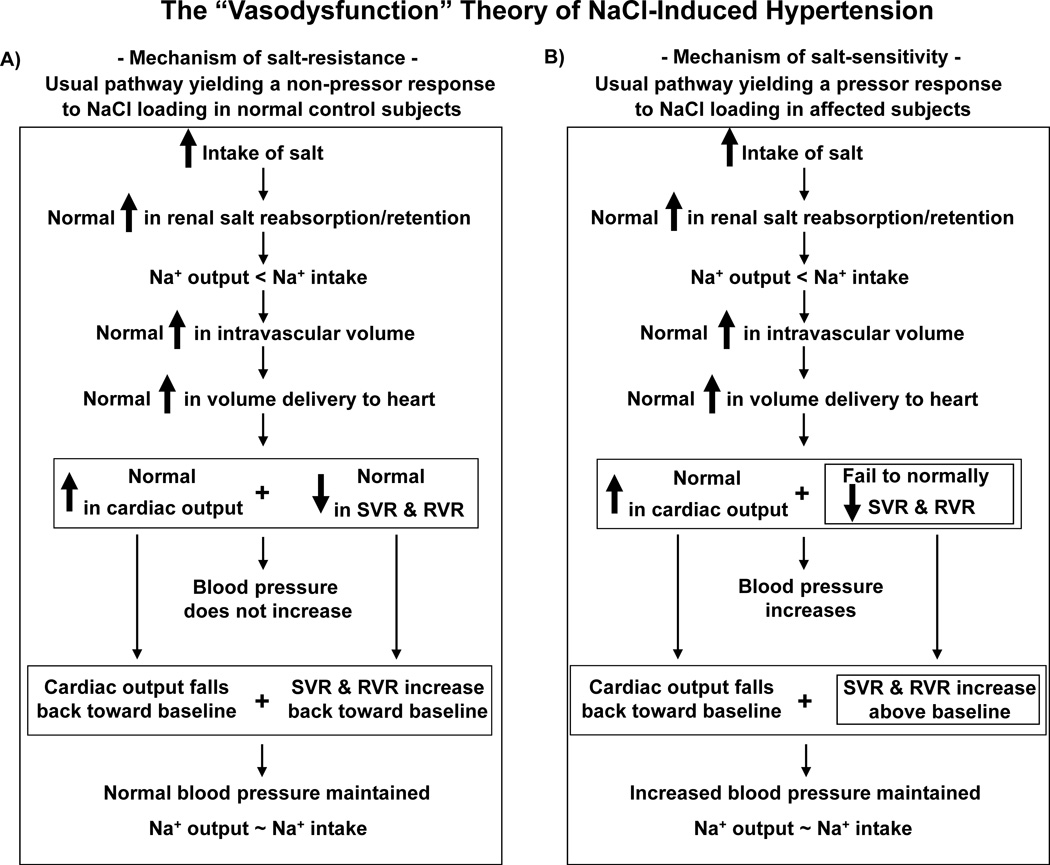
We propose the “vasodysfunction” theory (Figure 3) which holds that: 1) Substantial increases in salt intake induce little or no increase in blood pressure in salt-resistant normal subjects because they substantially reduce systemic vascular resistance in response to salt-loading, not because they excrete sodium more rapidly, retain less sodium, and undergo smaller increases in cardiac output than do salt-loaded salt-sensitive subjects. 2) Substantial increases in salt intake induce substantial increases in blood pressure in salt-sensitive subjects because they undergo “vasodysfunction,” i.e., they fail to normally reduce systemic vascular resistance in response to salt-loading, not because they retain more of a salt load and have greater increases in cardiac output than do salt-loaded normal subjects.
Figure 3.
The “vasodysfunction” theory of the pathogenesis of NaCl-induced hypertension. A) Usual pathway yielding a normal (non-pressor) response to NaCl loading (mechanism of salt-resistance). This pathway depicts the initial sodium balance and hemodynamic responses to increases in salt intake usually observed in salt-resistant normotensive control subjects. Note that the normal decrease in systemic vascular resistance (SVR) that occurs with salt loading offsets the potential pressor effect of a large increase in cardiac output (CO) that occurs with salt loading.37, 38, 44, 53 The normal decrease in SVR upon NaCl loading likely includes an early decrease in renal vascular resistance (RVR).45, 46 B) Usual pathway yielding an abnormal (pressor) response to NaCl loading (mechanism of salt-sensitivity). In salt-sensitive subjects, the salt-induced increases in cumulative sodium balance and CO are not abnormal, i.e., are usually not greater in magnitude than the salt-induced increases in cumulative sodium balance and CO that occur in normal, salt-resistant controls.37, 38, 44, 53 In the salt-sensitive subjects compared to the normal salt-resistant controls, the failure to normally decrease vascular resistance with salt loading is the first hemodynamic abnormality occurring in the chain of events mediating salt-induced increases in blood pressure (the abnormality doubly emboxed within the Figure).37, 38, 44, 53 The failure of salt-sensitive subjects to normally decrease SVR during initial NaCl loading may include unchanged or even increased renal vascular resistance (RVR),77–79 whereas in some other organ beds, vascular resistance may decrease.77 Although the current analysis focuses on the sodium balance and hemodynamic changes that occur upon initiation of increased salt intake, this figure also depicts the changes observed in humans when increased salt intake is sustained (Figure 2).37, 38 With continuation of high salt intake, salt-resistant normal subjects and salt-sensitive subjects begin to attain sodium balance through a variety of mechanisms which may differ between the two groups. For example, in normal salt-resistant subjects,46, 78, 79 salt-induced decreases in renal vascular resistance and or increases in renal plasma flow together with other factors may contribute to attainment of sodium balance. In salt-sensitive subjects, increases in renal perfusion pressure together with other factors, not decreases in renal vascular resistance and increases in renal plasma flow,78, 79 may contribute to attainment of sodium balance. The mechanisms mediating the changes in CO and SVR observed during continuation of high salt intake are not well defined. Neural mechanisms, nitric oxide related effects, or other factors regulating vascular tone could: 1) mediate the return of CO towards baseline by reducing peripheral venous tone, increasing vascular capacitance, and redistributing blood volume to the peripheral venous compartment112 in both salt-resistant and salt-sensitive subjects, and 2) mediate increases in SVR towards baseline in salt-resistant subjects, and increases in SVR above baseline in salt-sensitive subjects. Thick arrows indicate an increase above, or a decrease below, the baseline level present before initiation of salt retention. SVR, systemic vascular resistance; RVR, renal vascular resistance.
The observations of many investigators indicate that “vasodysfunction,” i.e., a failure to normally decrease systemic vascular resistance in response to salt loading, can contribute to the initiation of salt-induced increases in blood pressure in salt-sensitive subjects.15, 20–22, 24, 37, 38, 44, 53, 55, 56, 68–76 The roots of this concept extend back to at least 1975 when Mark et al noted that in normotensive salt-resistant subjects ingesting a low salt diet, increases in dietary salt induced decreases in forearm vascular resistance, whereas in salt-sensitive subjects, such salt loading induced increases in forearm vascular resistance.68
It should be noted that in salt-sensitive subjects, the failure of systemic vascular resistance (total peripheral resistance) to normally decrease in response to initiation of increased salt intake does not necessarily require the occurrence of a subnormal decrease in vascular resistance in all organs/tissues. For example, in some organs/tissues, vascular resistance may substantially decrease whereas in other organs/tissues, vascular resistance may fail to decrease, or even substantially increase.77 The inability of salt-sensitive subjects to normally decrease SVR during initial NaCl loading may include unchanged or even increased renal vascular resistance (RVR).77–79 Thus, the “vasodysfunction” theory depicted in Figure 3 allows for the view that an abnormal renal vascular resistance response to salt loading contributes to development of the abnormal systemic vascular resistance response to salt loading that, together with normal salt-induced increases in cardiac output, initiates the increase in arterial pressure (Figure 3). The abnormal renal vascular resistance response to salt does not cause salt-sensitive subjects to retain more of a salt load than salt-resistant normal controls.
We do not intend to imply that in salt-sensitive subjects, the kidney is uninvolved in the initiation of salt-induced increases in blood pressure. Rather, we contend that during salt-loading in salt-sensitive subjects: 1) the level of renal vascular resistance is abnormally high, i.e. is greater than that in salt-loaded normal subjects, and this contributes to the abnormally high level of systemic vascular resistance that hemodynamically initiates salt-induced hypertension, and 2) the level of renal sodium retention is not abnormally high, i.e., is not greater than that in salt-loaded normal subjects and thus, abnormally increased renal sodium retention is not involved in the initiation of salt-induced hypertension.
Vasodysfunction Is Likely Involved in the Initiation of All Forms of Salt-Sensitivity and Salt-Induced Hypertension
We have previously proposed that the “vasodysfunction” theory likely accounts for initiation of salt-induced increases in blood pressure that might occur in rare Mendelian forms of human hypertension, including those characterized by high mineralocorticoid levels.80 With one exception,81 all Mendelian disorders of hypertension have been found to be caused by mutations that are associated with increased renal tubular reabsorption of sodium.11 However, the association of these disorders of hypertension with mutations that increase renal tubular sodium reabsorption does not necessarily mean that mutation-dependent increased renal tubular reabsorption of sodium causes the salt-induced hypertension. The initiation of salt-induced hypertension in humans or animals with such mutations has not been demonstrated to be caused by greater increases in sodium retention and cardiac output in the mutant subjects than in salt-loaded normal controls.80 In experimental models relevant to Liddle syndrome,82–84 deficiency of 11β- hydroxysteroid dehydrogenase type 2,85, 86 familial hyperkalemic hypertension,87–90 and states of mineralocorticoid excess,80, 91–93 many studies have indicated that initiation of salt-induced hypertension may not be simply a sodium retention/volume loading phenomenon. As discussed in detail elsewhere,80 most if not all mutations affecting sodium transport in Mendelian disorders of hypertension have the potential to cause salt-induced vasodysfunction by affecting neural, hormonal, or vascular mechanisms that influence vascular resistance.
In the current analysis, we have reviewed information indicating that the vasodysfunction theory likely accounts for the hemodynamic initiation of common forms of salt-sensitivity. Thus, we contend that the vasodysfunction theory accounts for the hemodynamic initiation of salt-induced increases in blood pressure in most instances of salt-sensitivity. Even in animals rendered salt-sensitive by surgical reduction of renal mass, i.e., even in a model of hypertension that was classically thought to be “caused by pure excess volume loading,”62 the initiation of salt-induced increases in blood pressure does not appear to be due to retention of greater amounts of sodium in the salt-sensitive subjects than in salt-loaded normal controls.51
Mechanisms Mediating Normal and Abnormal Vascular Resistance Responses to Increases in Salt Intake
In salt-sensitive subjects, increases of salt intake usually induce normal increases in sodium retention and cardiac output, and subnormal decreases in systemic vascular resistance. What mediates this hemodynamic abnormality, i.e., the subnormal decrease in systemic vascular resistance in response to increased salt intake, that initiates the salt-induced increase in blood pressure? Multiple factors can affect arterial vascular resistance (Table) and the relative importance of different mechanisms in mediating failure to normally vasodilate in response to increases in salt intake remains to be established. Among the many candidate factors, it may be worthwhile to first consider those recognized to be involved in mediating the normal decrease in vascular resistance that occurs in response to increases in salt intake in normotensive, salt-resistant controls. For example, nitric oxide (NO) is a powerful vasodilator and it has been proposed that increases in salt intake normally cause increases in blood volume/blood flow that result in shear stress mediated release of NO from endothelial cells that may mediate vasodilation in response to increased salt intake.46
Table 1.
Examples of Systems/Factors That Affect Vascular Resistance
| Nitric oxide system |
| Sympathetic nervous system |
| Renin-angiotensin system |
| Adrenal hormone systems |
| Oxidant/Anti-oxidant systems |
| Immune system |
| Vasopressin system |
| Ouabain like factors |
| Eicosanoid system |
| Natriuretic peptide system |
| Endothelin system |
| Dopamine system |
| Kallkrein-kinin system |
| CGRP, substance P, adrenomedullin |
| γ-MSH and other neuropeptide systems |
| Transient receptor potential vanilloid channels |
| Structural factors affecting vessel mechanics and function |
| Various ion channels and cell signaling systems regulating MLC function |
CGRP, calcitonin gene related peptide;
γ-MSH, gamma melanocyte stimulating hormone
MLC, myosin light chain
The Role of Nitric Oxide Mechanisms in Mediating Normal and Abnormal Vascular Resistance Responses to Increases in Salt Intake
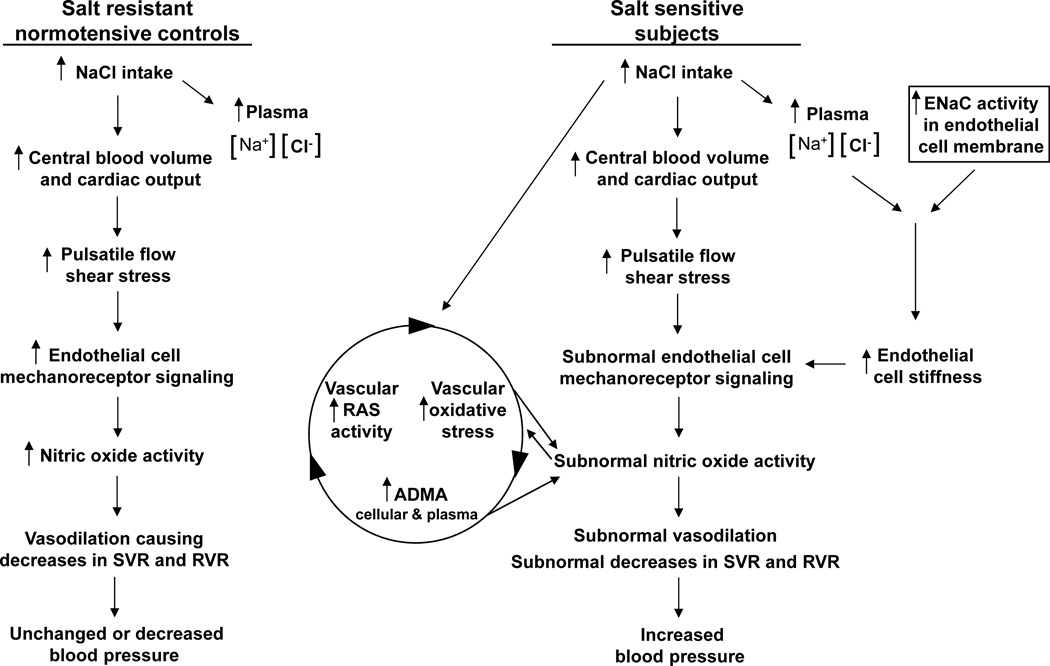
In Figure 4, we focus on several NO-related mechanisms influencing salt-sensitivity because of the apparent role of NO in contributing to normal vasodilatory responses to increases in salt intake, and because many studies have indicated that disturbances in NO activity play a major role in the pathogenesis of salt-induced hypertension,94 an idea proposed by Chen and Sanders at least 25 years ago.72 Recent studies from Schmidlin et al38 and from Oberleithner and others83, 92, 95–98 have provided new insights into abnormalities in the regulation of NO activity that likely contribute to the abnormal vascular resistance responses to salt that initiate various forms of salt-induced hypertension.
Figure 4.
Nitric oxide related mechanisms mediating vascular resistance responses to initiation of increased salt intake. A) In normal subjects (normotensive, salt-resistant subjects), increases in salt intake cause volume-induced increases in cardiac output, resulting in shear stress mediated release of nitric oxide from endothelial cells. The increases in nitric oxide activity contribute to vasodilation and decreases in systemic vascular resistance (SVR) that help offset potential pressor effects of the salt-induced increases in cardiac output such that blood pressure does not increase. This normal decrease in SVR upon NaCl loading includes a decrease in renal vascular resistance (RVR). B) In salt-sensitive subjects, increases in salt intake induce normal increases in cardiac output but subnormal decreases in SVR and RVR in part because of subnormal increases in nitric oxide activity caused by salt-induced increases in assymetrical dimethyl arginine (ADMA)38, 94, 102, 103 and endothelial cell stiffness.83, 92, 97, 98 Increases in cellular and plasma ADMA may be caused by several mechanisms including abnormally large salt-induced increases in oxidative stress that affect cellular export of ADMA and enzymatic production and clearance of ADMA.107 Note that increases in ADMA can also cause increases in vascular activity of the renin-angiotensin system (RAS) that promote oxidative stress.105, 106 Increases in endothelial cell stiffness may be caused by the combination of increases in plasma sodium concentrations with abnormal increases in activity of epithelial sodium channels (ENaCs) in the endothelium.83, 92, 97, 98
Salt-induced increases in blood pressure vary inversely with salt-induced increases in plasma99, 100 and urine101 biomarkers of NO, and vary directly with salt-induced increases in plasma38, 99, 100, 102 and urinary103 levels of asymmetrical dimethylarginine (ADMA), a major endogenous inhibitor of NO bioavailability through its capacity to inhibit NO synthase and also increase oxidative stress.104–107 Intravenously administered ADMA has been shown to acutely increase systemic vascular resistance and blood pressure in humans.108 In salt-sensitive humans, we have found that increases in plasma levels of ADMA occur within 24 hours of initiating increased salt intake, whereas in normal salt-resistant control subjects, the same salt-loading does not increase ADMA levels.38 In salt-sensitive subjects, increases in salt intake may induce increases in ADMA levels by both promoting oxidative stress- induced decreases of ADMA metabolism and increases in ADMA synthesis.107
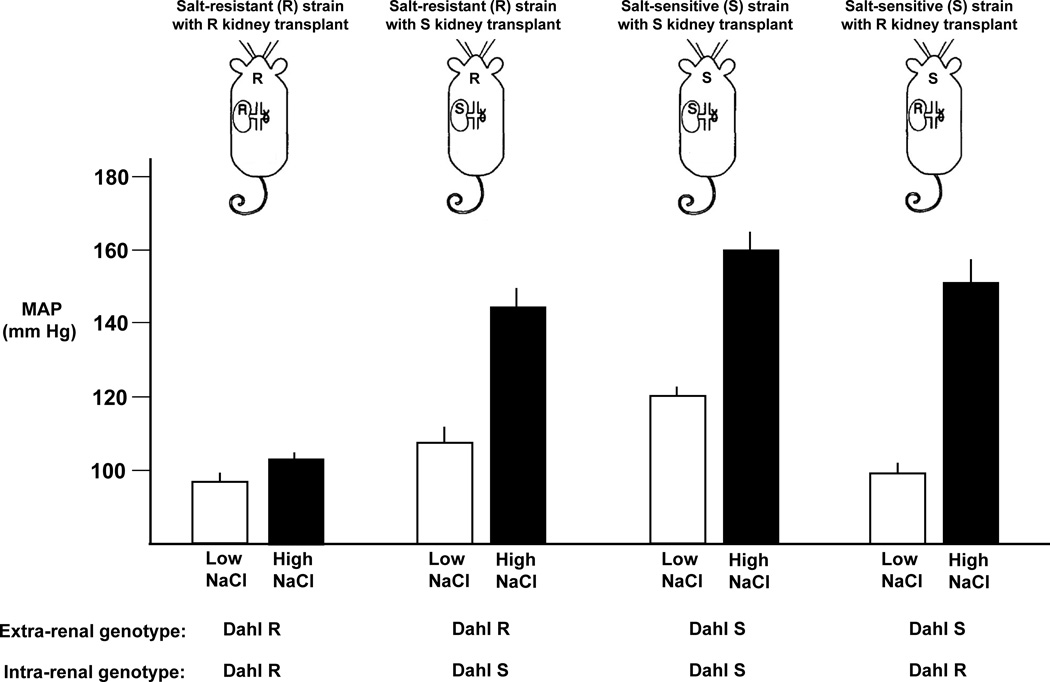
The role of ADMA-mediated decreases in NO activity in the abnormal vascular resistance response to salt is of particular interest because the involvement of ADMA might help to explain why the trait of salt-sensitivity does not always “follow the kidney” in transplantation studies.109 For example, the classic renal cross transplantation studies by Morgan, DiBona, and Mark indicate that the trait of salt-sensitivity in the Dahl rat model is determined not only by renal factors but also by extra-renal factors (Figure 5).109 ADMA is generated by multiple cell types in the cardiovascular system104 and circulating ADMA may originate from both extra-renal and intra-renal sources. Thus, in salt-sensitive animals, disturbances in NO activity caused by salt-induced increases in circulating ADMA from extra-renal sources, could help explain why transplanting a kidney from a Dahl salt-resistant rat into a bilaterally nephrectomized salt-sensitive recipient fails to correct salt-sensitivity in the recipient (Figure 5).109 Conversely, disturbances in NO activity caused by salt-induced increases in renal production of ADMA and decreases in renal clearance of ADMA, could help explain why transplanting a kidney from a Dahl salt-sensitive donor into a bilaterally nephrectomized salt-resistant recipient induces salt-sensitivity in the recipient.(Figure 5)109 It should also be noted that in humans, supplemental potassium appears to attenuate salt-sensitivity in part by preventing salt-induced increases in ADMA levels and decreases in NO activity.100
Figure 5.
Renal cross transplant studies in Dahl rats showing that the trait of salt-sensitivity does not always follow the kidney. Results from studies by Morgan, DiBona, and Mark demonstrating that Dahl salt-sensitive (S) recipients of kidneys from Dahl salt-resistant (R) donors are just as salt-sensitive as Dahl salt-resistant (R) recipients of kidneys from Dahl salt-sensitive (S) donors (adapted from Morgan et al109 with permission from the publisher). Some rats received the low salt diet (0.4% NaCl), some the high salt diet (8% NaCl). MAP, mean arterial pressure.
Emerging evidence from studies by Oberleithner and others83, 92, 95–98 has indicated that in some salt-sensitive models, the initiation of salt-induced hypertension is determined by decreases in vascular NO activity that are mediated by aberrant increases in the activity of epithelial like sodium channels in endothelial cells (termed “EnNaCs”).98 Specifically, it has been proposed that in some forms of salt-sensitivity, salt induced increases in plasma sodium together with aberrant increases in EnNaC activity induce increases in endothelial cell membrane stiffness by promoting sodium influx, cell swelling, and membrane actin polymerization (Figure 4).83, 92, 97, 98 The increases in endothelial cell membrane stiffness are said to reduce membrane deformability in a manner that interferes with the normal capacity of increases in pulsatile flow/shear stress to activate signaling pathways that promote increases in nitric oxide activity and vasodilation (Figure 4).92, 97, 98 Such increases in EnNaC activity that promote endothelial cell stiffness in response to salt-loading may be determined by increases in mineralocorticoid levels or by variants in genes that code for EnNaC subunits or other proteins that affect EnNaC activity.98 Accordingly, this novel mechanism for impairing normal vasodilatory responses to increases in salt intake may be particularly relevant to the initiation of salt-induced hypertension in patients with hyperaldosteronism, certain forms of congenital adrenal hyperplasia, Liddle syndrome, and the syndrome of apparent mineralocorticoid excess.80, 83
Exposure of endothelial cells to increases in potassium levels in the physiologic range can reduce endothelial cell stiffness and increase NO release.110 This phenomenon might contribute to the ability of supplemental dietary potassium to increase plasma biomarkers of NO and attenuate salt-induced increases in blood pressure.100 Conversely, in subjects with a low potassium intake and suboptimal plasma levels of potassium, the risk for salt-sensitivity may be increased owing to increases in endothelial stiffness and decreases in NO activity mediated by both salt-loading and decreases in plasma concentrations of potassium.
We recognize that disturbances in NO activity, and in many other factors that could influence vascular resistance responses to salt-loading, may also influence renal tubular reabsorption of sodium.111 However, during initiation of salt-loading, the effects of these factors on renal tubular sodium transport usually do not cause greater increases in sodium retention35–38, 48–51 and cardiac output37, 38, 44, 53, 55, 56 in salt-sensitive subjects than in salt-loaded normal subjects. Thus, effects of these factors on renal tubular sodium transport do not mechanistically account for the hemodynamic initiation of greater salt-induced increases in blood pressure in salt-sensitive subjects than in salt-loaded normal subjects.
The Role of the Sympathetic Nervous System and Other Mechanisms in Mediating Normal and Abnormal Vascular Resistance Responses to Increases in Salt Intake
We have highlighted disturbances in several mechanisms regulating nitric oxide pathways in the vasculature of salt-sensitive subjects compared to normal subjects (Figure 4). However, we recognize that in salt-sensitive humans and animal models, a variety of factors, both neural and non-neural (Table 1), likely play important roles in the abnormal vascular resistance response to initiation of salt-loading. Among these other mechanisms, some that have received the most attention include alterations in sympathetic nervous system activity,15, 20–22, 24, 68, 71, 73, 112 activity of the local and circulating renin-angiotensin systems,113–117 and the activities of transient receptor potential vanilloid channels.118–121 In addition to research on the many well known pathways involved in regulation of vascular resistance (Table 1), novel mechanisms involved in subnormal vasodilatory responses to increases in salt intake may be revealed by studies in which genetic alterations are selectively targeted to the vasculature122, 123
It should also be noted that the sodium and chloride components of salt may each have an independent capacity to affect vascular resistance and blood pressure.124–133 It is possible that in some salt-sensitive subjects, the sodium or chloride components might not only fail to cause a normal decrease in vascular resistance, either or both ionic components of salt might provoke increases in vascular resistance. It is also possible that alterations in the amount of sodium and or chloride stored in skin or other tissues,134, 135 including the vasculature or the brain, might play a role in abnormal vascular resistance responses to salt.
The Relevance of Acute Salt Loading Studies to the Initiation of Sustained Hypertension
In the current analysis, we draw inferences about the initiation of short-term salt-induced increases in blood pressure and the initiation of sustained hypertension from acute salt loading studies in salt-sensitive and salt-resistant normotensive humans.36–38, 44 The rationale for this is based on data indicating that properly controlled acute salt loading studies are predictive of the occurrence of sustained hypertension and of cardiovascular disease in humans.5–7 It should also be noted that acute salt loading studies are highly predictive of the development of sustained hypertension in the Dahl rat model of salt-sensitivity.55, 56
The current analysis focuses on hemodynamic abnormalities that mediate the initiation of salt-induced hypertension and is not intended to discuss the hemodynamic abnormalities involved in sustaining salt-induced hypertension. However, it should be noted that in chronic forms of salt-sensitive hypertension (e.g., primary aldosteronism), the level of systemic vascular resistance, not the level of cardiac output, is greater than that in normotensive controls.136 In addition, in long-term salt-loading studies in humans, Titze’s group has reported that variations in blood pressure are associated with variations in salt intake but are not associated with variations in total body sodium.137
Conclusion
There now exists a large body of evidence which demonstrates that a subnormal vasodilatory response to increased salt intake, not abnormally large increases in renal sodium retention and cardiac output in response to increased salt intake, accounts for the hemodynamic initiation of most instances of salt-induced hypertension. Multiple mechanisms including disturbances in nitric oxide activity, sympathetic nervous system activity, and activity of other pathways regulating vascular resistance likely mediate the impaired vasodilatory response to salt that hemodynamically initiates salt-induced increases in blood pressure. This conclusion shifts the pathophysiologic focus to disturbances in the regulation of vascular resistance and away from the conventional view that salt-induced hypertension is mediated by increases in renal retention of sodium in amounts greater than those which occur during salt loading in normal controls. We believe that a better understanding of the mechanisms of salt-sensitivity and salt-resistance will lead to more precisely targeted, and hence more effective, approaches to the prevention and treatment of hypertension and cardiovascular disease.
Supplementary Material
Acknowledgments
FUNDING SOURCES: This work was supported by the General Clinical Research Center, Moffitt-Long Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources, M0 RR-00079, US Public Health Service. This work was also supported by National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230, and gifts from the Emil Mosbacher, Jr Foundation, the Antel Foundation, and the Maier Family Foundation.
Footnotes
DISCLOSURES: None.
References
- 1.Ambard L, Beaujard E. Causes de l’hypertension arterielle. Arch Gen de Med. 1904;1:520–533. [Google Scholar]
- 2.de Leeuw PW, Kroon AA. Salt and Sensitivity. Hypertension. 2013;62:461–462. doi: 10.1161/HYPERTENSIONAHA.113.01831. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 6.Barba G, Galletti F, Cappuccio FP, Siani A, Venezia A, Versiero M, Della Valle E, Sorrentino P, Tarantino G, Farinaro E, Strazzullo P. Incidence of hypertension in individuals with different blood pressure salt-sensitivity: results of a 15-year follow-up study. J Hypertens. 2007;25:1465–1471. doi: 10.1097/HJH.0b013e3281139ebd. [DOI] [PubMed] [Google Scholar]
- 7.Mu J, Zheng S, Lian Q, Liu F, Liu Z. Evolution of blood pressure from adolescents to youth in salt sensitivies: a 18-year follow-up study in Hanzhong children cohort. Nutr J. 2012;11:70. doi: 10.1186/1475-2891-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 10.Carey RM. Pathophysiology of Primary Hypertension. Comprehensive Physiology 2011, Supplement 9: Handbook of Physiology, The Cardiovascular System, Microcirculation. :794–895. First published in print 2008. [Google Scholar]
- 11.Scholl UI, Lifton RP. Inherited disorders of renal salt homeostasis: Insights from molecular genetics studies. In: Alpern RJ, Moe OW, Caplan M, editors. Seldin and Giebisch’s The Kidney. 5. 1 (III) London: Elsevier; 2013. pp. 1213–1240. [Google Scholar]
- 12.Hall JE. Guyton and Hall Textbook of Medical Physiology. 13th. Philadelphia: Elsevier; 2015. [Google Scholar]
- 13.Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med. 2012;44(Suppl 1):S119–S126. doi: 10.3109/07853890.2012.671538. [DOI] [PubMed] [Google Scholar]
- 14.Rossier BC, Staub O, Hummler E. Genetic dissection of sodium and potassium transport along the aldosterone-sensitive distal nephron: importance in the control of blood pressure and hypertension. FEBS Lett. 2013;587:1929–1941. doi: 10.1016/j.febslet.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Adrogue HJ, Madias NE. Sodium surfeit and potassium deficit: Keys to the pathogenesis of hypertension. J Am Soc Hypertens. 2014;8:203–213. doi: 10.1016/j.jash.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest. 2014;124:2341–2347. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 18.Khalil RA. Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R509–R513. doi: 10.1152/ajpregu.00600.2005. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension--update on novel findings. Nephrol Dial Transplant. 2007;22:992–995. doi: 10.1093/ndt/gfl757. [DOI] [PubMed] [Google Scholar]
- 20.Leenen FH. The central role of the brain aldosterone-”ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–1139. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Gavras I, Gavras H. ‘Volume-expanded’ hypertension: the effect of fluid overload and the role of the sympathetic nervous system in salt-dependent hypertension. J Hypertens. 2012;30:655–659. doi: 10.1097/HJH.0b013e32834f6de1. [DOI] [PubMed] [Google Scholar]
- 22.Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Curr Opin Nephrol Hypertens. 2013;22:51–58. doi: 10.1097/MNH.0b013e32835b36ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RJ, Lanaspa MA, Gabriela Sanchez-Lozada L, Rodriguez-Iturbe B. The discovery of hypertension: evolving views on the role of the kidneys, and current hot topics. Am J Physiol Renal Physiol. 2015;308:F167–F178. doi: 10.1152/ajprenal.00503.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol. 2015;593:3065–3075. doi: 10.1113/jphysiol.2014.278317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borst JGG, Borst-De Geus A. Hypertension explained by Starling’s theory of circulatory homeostasis. Lancet. 1963;1:677–682. doi: 10.1016/s0140-6736(63)91443-0. [DOI] [PubMed] [Google Scholar]
- 26.Ledingham JM, Cohen RD. Changes in the Extracellular Fluid Volume and Cardiac Output during the Development of Experimental Renal Hypertension. Can Med Assoc J. 1964;90:292–294. [PMC free article] [PubMed] [Google Scholar]
- 27.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 28.Coleman TG, Guyton AC. Hypertension caused by salt loading in the dog. 3. Onset transients of cardiac output and other circulatory variables. Circ Res. 1969;25:153–160. doi: 10.1161/01.res.25.2.153. [DOI] [PubMed] [Google Scholar]
- 29.Guyton AC, Manning RD, Jr, Hall JE, Norman RA, Jr, Young DB, Pan YJ. The pathogenic role of the kidney. J Cardiovasc Pharmacol. 1984;6(Suppl 1):S151–S161. doi: 10.1097/00005344-198400061-00025. [DOI] [PubMed] [Google Scholar]
- 30.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis: A cause or a consequence of hypertension? Hypertension. 1990;15:547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 31.Brands MW. Chronic blood pressure control. Compr Physiol. 2012;2:2481–2494. doi: 10.1002/cphy.c100056. [DOI] [PubMed] [Google Scholar]
- 32.Ivy JR, Bailey MA. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol. 2014;592:3955–3967. doi: 10.1113/jphysiol.2014.271676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott P, Brown I. Sodium intakes around the world. Geneva: World Health Organization; 2007. [Google Scholar]
- 34.Weinberger MH, Luft FC, Bloch R, Henry DP, Pratt JH, Weyman AE, Rankin LI, Murray RH, Willis LR, Grim CE. The blood pressure-raising effects of high dietary sodium intake: racial differences and the role of potassium. J Am Coll Cardiol. 1982;1:139–148. doi: 10.1080/07315724.1982.10718981. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, Atarashi K, Ikeda T, Hirata Y, Igari T, Uehara Y, Takagi M, Matsuoka H, Takeda T, Murao S. Role of the aldosterone system in the salt-sensitivity of patients with benign essential hypertension. Jpn Heart J. 1983;24:79–89. doi: 10.1536/ihj.24.79. [DOI] [PubMed] [Google Scholar]
- 36.Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, Aronoff GR, Luft FC. Sodium kinetics in salt-sensitive and salt-resistant normotensive and hypertensive subjects. J Hypertens. 1992;10:663–669. [PubMed] [Google Scholar]
- 37.Schmidlin O, Sebastian AF, Morris RC., Jr What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–1039. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC., Jr Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385. doi: 10.1161/HYPERTENSIONAHA.111.170175. [DOI] [PubMed] [Google Scholar]
- 39.Heer M, Frings-Meuthen P, Titze J, Boschmann M, Frisch S, Baecker N, Beck L. Increasing sodium intake from a previous low or high intake affects water, electrolyte and acid-base balance differently. Br J Nutr. 2009;101:1286–1294. doi: 10.1017/S0007114508088041. [DOI] [PubMed] [Google Scholar]
- 40.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–F595. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 41.Sagnella GA, Markandu ND, Buckley MG, Miller MA, Singer DR, MacGregor GA. Hormonal responses to gradual changes in dietary sodium intake in humans. Am J Physiol. 1989;256:R1171–R1175. doi: 10.1152/ajpregu.1989.256.6.R1171. [DOI] [PubMed] [Google Scholar]
- 42.Brown WJJ, Brown FK, Krishan E. Exchangeable sodium and blood volume in normotensive and hypertensive humans on high and low sodium intake. Circulation. 1971;43:508–519. doi: 10.1161/01.cir.43.4.508. [DOI] [PubMed] [Google Scholar]
- 43.Lyons RH, Jacobson SD, Avery NL. Increases in the plasma volume following the administration of sodium salts. Am J Med Sci. 1944;208:148–154. [Google Scholar]
- 44.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1987;9:398–406. doi: 10.1161/01.hyp.9.4.398. [DOI] [PubMed] [Google Scholar]
- 45.Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239:F271–F280. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- 46.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol. 1998;274:F914–F923. doi: 10.1152/ajprenal.1998.274.5.F914. [DOI] [PubMed] [Google Scholar]
- 47.Krieger JE, Liard JF, Cowley AW., Jr Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol. 1990;259:H1629–H1636. doi: 10.1152/ajpheart.1990.259.6.H1629. [DOI] [PubMed] [Google Scholar]
- 48.Roman RJ, Osborn JL. Renal function and sodium balance in conscious Dahl S and R rats. Am J Physiol. 1987;252:R833–R841. doi: 10.1152/ajpregu.1987.252.5.R833. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K, Cowley AW., Jr Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- 50.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am J Physiol. 1995;268:H2375–H2383. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 51.Kanagy NL, Fink GD. Losartan prevents salt-induced hypertension in reduced renal mass rats. J.Pharmacol.Exp.Ther. 1993;265:1131–1136. [PubMed] [Google Scholar]
- 52.Gupta BN, Linden RJ, Mary DA, Weatherill D. The influence of high and low sodium intake on blood volume in the dog. Q J Exp Physiol. 1981;66:117–128. doi: 10.1113/expphysiol.1981.sp002539. [DOI] [PubMed] [Google Scholar]
- 53.Greene AS, Yu ZY, Roman RJ, Cowley AW., Jr Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol. 1990;258:H508–H514. doi: 10.1152/ajpheart.1990.258.2.H508. [DOI] [PubMed] [Google Scholar]
- 54.Rocchini AP, Cant JR, Barger AC. Carotid sinus reflex in dogs with low- to high-sodium intake. Am J Physiol. 1977;233:H196–H202. doi: 10.1152/ajpheart.1977.233.2.H196. [DOI] [PubMed] [Google Scholar]
- 55.Ganguli M, Tobian L, Iwai J. Cardiac output and peripheral resistance in strains of rats sensitive and resistant to NaCl hypertension. Hypertension. 1979;1:3–7. doi: 10.1161/01.hyp.1.1.3. [DOI] [PubMed] [Google Scholar]
- 56.Simchon S, Manger WM, Carlin RD, Peeters LL, Rodriguez J, Batista D, Brown T, Merchant NB, Jan K-M, Chien S. Salt-induced hypertension in Dahl salt-sensitive rats: Hemodynamics and renal responses. Hypertension. 1989;13:612–621. doi: 10.1161/01.hyp.13.6.612. [DOI] [PubMed] [Google Scholar]
- 57.Langston JB, Guyton AC, Douglas BH, Dorsett PE. Effect of changes in salt intake on arterial pressure and renal function in partially nephrectomized dogs. Circ Res. 1963;12:508–513. [Google Scholar]
- 58.Douglas BH, Guyton AC, Langston JB, Bishop VS. Hypertension Caused by Salt Loading. Ii. Fluid Volume and Tissue Pressure Changes. Am J Physiol. 1964;207:669–671. doi: 10.1152/ajplegacy.1964.207.3.669. [DOI] [PubMed] [Google Scholar]
- 59.Manning RD, Jr, Coleman TG, Guyton AC, Norman RA, Jr, McCaa RE. Essential role of mean circulatory filling pressure in salt-induced hypertension. Am J Physiol. 1979;236:R40–R47. doi: 10.1152/ajpregu.1979.236.1.R40. [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 61.Fujita T, Henry WL, Bartter FC, Lake CR, Delea CS. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am J Med. 1980;80:234. doi: 10.1016/0002-9343(80)90002-9. [DOI] [PubMed] [Google Scholar]
- 62.Guyton AC. Circulatory Physiology III: Arterial Pressure and Hypertension. Philadelphia: W.B. Saunders; 1980. [Google Scholar]
- 63.Hall JE, Mizelle HL, Brands MW, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 64.Mizelle HL, Montani J-P, Hester RL, Didlake RH, Hall JE. Role of pressure natriuresis in long-term control of renal electrolyte excretion. Hypertension. 1993;22:102–110. doi: 10.1161/01.hyp.22.1.102. [DOI] [PubMed] [Google Scholar]
- 65.Guyton AC, Hall JE, Coleman TG, Manning RD, Norman RA., Jr . The Dominant Role of the Kidneys in Long-Term Arterial Pressure Regulation in Normal and Hypertensive States. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2. Vol. 1. New York: Raven Press, Ltd; 1995. pp. 1311–1326. [Google Scholar]
- 66.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 67.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 68.Mark AL, Lawton WJ, Abboud FM, Fitz AE, Connor WE, Heistad DD. Effects of high and low sodium intake on arterial pressure and forearm vascular resistance in borderline hypertension. Circ Res. 1975;36/37(Supp 1):I-194–I-198. doi: 10.1161/01.res.36.6.194. [DOI] [PubMed] [Google Scholar]
- 69.Fink GD, Takeshita A, Mark AL, Brody MJ. Determinants of renal vascular resistance in the Dahl strain of genetically hypertensive rat. Hypertension. 1980;2:274–280. doi: 10.1161/01.hyp.2.3.274. [DOI] [PubMed] [Google Scholar]
- 70.Hatzinikolaou P, Gavras H, Brunner HR, Gavras I. Sodium-induced elevation of blood pressure in the anephric state. Science. 1980;209:935–936. doi: 10.1126/science.7403861. [DOI] [PubMed] [Google Scholar]
- 71.Mark AL. Sympathetic neural contribution to salt-induced hypertension in Dahl rats. Hypertension. 1991;17:I86–I90. doi: 10.1161/01.hyp.17.1_suppl.i86. [DOI] [PubMed] [Google Scholar]
- 72.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks VL, Osborn JW. Hormonal-sympathetic interactions in long-term regulation of arterial pressure: an hypothesis. Am J Physiol. 1995;268:R1343–R1358. doi: 10.1152/ajpregu.1995.268.6.R1343. [DOI] [PubMed] [Google Scholar]
- 74.Qi N, Rapp JP, Brand PH, Metting PJ, Britton SL. Body fluid expansion is not essential for salt-induced hypertension in SS/Jr rats. Am J Physiol. 1999;277:R1392–R1400. doi: 10.1152/ajpregu.1999.277.5.R1392. [DOI] [PubMed] [Google Scholar]
- 75.Boegehold MA. Microvascular structure and function in salt-sensitive hypertension. Microcirculation. 2002;9:225–241. doi: 10.1038/sj.mn.7800139. [DOI] [PubMed] [Google Scholar]
- 76.Beard DA, Mescam M. Mechanisms of pressure-diuresis and pressure-natriuresis in Dahl salt-resistant and Dahl salt-sensitive rats. BMC Physiol. 2012;12:6. doi: 10.1186/1472-6793-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liard JF. Regional blood flows in salt loading hypertension in the dog. Am J Physiol. 1981;240:H361–H367. doi: 10.1152/ajpheart.1981.240.3.H361. [DOI] [PubMed] [Google Scholar]
- 78.van Paassen P, de Zeeuw D, Navis G, de Jong PE. Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension. 1996;27:202–208. doi: 10.1161/01.hyp.27.2.202. [DOI] [PubMed] [Google Scholar]
- 79.Schmidlin O, Forman A, Tanaka M, Sebastian A, Morris RC., Jr NaCl-induced renal vasoconstriction in salt-sensitive African Americans: antipressor and hemodynamic effects of potassium bicarbonate. Hypertension. 1999;33:633–639. doi: 10.1161/01.hyp.33.2.633. [DOI] [PubMed] [Google Scholar]
- 80.Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC. Molecular based mechanisms of Mendelian forms of salt-dependent hypertension: Questioning the prevailing theory. Hypertension. 2015;65:932–941. doi: 10.1161/HYPERTENSIONAHA.114.05092. [DOI] [PubMed] [Google Scholar]
- 81.Maass PG, Aydin A, Luft FC, Schachterle C, Weise A, Stricker S, Lindschau C, Vaegler M, Qadri F, Toka HR, Schulz H, Krawitz PM, Parkhomchuk D, Hecht J, Hollfinger I, Wefeld-Neuenfeld Y, Bartels-Klein E, Muhl A, Kann M, Schuster H, Chitayat D, Bialer MG, Wienker TF, Ott J, Rittscher K, Liehr T, Jordan J, Plessis G, Tank J, Mai K, Naraghi R, Hodge R, Hopp M, Hattenbach LO, Busjahn A, Rauch A, Vandeput F, Gong M, Ruschendorf F, Hubner N, Haller H, Mundlos S, Bilginturan N, Movsesian MA, Klussmann E, Toka O, Bahring S. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat Genet. 2015;47:647–653. doi: 10.1038/ng.3302. [DOI] [PubMed] [Google Scholar]
- 82.Van Huysse JW, Amin MS, Yang B, Leenen FH. Salt-induced hypertension in a mouse model of Liddle syndrome is mediated by epithelial sodium channels in the brain. Hypertension. 2012;60:691–696. doi: 10.1161/HYPERTENSIONAHA.112.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–1059. doi: 10.1161/HYPERTENSIONAHA.111.199455. [DOI] [PubMed] [Google Scholar]
- 84.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bailey MA, Paterson JM, Hadoke PW, Wrobel N, Bellamy CO, Brownstein DG, Seckl JR, Mullins JJ. A switch in the mechanism of hypertension in the syndrome of apparent mineralocorticoid excess. J Am Soc Nephrol. 2008;19:47–58. doi: 10.1681/ASN.2007040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bailey MA, Craigie E, Livingstone DE, Kotelevtsev YV, Al-Dujaili EA, Kenyon CJ, Mullins JJ. Hsd11b2 haploinsufficiency in mice causes salt sensitivity of blood pressure. Hypertension. 2011;57:515–520. doi: 10.1161/HYPERTENSIONAHA.110.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to alpha 1-adrenergic stimulation in mice. Hypertension. 2011;58:439–445. doi: 10.1161/HYPERTENSIONAHA.111.172429. [DOI] [PubMed] [Google Scholar]
- 88.Susa K, Kita S, Iwamoto T, Yang SS, Lin SH, Ohta A, Sohara E, Rai T, Sasaki S, Alessi DR, Uchida S. Effect of heterozygous deletion of WNK1 on the WNK-OSR1/ SPAK-NCC/NKCC1/NKCC2 signal cascade in the kidney and blood vessels. Clin Exp Nephrol. 2012;16:530–538. doi: 10.1007/s10157-012-0590-x. [DOI] [PubMed] [Google Scholar]
- 89.Park HW, Kim JY, Choi SK, Lee YH, Zeng W, Kim KH, Muallem S, Lee MG. Serine-threonine kinase with-no-lysine 4 (WNK4) controls blood pressure via transient receptor potential canonical 3 (TRPC3) in the vasculature. Proc Natl Acad Sci U S A. 2011;108:10750–10755. doi: 10.1073/pnas.1104271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Obst M, Gross V, Luft FC. Systemic hemodynamics in non-anesthetized L-NAME- and DOCA-salt-treated mice. J Hypertens. 2004;22:1889–1894. doi: 10.1097/00004872-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dupont JJ, Hill MA, Bender SB, Jaisser F, Jaffe IZ. Aldosterone and vascular mineralocorticoid receptors: regulators of ion channels beyond the kidney. Hypertension. 2014;63:632–637. doi: 10.1161/HYPERTENSIONAHA.113.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415–424. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 95.Perez FR, Venegas F, Gonzalez M, Andres S, Vallejos C, Riquelme G, Sierralta J, Michea L. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension. 2009;53:1000–1007. doi: 10.1161/HYPERTENSIONAHA.108.128520. [DOI] [PubMed] [Google Scholar]
- 96.Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflugers Arch. 2014;466:851–859. doi: 10.1007/s00424-013-1356-3. [DOI] [PubMed] [Google Scholar]
- 97.Kusche-Vihrog K, Tarjus A, Fels J, Jaisser F. The epithelial Na+ channel: a new player in the vasculature. Curr Opin Nephrol Hypertens. 2014;23:143–148. doi: 10.1097/01.mnh.0000441054.88962.2c. [DOI] [PubMed] [Google Scholar]
- 98.Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, Jaisser F. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol. 2014;10:146–157. doi: 10.1038/nrneph.2013.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension : modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–861. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 100.Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive asians. Hypertension. 2006;48:724–729. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 101.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension. 1999;33:1008–1012. doi: 10.1161/01.hyp.33.4.1008. [DOI] [PubMed] [Google Scholar]
- 102.Cao Y, Mu JJ, Fang Y, Yuan ZY, Liu FQ. Impact of High Salt Independent of Blood Pressure on PRMT/ADMA/DDAH Pathway in the Aorta of Dahl Salt-Sensitive Rats. Int J Mol Sci. 2013;14:8062–8072. doi: 10.3390/ijms14048062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, Yasukawa H, Iwami G, Okuda S, Imaizumi T. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension. 1997;29:242–247. doi: 10.1161/01.hyp.29.1.242. [DOI] [PubMed] [Google Scholar]
- 104.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 105.Suda O, Tsutsui M, Morishita T, Tasaki H, Ueno S, Nakata S, Tsujimoto T, Toyohira Y, Hayashida Y, Sasaguri Y, Ueta Y, Nakashima Y, Yanagihara N. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1682–1688. doi: 10.1161/01.ATV.0000136656.26019.6e. [DOI] [PubMed] [Google Scholar]
- 106.Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension. 2008;52:960–966. doi: 10.1161/HYPERTENSIONAHA.108.116731. [DOI] [PubMed] [Google Scholar]
- 107.Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59:375–381. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 109.Morgan DA, DiBona GF, Mark AL. Effects of interstrain renal transplantation on NaCl-induced hypertension in Dahl rats. Hypertension. 1990;15:436–442. doi: 10.1161/01.hyp.15.4.436. [DOI] [PubMed] [Google Scholar]
- 110.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009;106:2829–2834. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol. 2012;2:2393–2442. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 112.Fink GD, Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension. 2009;53:307–312. doi: 10.1161/HYPERTENSIONAHA.108.119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams GH, Hollenberg NK. Non-modulating hypertension. A subset of sodium-sensitive hypertension. Hypertension. 1991;17:I81–I85. doi: 10.1161/01.hyp.17.1_suppl.i81. [DOI] [PubMed] [Google Scholar]
- 114.He FJ, MacGregor GA. Salt, blood pressure and the renin-angiotensin system. J Renin Angiotensin Aldosterone Syst. 2003;4:11–16. doi: 10.3317/jraas.2003.001. [DOI] [PubMed] [Google Scholar]
- 115.Chamarthi B, Williams JS, Williams GH. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens. 2010;28:1020–1026. doi: 10.1097/HJH.0b013e3283375974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leenen FH. Actions of Circulating Angiotensin II and Aldosterone in the Brain Contributing to Hypertension. Am J Hypertens. 2014;27:1024–1032. doi: 10.1093/ajh/hpu066. Epub 2014 Apr 17. [DOI] [PubMed] [Google Scholar]
- 117.Majid DS, Prieto MC, Navar LG. Salt-Sensitive Hypertension: Perspectives on Intrarenal Mechanisms. Curr Hypertens Rev. 2015;11:38–48. doi: 10.2174/1573402111666150530203858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 119.Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension. 2009;53:228–235. doi: 10.1161/HYPERTENSIONAHA.108.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Armando I, Jose PA. Sensing salt intake. Hypertension. 2009;53:118–119. doi: 10.1161/HYPERTENSIONAHA.108.125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hollis M, Wang DH. Transient receptor potential vanilloid in blood pressure regulation. Curr Opin Nephrol Hypertens. 2013;22:170–176. doi: 10.1097/MNH.0b013e32835c8d4c. [DOI] [PubMed] [Google Scholar]
- 122.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 123.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD, Faraci FM. Genetic interference with peroxisome proliferator-activated receptor gamma in smooth muscle enhances myogenic tone in the cerebrovasculature via A Rho kinase-dependent mechanism. Hypertension. 2015;65:345–351. doi: 10.1161/HYPERTENSIONAHA.114.04541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kurtz TW, Al-Bander HA, Morris RCJ. “Salt-sensitive” essential hypertension in men: Is the sodium ion alone important? N Engl J Med. 1987;317:1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- 126.Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol. 1993;108:106–110. doi: 10.1111/j.1476-5381.1993.tb13447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka M, Schmidlin O, Yi SL, Bollen AW, Morris RC., Jr Genetically determined chloride-sensitive hypertension and stroke. Proc Natl Acad Sci U S A. 1997;94:14748–14752. doi: 10.1073/pnas.94.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 129.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC., Jr Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension. 2005;45:867–873. doi: 10.1161/01.HYP.0000164628.46415.66. [DOI] [PubMed] [Google Scholar]
- 130.Kotchen TA. Contributions of sodium and chloride to NaCl-induced hypertension. Hypertension. 2005;45:849–850. doi: 10.1161/01.HYP.0000164629.94634.27. [DOI] [PubMed] [Google Scholar]
- 131.Schmidlin O, Forman A, Sebastian A, Morris RC., Jr Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50:1085–1092. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmidlin O, Tanaka M, Sebastian A, Morris RC., Jr Selective chloride loading is pressor in the stroke-prone spontaneously hypertensive rat despite hydrochlorothiazide-induced natriuresis. J Hypertens. 2010;28:87–94. doi: 10.1097/HJH.0b013e3283316cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hubner CA, Schroeder BC, Ehmke H. Regulation of vascular tone and arterial blood pressure: role of chloride transport in vascular smooth muscle. Pflugers Arch. 2015;467:605–614. doi: 10.1007/s00424-014-1684-y. [DOI] [PubMed] [Google Scholar]
- 134.Helle F, Karlsen TV, Tenstad O, Titze J, Wiig H. High-salt diet increases hormonal sensitivity in skin pre-capillary resistance vessels. Acta Physiol (Oxf) 2013;207:577–581. doi: 10.1111/apha.12049. [DOI] [PubMed] [Google Scholar]
- 135.Titze J. A different view on sodium balance. Curr Opin Nephrol Hypertens. 2015;24:14–20. doi: 10.1097/MNH.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 136.Frohlich ED, Tarazi RC, Dustan HP. Re-examination of the hemodynamics of hypertension. Am J Med Sci. 1969;257:9–23. doi: 10.1097/00000441-196901000-00002. [DOI] [PubMed] [Google Scholar]
- 137.Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, Vassilieva G, Lenkova L, Johannes B, Wabel P, Moissl U, Vienken J, Gerzer R, Eckardt KU, Muller DN, Kirsch K, Morukov B, Luft FC, Titze J. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.