Abstract
Satyrization, a form of asymmetric reproductive interference, has recently been shown to play a role in competitive displacements of Aedes aegypti (L.) by Aedes albopictus (Skuse). Furthermore, female Ae. aegypti from populations in sympatry with Ae. albopictus have evolved reproductive character displacement and changes in mating behavior to reduce interspecific mating. In this article, we examine evolutionary responses of males to interspecific mating and show that satyrization has also evoked reproductive character displacement in males. We demonstrate that the presence of heterospecific females negatively influences conspecific mating success in male Ae. aegypti, most likely due to misdirected courting or mating efforts, and that males of this species from populations in sympatry with Ae. albopictus have evolved to be less influenced by the presence of heterospecific females than their allopatric counterparts. Conversely, we suggest that the presence of conspecifics may, in some circumstances, increase interspecific mating. This study demonstrates that co-occurrences of these two invasive species may lead to evolution and adaptation of reproductive behaviors to changing circumstances. Understanding the processes driving development of mate choice preferences or avoidance mechanisms may help predict future changes in the distribution and abundance of insect vectors or pests.
Keywords: satyrization, interspecific courtship, Aedes aegypti, Aedes albopictus, male
Aedes aegypti (L.) and Aedes albopictus (Skuse) are considered the most invasive vectors in history (Juliano and Lounibos 2005) and, owing to their wide dispersal, often come in contact in their invasive ranges. Both species belong to the subgenus Stegomyia and share similar life histories and mating habits. Males and females aggregate at vertebrate hosts during similar diurnal peak activity periods (Hartberg 1971, Gubler and Bhattachaya 1972) and initiate mating in flight by following visual and auditory cues (Roth 1948, Cator et al. 2009). These common behaviors may contribute to interspecific mating between these two species, particularly after successful establishments lead to first encounters of invasive and resident populations. Interspecific matings, however, do not produce viable offspring (Leahy and Craig 1967, Lee et al. 2009) and leave females of Ae. aegypti, but not Ae. albopictus, refractory to further mating (Tripet et al. 2011). This satyrizing effect may be a powerful mechanism (Ribeiro and Spielman 1986, Ribeiro 1988, Nasci et al. 1989, Lounibos 2007, Tripet et al. 2011, Bargielowski et al. 2013, Bargielowski and Lounibos 2014) in the displacement of Ae. aegypti populations by invading Ae. albopictus. Furthermore, recent work has shown bidirectional mating in this species pair to be asymmetrical, with Ae. aegypti females being more susceptible to interspecific insemination than Ae. albopictus females (Nasci et al. 1989, Bargielowski et al. 2013, Bargielowski and Lounibos 2014). The selection pressure for Ae. aegypti females to avoid such errant mating is therefore great, and rapid evolution of resistance to satyrization has been documented in previous studies (Bargielowski et al. 2013, Bargielowski and Lounibos 2014). Female Ae. aegypti from populations allopatric to Ae. albopictus in the field were more susceptible to interspecific mating than females from sympatric populations, and selection experiments in cages confirmed the rapid development of resistance to satyrization in the laboratory, as well as changes in behavior toward conspecifics associated with increased satyrization resistance (Bargielowski and Lounibos 2014). In contrast, little is known about male mating behavior of these species in relation to interspecific encounters.
In this article, we examine evolutionary responses of both Ae. aegypti and Ae. albopictus males to interspecific mating by measuring whether the presence of heterospecific females may impact conspecific mating success in cages.
The mating systems of Ae. aegypti and Ae. albopictus have traditionally been thought to be governed largely by male scramble competition. However, recent findings suggest female mate choice (at least in Ae. aegypti) to be important in these species (Cator and Harrington 2011, Bargielowski et al. 2013, Bargielowski and Lounibos 2014). Males may therefore not only need to reach a female before their competitors, but also elicit female acceptance once they have located a potential mate. Furthermore, studies suggest males may be limited in the number of females they can inseminate in a day and over a lifetime (Bargielowski et al. 2011). Interspecific courtship will therefore waste time, energy, and possibly gametes, as well as exposing males to increased predation risk or host defenses. Male courtship costs have been established in a number of arthropods (e.g., fruit flies (Cordts and Partridge 1996), drumming wolf spiders (Mappes et al. 1996), tsetse flies (Clutton-Brock and Parker 1992), dobsonflies (Hayashi 1993), and crickets (Sakaluk 1985)) as well as for the mosquitoes Sabethes cyaneus (South et al. 2009) and Anopheles freeborni (Yuval and Bouskila 1993, Yuval et al. 1993). From an evolutionary perspective, it is therefore important for males, as well as females, to direct their courtship toward conspecifics instead of incompatible heterospecifics.
In this article, we test two predictions, that—1) the presence of heterospecific females will impact the success of conspecific mating (as males will waste reserves courting and possibly mating heterospecific females) and 2) adaptations in male behavior will have evolved, analogous to those in female behavior established by our earlier work (Bargielowski et al 2013, Bargielowski and Lounibos 2014), when comparing males from sympatric and allopatric populations. Since mating “errors” as predicted in 1) are costly to males, we expect males from sympatric populations to avoid such behavior and therefore to have higher intraspecific mating success than males from allopatric populations.
Furthermore, we examine the effect that the presence of conspecifics has on the rate of interspecific insemination. To date, interspecific mating has commonly been assessed in nonchoice trials (i.e., female Ae. aegypti caged with male Ae. albopictus, or vice versa). However, it is possible that the presence of conspecifics may influence the dynamics of interspecific interactions and change the frequency of interspecific courtship and mating.
Methods
Laboratory and Rearing Conditions
Ae. aegypti and Ae. albopictus colonies were established in 2011 from field collections of aquatic immatures from artificial containers, such as discarded tires or cemetery vases. Sympatric lines were derived from collections at a salvage yard in Vero Beach (VB), FL, where the two species have co-occurred for at least two decades (O’Meara et al. 1993), while the allopatric line of Ae. aegypti was collected in Key West (KW), FL, and the allopatric line of Ae. albopictus was established from collections in East St. Louis (ESL), IL. Adults used in the experiments had spent three to five generations in the laboratory (F3–5), except for the allopatric strain of Ae. albopictus, which was F9. Experiments were carried out in screened, plastic Bug Dorm (Bioquip) cages (30 by 30 by 30 cm3) in an insectary maintained at 27 (±0.62)°C and 89 (±5.28)% relative humidity (RH) under a photoperiod of 14:10 (L:D) h. Larvae were reared from hatch to pupation in pans containing one liter of tap water (100 larvae per pan) and provided 0.6 g of a 1:1 brewer’s yeast and egg albumin mix on day one. Pupae were sexed according to morphological differences in their external genitalia (Vargas 1968) and segregated by species and sex in small containers (30 per container) for emergence. If a mistake during sexing was detected after emergence, the container was discarded. All adult mosquitoes were provided continuous access to 10% sugar solution and were 3–4 d old when used in experiments.
Mate Choice in the Presence of Heterospecifics
For each test, 25 males were aspirated into a cage containing 50 conspecific and 50 heterospecific females. They were left to cohabit for 24 h before the females were removed, dissected, and scored for the presence of sperm in their spermathecae. The sex ratio used in this experiment was chosen based on preliminary test results showing this ratio to be low enough for differences in conspecific mating to be detected, but still high enough for heterospecific mating to occur. Three repeats were carried out for Ae. aegypti and Ae. albopictus males from allopatric and sympatric populations with conspecific females of the same colony and heterospecific females from either sympatric or allopatric lines. Control treatments comprised 25 males caged with 100 conspecific females.
Effect of Exposure Time on Interspecific Mating
To establish a baseline of mating frequency in nonchoice trials, 100 Ae. albopictus males (ESL) and 100 Ae. aegypti females (KW) were aspirated into cages and left to cohabit for either 24 h, 1 wk, 2 wk, or 3 wk (three cages per treatment) before the females were removed, dissected, and scored for the presence of sperm in their spermathecae.
Statistical Analysis
The proportions of females inseminated were arcsine transformed, which gave normally distributed residuals when analyzed. Data were analyzed with a nominal logistic model in JMP (version 8; http://www.jmpdiscovery.com, accessed 27 January 2015) for effects of the independent variables population origin (sympatric vs allopatric) of males and females on the dependent variable likelihood of cross-mating. Variation among groups was analyzed by ANOVA and post hoc means comparisons.
Results
Mate Choice in the Presence of Heterospecifics
Insemination of Conspecific Females
Ae. aegypti (males)
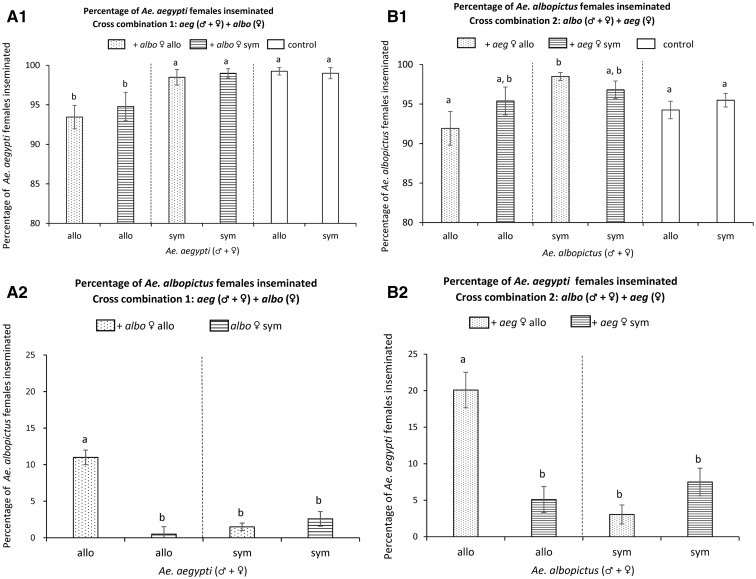
The results showed that the origin of the Ae. aegypti males (F = 8.93, df = 1, P = 0.01), but not that of the heterospecific females (F = 0.49, df = 1, P = 0.50; or the interaction term (F = 0.16, df = 1, P = 0.70)) influenced the percentage of conspecific Ae. aegypti females inseminated, suggesting adaptation in male mating behavior (Fig. 1A1).
Fig. 1.
Mate choice in the presence of heterospecifics. Each treatment within cross combinations was repeated three times. Abbreviations: aeg, Ae. aegypti; albo, Ae. albopictus; allo, allopatric; sym, sympatric. Origins: allopatric Ae. aegypti—Key West, sympatric Ae. aegypti and Ae. albopictus—Vero Beach, allopatric Ae. albopictus—East St. Louis. Error bars denote standard error. (A) Panels describe the results of the cross combination: Ae. aegypti males and females caged with Ae. albopictus females; (A1) showing the percentage of conspecific females inseminated, (A2) showing the percentage of heterospecific females inseminated. (B) Panels describe equivalent results for the cross combination: Ae. albopictus males and females caged with Ae. aegypti females. X-axes labels show the origin of conspecifics, while the fill patterns of bars show the origin of heterospecific females (see figure legend). In all panels, significant differences (P < 0.05) among arcsine-transformed proportions within species are denoted by different letters (post hoc means comparisons (Student-t) following ANOVA).
Compared with purely conspecific crosses (no heterospecific females; sympatric population: 99.0% ± SE 0.7, allopatric population: 99.25% ± SE 0.48), Ae. aegypti males from populations sympatric with Ae. albopictus in the field inseminated a similar proportion of conspecifics (98.73% ± SE 0.78; F = 0.13, df = 1, P = 0.73) in the presence of heterospecific females, while males from populations that were allopatric to Ae. albopictus in the field inseminated significantly fewer conspecific females (94.13% ± SE 1.64; F = 7.20, df = 1, P = 0.02) in the presence of heterospecifics (Fig. 1A1).
Ae. albopictus (males)
Neither the origin of Ae. albopictus males (F = 4.54, df = 1, P = 0.06), the origin of the heterospecific females (F = 0.11, df = 1, P = 0.75), nor their interaction (F = 3.38, df = 1, P = 0.09) significantly affected the percentage of conspecific females inseminated (Fig. 1B1).
Compared with purely conspecific crosses (sympatric population: 95.49% ± SE 0.86, allopatric population: 94.25% ± SE 1.11), Ae. albopictus males from populations sympatric with Ae. aegypti (93.66% ± SE 1.95; F = 3.56, df = 1, P = 0.09) as well as from populations allopatric to Ae. aegypti (97.65% ± SE 0.8) inseminated a similar percentage (F = 3.74, df = 1, P = 0.09) of conspecific females in the presence of heterospecifics (Fig. 1B1).
Insemination of Heterospecific Females
Ae. aegypti (males)
The origin of Ae. aegypti males (F = 27.87, df = 1, P < 0.001), that of the heterospecific females (F = 17.30, df = 1, P = 0.001), as well the interaction term were significant (F = 42.16, df = 1, P < 0.01; (Fig. 1A2). These differences are driven largely by the high number of heterospecific females inseminated in the allopatric (Ae. aegypti males + conspecific females) × allopatric (Ae. albopictus females) combination (11% ± SE 1.00) compared with all other combinations (range: 0.51–2.59%; Fig. 1A2).
Ae. albopictus (males)
The origin of Ae. albopictus males (F = 7.85, df = 1, P = 0.02), that of the heterospecific females (F = 15.05, df = 1, P < 0.001), as well the interaction term (F = 26.42, df = 1, P < 0.01) were significant (Fig. 1B2). Again, these differences are driven largely by the high number of heterospecific females inseminated in the allopatric (Ae. albopictus males + conspecific females) × allopatric (Ae. aegypti females) combination (20.09% ± SE 2.43) compared with all other combinations (range: 3.05–7.50%; Fig. 1B2).
Effect of Exposure Time on Interspecific Mating
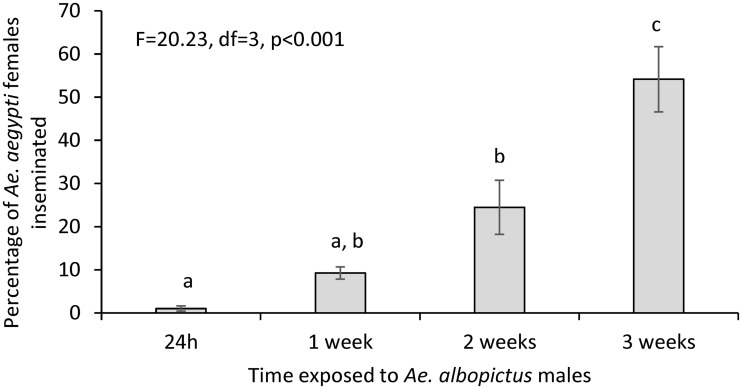
Exposure time significantly affected interspecific insemination (F = 20.23, df = 3, P < 0.001), with insemination rates increasing from 1.04 (±SE 0.61)% after 24 h to 54.13 (±SE 7.56)% after 3 wk (Fig. 2).
Fig. 2.
Effect of exposure time on interspecific mating. Three repeats were carried out for each time point. Significant differences among arcsine-transformed proportions at measured time points are denoted by different letters (post-hoc means comparisons (Student-t) following ANOVA). Error bars denote standard error.
Discussion
Despite extensive literature on Ae. aegypti and Ae. albopictus, owing largely to their status as important vectors of both dengue and chikungunya viruses (Vazeille et al. 2007, Kyle and Harris 2008, Pages et al. 2009, Paupy et al. 2010), comparatively little is known about their mate recognition systems, particularly with regard to male behavior (reviewed in Oliva et al 2014). Most studies cover female aspects of reproduction and date from the 1970s. However, more recent reports include the possible existence of an “aggregation pheromone” (produced by both males and females) that may modulate swarming behavior in Ae. aegypti (Cabrera and Jaffe 2007) and the discovery that male and female mosquitoes synchronize their flight tones (wing beat frequencies) before mating (Cator et al 2009). While it has been established that the flight tones of Ae. aegypti and Ae. albopictus females differ significantly (Brogdon 1994) and flight tone recognition seems to be involved in species recognition in anophelines (Pennetier et al 2010), it is still unclear whether this is the case in aedines (Roth 1948, Nijhout and Craig 1971). Nijhout and Craig (1971) instead suggest the involvement of a species-specific pheromone that enables recognition following contact, although subsequent investigators have been unable to confirm this.
Though it is unclear which traits are subject to change, we here demonstrate that mate recognition systems can evolve in the presence of closely related species whose geographic distribution did not overlap until ranges expanded by means of human-aided invasions. If mating attempts directed at heterospecifics are costly in terms of reproductive success, either through direct or indirect measures, natural selection may increase divergence between sympatric taxa by selecting against these “misdirected” mating attempts. This process may result in reproductive character displacement (Howard 1993), where sympatric populations of closely related (interacting) species diverge in mate recognition to a greater extent than allopatric populations (Higgie and Blows 2008). Our recent work on satyrization in Ae. aegypti and Ae. albopictus (Bargielowski et al. 2013, Bargielowski and Lounibos 2014) has shown such reproductive character displacement in the mating behavior of females, with Ae. aegypti females from allopatric origins being more likely to engage in interspecific mating than females from sympatric origins. Here we document a similar behavioral shift in males. We note that the experimental design employed does not readily distinguish between a response in male behavior versus a response in conspecific female behavior. However, as discussed below we propose the biologically most probable interpretation is indeed that of male adaptation. Ae. aegypti males from sympatric populations mate significantly more conspecific females in the presence of heterospecific females than do males of allopatric origin. Males from sympatric populations may have developed a more specialized species recognition mechanism allowing them to better distinguish conspecific from heterospecific females. Their allopatric counterparts, lacking evolution of this trait, may thus waste time and energy courting and inseminating heterospecific females, instead of directing their attentions to conspecifics, in the process diminishing their reproductive potential. Our earlier work (Bargielowski et al. 2013, Bargielowski and Lounibos 2014) showed that the same does not apply for sympatric Ae. albopictus females, which actually mated interspecifically more frequently than their allopatric counterparts. Conversely, in this article, we saw that Ae. albopictus males showed a trend, though not statistically significant, similar to male Ae. aegypti. We speculate that this trend was not significant because of the unexpectedly low mating success of the control cages (compared with similar measures of Ae. albopictus interspecific mating rates observed in our laboratory) and suggest that this trend may indeed represent a biologically relevant phenomenon. Therefore, for Ae. albopictus, the two sexes demonstrate contrary responses to interactions with Ae. aegypti. One speculative explanation may be in the respective cost of misdirected mating for either sex. Ae. albopictus females may lose a small amount of time and energy engaging in interspecific mating, but ultimately are able to re-mate a conspecific male with no (documented) loss of reproductive potential, as females require only one compliment of sperm to fertilize a lifetime’s supply of eggs. Males on the other hand, have been shown to have limited mating potential over the course of their lifetimes. Boyer et al. (2011) report that in laboratory trials, Ae. albopictus males mated on average 8.6 females over a 2-wk period (once it had been established that mating ceased following this time point). Thus, males lose time, energy and reproductive potential with each interspecific mating.
For both species combinations, the heterospecific insemination rates were highest in the (F/M) “allopatric” + (F) “allopatric” combinations. Given the fact that the lineages of both the females and males in these cross combinations had no histories of interspecific encounters, this result is not unexpected. However, the magnitude of the difference compared with all other cross combinations was large. Furthermore, the relatively high rates of heterospecific insemination observed in 24 h were surprising (up to 11% for Ae. albopictus females (Fig. 1A2) and 20.09% for Ae. aegypti females (Fig. 1B2) compared with only 1.04% for Ae. aegypti females in the nonchoice trial (Fig. 2)). The nonchoice trials ((F) Ae. aegypti (KW) × (M) Ae. albopictus (ESL)) showed that increasing cohabitation time significantly increased interspecific insemination rates. Though the proportion of males versus females differed in the choice versus nonchoice trials (1:4 vs 1:1), it appears that the presence of conspecifics increased heterospecific mating. It is conceivable that the presence of conspecifics elicited courting behavior and perhaps, partially due to their confinement in cages, these courting attempts were more frequently misdirected at heterospecifics than in nonchoice scenarios.
This study demonstrates that co-occurrences of these two invasive species lead to evolution and adaptation of reproductive behaviors to changing circumstances. Understanding the processes driving development of mate choice preferences or avoidance mechanisms may help predict future changes in the distribution and abundance of vector populations.
Acknowledgments
We thank Steve Juliano for providing eggs of allopatric Ae. albopictus and Barry Alto for providing Ae. aegypti eggs from Key West. This research was supported by National Institutes of Health R21 grant AI095780 (to L.P.L.).
References Cited
- Bargielowski I., Alphey L., Koella J. C. 2011. Cost of mating and insemination capacity of a genetically modified mosquito Aedes aegypti OX513A compared to its wild type counterpart. PLoS One 6: e26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski I., Lounibos L. P. 2014. Rapid evolution of reduced receptivity to interspecific mating in the dengue vector Aedes aegypti in response to satyrization by invasive Aedes albopictus. Evol. Ecol. 28: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski I. E., Lounibos L. P., Carrasquilla M. C. 2013. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA 110: 2888–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S., Gilles J., Merancienne D., Lemperiere G., Fontenille D. 2011. Sexual performance of male mosquito Aedes albopictus. Med. Vet. Entomol. 25: 454–459. [DOI] [PubMed] [Google Scholar]
- Brogdon W. G. 1994. Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae). J. Med. Entomol. 31: 700–703. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Jaffe K. 2007. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 23: 1–10. [DOI] [PubMed] [Google Scholar]
- Cator L. J., Harrington L. C. 2011. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim. Behav. 82: 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator L. J., Arthur B. J., Harrington L. C., Hoy R. R. 2009. Harmonic convergence in the love songs of the dengue vector mosquito. Science 323: 1077–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Parker G. H. 1992. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans Westwood (Diptera: Glossinidae). Behav. Ecol. 8: 392–395. [Google Scholar]
- Cordts R., Partridge L. 1996. Courtship reduces longevity of male Drosophila melanogaster. Anim. Behav. 52: 269–278. [Google Scholar]
- Gubler D. J., Bhattachaya N. C. 1972. Swarming and mating of Aedes (S.) albopictus in nature. Mosq. News 32: 219–223. [Google Scholar]
- Hartberg W. K. 1971. Observations on the mating behaviour of Aedes aegypti in nature. Bull. World Health Organ. 45: 847–850. [PMC free article] [PubMed] [Google Scholar]
- Hayashi F. 1993. Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim. Behav. 45: 343–349. [Google Scholar]
- Higgie M., Blows M. W. 2008. The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62: 1192–1203. [DOI] [PubMed] [Google Scholar]
- Howard D. J. 1993. Reinforcement: Origin, dynamics, and fate of an evolutionary hypothesis. In Harrison R. G. (ed.), Hybrid zones and evolutionary processes. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Juliano S. A., Lounibos L. P. 2005. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 8: 558–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle J. L., Harris E. 2008. Global spread and persistence of dengue. Ann. Rev. Microbiol. 62: 71–92. [DOI] [PubMed] [Google Scholar]
- Leahy M. G., Craig G. B. 1967. Barriers to hybridization between Aedes aegypti and Aedes albopictus. Evolution 21: 41–58. [DOI] [PubMed] [Google Scholar]
- Lee H. L., Aramu M., Nazni W. A., Selvi S., Vasan S. 2009. No evidence for successful interspecific cross-mating of transgenic Aedes aegypti (L.) and wild type Aedes albopictus Skuse. Trop. Biomed. 26: 312–319. [PubMed] [Google Scholar]
- Lounibos L. P. 2007. Competitive displacement and reduction. In Floore T. E. (ed.), Biorational control of mosquitoes. Bull. No. 7 Am. Mosq. Control Assoc. 23: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mappes J., Atalo R. V., Kotiaho J. S., Parri S. 1996. Viability costs of condition-dependent sexual male display in a drumming wolf spider. Proc. R. Soc. B 263: 785–789. [Google Scholar]
- Nasci R. S., Hare S. G., Willis F. S. 1989. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and in laboratory. J. Am. Mosq. Control Assoc. 5: 416–421. [PubMed] [Google Scholar]
- Nijhout H. F., Craig G. B., Jr. 1971. Reproductive isolation in Stegomyia mosquitoes. III Evidence for a sexual pheromone. Entomol. Exp. Appl. 14: 399–412. [Google Scholar]
- Oliva C. F., Damiens D., Benedict M. Q. 2014. Male reproductive biology of Aedes mosquitoes. Acta Trop. 132S: S12–S19. [DOI] [PubMed] [Google Scholar]
- O’Meara G. F., Gettman A. D., Evans L. F., Curtis G. A. 1993. The spread of Aedes albopictus in Florida. Am. Entomol. 39: 163–173. [Google Scholar]
- Pages F., Peyrefitte C. N., Mve M. T., Jarjaval F., Brisse S., Iteman I., Gravier P., Nkoghe D., Grandadam M. 2009. Aedes albopictus mosquito: the main vector of the 2007 chikungunya outbreak in Gabon. PLoS ONE 4: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C., Ollomo B., Kamgang B., Moutailler S., Rousset D., Demanou M., Herve J. P., Leroy E., Simard F. 2010. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in Central Africa. Vector Borne Zoonotic. Dis. 10: 259–266. [DOI] [PubMed] [Google Scholar]
- Pennetier C., Warren B., Dabiré K. R., Russell I. J., Gibson G. 2010. Singing on the wing as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr. Biol. 20: 131–136. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Spielman A. 1986. The satyr effect: A model predicting parapatry and species extinction. Am. Nat. 128: 513–528. [Google Scholar]
- Ribeiro J. M. 1988. Can satyrs control pests and vectors? J. Med. Entomol. 25: 431–440. [DOI] [PubMed] [Google Scholar]
- Roth L. M. 1948. A study of mosquito behavior. An experimental laboratory study of the sexual behavior of Aedes aegypti Linnaeus. Am. Midl. Nat. 40: 265–352. [Google Scholar]
- Sakaluk S. K. 1985. Spermatophore size and its role in the reproductive behaviour of the cricket, Gryllodes supplicans (Orthoptera: Grylladae). Can. J. Zool. 63: 1652–1656. [Google Scholar]
- South S. H., Steiner D., Arnqvist G. 2009. Male mating costs in a polygynous mosquito with ornaments expressed in both sexes. Proc. R. Soc. B 276: 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet F., Lounibos L. P., Robbins D., Moran J., Nishimura N., Blosser E. M. 2011. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am. J. Trop. Med. Hyg. 85: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M. 1968. Sexual dimorphism of larvae and pupae of Aedes aegypti (Linn.). Mosq. News 28: 374–380. [Google Scholar]
- Vazeille M., Moutailler S., Coudrier D., Rousseaux C., Khun H., Huerre M., Thiria J., Dehecq J. S., Fontenille D., Schuffenecker I., et al. 2007. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval B., Bouskila A. 1993. Temporal dynamics of mating and predation in mosquito swarms. Oecologia 95: 65–69. [DOI] [PubMed] [Google Scholar]
- Yuval B., Wekesa J. W., Washino R. K. 1993. Effect of body size on swarming behavior and mating success of male Anopheles freeborni (Diptera: Culicidae). J. Insect Behav. 6: 333–342. [Google Scholar]