SUMMARY
Indolent CNS lymphomas (CNSLs) are rare and no guidelines exist for management. Recent literature highlights the potential for safe and tolerable intrathecal (IT) delivery of rituximab, a large anti-CD20 monoclonal antibody, for aggressive CNSL. We report a patient with relapsed indolent CNSL who failed systemic rituximab and could not tolerate IT chemotherapies, but had an objective response of 6 months duration to IT rituximab.
KEYWORDS : CNS lymphoma, indolent lymphoma, intrathecal rituximab, low-grade lymphoma
Practice points .
CNS involvement with lymphoma may occur with or without systemic disease.
The majority of CNS lymphomas are aggressive large B-cell lymphomas but rarely indolent forms of the disease may exist.
Rituximab administered intrathecally is well tolerated in patients with CNS lymphoma, including those with indolent forms of this disease.
Cerebrospinal fluid parameters can provide a measure of disease response and in this case, lumbar cerebrospinal fluid glucose trended most closely with response to intrathecal-rituximab (IT-R).
Despite progression with intravenous rituximab, responses to IT-R do occur. Further study is required to define optimal use of IT-R.
Approximately 95% of CNS lymphomas (CNSLs) are aggressive large B-cell lymphomas with only a small minority presenting as indolent neoplasms. Given their rarity, no set guidelines for management exist. Prognosis for these indolent neoplasms remains more favorable with prolonged survival despite less aggressive therapies [1]. This is the first report of the safety and tolerability of intrathecal rituximab (IT-R) in an indolent CNSL.
Case report
• Initial presentation
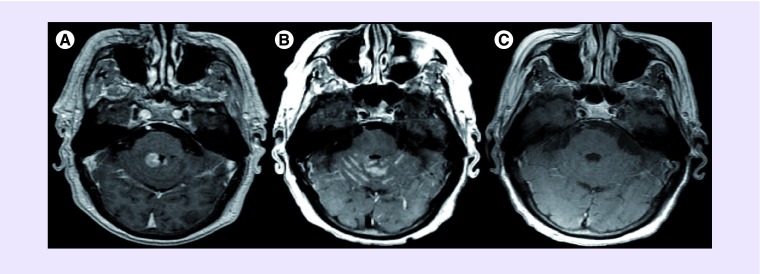
A 78-year-old man presented in February 2008 with 2 months of progressive ataxia. Neuroimaging revealed bilateral, homogenously enhancing periventricular masses in the posterior fossa (Figure 1A). Cerebrospinal fluid (CSF) flow cytometry revealed a CD20+ monoclonal B-cell population with lambda light-chain restriction and the patient was started on dexamethasone (4 mg thrice daily) and transferred to our institution for evaluation. Systemic workup including PET and bone marrow biopsy was unremarkable and there was strong suspicion for CNSL. Biopsy was planned to confirm the presence of a lymphoproliferative process; however, after treatment with glucocorticoids a complete remission was observed. The yield of subsequent diagnostic biopsy was felt to be low and though initial CSF flow cytometry was worrisome for a lymphoproliferative disorder it was not diagnostic and radiographic surveillance was pursued without further treatment. The patient remained disease free for over 2 years.
Figure 1. . Serial neuroimaging results.
Axial T1-weighted gadolinium enhanced brain MRI showing (A) bilateral, homogenously enhancing periventricular masses in the posterior fossa in February 2008; (B) recurrent leptomeningeal enhancement within the cerebellar folia in June 2010 and (C) no abnormal enhancement or parenchymal involvement in April 2013 with isolated cerebrospinal fluid disease.
• First & second recurrence – systemic treatment
In April 2010, he suffered first relapse manifesting as progressive ataxia with nodular leptomeningeal enhancement on MRI (Figure 1B) and CSF which demonstrated monocytic pleocytosis (73 cells/mm3), glucose 6 mg/dl and protein 115 mg/dl. CSF cytology showed two abnormal monoclonal B-cell populations including a small CD5+, CD19+, CD20+, dim CD38+ kappa-light-chain-restricted population and a medium-to-large CD19+, CD20+, CD5-, CD10- lambda-restricted population. Serum studies showed corresponding IgM biclonal gammopathy and macroglobulinemia (IgM: 1240 mg/dl). Bing–Neel syndrome, a rare form of Waldenstrom's macroglobulinemia characterized by neoplastic infiltration into the CNS, was considered though bone marrow biopsy showed only hypercellular marrow with a single small lymphoid aggregate of small lymphocytes and predominance of kappa-light-chain-positive B cells without neoplasia [2]. As his clinical course and CSF results strongly supported an indolent CNSL, he was started on weekly systemic rituximab (375 mg/m2) and glucocorticoids (dexamethasone 4 mg four times daily for 2 weeks) with clinical, cytologic and radiographic partial response after four treatments but subsequent cytologic progression by 8 weeks. Serum studies to determine the status of the systemic monoclonal gammopathy were not repeated and biweekly intrathecal-methotrexate (IT-MTX) was initiated by Ommaya reservoir. This resulted in a complete radiographic remission which persisted after eight doses despite discontinuing therapy after 12 doses due to severe infusional reaction (e.g., confusion, agitation, nausea and hyperthermia).
• Third recurrence – intrathecal treatment
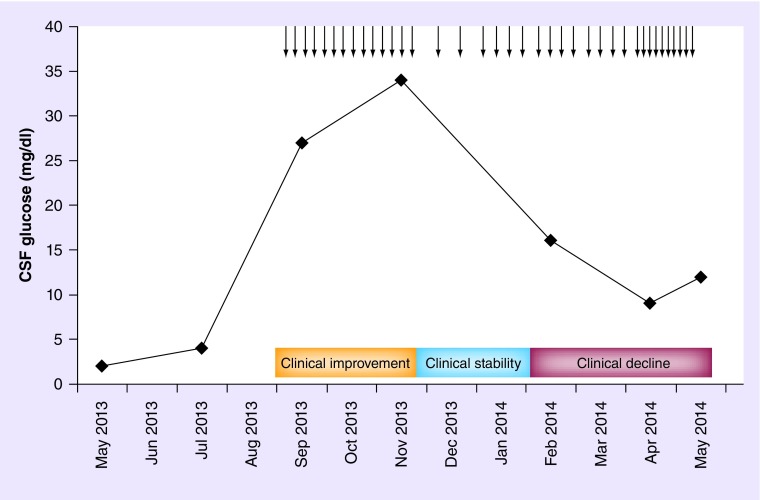
He remained clinically, radiographically and cytologically stable until May 2013, when he developed recurrent progressive ataxia, aphasia and abulia. Neuroimaging was unremarkable (Figure 1C) but CSF showed monocytic pleocytosis (32 cells/mm3), protein 83 mg/dl and markedly reduced glucose of 2 mg/dl. CSF cultures were negative for infection. Flow cytometry revealed 10% phenotypically abnormal monoclonal B-cell and lambda-light-chain-restricted population consistent with recurrent disease. Radiation therapy and systemic chemotherapy (i.e., high-dose methotrexate) were considered; however, given his age, modest renal insufficiency, intolerance to prior IT-MTX, isolated leptomeningeal dissemination without bulky or radiographically measurable disease and indolent course, IT-R (25 mg once weekly, 5ml of a 10 mg/ml solution without dilution, no concurrent glucocorticoids) was initiated in August 2013. Treatments were extremely well tolerated without toxicity. Cytology, CSF protein and cells did not normalize but marked improvement in CSF glucose trended most closely with clinical improvement (Figure 2 & Table 1). At peak clinical improvement, CSF by lumbar dural puncture showed WBC 11 cells/mm3, glucose 27 mg/dl, and protein 70 mg/dl.
Figure 2. . Association between cerebrospinal fluid glucose by lumbar cistern and clinical symptomatology.
Cerebrospinal glucose concentration by lumbar cistern dural puncture prior to, during and following the 38 treatments with intrathecal rituximab (25 mg) showing the close association with clinical symptomatology.
↓:Indicates intrathecal rituximab (25 mg) administration.
CSF: Cerebrospinal fluid.
Table 1. . Serial cerebrospinal fluid analysis by lumbar cistern dural puncture.
| Date of dural puncture | Cells (per mm3) | Monocytes (cells/mm3) | Protein (mg/dl) | Glucose (mg/dl) | Flow (%) | Cytopathology |
|---|---|---|---|---|---|---|
| May 2013 | 32 | 34 | 83 | 2 | 0 | ATY |
| July 2013 | 16 | 16 | 73 | 4 | 10 | ATY |
| September 2013 | 11 | 11 | 70 | 27 | 0 | ATY |
| November 2013 | 27 | 26 | 73 | 34 | 0 | ATY |
| February 2014 | 14 | 14 | 70 | 16 | 2 | ATY |
| April 2014 | 19 | 19 | 93 | 9 | 2 | ATY |
| May 2014 | 15 | 20 | 89 | 12 | 1 | ATY |
Serial cerebrospinal fluid results by lumbar cistern dural puncture showing changes in cell count, monocytes, protein, glucose, flow cytometry and cytopathology results over the course of treatment of which cerebrospinal fluid glucose and no other markers trended most closely with clinical symptomatology.
ATY: Cytology showing atypical cells without definitive malignancy but concerning.
• Final outcome
He remained clinically and cytologically stable until February 2014 when his CSF glucose declined to 16 mg/dl and despite dose escalation, clinical symptoms and CSF studies worsened. He subsequently failed IT-thiotepa and did not tolerate IT-cytarabine. He subsequently elected to transition to hospice care and died in December 2014.
Discussion
Rituximab has revolutionized the management of both aggressive and indolent systemic lymphomas [3,4]. Over the past decade, interest has increased in its role in CNSL as it appears to increase survival in recurrent and newly diagnosed aggressive primary CNSL [5,6]. Despite these favorable results, only about 0.1% of this large monoclonal antibody penetrates the blood–brain barrier [7]. Recently, Phase I investigation has established the safety and tolerability of IT-R in aggressive, recurrent primary CNSL as a single agent and in combination with methotrexate [8,9]. To date, leptomeningeal predominant disease has been the primary focus of study. Cytologic remission has been demonstrated in around 50–75% of patients [9,10] with long-term responses of 6–8 months in two patients [9,10] and 6 years in another [11]. Brain parenchymal responses have also been reported but have been less robust [9].
Approximately 95% of primary CNSLs are aggressive diffuse large B-cell lymphomas with only a small minority presenting as indolent, low-grade neoplasms. Given their rarity, no set guidelines for management exist. Prognosis for these indolent neoplasms remains more favorable with prolonged survival despite less aggressive therapies [12]. As in systemic indolent lymphomas, objective response criteria and side effect profile remain important as the risks of long-term therapies must be balanced against the risk of continued administration.
The current report highlights several unique aspects of CNSL. While tissue acquisition by biopsy is the favored approach to diagnosis, as in this case, response to glucocorticoids can confound the initial workup. A systematic algorithm which includes imaging, CSF and ophthalmologic evaluation in addition to biopsy can be used to establish a diagnosis [13]. In our patient, CSF cytology was critical and while a small kappa-restricted population was observed at second recurrence, the patient's disease was consistently associated with a monoclonal lambda-restricted B-cell population. Prior studies have demonstrated that combined elevations of both kappa and lambda light chains can exist in lymphoproliferative disorders involving the CNS though a single light chain does tend to predominate in most cases [14,15]. Additionally, extent of disease is a consideration in treatment selection. While our patient initially presented with parenchymal lesions, at recurrence the patient's symptomatic disease was confined to the leptomeninges and given the intolerance to alternative intrathecal agents, IT-R was felt to be the preferred option.
This case is the first report of the safety and tolerability of IT-R in an indolent CNSL. As has been demonstrated in the early phase studies in aggressive, recurrent CNSL, IT-R was extremely well tolerated. Despite the patient's age of 83 years and his severe intolerance to IT-MTX and IT-cytarabine, he suffered no serious toxicity tolerating 38 sequential treatments with IT-R. Furthermore, he had an objective response that persisted for 6 months despite prior partial response and progression on systemic rituximab. In indolent lymphoma where cure is not expected but long-term survival is observed, IT-R appears to offer a favorable risk-benefit profile.
As in indolent systemic lymphomas, objective response criteria and favorable side effect profile are important in indolent CNSL as the risks of long-term therapies must be balanced against continued administration of drug. In our patient, CSF cytology remained positive throughout the patient's treatment course providing a poor indicator of disease response. CSF cell count, differential, protein, intraventricular glucose, and flow cytometry varied over the course of treatment but none proved an early indicator of clinical-disease status. In contrast, lumbar CSF glucose trended most closely with clinical symptomatology and provided early evidence of disease response and progression.
Conclusion & future perspective
Intrathecal delivery of rituximab is an emerging therapeutic modality in the management of CNS lymphomas that appears to be well-tolerated and safe in patients with CNS lymphoma. Existing Phase I studies have identified a maximally tolerated dose. In this report of an indolent CNSL, salvage IT-R resulted in 6 months of objective clinical and CSF response without toxicities despite prior failure to systemic rituximab and severe intolerance to other IT chemotherapeutics. Further study is required to prospectively define efficacy and identify optimal compartments (i.e., CSF vs parenchymal) for response. The current and other existing reports support its tolerability in patients with CNS lymphoma and highlight potential markers of disease response.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Abbi KK, Muzaffar M, Gaudin D, et al. Primary CNS lymphoplasmacytic lymphoma: a case report and review of literature. Hematol. Oncol. Stem Cell Ther. 2013;6(2):76–78. doi: 10.1016/j.hemonc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Grewal JS, Brar PK, Sahijdak WM, Tworek JA, Chottinger EG. Bing-Neel syndrome: a case report and systematic review of clinical manifestations, diagnosis and treatment options. Clin. Lymphoma Myeloma. 2009;9(6):462–466. doi: 10.3816/CLM.2009.n.091. [DOI] [PubMed] [Google Scholar]
- 3.Bendandi M. Aiming at a curative strategy for follicular lymphoma. CA Cancer J. Clin. 2008;58(5):305–317. doi: 10.3322/CA.2008.0011. [DOI] [PubMed] [Google Scholar]
- 4.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J. Clin. 2010;60(6):393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 5.Holdhoff M, Ambady P, Abdelaziz A, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83(3):235–239. doi: 10.1212/WNL.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain MC, Johnston SK. High-dose methotrexate and rituximab with deferred radiotherapy for newly diagnosed primary B-cell CNS lymphoma. Neuro Oncol. 2010;12(7):736–744. doi: 10.1093/neuonc/noq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batchelor T, Grossman S, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubenstein J, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. JCO. 2007;25(11):1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]; •• Describes the first Phase I single-center study of the feasibility, safety and maximally tolerated dose of intrathecal rituximab.
- 9.Rubenstein J, Li J, Chen L, et al. Multicenter Phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745–751. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the first Phase I multi-institution study of the feasibility and tolerability of intrathecal rituximab in combination with high-dose systemic methotrexate.
- 10.Schulz H, Pels H, Schmidt-Wolf I, Zeelen U, Germing U, Engert A. Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematologica. 2004;89(6):753–754. [PubMed] [Google Scholar]
- 11.Birnbaum T, Baumgarten LV, Dudel C, Straube A. Successful long-term control of lymphomatous meningitis with intraventricular rituximab. J. Clin. Neurosci. 2014;21(2):356–358. doi: 10.1016/j.jocn.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Jahnke K, Thiel E, Schilling A, et al. Low-grade primary central nervous system lymphoma in immunocompetent patients. Br. J. Haematol. 2005;128(5):616–624. doi: 10.1111/j.1365-2141.2004.05361.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311–319. doi: 10.1001/jamaneurol.2013.606. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of published literature on CNS lymphoma from 1996 to 2011 that provides a summary of key diagnostic studies employed in the evaluation of CNS lymphoma and a proposed diagnostic algorithm for approaching these patients.
- 14.Schroers R, Baraniskin A, Heute C, et al. Detection of free immunoglobulin light chains in cerebrospinal fluids of patients with central nervous system lymphomas. Eur. J. Haematol. 2010;85(3):236–242. doi: 10.1111/j.1600-0609.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- 15.Delville JP, Heimann P, El Housni H, et al. Biclonal low grade B-cell lymphoma confirmed by both flow cytometry and karyotypic analysis, in spite of a normal kappa/lambda Ig light chain ratio. Am. J. Hematol. 2007;82(6):473–480. doi: 10.1002/ajh.20850. [DOI] [PubMed] [Google Scholar]