Abstract
OBJECTIVES
To report prevalence, correlates, and medication management of pain in community-dwelling older adults with dementia.
DESIGN
Cross-sectional.
SETTING
In-person interviews with self- or proxy respondents living in private residences or non-nursing home residential care settings.
PARTICIPANTS
Nationally representative sample of community-dwelling Medicare beneficiaries aged 65 and older enrolled in the National Health and Aging Trends Study 2011 wave.
MEASUREMENTS
Dementia status was determined using a modified previously validated algorithm. Participants were asked whether they had had bothersome and activity-limiting pain over the past month. A multivariable Poisson regression model was used to determine the relationship between bothersome pain and sociodemographic and clinical characteristics.
RESULTS
Of the 7,609 participants with complete data on cognitive function, 802 had dementia (67.2% aged ≥80, 65.0% female, 67.9% white, 49.7% proxy response, 32.0% lived alone, 18.8% lived in residential care); 670 (63.5%) participants with dementia experienced bothersome pain, and 347 (43.3%) had pain that limited activities. These rates were significantly higher than in a propensity score–matched cohort without dementia (54.5% bothersome pain, P < .001, 27.2% pain that limited activity, P < .001). Proxies reported slightly higher rates of pain than self-respondents, but differences were statistically significant only for activity-limiting pain (46.6% proxy vs 40.1% self, P = .03). Correlates of bothersome pain included arthritis, heart and lung disease, less than high school education, activity of daily living disability, depressive and anxiety symptoms, and low energy. Of those reporting pain, 30.3% stated that they rarely or never took any medications for pain.
CONCLUSION
Community-living older adults with dementia are at high risk of having pain. Creative interventions and programs are needed to manage pain adequately in this vulnerable population.
Keywords: NHATS, community-dwelling, dementia, pain
Dementia is a progressive, eventually terminal disease that currently affects more than 4.5 million people in the United States. With impending demographic shifts, the number of people with dementia in the United States is expected to triple, to almost 14 million people, in the next 25 years.1 Although more than 75% of this population resides in the community (e.g., private residences or non-nursing home residential care facilities),2,3 little is known about the health of community-dwelling individuals with dementia, particularly in terms of their experiences with pain.
Pain has profound effects on quality of life4,5 and is associated with numerous adverse outcomes, such as high levels of disability and mortality.6,7 Although the importance of pain in individuals with dementia has increasingly attracted the attention of researchers in recent decades, the majority of research has been conducted using samples from nursing homes. These nursing home studies reveal prevalence rates of pain in older adults with dementia generally in the range of 40% to 80%.8–14 Their small non-representative samples15,16 or imprecise assessment of dementia status17 have limited the few studies that have focused on pain specifically in community-dwelling older adults with dementia in the United States.
An analysis of the data from the National Health and Aging Trends Study (NHATS), a survey of a nationally representative sample of older adults in the United States, was therefore conducted to provide an epidemiological perspective on the prevalence of pain in the important but understudied population of community-dwelling older adults with dementia. The prevalence of pain in the cohort of individuals with dementia was compared with that of a propensity score–matched cohort of individuals without dementia. To identify differences within the dementia cohort and to address methodological concerns related to measuring pain in this population, results were categorized according to reporting status (self vs proxy) and according to scores on performance-based cognitive testing. Finally, to aid in the identification of older adults with dementia who were at risk of being in pain, the study sought to model which socioeconomic characteristics, level of disability, and other comorbidities were associated with greater risk of pain.
METHODS
Population and Setting
NHATS is a longitudinal, nationally representative, prospective cohort study developed to provide a database to support the scientific study of how daily life changes as people age. NHATS participants are drawn from the list of all Medicare enrollees in the United States aged 65 and older. Persons are selected from all age groups from the youngest (65–69) to the oldest (≥90), with oversampling of the oldest age groups and of non-Hispanic black persons. Details of the complex study design and sampling methodology are available elsewhere.18 In-person interviews are conducted annually in study participants’ homes and include questionnaires as well as measurement of physical and cognitive characteristics. Whenever possible, sample persons act as their own respondents. When they are unable to respond for themselves because of memory or health problems, as assessed in the interview process, persons familiar with the sample person’s daily routines act as proxy respondents. If the sample person has a proxy respondent, attempts are made to complete the cognitive and physical portion of the interview. The first round of NHATS data collected in 2011 that included 8,245 individuals (weighted response rate 71.3%) was used. Four hundred sixty-eight nursing home residents who did not have interview data collected were excluded. Of the remaining 7,777 individuals, 7,609 with complete data on cognitive functioning were included in the analysis.
A previously described algorithm was modified to define the cohort of older adults with dementia.19 The previously defined algorithm relies on three types of information. First, self- and proxy respondents are asked whether a doctor has ever told the sample person that he or she has dementia or Alzheimer’s disease. If they respond “yes,” the sample person is placed into the probable dementia category. Second, all proxy respondents are asked to complete an AD8 dementia screening questionnaire, an eight-item tool that measures the sample person’s memory, temporal orientation, judgment, and function.20 Proxy respondents not reporting a diagnosis of dementia from a doctor who give answers to the AD8 that meet criteria for likely dementia (score ≥2) are classified as having probable dementia. Third, cognitive testing cut-points are used for classification. The cognitive testing assesses four areas of cognitive functioning: memory (self-rated, whether memory interferes with daily activities and immediate and delayed 10 word recall), orientation (date, month, year, day of week; naming president and vice president), executive function (clock drawing test), and retrieval of information (delayed 10-word recall). Factor analysis of the cognitive tests identified three domains of cognitive functioning: memory, orientation, and executive functioning. Self-respondents who score 1.5 standard deviations below the mean in two domains are classified as having probable dementia, and those below the mean in one domain are classified as having possible dementia. All others fall into the no dementia category. The cutoff of 1.5 standard deviations is based on commonly used guidelines for determining cognitive impairment previously reported in the literature.21,22
Upon further analysis of the characteristics of persons in the probable dementia category, it was found that some did not meet nationally recognized diagnostic criteria for all-cause dementia that stipulate that persons with dementia should exhibit evidence of significant functional and cognitive impairment.23,24 Individuals whose scores on the cognitive testing were above the cutoffs for impairment in any domain and those who reported no impairment in activities of daily living (ADLs) or instrumental activities of daily living (IADLs) were therefore excluded.
Using the rigorous research diagnosis criteria of the Aging, Demographics, and Memory Study as the criterion standard, sensitivity of the NHATS probable dementia criteria is 65.7%, and specificity is 87.2%. It was decided to modify the NHATS algorithm, as described above, in an attempt to increase the specificity of the algorithm and maximize the likelihood that those categorized as having dementia actually have dementia. To provide a comparison group for pain outcomes, a cohort of individuals was created from the group with no dementia, matched for age and sex using propensity score matching methods (n = 802).
Because this study included deidentified data only, it did not qualify as human subject research and was exempt from institutional review board approval per protocol at University of California at San Francisco.
Outcome Measures
Pain
The presence of pain was measured using a two-question verbal descriptor scale (VDS). Respondents are first are asked whether they had any bothersome pain in the last month. Those who answer “yes” are then asked whether they have activity limitations due to pain. A number of studies have demonstrated that persons with mild to moderate dementia are able to self-report pain using similar VDSs with good reliability and validity.12,25–30 Because of concerns regarding the ability of persons with severe dementia to answer even simple questions regarding pain, a sensitivity analysis was conducted to estimate prevalence of pain excluding self-reporting individuals with impairment in more than three domains on the performance-based cognitive testing. Prevalence rates were further categorized according to reporting status (self or proxy) and number of impairments on the performance-based cognitive testing.
Other Measures
Sociodemographic Characteristics
Sociodemographic characteristics included age (65–69, 70–74, 75–79, 80–84, 85–89, ≥90), sex, race (white, black, Hispanic, don’t know or refused), income, education (< vs > high school); reporting status (self or proxy), and living situation (alone, with spouse only, with spouse and others, with others only). Participants were also classified according to residential care status (private residence or residential care setting, including assisted living facility, continuing care retirement community, board and care home, other group home).
Comorbidities
Participant report of receiving a physician’s diagnosis of arthritis, heart disease, lung diseases, cancer, diabetes mellitus, or stroke was used.
Disability
Disability in ADLs was defined as self- or proxy report of requiring assistance from another person for dressing, transferring, walking, bathing, toileting, or eating, and participants were categorized into three groups: no ADL disability, moderate ADL disability (require assistance with 1 or 2 ADLs), and severe (more than 3 ADL disabilities). IADL disability was defined as requiring assistance because of impairment in health or functioning for laundry, shopping, preparing meals, managing finances, or taking medications, and participants were categorized as having impairment in one IADL or two or more IADLs.
Physical Symptoms
Shortness of breath and fatigue were measured according to a question asking whether participants had had breathing problems or low energy or exhaustion in the last month. Those who respond “yes” were asked whether these problems resulted in limitations in activity.
Psychological Symptoms
Depression was measured using the Patient Health Questionnaire (PHQ)-2 and anxiety using the General Anxiety Disorder (GAD)-2, both brief screening instruments that have high reliability and validity.31–33 Scores of 3 or greater on the PHQ-2 or the GAD-2 instruments (both instruments have scores ranging from 0–6) indicated clinically significant symptoms of depression or anxiety, respectively, based on previous cutoffs reported in the literature.
Statistical Analysis
Descriptive statistics were used to describe the characteristics of the sample and prevalence rates of pain according to cognitive testing scores and proxy status. Chi-square analysis was used to compare the prevalence of pain in self-reports with that in proxy reports and the cohort with dementia with the matched cohort without. To evaluate predictors of pain, a multivariable regression model was created to estimate whether the sociodemographic, health, and functional characteristics described above were associated with greater risk of pain. A relative risk estimation using Poisson regression with balanced repeated replication error variance was used for the model. This type of analysis was chosen because prevalence of pain was high, and a logistic regression would have overestimated risk. The model was adjusted for age, sex, and race. All reported analyses were weighted for the differential probability of selection and took into account the complex design of NHATS. Statistical analyses were conducted using Stata software, version 10.1 (Stata Corp., College Station, TX) and SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Using the NHATS algorithm for determining dementia status, 1,038 people were identified as having probable dementia, 996 possible dementia, and 5,575 no dementia. After excluding individuals from the probable dementia category with no evidence of cognitive (n = 78) or functional impairment (n = 158), the final sample included 802 individuals. Of those participants, 67.2% were aged 80 and older, 65.0% were female, and 50.4% were self-respondents. Thirty-two percent of study participants lived alone, and 68% lived with their spouse or others. Eighteen percent lived in a residential care setting. Participants with a proxy respondent tended to be more likely to be older than 85, live in a residential care facility, have higher levels of functional impairment, have a history of heart disease and stroke, and report more physical and psychological symptoms than self-respondents (Table 1).
Table 1.
Participant Characteristics in the National Health and Aging Trends Study Dementia Cohort
| Characteristic | Total Sample, N = 802 | Self-Report, n = 395 (50.4%) | Proxy Report, n = 407 (49.6%) |
|---|---|---|---|
| Sociodemographic | |||
| Age, n (%) | |||
| 65–69 | 34 (8.0) | 21 (56.7) | 13 (43.3) |
| 70–74 | 49 (8.6) | 25 (53.5) | 24 (46.5) |
| 75–79 | 113 (16.2) | 59 (51.4) | 54 (48.6) |
| 80–84 | 178 (22.6) | 94 (55.4) | 84 (44.6) |
| 85–89 | 212 (25.6) | 93 (43.4) | 119 (56.6) |
| ≥90 | 216 (19.0) | 103 (49.0) | 113 (51.0) |
| Female, n (%) | 535 (65.0) | 259 (50.0) | 276 (50.0) |
| Marital status, n (%) | |||
| Married or partnered | 255 (37.1) | 133 (51.9) | 122 (48.1) |
| Widowed | 419 (47.2) | 198 (48.4) | 221 (51.6) |
| Never married, divorced, separated | 126 (15.6) | 64 (53.3) | 62 (46.8) |
| Race, n (%) | |||
| White | 435 (67.9) | 218 (49.6) | 217 (50.4) |
| Black | 238 (12.6) | 106 (45.0) | 132 (55.0) |
| Hispanic | 84 (12.3) | 48 (62.6) | 36 (37.4) |
| Other | 27 (4.8) | 8 (28.6) | 19 (71.4) |
| Don’t know or refused | 18 (2.4) | 15 (83.3) | 3 (16.7) |
| Education, n (%) | |||
| Less than high school | 388 (45.4) | 200 (53.1) | 188 (46.9) |
| More than high school | 379 (54.6) | 178 (47.9) | 201 (52.1) |
| Income, $, median (interquartile range) | 14,400 (9,384–27,000) | 15,000 (9,600–27,000) | 14,000 (9,000–24,099) |
| Living situation, n (%) | |||
| Alone | 240 (32.0) | 136 (55.0) | 104 (45.0) |
| With spouse or partner only | 168 (24.5) | 95 (57.9) | 73 (42.1) |
| With spouse or partner and others | 77 (10.8) | 36 (43.8) | 41 (56.2) |
| With others only | 315 (32.7) | 126 (42.2) | 189 (57.8) |
| Residential care status, n (%) | |||
| Private residence | 692 (81.2) | 342 (51.3) | 350 (48.7) |
| Residential care | 110 (18.8) | 53 (46.5) | 57 (53.5) |
| Health conditions, n (%) | |||
| Arthritis | 518 (61.7) | 259 (52.5) | 259 (47.5) |
| Heart disease | 208 (25.8) | 91 (45.9) | 117 (54.1) |
| Lung disease | 130 (17.1) | 73 (55.9) | 57 (44.1) |
| Cancer | 186 (22.8) | 82 (45.9) | 104 (54.1) |
| Stroke | 199 (24.8) | 80 (41.7) | 119 (58.3) |
| Diabetes mellitus | 228 (30.0) | 114 (52.9) | 114 (47.1) |
| History of falls in the last month | 208 (27.7) | 88 (42.8) | 120 (57.2) |
| History of hip fracture since age 50 | 97 (11.7) | 43 (43.1) | 54 (56.9) |
| Disabilities, n (%) | |||
| ≥2 instrumental ADL impairments | 651 (75.7) | 292 (45.1) | 359 (54.9) |
| ≥1 ADL impairment | 478 (59.4) | 169 (35.7) | 309 (64.3) |
| >3 ADL impairments | 299 (37.6) | 69 (23.7) | 230 (76.3) |
| Difficulty chewing or swallowing | 171 (21.5) | 65 (40.4) | 106 (59.6) |
| Difficulty speaking | 209 (26.7) | 60 (28.0) | 149 (72.0) |
| Never or rarely goes outside | 274 (32.6) | 95 (34.2) | 179 (65.8) |
| Uses a cane, walker, or wheelchair | 539 (64.2) | 257 (47.7) | 282 (52.3) |
| Physical symptoms, n (%) | |||
| Low energy | 525 (65.2) | 235 (45.3) | 290 (54.7) |
| Low energy limits activities | 436 (53.6) | 186 (42.7) | 250 (57.3) |
| Difficulty breathing | 219 (28.9) | 104 (46.3) | 115 (53.8) |
| Difficulty breathing limits activities | 142 (19.1) | 62 (44.7) | 80 (55.3) |
| Psychological symptoms, n (%) | |||
| Depressive symptomsa | 293 (39.4) | 117 (41.6) | 176 (58.4) |
| Anxiety symptomsb | 228 (30.0) | 109 (47.9) | 119 (52.1) |
Reported data incorporated with the complex survey design (analytical weights).
Patient Health Questionnaire-2 score ≥3/6.
Generalized Anxiety Disorder-2 score ≥3/6.
ADL = activity of daily living.
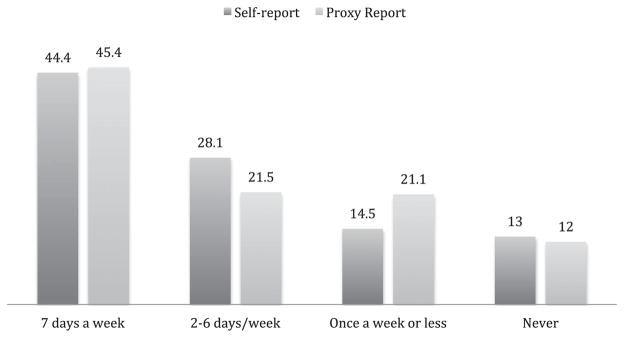
Table 2 shows that community-dwelling older adults with dementia had a higher prevalence of bothersome pain (63.5%) than the matched cohort without dementia (54.5%) (P < .001). In addition, a higher proportion of participants with dementia reported activity-limiting pain (43.3%) than of participants without dementia (27.2%) (P < .001). There were no statistically significant differences in bothersome or activity-limiting pain prevalence rates for all respondents or self-respondents when self-respondents with impairment in more than three domains of the performance-based cognitive testing were excluded from analysis (Table 2 footnotes). Proxies tended to report slightly higher levels of pain than self-respondents. These differences were statistically significant for activity-limiting pain (46.6% proxy, 40.1% self-report, P = .03) but not for bothersome pain (64.4% proxy, 62.7% self-report, P = .59). Of respondents who reported bothersome pain, 30.3% reported rarely or never taking pain medication. This was slightly higher for participants with a proxy report (33.1%) than a self-report (27.5%) (Figure 1).
Table 2.
Prevalence of Pain in Participants with and without Dementia
| Pain | Dementia Cohort | Matched Cohort All Respondents, n = 802 |
||
|---|---|---|---|---|
| All Respondents, n = 802a | Self-Report, n = 395b | Proxy Report, n = 407 | ||
| % (95% CI) | ||||
| Bothersome | 63.5 (60.5–66.4) | 62.7 (58.7–66.6) | 64.4 (59.8–68.7) | 54.5 (51.4–57.7)c |
| Activity limiting | 43.3 (40.2–46.5) | 40.1 (35.7–44.6) | 46.6 (42.5–50.7) | 27.2 (25.2–29.2)c |
Excluding self-respondents with impairment in three or more domains on cognitive testing (n = 96), values were 63.6% (95% confidence interval (CI) = 60.5–66.6) for bothersome pain and 43.3% (95% CI = 40.1–46.5) for activity-limiting pain for all respondents (n = 706).
Excluding self-respondents with impairment in three or more domains on cognitive testing (n = 96), values were 62.7% (95% CI = 58.5–66.7) for bothersome pain and 39.1% (95% CI = 34.5–43.9) for activity-limiting pain for self-respondents (n = 299).
P < .001 vs all respondents with dementia.
Figure 1.
Frequency of reported pain medication use in the month according to reporting status (%).
In examining pain reports according to level of cognitive impairment and reporting status (self vs proxy report), some variation was found (Table 3). Proxies for participants with impairment in three domains or those who were unable to complete testing reported higher levels of activity-limiting pain than self-respondents with impairment in one or two domains. Proxies reported higher levels of bothersome pain for participants with impairment in two domains and higher levels of activity-limiting pain for participants with impairment in one domain.
Table 3.
Pain According to Level of Impairment in Performance-Based Cognition Testing and Reporting Status
| Pain | Impairment in 1 Domain
|
Impairment in 2 Domains
|
Impairment in 3 Domains
|
Unable to Complete Testing
|
||||
|---|---|---|---|---|---|---|---|---|
| Self, n = 34 | Proxy, n = 28 | Self, n = 265 | Proxy, n = 43 | Self, n = 96 | Proxy, n = 136 | Self, n = 0 | Proxy, n = 197 | |
| Bothersome | 20 (64.2) | 18 (67.7) | 167 (62.5) | 21 (48.3) | 63 (62.7) | 88 (63.9) | 0 (0.0) | 132 (67.5) |
|
| ||||||||
| Activity limiting | 13 (37.9) | 16 (55.7) | 105 (39.3) | 16 (38.0) | 46 (43.3) | 60 (42.8) | 0 (0.0) | 97 (49.0) |
The three domains included in the cognitive testing were memory, orientation, and executive functioning. Impairment was defined as 1.5 standard deviations or more below the mean score for each domain. Reported data incorporated with the complex survey design (analytical weights).
Table 4 details the association between a report of bothersome pain and dementia cohort characteristic according to reporting status. Some characteristics were associated with greater risk of bothersome pain for self-and proxy respondents. For instance, arthritis had a strong association with bothersome pain (aRR = 1.83, 95% CI = 1.59–2.12 for self-respondents; aRR = 1.74, 95% CI = 1.47–2.07 for proxy respondents). Other conditions associated with greater risk of bothersome pain for self-and proxy respondents in the model included heart and lung disease, ADL disability, low energy, difficulty breathing, and depressive and anxiety symptoms. Cancer was associated with greater risk of pain for proxies but not self-respondents. Differences between self- and proxy respondents also emerged for less education (aRR = 1.28, 95% CI = 1.11–1.49 for self-respondents; aRR = 1.06, 95% CI = 0.93–1.21 for proxy respondents) and for living with a spouse versus living alone (aRR = 1.12, 95% CI = 0.93–1.34 for self-respondents; aRR = 0.64, 95% CI = 0.53–0.79 for proxy respondents).
Table 4.
Association Between Report of Bothersome Pain and Dementia Cohort Characteristic (N = 802) According to Reporting Status
| Characteristic | Self-Report (n = 395)
|
Proxy Report (n = 407)
|
||
|---|---|---|---|---|
| % (95% CI) | aRR (95% CI) | % (95% CI) | aRR (95% CI) | |
| Sociodemographic | ||||
|
| ||||
| Age | ||||
|
| ||||
| 65–69 | 65.9 (49.2–79.5) | Reference | 60.5 (37.0–80.0) | Reference |
|
| ||||
| 70–74 | 68.8 (51.4–82.2) | 1.04 (0.72–1.50) | 71.8 (53.0–85.2) | 1.19 (0.71–2.00) |
|
| ||||
| 75–79 | 70.1 (60.1–77.9) | 1.07 (0.80–1.42) | 76.5 (65.7–84.7) | 1.28 (0.85–1.94) |
|
| ||||
| 80–84 | 53.7 (46.5–60.8) | 0.86 (0.65–1.15) | 57.0 (46.7–66.6) | 0.95 (0.64–1.41) |
|
| ||||
| 85–89 | 63.4 (54.2–71.8) | 0.97 (0.73–1.30) | 64.5 (57.1–71.3) | 1.08 (0.72–1.63) |
|
| ||||
| ≥90 | 62.7 (55.5–69.5) | 0.98 (0.75–1.26) | 60.6 (51.1–69.3) | 1.07 (0.72–1.60) |
|
| ||||
| Sex | ||||
|
| ||||
| Male | 58.2 (50.9–65.1) | Reference | 62.4 (54.4–69.8) | Reference |
|
| ||||
| Female | 65.2 (60.7–69.4) | 1.13 (0.99–1.30) | 65.4 (60.3–70.1) | 1.04 (0.90–1.22) |
|
| ||||
| Race | ||||
|
| ||||
| White | 61.9 (57.0–66.7) | Reference | 60.9 (55.0–66.4) | Reference |
|
| ||||
| Black | 58.9 (50.8–66.7) | 0.86 (0.73–1.01) | 60.5 (52.6–67.9) | 0.96 (0.82–1.13) |
|
| ||||
| Hispanic | 69.9 (57.5–79.9) | 0.99 (0.80–1.22) | 76.5 (63.2–86.1) | 1.21 (1.00–1.46)c |
|
| ||||
| Other | 60.4 (43.6–75.1) | 0.85 (0.47–1.54) | 87.7 (76.7–93.9) | 1.43 (1.20–1.72)e |
|
| ||||
| Education | ||||
|
| ||||
| ≥High school | 55.8 (50.1–61.4) | Reference | 61.3 (56.2–66.2) | Reference |
|
| ||||
| <High school | 70.4 (65.0–75.3) | 1.28 (1.11–1.49)d | 69.0 (61.4–75.8) | 1.06 (0.93–1.21) |
|
| ||||
| Living situation | ||||
|
| ||||
| Alone | 61.0 (53.8–67.7) | Reference | 69.6 (61.1–76.9) | Reference |
|
| ||||
| With spouse or partner only | 64.6 (55.7–72.6) | 1.12 (0.93–1.34) | 49.7 (41.2–58.2) | 0.64 (0.53–0.79)e |
|
| ||||
| With spouse or partner and others | 65.2 (51.0–77.2) | 1.15 (0.88–1.50) | 74.0 (59.9–84.4) | 0.83 (0.68–1.01) |
|
| ||||
| With others only | 61.4 (55.0–67.5) | 0.95 (0.80–1.13) | 65.5 (58.7–71.7) | 0.86 (0.75–0.98)c |
|
| ||||
| Residential care | ||||
|
| ||||
| Private residence | 63.8 (59.7–67.6) | Reference | 64.5 (60.0–68.8) | Reference |
|
| ||||
| Residential care | 57.7 (47.1–67.7) | 0.95 (0.79–1.15) | 63.8 (53.3–73.1) | 1.11 (0.95–1.29) |
|
| ||||
| Health conditions | ||||
|
| ||||
| Arthritis | ||||
|
| ||||
| No | 38.6 (33.4–44.1) | Reference | 46.8 (39.5–54.2) | Reference |
|
| ||||
| Yes | 76.1 (71.7–80.1) | 1.83 (1.59–2.12)e | 76.5 (71.8–80.7) | 1.74 (1.47–2.07)e |
|
| ||||
| Heart disease | ||||
|
| ||||
| No | 59.9 (55.4–64.2) | Reference | 61.0 (55.3–66.5) | Reference |
|
| ||||
| Yes | 72.3 (64.6–78.9) | 1.28 (1.15–1.43)e | 72.0 (64.4–78.5) | 1.21 (1.06–1.37)e |
|
| ||||
| Lung disease | ||||
|
| ||||
| No | 59.4 (55.2–63.6) | Reference | 60.1 (55.3–64.6) | Reference |
|
| ||||
| Yes | 76.7 (64.7–85.6) | 1.28 (1.12–1.46)e | 88.2 (82.1–92.4) | 1.51 (1.34–1.69)e |
|
| ||||
| Cancer | ||||
|
| ||||
| No | 62.7 (58.1–67.1) | Reference | 62.7 (57.7–67.4) | Reference |
|
| ||||
| Yes | 62.8 (55.2–69.9) | 1.02 (0.88–1.18) | 69.4 (61.1–76.7) | 1.21 (1.06–1.37)d |
|
| ||||
| Stroke | ||||
|
| ||||
| No | 63.2 (58.6–67.5) | Reference | 63.7 (58.6–68.6) | Reference |
|
| ||||
| Yes | 60.8 (51.2–69.7) | 0.99 (0.83–1.18) | 65.9 (57.0–73.8) | 1.06 (0.91–1.24) |
|
| ||||
| Diabetes mellitus | ||||
|
| ||||
| No | 59.5 (54.6–64.2) | Reference | 59.9 (55.1–64.6) | Reference |
|
| ||||
| Yes | 69.7 (61.1–77.1) | 1.12 (0.96–1.30) | 75.4 (67.0–82.2) | 1.22 (1.09–1.38)d |
|
| ||||
| Disability | ||||
|
| ||||
| Number of activity of daily living impairments | ||||
|
| ||||
| 0 | 57.5 (53.3–61.7) | Reference | 50.8 (41.5–59.9) | Reference |
|
| ||||
| 1–2 | 66.3 (57.8–73.8) | 1.16 (1.00–1.34)c | 68.4 (58.6–76.9) | 1.43 (1.14–1.80)d |
|
| ||||
| 3–6 | 72.8 (62.0–81.5) | 1.33 (1.13–1.57)d | 68.8 (62.9–74.1) | 1.42 (1.17–1.72)d |
|
| ||||
| Number of instrumental activity of daily living impairments | ||||
|
| ||||
| 1 | 61.7 (54.0–68.9) | Reference | 68.2 (56.2–78.1) | Reference |
|
| ||||
| ≥2 | 63.2 (58.8–67.4) | 1.07 (0.92–1.25) | 63.6 (58.5–68.5) | 1.01 (0.82–1.23) |
|
| ||||
| Physical and psychological symptoms | ||||
|
| ||||
| Low energy | ||||
|
| ||||
| No | 43.9 (38.0–50.0) | Reference | 41.2 (33.6–49.2) | Reference |
|
| ||||
| Yes | 76.3 (71.6–80.5) | 1.74 (1.51–2.01)e | 73.5 (69.1–77.4) | 1.83 (1.48–2.27)e |
|
| ||||
| Breathing problems | ||||
|
| ||||
| No | 58.8 (54.2–63.2) | Reference | 56.4 (51.1–61.6) | Reference |
|
| ||||
| Yes | 74.1 (66.0–80.8) | 1.23 (1.09–1.39)d | 81.8 (76.2–86.3) | 1.44 (1.28–1.63)e |
|
| ||||
| Depressive symptomsa | ||||
|
| ||||
| No | 57.3 (52.3–62.2) | Reference | 54.5 (49.2–59.7) | Reference |
|
| ||||
| Yes | 74.6 (67.9–80.2) | 1.27 (1.09–1.48)d | 76.2 (70.8–80.9) | 1.43 (1.28–1.59)e |
|
| ||||
| Anxiety symptomsb | ||||
|
| ||||
| No | 56.2 (51.9–60.5) | Reference | 59.1 (53.8–64.2) | Reference |
|
| ||||
| Yes | 80.1 (72.9–85.7) | 1.40 (1.26–1.57)e | 75.4 (68.2–81.3) | 1.25 (1.10–1.43)d |
Adjusted for age group, sex, race, and proxy status. Used survey weights (analytical) to account for complex survey design. Relative risk estimation by Poisson regression with balanced repeated replication error variance.
Patient Health Questionnaire-2 score ≥3/6.
Generalized Anxiety Disorder-2 score ≥3/6.
P<.05,
P<.01,
P<.001.
CI = confidence interval; aRR = adjusted relative risk.
DISCUSSION
To the knowledge of the authors, this is the first study to examine the prevalence and correlates of pain in a nationally representative cohort of community-dwelling older adults with dementia in the United States. Most studies of pain in individuals with dementia have been conducted in nursing home residents,9–14 even though the majority older adults with dementia reside in the community.2,3 Community-dwelling individuals with dementia have a high burden of pain, with more than six of 10 respondents reporting bothersome pain and more than four of 10 reporting pain severe enough to limit activity. In addition, comorbid disease, functional disability, and physical and psychological symptoms are common in community-dwelling individuals with dementia and are associated with higher risk of pain. Participants with dementia also reported more pain than a matched cohort without dementia. The exact reasons for this are unknown, but it was probably because those without dementia were matched only for sex and age and thus probably had fewer pain-causing conditions than participants with dementia. Overall, similar prevalence rates of pain, generally in the range of 50% to 60% for any pain and 30% to 40% for moderate to severe pain found in several large population-based surveys of older adults with and without dementia in the United States and other countries confirm the validity of the current study’s findings.17,34,35
The results of the current study must be understood within the context of several major limitations. First, identifying persons with dementia in large population-based surveys in which clinical evaluations are not feasible poses difficulties. NHATS attempted to address these difficulties by developing an algorithm based on self-report of a dementia diagnosis, cognitive testing, and proxy reports of cognitive function. The NHATS algorithm results in population-based estimates of dementia that are similar to those found in other major population-based studies.1,36 The NHATS dementia algorithm was further modified to ensure that the current study sample met nationally recognized diagnostic criteria for dementia. It was attempted to categorize participants according to level of cognitive impairment using cutoff scores on the performance-based cognitive testing, although this method of categorization has not been previously validated.
Second, communication, cognitive, and memory impairments that influence the ability to self-report complicate the assessment of pain and other subjective states such as depression in persons with dementia.37 In particular, the fact that the VDS used to measure pain has not been validated in the dementia population with self- or proxy respondents limited the current study. Additionally, the VDS asks respondents to describe their pain over the last month, which given problems with memory, may be more difficult for persons with dementia to assess, although multiple studies have found that persons with mild to moderate dementia are able to respond to a variety of pain measurement tools with varying temporal elements with good validity and reliability and that VDSs are the most easily understood tool in this population.11,24–29,38 The results from the sensitivity analysis were unchanged when self-respondents with more-severe cognitive impairment were excluded.
Another concern is the large reliance on proxy respondents. Self-response is considered the criterion standard for pain assessment, but many people with dementia are unable to self-report. Previous studies examining proxy versus self-report of pain in people with dementia have found only fair agreement with self-report of pain in persons with dementia, with bias in both directions depending on the study, population, and type of proxy respondent (e.g., health professional or family caregiver).16,39–42 Despite methodological concerns, it was decided to include participants with proxy respondents in the analysis because it allowed for the inclusion of a broader and possibly more-representative range of participants.43 Prevalence rates of pain were slightly higher for proxy respondents than self-respondents. This may be the result of biased proxy overreporting. Alternatively, participants with a proxy respondent were frailer and more likely to have multiple diseases and impaired function. This, combined with the large sample size, may have cancelled out proxy bias and resulted in more-reliable proxy report.
Despite these limitations, these findings are strongly suggestive that the majority of community-dwelling older adults with dementia in the United States have pain. These results have several important clinical and policy implications. First, the findings reinforce previously published clinical guidelines for assessing and treating pain in persons with dementia.44,45 These guidelines recommend screening all persons with dementia for pain with self-reported VDSs if possible, corroborated with proxy report or observational data for individuals with more-severe cognitive impairment. They also recommend evaluating persons with dementia for a pathological source of pain, such as arthritis or cancer. Even conditions that are not thought of as typically painful, such as heart and lung disease, were associated with greater risk of pain. One explanation for this is that these conditions are predecessors of typically painful conditions, such as peripheral vascular disease. Although these findings are based on cross-sectional data, and thus causality cannot be determined, it advisable for clinicians to consider persons with dementia with multiple comorbidities, functional impairment, and other psychological and physical symptoms such as depression and fatigue, as being at high risk of pain.
Although it was not possible with the data to assess how well pain is managed in community-dwelling older adults with dementia, the fact that more than 30% of individuals with bothersome pain were infrequently or never taking pain medication suggests that there is room for improvement in the management of pain in community-dwelling older adults with dementia. Results of studies in long-term care settings that have shown that pain is not being appropriately addressed and treated in individuals with dementia corroborate this,46,47 although the lack of effective and safe nonpharmacological and pharmacological treatments for use in this population and lack of knowledge among clinicians and caregivers about evaluation and treatment of pain in this population hamper pain management in this population.48 There is a great need for methodological and intervention studies using validated and responsive pain measurement tools that can provide more information about differences between raters, changes in pain over time, and pain treatment effects.
Physical and environmental barriers in connecting older adults with dementia with healthcare providers add additional obstacles to treating pain in community-dwelling individuals with dementia. As evidenced by the fact that more than 30% of NHATS participants with dementia reported never going outside and more than 60% reported using assistive devices, functional and mobility declines make it difficult for older adults with dementia to travel to clinician offices, and few clinicians offer home visits. For the increasing number of older adults with dementia living in non-nursing home residential care facilities, variations in the size and type of facilities, as well as regulatory variations across states, may result in vast differences in the quality of health care and pain management that individuals are receiving in these settings.49 Given the difficulties of providing care to community-dwelling older adults with dementia, policy-makers and healthcare providers should be evaluating existing models of care and, when necessary, creating new models of care that address pain, symptoms, and other supportive needs. For example, home-based palliative care is one new model that may offer a solution for addressing pain and symptoms in community-dwelling individuals with dementia.50
In conclusion, community-dwelling older adults with dementia experience a high burden of bothersome pain and activity-limiting pain. The extensive challenges associated with the assessment and treatment of pain in older adults with dementia will require creative solutions from researchers, clinicians, and policy-makers to ensure pain is being adequately managed in this vulnerable population.
Acknowledgments
LJH is supported by Grant T32-NR07088 from the National Institute of Nursing Research. AKS is supported by Paul Beeson Career Development Award in Aging 1K23 AG040772 from the National Institute on Aging (NIA). KEC is supported by Mentoring Award 1 K24 AG041180 from the NIA. KY is supported by Mentoring Award 2 K24 AG031155 from the NIA. CES is supported by Clinical and Translational Sciences Institute Career Development Award 8 KL2 TR000143–08. NHATS is sponsored by National Institute on Aging Grant U01AG032947 through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contribution: Hunt, Covinsky, Yaffe, Stephens, Miao, Boscardin, Smith: study concept and design, analysis and interpretation of data. Hunt, Miao, Smith: acquisition of subjects and data. Hunt, Covinsky, Yaffe, Stephens, Miao, Smith: preparation of manuscript.
Sponsor’s Role: Neither the National Institute of Aging nor the National Institute on Nursing Research had any role in the design, methods, participant recruitment, data collection, analysis, or preparation of the paper.
References
- 1.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones AL, Dwyer LL, Bercovitz AR, et al. The National Nursing Home Survey: 2004 overview. Vital Health Stat. 2009;13:167. [PubMed] [Google Scholar]
- 3.Magaziner J, German P, Zimmerman SI, et al. The prevalence of dementia in a statewide sample of new nursing home admissions aged 65 and older: Diagnosis by expert panel. Epidemiology of Dementia in Nursing Homes Research Group. Gerontologist. 2000;40:663–672. doi: 10.1093/geront/40.6.663. [DOI] [PubMed] [Google Scholar]
- 4.Snow AL, Chandler JF, Kunik ME, et al. Self-reported pain in persons with dementia predicts subsequent decreased psychosocial functioning. Am J Geriatr Psychiatry. 2009;17:873–880. doi: 10.1097/JGP.0b013e3181ad4f73. [DOI] [PubMed] [Google Scholar]
- 5.Torvik K, Kaasa S, Kirkevold O, et al. Pain and quality of life among residents of Norwegian nursing homes. Pain Manag Nurs. 2010;11:35–44. doi: 10.1016/j.pmn.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Covinsky KE, Lindquist K, Dunlop DD, et al. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57:1556–1561. doi: 10.1111/j.1532-5415.2009.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews JS, Cenzer IS, Yelin E, et al. Pain as a risk factor for disability or death. J Am Geriatr Soc. 2013;61:583–589. doi: 10.1111/jgs.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10:591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 9.Takai Y, Yamamoto-Mitani N, Okamoto Y, et al. Literature review of pain prevalence among older residents of nursing homes. Pain Manag Nurs. 2010;11:209–223. doi: 10.1016/j.pmn.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Zwakhalen SM, Koopmans RT, Geels PJ, et al. The prevalence of pain in nursing home residents with dementia measured using an observational pain scale. Eur J Pain. 2009;13:89–93. doi: 10.1016/j.ejpain.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Husebo BS, Strand LI, Moe-Nilssen R, et al. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) Pain Scale in a clinical setting. Scand J Caring Sci. 2010;24:380–391. doi: 10.1111/j.1471-6712.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Monroe TB, Misra SK, Habermann RC, et al. Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatr Gerontol Int. 2014;14:541–548. doi: 10.1111/ggi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horgas AL, Elliott AF, Marsiske M. Pain assessment in persons with dementia: Relationship between self-report and behavioral observation. J Am Geriatr Soc. 2009;57:126–132. doi: 10.1111/j.1532-5415.2008.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shega JW, Hougham GW, Stocking CB, et al. Pain in community-dwelling persons with dementia: Frequency, intensity, and congruence between patient and caregiver report. J Pain Symptom Manage. 2004;28:585–592. doi: 10.1016/j.jpainsymman.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Murray TM, Sachs GA, Stocking C, et al. The symptom experience of community-dwelling persons with dementia: Self and caregiver report and comparison with standardized symptom assessment measures. Am J Geriat Psychiatry. 2012;20:298–305. doi: 10.1097/JGP.0b013e318235b758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel KV, Guralnik JM, Dansie EJ, et al. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154:2649–2657. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montaquila J, Freedman VA, Edwards B, et al. National Health and Aging Trends Study Round 1 Sample Design and Selection. Baltimore: Johns Hopkins University School of Public Health; 2012. NHATS Technical Paper #1. [Google Scholar]
- 19.Kasper JD, Freedman VA, Spillman B. Technical Paper #5. Baltimore: Johns Hopkins University School of Public Health; 2013. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. [Google Scholar]
- 20.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 21.Schinka JA, Loewenstein DA, Raj A, et al. Defining mild cognitive impairment: Impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry. 2010;18:684–691. doi: 10.1097/JGP.0b013e3181e56d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch Neurol. 2012;69:700–708. doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 25.Lukas A, Niederecker T, Gunther I, et al. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment: Experiences gained in a geriatric hospital. Z Gerontol Geriatr. 2013;46:214–221. doi: 10.1007/s00391-013-0475-y. [DOI] [PubMed] [Google Scholar]
- 26.Pautex S, Herrmann F, Le Lous P, et al. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalized elderly demented patients. J Gerontol A Biol Sci Med Sci. 2005;60A:524–529. doi: 10.1093/gerona/60.4.524. [DOI] [PubMed] [Google Scholar]
- 27.Closs SJ, Barr B, Briggs M, et al. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage. 2004;27:196–205. doi: 10.1016/j.jpainsymman.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Taylor LJ, Harris J, Epps CD, et al. Psychometric evaluation of selected pain intensity scales for use with cognitively impaired and cognitively intact older adults. Rehabil Nurs. 2005;30:55–61. doi: 10.1002/j.2048-7940.2005.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 29.Krulewitch H, London MR, Skakel VJ, et al. Assessment of pain in cognitively impaired older adults: A comparison of pain assessment tools and their use by nonprofessional caregivers. J Am Geriatr Soc. 2000;48:1607–1611. doi: 10.1111/j.1532-5415.2000.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 30.Werner P, Cohen-Mansfield J, Watson V, et al. Pain in participants of adult day care centers: Assessment by different raters. J Pain Symptom Manage. 1998;15:8–17. doi: 10.1016/S0885-3924(97)00274-1. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB, et al. An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics. 2009;50:613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55:596–602. doi: 10.1111/j.1532-5415.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith AK, Cenzer IS, Knight SJ, et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563–569. doi: 10.1059/0003-4819-153-9-201011020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shega JW, Paice JA, Rockwood K, et al. Is the presence of mild to moderate cognitive impairment associated with self-report of non-cancer pain? A cross-sectional analysis of a large population-based study. J Pain Symptom Manage. 2010;39:734–742. doi: 10.1016/j.jpainsymman.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shega J, Emanuel L, Vargish L, et al. Pain in persons with dementia: Complex, common, and challenging. J Pain. 2007;8:373–378. doi: 10.1016/j.jpain.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Weiner DK, Peterson BL, Logue P, et al. Predictors of pain self-report in nursing home residents. Aging (Milano) 1998;10:411–420. doi: 10.1007/BF03339888. [DOI] [PubMed] [Google Scholar]
- 39.Jensen-Dahm C, Vogel A, Waldorff FB, et al. Discrepancy between self-and proxy-rated pain in Alzheimer’s disease: Results from the Danish Alzheimer Intervention Study. J Am Geriatr Soc. 2012;60:1274–1278. doi: 10.1111/j.1532-5415.2012.04036.x. [DOI] [PubMed] [Google Scholar]
- 40.Leong IY, Chong MS, Gibson SJ. The use of a self-reported pain measure, a nurse-reported pain measure and the PAINAD in nursing home residents with moderate and severe dementia: A validation study. Age Ageing. 2006;35:252–256. doi: 10.1093/ageing/afj058. [DOI] [PubMed] [Google Scholar]
- 41.Boyer F, Novella JL, Morrone I, et al. Agreement between dementia patient report and proxy reports using the Nottingham Health Profile. Int J Geriatr Psychiatry. 2004;19:1026–1034. doi: 10.1002/gps.1191. [DOI] [PubMed] [Google Scholar]
- 42.van Herk R, van Dijk M, Biemold N, et al. Assessment of pain: Can caregivers or relatives rate pain in nursing home residents? J Clin Nurs. 2009;18:2478–2485. doi: 10.1111/j.1365-2702.2008.02776.x. [DOI] [PubMed] [Google Scholar]
- 43.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: Lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48:1646–1654. doi: 10.1111/j.1532-5415.2000.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 44.Hadjistavropoulos T, Herr K, Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(1 Suppl):S1–S43. doi: 10.1097/AJP.0b013e31802be869. [DOI] [PubMed] [Google Scholar]
- 45.Herr K, Coyne PJ, Key T, et al. Pain assessment in the nonverbal patient: Position statement with clinical practice recommendations. Pain Manag Nurs. 2006;7:44–52. doi: 10.1016/j.pmn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Balfour JE, O’Rourke N. Older adults with Alzheimer disease, comorbid arthritis and prescription of psychotropic medications. Pain Res Manag. 2003;8:198–204. doi: 10.1155/2003/105459. [DOI] [PubMed] [Google Scholar]
- 47.Achterberg WP, Pieper MJ, van Dalen-Kok AH, et al. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471–1482. doi: 10.2147/CIA.S36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones KR, Fink R, Pepper G, et al. Improving nursing home staff knowledge and attitudes about pain. Gerontologist. 2004;44:469–478. doi: 10.1093/geront/44.4.469. [DOI] [PubMed] [Google Scholar]
- 49.Beeber AS, Zimmerman S, Reed D, et al. Licensed nurse staffing and health service availability in residential care and assisted living. J Am Geriatr Soc. 2014;62:805–811. doi: 10.1111/jgs.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritchie CS. Ushering in an era of community-based palliative care. J Palliat Med. 2013;16:818–819. doi: 10.1089/jpm.2013.9493. [DOI] [PubMed] [Google Scholar]