Abstract
Background
Opioid dependence is associated with high levels of morbidity, yet sparse data exists regarding the health-related quality of life (HRQoL) of individuals with opioid dependence, particularly following treatment initiation. To inform cost-effectiveness analyses of treatment modalities, this study investigates short-term changes in HRQoL following enrollment into opioid agonist treatment (OAT), across treatment modalities and patient subgroups.
Methods
Data was analyzed from the Starting Treatment with Agonist Replacement Therapies (START) and Prescription Opioid Addiction Treatment Studies (POATS) randomized controlled trials. Participants included individuals dependent on prescription opioids (POs) or heroin, receiving limited-term or time-unlimited treatment. PO- or heroin-users in START received buprenorphine/naloxone (BUP/NX) or methadone (MET) over 24 weeks. PO-users in POATS received psychosocial care and short-term (4-week) taper with BUP/NX, with non-responders offered subsequent extended (12-week) stabilization and taper. HRQoL was assessed using the short-form SF-6D while in and out of OAT, with distinction between MMT and BUP/NX in START. Linear mixed effects regression models were fitted to determine the independent effects of OAT on HRQoL and characterize HRQoL trajectories.
Results
Treatment had a similar immediate and modest positive association with HRQoL in each patient subgroup. The association of OAT on HRQoL was statistically significant in each model, with effect sizes between 0.039 (Heroin-users receiving BUP/NX) and 0.071 (PO-users receiving MET). After initial improvement, HRQoL decreased slightly, or increased at a diminished rate.
Conclusions
OAT, whether delivered in time-limited or unlimited form, using BUP/NX or MET, is associated with modest immediate HRQoL improvements, with diminishing benefits thereafter.
Keywords: Health related quality of life, Opioid Agonist Treatment, health utility, HRQoL, opioid use disorder, buprenorphine/naloxone, suboxone, methadone
1. INTRODUCTION
As of 2012, there were approximately 2.5 million people in the United States who abused or were dependent on opioids; 2.1 million were dependent on prescription opioids (PO) such as oxycodone, but rates of heroin use may be increasing (Kuehn, 2013; Substance Abuse and Mental Health Services Administration, 2012). Opioid overdose is now the second leading cause of accidental death in the United States - surpassed only by motor vehicle accidents - and has been labeled a national epidemic (Centers for Disease Control and Prevention, 2011).
Opioid agonist treatment (OAT) with methadone or buprenorphine has been shown to be effective in numerous randomized trials, meta-analyses, and large-scale longitudinal studies (Amato et al., 2005; Faggiano et al., 2003; Mattick et al., 2008). Methadone costs less and is more effective in retaining clients in treatment, while buprenorphine has a better safety profile and can be used in office-based practices in the US (Nosyk et al., 2013). Prolonged retention in treatment typically results in reductions in illicit drug use, behaviors that increase the risk of contracting HIV, and criminal activity (Amato et al., 2005). There is evidence that prescription opioid users are more likely to respond and be retained in OAT compared to heroin users (Moore et al., 2007; Nosyk et al., 2014; Soeffing et al., 2009). Discontinuing treatment typically results in relapse and elevated risk of mortality, with the risk of death after discontinuing treatment estimated to be 2.4 times greater than during treatment (Degenhardt et al., 2011).
Beyond clinical effectiveness, OAT has the potential to improve patients’ health-related quality of life (HRQoL) through reduction in drug use and withdrawal symptoms, decreased drug-seeking behaviour and increased access to psychosocial and pharmacological treatment for co-morbid conditions, as recommended in best practices guidelines (Health Canada, 2005; National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998). In recent years, evidence has accumulated for the improvement of HRQoL during long-term opioid agonist treatment for heroin-dependent individuals (Giacomuzzi et al., 2003; Karow et al., 2010; Korthuis et al., 2011; Nosyk et al., 2011; Ponizovsky and Grinshpoon, 2007; Winklbaur et al., 2008).
In most instances, however, HRQoL gains have been modest in magnitude. For instance, a prior study of chronic heroin-dependent individuals revealed modest immediate HRQoL improvement, which declined slightly over time during OAT. Critically, these gains were only observed in a subset of 61% of the study cohort, with 20% demonstrating no HRQoL response despite sustained engagement in treatment (Nosyk et al., 2011). Furthermore, an increasing proportion of OAT clients in the US are presenting with PO dependence (Nosyk et al., 2014), often receiving buprenorphine treatment in office-based settings (Kleber, 2008) in either time-limited (i.e., detoxification) or time-unlimited treatment regimens. There is a paucity of research on the potential differential effects on HRQoL of disparate treatment modalities, or whether greater gains are achievable in PO dependence compared to heroin dependence, considering the latter may be associated with more concurrent medical problems such as hepatitis C (Suryaprasad et al., 2014).
These distinctions may be consequential in the context of health economic evaluations, which rely on health state-specific HRQoL measures to evaluate quality-adjusted life year benefits in comparative analyses of competing treatment regimens. Substance use disorder treatment is typically accessed several times over an individual’s drug use career given the chronic, recurrent nature of opioid dependence (McLellan et al., 2000). Information on the impact of repeated treatment attempts, and the durability of the impact of treatment on HRQoL, are critical to the accurate estimation of relative value for money of alternative substance use disorder treatment modalities, including medications. Uncertainty surrounding HRQoL valuations had an effect on the findings in at least one prior cost-effectiveness analysis for the treatment of opioid dependence (Schackman et al., 2012). Our objective was therefore to characterize short-term changes in HRQoL following enrollment into OAT across different modalities (detoxification, or tapered-dose treatment, maintenance, or time-unlimited treatment), medications (buprenorphine, methadone) and patient subgroups (heroin, PO dependence).
2. METHODS
2.1. Study Populations
This study was a secondary analysis of two multi-site, US-based randomized controlled trials executed by the NIDA-supported Clinical Trials Network. Characteristics of the trials and participants are described in Table 1, and results of the trials are summarized elsewhere (Potter et al., 2013; Weiss et al., 2011). The Starting Treatment with Agonist Replacement Therapies (START) trial was a 24-week multi-site phase-IV trial designed to compare OAT with methadone and buprenorphine/naloxone (suboxone®) (BUP/NX) in their effects on changes in liver enzymes among individuals dependent on heroin or prescription opioids. The Prescription Opioid Addiction Treatment Study (POATS) was a 2-phase trial designed to compare different lengths of BUP/NX and different intensities of counseling for individuals with prescription opioid dependence.
Table 1.
Comparison of START, POATS Trials
| CTN0027: Starting treatment with agonist replacement therapies (START) | CTN0030: Prescription Opioid Abuse Treatment Study (POATS) | |
|---|---|---|
| Study Design | Randomized, open-label, multi-center phase 4 study to assess changes in liver enzymes related to treatment with BUP/NX vs. MET in patients entering OAT. Randomization stratified by site according to liver abnormality. |
Two-phase, randomized, multi-center study. All participants were administered BUP/NX in a 1 day induction regimen, which began the 4-week BUP/NX treatment phase. Participants were also randomly assigned to either SMM or EMM. Randomization was stratified within sites with respect to chronic pain and lifetime heroin use. |
| Primary objective | Compare changes in liver enzymes related to tx with BUP/NX vs. MET during 24 weeks of tx of opioid dependent individuals. | Determine whether the addition of individual drug counseling to the prescription of BUP/NX along with standard medical management (SMM) improves outcome during (a) an initial 4-week treatment with taper and (b) 12-week stabilization treatment for those not responding to taper at 4 weeks. |
| Treatment options | BUP/NX, methadone; aim to ensure ‘adequate dosing’ BUP/NX: 26/4 to start, up to 32/8 on day 2. Methadone: 40mg on day 1, +10 on days 2, 3, adjusted as necessary thereafter. | BUP/NX+SMM vs. BUP/NX+ extended medical management (EMM); BUP/NX dose: 12mg; max 32mg. |
| Study duration | 24 weeks | Phase 1: 12 weeks; Phase 2: 24 weeks (36 weeks total) |
| Patient selection criteria |
Inclusion: Men and women seeking opioid agonist treatment; ≥18; DSM-IV opioid dependence; ability to provide written, informed consent Exclusion: medical condition making treatment hazardous; allergy/sensitivity to study meds, acute psychosis, severe depression, immediate suicide risk; stimulant dependence; pending legal sanctions. |
Inclusion: Subjects dependent on prescription opioid analgesics; ≥ 18; agreement to use birth control; DSM-IV opioid dependence; ability to provide written, informed consent Exclusion: medical cross-indication, acute psychosis/depression, suicide risk; stimulant dependence; heroin use>4 days in past 30; ever injected heroin; pregnant females, pending legal action preventing study completion within 30 days of enrollment; pain indication for opioids. |
BUP/NX: buprenorphine/naloxone; OAT: opioid agonist treatment; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
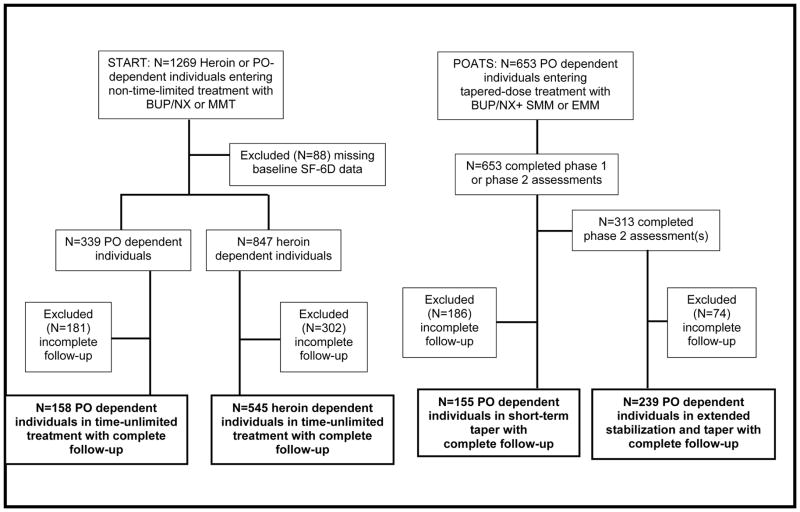
Four distinct subject groups were defined from the START and POATS study populations for this analysis: (i) PO-dependent and (ii) heroin-dependent individuals receiving maintenance, or time-unlimited treatment with either BUP/NX or methadone in START; and PO-dependent individuals receiving either (iii) short-term taper or (iv) taper after 12 weeks of BUP/NX stabilization in POATS. Groups (iii) and (iv) pertain to phases 1 and 2 of the POATS trial, respectively. Heroin or PO classification was established based on the greatest frequency of use at baseline; individuals can therefore be considered ‘primary’ users of heroin or PO, respectively. Individuals were included in the analyses if they completed the final study assessment (week 24 assessment for groups (i) and (ii), week 4 assessment for group (iii) and week 36 assessment for group (iv)), and otherwise excluded if they had incomplete capture of baseline opioid use data.
2.2. Measures
Health related quality of life, or health utility, was measured using the SF-6D. The SF-6D is a generic, preference-weighted measure of health status derived from the Short Form-36 and Short-Form-12 health surveys, which are widely used psychometric measures of health and psychological functioning (Brazier et al., 2002, 2004; Ware and Sherbourne, 1992; Brazier and Roberts, 2004). The psychometric properties of the SF-6D have been tested in numerous disease areas (Brazier et al., 2004). Each of the six domains included in the SF-6D scoring algorithm (‘physical limitations’, ‘role limitations’, ‘social limitations’, ‘pain’, ‘mental health problems’ and ‘vitality’), contains between four and six levels of response, assessed on the basis of the patients’ self-reported health on the day of the interview with a 14-day recall period. The combinations of possible responses within these six domains generates 18,000 unique health states, a sample of which were valued by a representative sample of the UK general population using the standard gamble valuation method (Ware and Sherbourne, 1992), thus providing empirically-derived societal preference weights.
Our primary interest was to characterize HRQoL improvements during OAT. We therefore constructed measures of treatment receipt based on the therapies studied in each trial and pertaining to each patient subgroup. In the POATS study, we first considered distinct standard medical management (SMM)+BUP/NX and extended medical management (EMM)+BUP/NX health states, and added a ‘post-treatment’ indicator variable to determine whether HRQoL declined after concluding detoxification treatment. In the START study, we constructed distinct covariates for those receiving methadone versus BUP/NX. Baseline assessments, completed prior to initiating trial medications, served as the referent case in all regression models. As such, coefficients on the on-treatment and post-treatment variables can be interpreted as changes in HRQoL associated with treatment, independent of the effects of covariates included in the final model. The ‘post-treatment’ covariate represented follow-up assessments completed after medication discontinuation – either treatment dropout in the START trial, or after a planned stabilization and taper in phase 2 of the POATS trial.
Prior cross-sectional and longitudinal studies informed selection of additional determinants of HRQoL among opioid-dependent individuals (Astals et al., 2008; Falck et al., 2000; Kertesz et al., 2005; Millson et al., 2006; Puigdollers et al., 2004). The START trial had complete capture of the following covariates: age, gender, ethnicity, comorbid mental health conditions, baseline indication of viral hepatitis and time-varying indicators of positive urine screens for illicit opiates and stimulants. Within the POATS analyses, the following variables were considered: age, gender, ethnicity, education, criminal activity, marital status, employment, indicators of psychiatric conditions, use of a range of illicit drugs captured in the Addiction Severity Index (ASI-Lite), as well as chronic medical problems. We note that mental health conditions were defined as ‘ever being treated or having a history of Schizophrenia, Major Depressive Disorder, Bipolar Disorder, Anxiety or Panic Disorder or clinically significant neurological damage’ in the START trial, whereas it was operationalized as ‘psychiatric problems in the past 30 days’ in the POATS trial. Time-dependent urine screen measures of stimulant use were considered, while all other covariates were measured at baseline assessment.
2.3. Statistical Analysis
We analyzed four separate sub-groups of patients within the START and POATS trials in parallel, to provide a basis of comparison of HRQoL changes across the disparate treatment options and patient populations enrolled in the studies. Analyses were executed on study completers to adequately characterize longitudinal HRQoL response among treatment recipients. We first summarized the characteristics of patients within each of four sub-groups according to the data available within each study. Linear mixed effects regression models were estimated to determine the marginal effect of OAT receipt on HRQoL, controlling for potential confounding factors. Given the low frequency of ceiling-valued responses and noted problems in the interpretation of results from Tobit or Censored Least Absolute Deviations, (Pullenayegum et al., 2010; Pullenayegum et al., 2011) linear regression models were estimated. In addition, least-squares means, or fitted values from mixed effects models of the form: HRQoLit ~ f(timeit, Xit) for individuals i at time t, where X included potential confounders, were estimated to characterize and compare temporal changes in HRQoL during the course of treatment (gaussian distribution, identity link). We note here that observations classified as ‘post-treatment’ were excluded in this analysis. Finally, domain-specific changes in health state valuations between baseline and the final assessment in each patient subgroup were plotted to characterize the observed improvements in HRQoL within each of the four patient subgroups. All analyses were executed with SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
Patient subgroup selection from the START and POATS trials is described in Figure 1. Of note, 88 individuals from the START trial were excluded due to missing baseline data on self-reported opioid use. Among selected participants providing final follow-up assessments, 158 were PO-dependent while 545 were heroin dependent individuals. A total of 653 POATS participants completed assessments during short-term taper, with 313 completing assessments during extended stabilization and taper. After excluding those with incomplete follow-up, 155 individuals were included in the short-term taper study group, and 239 included in the extended stabilization and taper study group. We note that individuals characterized as ‘short-term taper treatment completers’ may have had either successful or unsuccessful response to treatment but completed the 12-week assessment and did not continue to extended stabilization and taper.
Figure 1. Study sample selection.
Complete follow-up entailed completion of the final follow-up assessment in the respective trial and phase; participants could miss intermediate assessment and still be included in the study. PO: prescription opioids; MET: methadone treatment; BUP/NX: Buprenorphine/naloxone (suboxone); SMM: standard medical management; EMM: extended medical management;
Characteristics of individuals in each of the patient subgroups are presented in Table 2. Those enrolled in time-unlimited treatment had high levels of mental health comorbidity (89.5% and 89.9% of heroin and PO users, respectively), and a higher percentage of heroin users had positive opioid and stimulant urine screens at baseline assessment (96.1% and 45.1% versus 86.1% and 22.1%, respectively). PO dependent individuals enrolled in brief treatment or extended treatment, with taper were primarily white and were younger than those receiving -time-unlimited treatment, and fewer reported mental health conditions (34.9% and 36.2% of short term and extended detoxification participants, respectively).
Table 2.
Baseline summary statistics on selected patient subgroups
| Heroin dependent individuals in time-unlimited treatment a(N=545) | PO dependent individuals in time-unlimited treatment a (N=158) | PO dependent individuals in short-term taper b (N=155) | PO dependent individuals in extended stabilization and taper b (N=239) | |
|---|---|---|---|---|
|
| ||||
| N (%) | N (%) | N (%) | N (%) | |
| Female | 166 (30.4) | 60 (38.0) | 69 (44.5) | 93 (38.9) |
| Age (median, IQR) | 39.2 (29.2, 48.2) | 32.9 (26.0, 45.7) | 32 (24, 42) | 29 (25,38) |
| White | 396 (72.6) | 136 (86.1) | 141 (91.0) | 218 (91.2) |
| Married | - | - | 48 (31.0) | 68 (28.5) |
| High school education | - | - | 133 (85.8) | 199 (83.3) |
| Mental health condition c | 488 (89.5) | 142 (89.9) | 76 (49.0) | 103 (43.1) |
| Chronic medical problem | - | - | 67 (43.2) | 89 (37.2) |
| Hepatic disease | 195 (35.8) | 25 (15.8) | - | - |
| Positive urine screen-opioid d | 524 (96.1) | 136 (86.1) | - | - |
| Positive urine screen-stimulant d | 246 (45.1) | 36 (22.8) | - | - |
| Ever treated for opioid dependence | - | - | 58 (37.4) | 78 (32.6) |
| Heroin use, past 30 days | 30 (27, 30) | 0 (0, 0) | 8 (5.2) | 18 (7.5) |
| Prescribed methadone, past 30 days | - | - | 5 (3.2) | 5 (2.1) |
| Illicit methadone, past 30 days | - | - | 18 (11.6) | 64 (26.8) |
| Prescription opioid use, past 30 days | 0 (0, 1) | 28 (19, 30) | 152 (98.1) | 233 (97.5) |
| Stimulant use, past 30 days | - | - | 43 (27.7) | 45 (18.8) |
|
| ||||
| Classification during follow-upe | N=1573 | N=442 | N=310 | N=928 |
|
| ||||
| Pre-treatment (t) | 545 (34.7) | 158 (35.8) | 155 (54.2) | 239 (25.8) |
| On treatment (t) | -- | -- | 142 (45.8) | 661 (71.2) |
| On treatment-MET (t) | 476 (30.3) | 109 (24.7) | -- | -- |
| On treatment-BUP/NX (t) | 444 (28.2) | 133 (30.1) | -- | -- |
| Post treatment(t) | 111 (7.6) | 43 (9.7) | 13 (4.2)f | 28 (3.0) |
PO: Prescription Opioids
Participants in the START trial.
Participants in the POATS trial.
Defined as ‘ever being treated or having a history of Schizophrenia, Major Depressive Disorder, Bipolar Disorder, Anxiety or Panic Disorder or Clinically significant neurological damage’ in the START trial. Defined as ‘psychiatric problems, past 30 days’ in the POATS trial.
Across all study assessments.
all figures represent numbers and percentages of the total number of observations per study stratum.
The 13 ‘post-treatment’ observations in this study stratum were combined with pre-treatment observations in multiple regression analysis (Table 3).
Results of the multiple regression analyses on HRQoL scores in each of the four participant subgroups are presented in Table 3. Treatment had a uniformly positive and statistically significant association with HRQoL in each patient subgroup. The association of treatment on HRQoL was strongest among PO dependent individuals receiving time-unlimited treatment with methadone (0.071; P<0.01), and weakest among heroin-dependent individuals receiving maintenance treatment with BUP/NX (0.039; p<0.001). The difference in HRQoL response to time-unlimited treatment with methadone or BUP/NX was not statistically significant in either PO- or heroin-dependent individuals (PO:0.011; p=0.48; Heroin:0.004; p=0.59). HRQoL scores were also not statistically significantly different from baseline (pre-treatment) scores following discontinuation of time-unlimited treatment. Otherwise, baseline psychiatric and chronic medical problems also had relatively large negative impacts on HRQoL in POATS patient subgroups.
Table 3.
Results of multiple regression analyses on HRQoL scores in each of the four participant subgroups
| Heroin dependent individuals in time-unlimited treatment a (N=545) | PO dependent individuals in time-unlimited treatment a (N=158) | PO dependent individuals in short-term taperb (N=155) | PO dependent individuals in extended stabilization and taper b (N=239) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Beta | SE | P-value | Beta | SE | P-value | Beta | SE | P-value | Beta | SE | P-value | |
| Intercept | 0.756 | 0.025 | <.001 | 0.708 | 0.057 | <.001 | 0.791 | 0.034 | <.001 | 0.789 | 0.024 | <.001 |
| Pre-treatment | ref | ref | ref | ref | ||||||||
| On treatment (t) | - | - | - | - | - | - | 0.044 | 0.011 | <.001 | 0.051 | 0.007 | <.001 |
| On treatment-MET (t) | 0.044 | 0.006 | <.001 | 0.071 | 0.012 | <.001 | - | - | - | - | - | - |
| On treatment-BUP/NX (t) | 0.039 | 0.006 | <.001 | 0.059 | 0.011 | <.001 | - | - | - | - | - | - |
| Post treatment(t) | 0.007 | 0.016 | 0.672 | 0.045 | 0.031 | 0.148 | - | - | - | 0.050 | 0.021 | 0.014 |
| Female gender (vs. male) | −0.017 | 0.010 | 0.089 | −0.039 | 0.018 | 0.032 | −0.033 | 0.017 | 0.063 | −0.034 | 0.012 | 0.006 |
| Age (deciles) (t0) | −0.010 | 0.008 | 0.032 | −0.010 | 0.012 | 0.205 | −0.022 | 0.008 | 0.008 | −0.016 | 0.006 | 0.015 |
| White (t0) (vs. Non-white) | −0.014 | 0.011 | 0.218 | 0.019 | 0.026 | 0.481 | - | - | - | - | - | - |
| Married (t0) (vs. other) | - | - | - | - | - | - | −0.004 | 0.019 | 0.838 | 0.030 | 0.013 | 0.027 |
| Employment (t0) (vs. other) | - | - | - | - | - | - | 0.013 | 0.017 | 0.446 | −0.026 | 0.007 | <.001 |
| Mental health condition (t0)c (vs. no condition) | −0.014 | 0.016 | 0.373 | −0.022 | 0.029 | 0.454 | −0.046 | 0.018 | 0.010 | −0.054 | 0.012 | <.001 |
| Chronic med. condition (t0) (vs. no condition) | - | - | - | - | - | - | −0.052 | 0.018 | 0.004 | −0.073 | 0.013 | <.0001 |
| Hepatic disease (t0) (vs. no Hepatic disease) | −0.026 | 0.010 | 0.012 | 0.001 | 0.026 | 0.958 | - | - | - | - | - | - |
| Stimulant-positive urinalysis (t) (vs. negative urinalysis) | −0.010 | 0.007 | 0.126 | 0.020 | 0.014 | 0.151 | - | - | - | - | - | - |
| Sedative/hypnotic use (t0) (vs. no sedative/hypnotic use) | - | - | - | - | - | - | −0.037 | 0.018 | 0.038 | −0.031 | 0.012 | 0.010 |
| Stimulant use (t) (vs. no stimulant use) | - | - | - | - | - | - | −0.027 | 0.017 | 0.115 | −0.018 | 0.010 | 0.085 |
Ref: reference category; SE: standard error. MET: methadone; BUP/NX: buprenorphine-naloxone (Suboxone). (t) indicates time-varying covariates, (t0) indicates fixed covariates.
Enrolled in the START trial.
Enrolled in the POATS trial.
Defined as ‘ever being treated or having a history of Schizophrenia, Major Depressive Disorder, Bipolar Disorder, Anxiety or Panic Disorder or clinically significant neurological damage’ in the START trial. Defined as ‘psychiatric problems, past 30 days’ in the POATS trial.
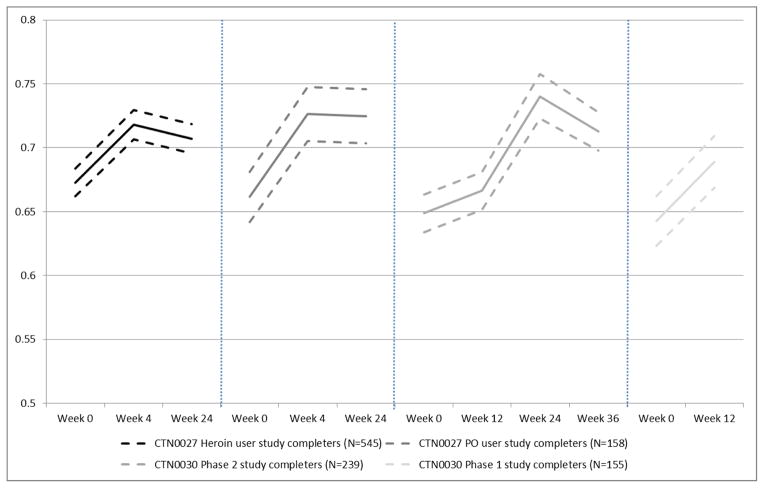
Mean longitudinal trajectories of HRQoL while on treatment during the observation period for each patient subgroup are plotted in Figure 2. HRQoL improvement following treatment enrollment was similar across each of the patient subgroups (Figure 1); in each case, both baseline assessment and HRQoL gains were similar. Improvements were immediate and modest, and either decreased slightly after initial improvement, or increased at a diminished rate. Among PO dependent individuals entering phase 2 of the POATS trial, HRQoL gains following initial (failed) short-term taper (at the week 12 assessment, compared to baseline) were minimal, and not statistically significant; HRQoL gains were most pronounced at the week 24 assessment, following phase 2 initiation at week 12, then decreased after the taper had completed by week 36.
Figure 2. Mean trajectories of HRQoL following study enrollment, conditional on treatment adherence.
CTN0027: Starting treatment with agonist replacement therapies (START); CTN0030: Prescription Opioid Abuse Treatment Study (POATS). PO: prescription opioids.
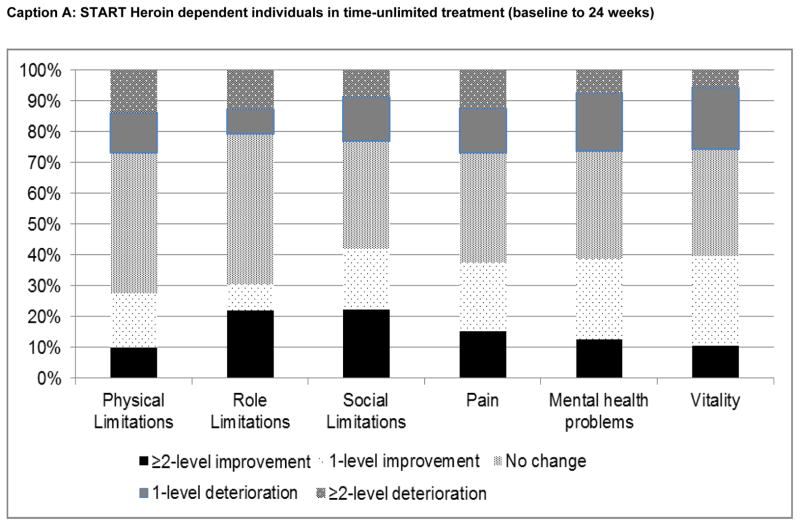
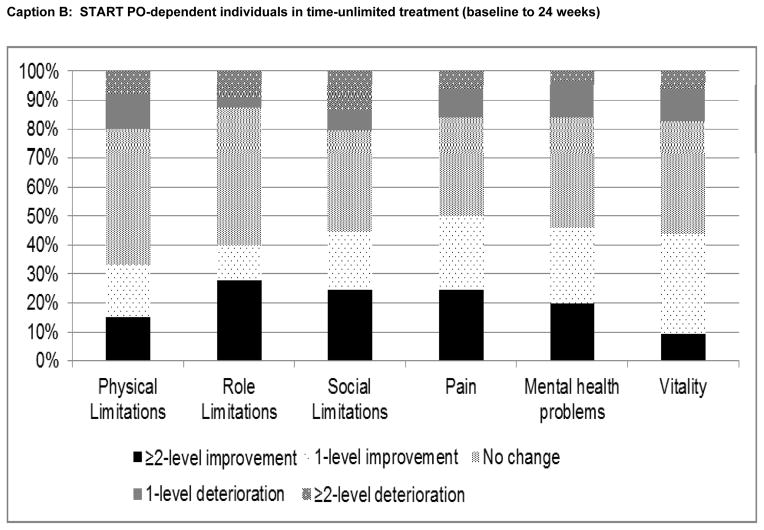
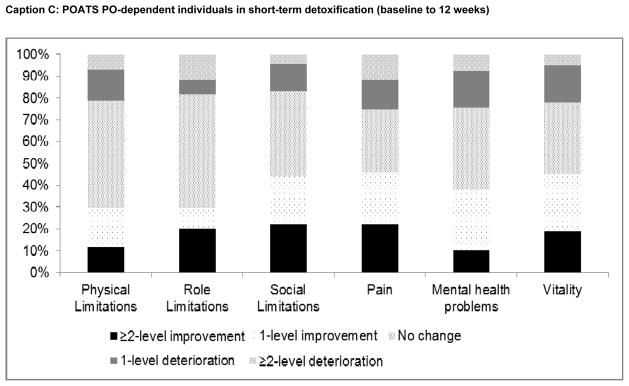
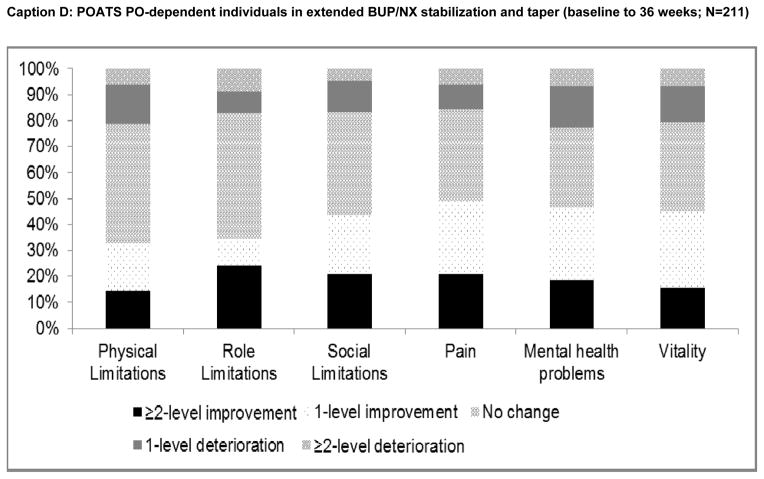
Finally, changes in SF-6D domain scores, from baseline to final assessment for each patient subgroup, are plotted in Figure 3. Among heroin users in time-unlimited treatment, the greatest proportional improvements were observed in the ‘social limitations’, ‘pain’, ‘mental health problems’ and ‘vitality’ domains (Caption A). Changes in physical and role limitations were relatively uncommon. Similar patterns were observed for PO dependent individuals (Caption B), with the greatest level of improvement observed in the ‘pain’ domain. Among PO- dependent individuals receiving short-term (Caption C) or extended taper (Caption D), responses to physical and role limitations were also least likely to change, while the highest percentage improvements were observed in the ‘pain’ ‘vitality’, ‘mental health problems’ and ‘social limitations’ domains.
Figure 3. Changes in SF-6D HRQoL domain scores from baseline to final assessment.
START: Starting treatment with agonist replacement therapies trial; POATS: Prescription Opioid Abuse Treatment Study; PO: prescription opioids; BUP/NX: buprenorphine/naloxone (suboxone).
4. DISCUSSION
We found that OAT, whether delivered in time-unlimited (maintenance) or detoxification, using buprenorphine/naloxone or methadone, is associated with modest improvements in HRQoL soon after treatment initiation that is near conventional minimally important difference level for the SF-6D (0.041; Walters and Brazier, 2005), with diminishing, and possibly deteriorating benefits thereafter. This observed pattern of immediate increase and diminishing or levelling off is consistent with at least two other studies considering HRQoL trajectories over 12- and 18-month periods. Using the Brief Version of the World Health Organization Quality of Life Instrument (WHOQOL-BREF) in an 18-month study of heroin users in methadone treatment in Vietnam, Wang et al. (2012) noted a rapid improvement in HRQoL in the three months following OAT initiation, with domain-specific QoL measures levelling-off thereafter. With a 12-month observation period, Nosyk et al. (2011) observed a similar pattern of early improvement and subsequent deterioration in one of three identified latent classes (comprising nearly 2/3 of the study sample) of chronic heroin dependent individuals in OAT.
Taken together, these results suggest a possible threshold level of HRQoL response to OAT. Co-occurring mental health conditions, infectious diseases such as HIV and HCV, as well as other chronic medical conditions are highly prevalent in opioid dependent individuals and may contribute to the threshold level of HRQoL response to OAT (Volkow and Montaner, 2011). While HIV status was not consistently collected in the START and POATS studies, mental health conditions, chronic medical conditions and HCV had independent negative associations with HRQoL in this study. Although improvements in vitality and mental health domains were observed, improvements were incomplete within the population under study. Further, improvements in physical functioning and role limitations were uncommon, contributing to the incomplete HRQoL response to treatment. These results are consistent with a perspective that OAT alone should not be considered comprehensive medical care for opioid dependent individuals. Rather, OAT can provide an opportunity for additional medical and psychosocial care to address other deficits in health-related quality of life.
Our analyses have several limitations which should be considered. First, the patient subgroups we considered were all selected from clinical trials with multiple exclusion criteria, including stimulant dependence (START), pending incarceration or legal issues, pain indication for opioids, and severe mental health conditions, which are all commonly observed in opioid-dependent populations. While our results were consistent with others from diverse patient populations, they may be interpreted as best-case responses for a selective group of treatment initiators. Second, capture of covariates which may have been associated with both treatment receipt and HRQoL was limited in the START trial. While any potential unmeasured confounding may affect the point estimates of HRQoL gains during treatment, their consistency with POATS and external studies suggests the level of bias may have been small.
We note that measures of illicit opioid use during follow-up were not included in the models presented, as continued use would necessarily be the result of the success, or failure of OAT given the pharmacological properties and objectives of OAT (i.e., illicit opioid use is in the causal pathway of the OAT-HRQoL relationship). When included in alternate model specifications, continued illicit opioid use had a strong negative association with HRQoL, but OAT continued to have a uniformly positive and statistically significant association with HRQoL and coefficient values for HRQoL while on treatment were 0.004–0.02 lower compared to baseline models (results not presented). This is indicative of the positive HRQoL impact of OAT above and beyond associated decreases in illicit opioid use – albeit at a level below the minimally important difference, suggesting the primary benefits of OAT were attributable to the reduction of illicit opioid use. We also note that our descriptive analysis of domain change scores needs to be interpreted with caution, as the preference weights applied to responses within and across domains are not equivalent when HRQoL scores are calculated; thus the relative contribution of each response increment and each domain to overall HRQoL scores cannot be derived from the results presented in Figure 3.
This study demonstrated a consistent positive association between OAT and health-related quality of life across four diverse subgroups of opioid-dependent individuals treated with diverse OAT modalities. OAT should be considered as part of a broader set of health interventions for opioid dependent individuals, who tend to be socially marginalized and often present with multiple comorbid medical conditions.
Highlights.
We examine short-term health utility of patients on opioid agonist treatment (OAT)
OAT initially improves health utility across all treatment modalities and patients
Health utility subsequently decreases slightly or increases at diminished rate
Results suggest a threshold level in health-related quality of life response to OAT
Acknowledgments
Role of Funding Source: This research was supported in part by the National Institute on Drug Abuse (NIDA) (R01-DA033424 and R01-DA031727). Dr. Weiss was otherwise supported by grants U10-DA15831 and K24-DA022288 from NIDA.
We gratefully acknowledge the editorial support of Michelle Olding and assistance of representatives of the CTN study teams, including Maureen Hillhouse and Albert Hasson, for their helpful insight into the studies and databases.
Footnotes
Author Contributions: BN, JWB, EW, BA, AAE and BRS contributed to the design of the study. BN led the analysis and preparation of the manuscript. JWB contributed to analysis and interpretation of results. RDW, JP, AA, YI and WL led procurement of the databases. All authors provided critical revisions to the article and approved the final draft.
Conflict of Interests: none.
The funders had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat. 2005;28:321–329. doi: 10.1016/j.jsat.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Astals M, Domingo-Salvany A, Buenaventura CC, Tato J, Vazquez JM, Martin-Santos R, Torrens M. Impact of substance dependence and dual diagnosis on the quality of life of heroin users seeking treatment. Subst Use Misuse. 2008;43:612–632. doi: 10.1080/10826080701204813. [DOI] [PubMed] [Google Scholar]
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13:873–884. doi: 10.1002/hec.866. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: Overdoses of prescription opioid pain relievers - United States, 1999–2008. MMWR. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Falck RS, Wang J, Siegal HA, Carlson RG. Longitudinal application of the medical outcomes study 36-item short-form health survey with not-in-treatment crack-cocaine users. Med Care. 2000;38:902–910. doi: 10.1097/00005650-200009000-00004. [DOI] [PubMed] [Google Scholar]
- Giacomuzzi SM, Riemer Y, Ertl M, Kemmler G, Rossler H, Hinterhuber H, Kurz M. Buprenorphine versus methadone maintenance treatment in an ambulant setting: a health-related quality of life assessment. Addiction. 2003;98:693–702. doi: 10.1046/j.1360-0443.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- Health Canada. [accessed on February 21, 2014];Best Practices: Methadone Maintenance Treatment. 2005 http://www.hc-sc.gc.ca/hc-ps/pubs/adp-apd/methadone-bp-mp/index-eng.php.
- Karow A, Reimer J, Schafer I, Krausz M, Haasen C, Verthein U. Quality of life under maintenance treatment with heroin versus methadone in patients with opioid dependence. Drug Alcohol Depend. 2010;112:209–215. doi: 10.1016/j.drugalcdep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Kertesz SG, Larson MJ, Horton NJ, Winter M, Saitz R, Samet JH. Homeless chronicity and health-related quality of life trajectories among adults with addictions. Med Care. 2005;43:574–585. doi: 10.1097/01.mlr.0000163652.91463.b4. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Methadone maintenance 4 decades later: thousands of lives saved but still controversial. JAMA. 2008;300:2303–2305. doi: 10.1001/jama.2008.648. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, Botsko M, Acosta A, Gourevitch MN, Hersh D, Hsu J, Boverman J, Altice FL, Bhives Collaborative. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S39–45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. SAMHSA: Pain medication abuse a common path to heroin: experts say this pattern likely driving heroin resurgence. JAMA. 2013;310:1433–1434. doi: 10.1001/jama.2013.278861. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, Shore R, Hopkins S. Determinants of health-related quality of life of opiate users at entry to low-threshold methadone programs. Eur Addict Res. 2006;12:74–82. doi: 10.1159/000090426. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. Effective medical treatment of opiate addiction. JAMA. 1998;280:1936–1943. [PubMed] [Google Scholar]
- Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh D, Schackman B, Wood E, Montaner JSG. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff (Millwood) 2013;32:1–8. doi: 10.1377/hlthaff.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Guh DP, Sun H, Oviedo-Joekes E, Brissette S, Marsh DC, Schechter MT, Anis AH. Health related quality of life trajectories of patients in opioid substitution treatment. Drug Alcohol Depend. 2011;118:259–264. doi: 10.1016/j.drugalcdep.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Nosyk B, Li L, Evans E, Huang D, Urada D, Wood E, Rawson R, Hser YI. Utilization and outcomes of detoxification and maintenance treatment for opoiod dependence in publicly-funded facilities in California, US: 1991–2012. Drug Alcohol Depend. 2014;143:149–157. doi: 10.1016/j.drugalcdep.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33:631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START) J Stud Alcohol Drugs. 2013;74:605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigdollers E, Domingo-Salvany A, Brugal MT, Torrens M, Alvaros J, Castillo C, Magri N, Martin S, Vazquez JM. Characteristics of heroin addicts entering methadone maintenance treatment: quality of life and gender. Subst Use Misuse. 2004;39:1353–1368. doi: 10.1081/ja-120039392. [DOI] [PubMed] [Google Scholar]
- Pullenayegum EM, Tarride JE, Xie F, Goeree R, Gerstein HC, O’Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: are Tobit and CLAD models appropriate? Value Health. 2010;13:487–494. doi: 10.1111/j.1524-4733.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- Pullenayegum EM, Tarride JE, Xie F, O’Reilly D. Calculating utility decrements associated with an adverse event: marginal Tobit and CLAD coefficients should be used with caution. Med Decis Making. 2011;31:790–799. doi: 10.1177/0272989x11393284. [DOI] [PubMed] [Google Scholar]
- Schackman BR, Leff JA, Polsky D, Moore BA, Fiellin DA. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med. 2012;27:669–676. doi: 10.1007/s11606-011-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37:426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. [accessed on February 21, 2014];Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. 2012 http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.htm.
- Suryaprasad A, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Hamdounia SB, Church DR, Barton K, Fisher C, Macomber K, Stanley M, Guilfoyle SM, Sweet K, Liu S, Iqbal K, Tohme R, Sharapov U, Kupronis BA, Ward JW, Holmberg SD. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59:1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff (Millwood) 2011;30:1411–1419. doi: 10.1377/hlthaff.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- Wang PW, Wu HC, Yen CN, Yeh YC, Chung KS, Chang HC, Yen CF. Change in quality of life and its predictors in heroin users receiving methadone maintenance treatment in Taiwan: an 18-month follow-up study. Am J Drug Alcohol Abuse. 2012;38:213–219. doi: 10.3109/00952990.2011.649222. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbaur B, Jagsch R, Ebner N, Thau K, Fischer G. Quality of life in patients receiving opioid maintenance therapy. A comparative study of slow-release morphine versus methadone treatment. Eur Addict Res. 2008;14:99–105. doi: 10.1159/000113724. [DOI] [PubMed] [Google Scholar]