Abstract
Purpose of review
To review the discoveries in molecular pathophysiology contributing to the development of neurofibromatosis type 2 (NF2)-associated vestibular schwannomas and the recent experiences with drug therapies for these tumors. The review includes discussion of diagnostic criteria for NF2, populations to clinically consider for drug therapy and drug targets currently under consideration for NF2.
Recent findings
Increased insight into the complex pathways that underlie both the genetic syndrome of NF2 and the specific pathogenesis of vestibular schwannomas has highlighted multiple potential therapeutic targets. These discoveries have been translated into clinical trials with some early promising results. Inhibition of angiogenesis as well as regulation of mammalian target of rapamycin and the epidermal growth factor receptor family of receptors are the focus of current clinical investigations.
Summary
Although a great deal of work is ongoing to understand the multiple effects of the lack of the regulating protein Merlin on tumorgenesis in patients with NF2, advances are ongoing with clinical therapeutics. There is cause for enthusiasm based on recent results with antiangiogenesis therapy in select patients with NF2 and progressive vestibular schwannomas; however, awareness of the notable risks and limitations of therapies currently in development is required.
Keywords: antiangiogenesis, drug, mTOR, neurofibromatosis type 2
INTRODUCTION
Neurofibromatosis 2 (NF2) is an autosomal dominant tumor-suppressor syndrome characterized by schwannomas, meningiomas, and ependymomas that develop throughout the central and peripheral nervous system. The birth prevalence is estimated to be 1 in 25 000 births [1]. Patients with the most common form of NF2 (germline mutation in the NF2 gene) frequently present with either a focal neurologic deficit or progressive hearing loss in their late teens and early twenties. However, there is a wide range of symptomatic severity in NF2 ranging from multiple cranial and peripheral neuropathies resulting in significant morbidity early in life to mild hearing loss late in life [2]. The clinical heterogeneity is partially accounted for by a high rate of mosaicism. Mosaicism means that there are two cell populations, one with the NF2 deletion and one with the normal NF2 alleles, in a single patient. In these patients, only a subset of cells have the NF2 gene deletion and these patients often have fewer tumors, milder symptoms, and therefore a generally more mild course and may present later in life [2]. In contrast, development of neurologic deficits associated with NF2 early in life generally corresponds with a worse prognosis often related to multiple tumors and increased neurologic morbidity [3,4].
The tumors associated with NF2 are benign in histology; however, NF2 patients experience significant morbidity and mortality related to their disease related to the location of the tumors and the effects of treatments. Previous actuarial survival after diagnosis of NF2 is 85% at 5 years, 67% at 10 years, and 38% at 20 years [4]. Importantly, more recent population studies suggest that life expectancy in NF2 is better in the modern era than previously reported. Specifically, using a population registry for Northwest England, the median life expectancy for patients with NF2 was 69.0 years [95% confidence interval (CI) 58.9–79.0 years]. Although this is less than the general population, it is far better than prior reports [5]. This improvement in survival statistics may reflect improvement in management or it may represent ascertainment bias with patients with mosaic NF2 having improved prognosis increasingly being recognized.
There are four established diagnostic criteria for NF2, National Institutes of Health (NIH) 1987 and 1991 [6], Manchester criterion, and the National Neurofibromatosis Foundation (NNFF) criterion (Table 1) [7]. The diagnosis can be readily confirmed if there are bilateral vestibular schwannomas or a confirmed first degree relative and the presence of an NF2-associated tumor. However, there is diagnostic uncertainty outside of these two scenarios. Given that roughly 50% of patients with NF2 have a spontaneous mutation and therefore no family history, over time the diagnostic criterion has become increasingly more specific to increase the sensitivity of diagnosing patients with non-vestibular schwannomas findings of NF2. However, the variability in symptom presentation and syndrome severity made fulfilment of all diagnostic criteria at initial evaluation relatively rare [7]. For example, although the Manchester criterion was the most sensitive, only 14% of patients ultimately confirmed to have NF2 could be diagnosed at initial consultation with this criterion. Recently, the Baser criterion was proposed in order to preserve high specificity, but increased diagnostic sensitivity at early presentation [8▪▪]. This criterion is based on an algorithm that accounts for age of symptom onset, the constellation of symptoms, and genetic testing results by assigning different number of points for each of these features. If a patient is given a score of 6 or greater, that is consistent with a definite diagnosis of NF2. Importantly, this criterion incorporates consideration of mosaic NF2. Although an excellent addition to the field, the Baser criterion has not yet been widely incorporated into clinical practice or into inclusion criteria for clinical trials.
Table 1.
Clinical diagnostic criteria for neurofibromatosis 2
| NIH criteria for NF2 | Manchester criteria | NNFF criteria | Baser criteria | ||
|---|---|---|---|---|---|
| Presentation age <30 years (points) |
Presentation age >30 years (points |
||||
| Bilateral 8th nerve masses seen by MRI with gadolinium | Bilateral vestibular schwannomas or | Confirmed definite NF2: | First-degree family relative with NF2 | 2 | 2 |
| A parent, sibling, or child with NF2 and either unilateral 8th nerve mass or any one of the following | Family history of neurofibromatosis type 2 and | Bilateral VS | Unilateral VS | 4 | 1 (if <70 years) |
| Neurofibroma | (1) Unilateral acoustic or | First-degree family relative with NF2 and unilateral VS, 30 years or any two of meningioma, glioma, schwannoma, juvenile lens opacity (posterior subcapsular cataract or cortical cataract) | Second VS | 2 | 3 (if <70 years) |
| Meningioma | (2) Any two of: meningioma, glioma, neurofibroma, schwannoma, posterior subcapsular lenticular opacities | Presumptive or probable NF2: | One meningioma | 2 | 1 |
| Glioma | (3) Unilateral VS and any two of neurofibroma, meningioma, glioma, schwannoma, or posterior subcapsular lenticular opacities | Unilateral VS <30 years and | Second meningioma | 2 | 1 |
| Schwannoma | (4) Multiple meningiomas (two or more) and unilateral VS or any two of neurofibroma, glioma, schwannoma or cataract | At least one of meningioma, glioma, schwannoma, juvenile lens opacity | Cutaneous schwannoma | 2 | 1 |
| Posterior capsular cataract or opacity at a young age | Multiple meningiomas (two or more) and unilateral VS <30 years or at least one of meningioma, glioma, schwannoma, juvenile lens opacity | Cranial nerve tumor; one or more; excluding VS | 2 | 1 | |
| Mononeuropathy | 2 | 1 | |||
| Cataract (one or more) | 2 | 0 | |||
| >6 points = definite NF2 | |||||
NF2, neurofibromatosis type 2; NNFF, National Neurofibromatosis Foundation; VS, vestibular schwannomas.
Vestibular schwannomas in neurofibromatosis type 2
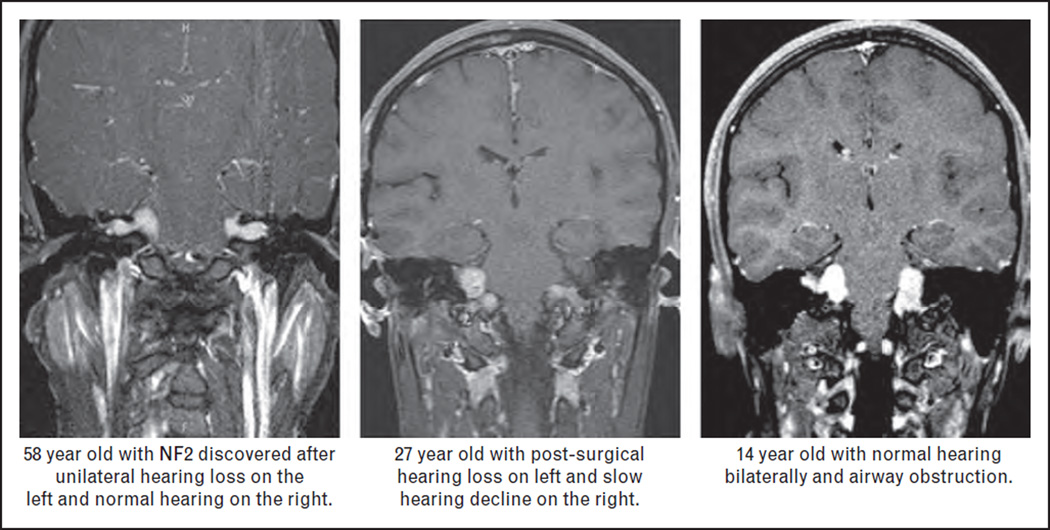
Although schwannomas can occur on any peripheral nerve (cranial, spinal, or distal), the pathognomonic finding of NF2 is bilateral vestibular schwannomas. These tumors are clinically challenging as they are often bilateral, may be part of ‘collision tumors’ resulting from intersection with neighboring schwannomas or meningiomas, they tend to grow in ‘clusters’ along the nerve rather than as a single schwannoma, and size does not correlate with clinical function [9]. The most frequent symptom associated with vestibular schwannomas is sensorineural hearing loss leading to complete deafness. However, vestibular schwannomas also commonly cause tinnitus and poor balance, and as they enlarge they involve the facial nerve causing facial palsy and impact the brainstem and lower cranial nerves. The morbidity related to NF2 vestibular schwannomas can be relatively mild and limited to unilateral hearing loss or severe, including early deafness, facial weakness, and lower cranial nerve and brainstem dysfunction (often resulting in poor chewing and swallowing requiring nonenteral nutrition and hemiparesis) (Fig. 1).
FIGURE 1.
Case examples illustrating the diversity in symptoms severity in patients with neurofibromatosis type 2 (NF2) ranging from unilateral hearing loss in a patient presenting in the 5th decade and lower cranial nerve dysfunction in a child. These cases also illustrate the lack of relationship between tumor size and hearing function in NF2.
Current standard therapy for patients with NF2-associated vestibular schwannomas is observation for radiographically stable tumors without symptoms. Surgery is the standard of care for progressive or symptomatic vestibular schwannomas [9]. In addition, there may be a role for early surgical resection with hearing preservation for some NF2 patients [10]. However, due to the large number of tumors in the brain and spinal cord in most patients with NF2, surgical removal of all tumors is not possible or advisable as there can be significant operative morbidity due to the location and nature of the tumors.
Radiation has been used in a subset of tumors that progress despite surgical treatment or in individuals who are considered high risk for surgical complications. However, this modality should be used with caution since the relationship between radiation therapy and secondary malignancy in patients with NF2 is not fully understood [11,12]. Moreover, the efficacy of radiosurgery for NF2-associated vestibular schwannomas appears to be modest with only 50% of patients having tumor control at 8 years and only 40% of patients having preserved hearing after radiotherapy at 3 years in the largest series reported for patients with NF2 [13]. Although surgery (and in some cases radiotherapy) is the mainstay of current therapy for NF2-associated vestibular schwannomas, the limitations of these approaches for patients with NF2 have prompted exciting developments in medical therapeutics for NF2-associated vestibular schwannomas. This review will discuss the major classes of drugs in development for NF2-associated vestibular schwannomas, the underlying biology that supports their clinical development, and the factors that influence the clinical pathway for rare tumor syndromes.
THERAPEUTIC TARGETS FOR NEUROFIBROMATOSIS TYPE 2-ASSOCIATED VESTIBULAR SCHWANNOMAS
The NF2 gene is located on chromosome 22q11.2 and encodes the protein Merlin [14]. Merlin is a ubiquitous protein that acts as a regulator of cell growth and cell–cell interactions, and is expressed across several cell types including schwann cells, meningeal cells, mesothelial cells, and lens cells [15]. Recent discoveries have shown that Merlin acts both at the cell membrane and in the nucleus [16,17].
At the cell membrane, Merlin has been shown to regulate multiple pathways implicated in tumorgenesis including: Ras/Raf/MEK/extracellular-signal-regulated kinases (ERK) [18], mTORC1 and 2 [19▪▪], Rac/p21-activated kinase/C-Jun kinase [20,21], PI3K/AKT [22], and Wnt/β-catenin [23]. All of these proposed sites of Merlin-directed regulation are potential therapeutic targets. In addition, the ability of Merlin to regulate the intranuclear E3 ubiquitin ligase CRL4 (DCAF1) suggests that decreasingDCAF1 is yet another target to modulate the pro-growth phenotype associated with inactive Merlin [16]. These recent works represent how complex the underlying molecular pathophysiology of NF2 is and suggest that there are multiple potential therapeutic targets (Table 2). However, translating these discoveries into effective therapies will require basic scientists and clinical scientists to work closely together to identify the targets that have the best portfolio for potential efficacy, tolerability and feasibility for the desired patient outcome.
Table 2.
Drugs in development for neurofibromatosis type 2
| Drug | Mechanism | Stage of development | Target tumor |
|---|---|---|---|
| Lapatinib | Oral small tyrosine kinase receptor inhibitor of EGFR/Erb2 | Phase 0 | Vestibular schwannoma |
| Phase 2 | |||
| RAD001 (Everolimus) | Oral mTOR inhibition | Phase 0 | Vestibular schwannoma |
| Phase 2 | Meningioma | ||
| Bevacizumab | Intravenous monoclonal antibody against vascular endothelial growth factor (VEGF) | Phase 2 | Vestibular schwannoma |
| Nilotinib | Oral multitarget kinase inhibitor including Bcr-Abl, c-kit, PDGFRbeta | Phase 0 | Cutaneous schwannomas |
| AR-42 | Oral histone deacetylase inhibitor | Phase 0 | Vestibular schwannoma |
| Phase 1 | Meningioma |
Pathways under investigation in clinical trials for neurofibromatosis type 2-associated vestibular schwannomas
The mammalian target of rapamycin (mTOR) is a tyrosine kinase that serves as a hub in the intracellular communication cascade integrating signals from multiple upstream pathways as well as the local intracellular environment. It belongs to two complexes: mTORC1 and mTORC2. mTORC1 is constitutively activated in Merlin-deficient schwannomas [24]. mTORC1 activation in turn leads to phosphorylation of ribosomal S6 kinase (S6K) and the eukaryotic initiation factor 4E binding protein-1 (4EBP-1), ultimately increasing translation, protein production, and cell growth [25]. Subsequent studies have shown decreased phosphorylation of downstream targets with mTOR inhibitors such as rapamycin. This and recent supporting preclinical animal data suggesting mTOR inhibition influences vestibular schwannomas cell proliferation have led to three ongoing clinical trials testing everolimus in patients with radiographic progression of NF2-associated vestibular schwannomas (NCT01345136, NCT01490476, NCT01419639). One study has completed enrollment and the other two are in active enrollment.
Everolimus (RAD001) is an attractive agent as there is strong preclinical rationale in NF2-specific models, a great deal of clinical experience with the drug from other disease states, and it is a relatively well tolerated and accessible drug. However, it is worth noting that recent data suggest that both mTORC1 and mTORC2 complexes are differentially deregulated in the setting of Merlin deficiency such that targeting both mTOR complexes with the newer mTORC1/mTORC2 inhibitors or with combined mTOR/PI3K inhibitors may be required for efficacy [19▪▪].
Epidermal growth factor receptor family receptors
The loss of Merlin protein in NF2 has been shown to result in abnormal activation of the epidermal growth factor receptor (EGFR) receptor tyrosine kinases (RTKs) proteins. The proteins implicated include EGFR, ErbB2, and ErbB3. These proteins all span the cell membrane and contribute to feed-back loops that regulate both cell death and cell division. When Merlin is inactive, EGFR, ErbB2, and ErbB3 remain constitutively active allowing increased cell proliferation and resistance to cell death [26]. In vitro, Merlin-deficient schwann cells with aberrant EGFR activation respond to EGFR inhibition [22,27]. EGFR is a common target across many solid tumors and hence, there are many available drugs for repurposing to address this signaling pathway in NF2-associated vestibular schwannomas. However, clinical experience with the oral EGFR inhibitor erlotinib did not result in tumor response in patients with NF2 [28]. This raised the questions of whether inhibition of other members of the EGFR family was more important for NF2-associated vestibular schwannomas growth regulation.
Lapatinib is an oral dual EGFR/ErbB2 inhibitor approved for use in breast cancer. In preclinical studies lapatinib was shown to have substantial inhibition of both cell proliferation and vestibular schwannomas growth [29,30]. On the basis of the favorable clinical profile of lapatinib (oral, well tolerated, known pharmacokinetics) and these preclinical results, it is now being investigated in two ongoing clinical studies in patients with NF2. In a phase 0 study, lapatinib is given to patients prior to planned tumor resection and at the time of surgery, the tissue is collected to assess both tumor drug concentration at steady state and assess the local effect of the drug via target phosphorylation assays (NCT00863122). The second study is a phase 2 study assessing the efficacy of daily lapatinib to stop progression of growing NF2-associated vestibular schwannomas (NCT00973739). The phase 2 study has completed enrollment, and results are expected in the near future. The phase 0 study remains open to accrual.
Antiangiogenesis pathways
Neoangiogenesis is a well recognized feature of all malignancies. However, the contribution of abnormal angiogenesis has recently been recognized in benign tumors as well and provides a novel therapeutic target for NF2-associated vestibular schwannomas. Vestibular schwannomas and peripheral schwannomas have been shown to express vascular endothelial growth factor (VEGF) in both tumor cells and associated endothelial cells [21,31]. In addition, radiographically vestibular schwannomas are known to have high uptake of gadolinium contrast suggesting high perfusion. Schwannomas have increased mean vessel density and perivascular cell coverage demonstrating an active angiogenesis phenotype with more and larger vessels and abnormal cellular and molecular features due to excessive VEGF [21]. Finally, recent studies indicated that Merlin also regulates angiogenesis via semaphorin 3F (SEMA3F), a protein that inhibits angiogenesis [32]. In the absence of Merlin, SEMA3F is down-regulated allowing angiogenesis [33▪▪]. These data suggest that the lack of Merlin in NF2 is related to abnormal angiogenesis via increased Rac expression.
On the basis of these observations, 10 NF2 patients at risk for complete hearing loss due to progressive vestibular schwannomas without other promising treatment options were offered bevacizumab through compassionate use [31]. The results were very promising with 6 of the 10 treated patients experiencing at least 20% reduction in tumor size as assessed with volumetrics [31,34]. Moreover, there was functional improvement in hearing such that four of the seven evaluable patients had improved word recognition scores. These clinical data combined with the pathological and preclinical data supporting a role for angiogenesis in NF2 vestibular schwannomas pathophysiology has led to two prospective clinical trials assessing the efficacy of bevacizumab in patients with NF2 and progressive hearing loss due to vestibular schwannomas.
The first study includes patients 14–70 years old with progressive hearing loss due to vestibular schwannomas. Bevacizumab is dosed at 7.5 mg/kg intravenously every 3 weeks for 12 months. Improvement in hearing function is the primary endpoint (NCT01207687). Importantly, this study includes continued clinical measures of hearing and tumor size off bevacizumab for 6 months in order to assess the duration of effect of bevacizumab. This is of clinical interest as bevacizumab has a long half life (up to 6 weeks) and it may be that less frequent dosing can be used for long-term maintenance of hearing in patients with NF2-associated progressive vestibular schwannomas. However, individual studies have indicated that the therapeutic effect with bevacizumab requires ongoing drug administration [35]. This study also includes plasma and radiographic biomarker data in an effort to identify which patients are likely to have clinical benefit from bevacizumab. Finally, quality of life data are collected before and after treatment. Enrollment is completed and results are expected in March 2013.
A second study will be focused exclusively on young patients aged 12–30 years with either growing tumor or progressive hearing loss. In this study, patients will be treated with an ‘induction’ dose followed by a maintenance dose for a total of 2 years. This study is planned to initiate enrollment in the fourth quarter of 2012. These two studies will provide the medical community with important data about the predictors of response to bevacizumab, drug tolerability over long treatment intervals, and the differential effects of various dosing strategies in patients with NF2 and progressive vestibular schwannomas. Lastly, there is an ongoing trial assessing the local delivery of bevacizumab via inter-arterial catheterization to vestibular schwannomas. An initial study suggests this is feasible and well tolerated; however, outcome data are not yet available [36].
Although there is tremendous enthusiasm for the success in hearing preservation and restoration with bevacizumab in patients with NF2-associated progressive vestibular schwannomas, this therapy must be used with caution. Bevacizumabis associated with rare, but well recognized toxicities including thrombosis, hemorrhage, and visceral proliferation, as well as longer-term less serious effects including hypertension and proteinuria [37]. Hence, ideally patients should be treated with bevacizumab only on clinical trials or if there are no viable treatment options due to high surgical risk for morbidity. In all instances, bevacizumab should be administered in a center with experience with both bevacizumab and NF2.
Ras and downstream pathways
Ras represents a family of proteins responsible for intracellular communication. Ras proteins are frequently altered in the setting of tumors. Merlin has been shown to regulate the interaction of Ras and various growth factors [38]. As presented above, in the past year, multiple targets in the Ras pathway have been implicated as part of the pathogenesis of NF2-associated tumors. Some of these discoveries are now being translated into clinical trials.
Sorafenib is an oral multitarget inhibitor of several targets downstream from Ras including Raf/Mek/Erk as well as platelet-derived growth factor (PDGF), VEGF, and c-kit. A phase 0 investigation of sorafenib patients with NF2 is ongoing to assess if the preclinical biologic markers of efficacy are seen in patients in order to justify a larger efficacy study. Similar to sorafenib, nilotinib is a receptor tyrosine kinase inhibitor of breakpoint cluster region-abelson that also targets PDGF receptor and c-kit. It has also shown promising NF2 in vitro and is entering a phase 0 trial with a similar design as described for sorafenib [39]. This includes a small number of patients (15) receiving 2 weeks of oral nilotinib to assess the biologic effect of nilotinib in tumors. The results of this study will help decide if the commitment should be made for an efficacy study of nilotinib in NF2.
Additional therapies in development of vestibular schwannomas
Curcumin is a natural herb that has been proposed to have anticancer properties. It has long been proposed as a therapy for NF2, but only recently is there evidence to support a mechanism of action. Curcumin monotherapy inhibited growth of schwannoma cells and induced apoptosis. Interestingly, in this setting, hsp70 was up-regulated [40▪▪]. Further, combination therapy with curcumin and the hsp90 inhibitor KNK437 worked together to suppress cell growth in schwannomas, suggesting that combination therapy with hsp90 inhibitors and curcumin may be a promising strategy for schwannomas.
The phosphoiniositide (PI)3-kinase/Akt pathway is dysregulated in NF2 and may be an important therapeutic target. The recently developed histone deacetylase (HDAC) inhibitor AR42 works in part via PI3K/Akt inhibition. Preclinical studies with AR42 in schwannoma cells show decreased phosphor-Akt, apoptosis, and decreased proliferation [41]. These promising preclinical studies have prompted a phase 1 study of AR42 in which patients with NF2 and progressive tumors are encouraged to enroll (NCT01129193).
Given the number of possible therapeutic targets affected by a lack of functional Merlin, an interesting development is the enrollment of patients with NF2 with symptomatic tumors on various phase 1 trials with rationally targeted therapies. This has allowed evaluation of Ras, MEK, EGFR, HDAC, mTOR, and VEGF inhibitors across a series of patients [42].
CONCLUSION
A tremendous amount of work has been produced in the past year about the mechanisms by which loss of Merlin in the setting of NF2 influences vestibular schwannomas proliferation. Some of this work has been rapidly translated into clinical trials. Although there is not yet an approved drug therapy for NF2-associated vestibular schwannomas, the continued close collaboration between basic and clinical scientists, as well as the increasing insights into the multiple pathways that regulate tumor growth in NF2-associated vestibular schwannomas are likely to yield a range of therapies in the next 10 years. Careful consideration of the optimal therapies for individual patients and the long-term requirements for treatment will be required to screen drugs for consideration as more potential therapies become available for consideration.
KEY POINTS.
Neurofibromatosis type 2 (NF2)-associated vestibular schwannomas are caused by loss of function of the tumor-suppressor Merlin.
Merlin has multiple actions both at the cell membrane and in the nucleus.
Early clinical efficacy has been observed with the antiangiogenesis therapy bevacizumab in patients with NF2 and progressive vestibular schwannomas. This has resulted in two prospective efficacy studies assessing bevacizumab for patients with NF2 that are ongoing.
Small tyrosine kinase inhibitors of targets in the Ras and Rac pathway are also in active clinical evaluation.
There are currently no approved therapies for progressive or symptomatic vestibular schwannomas in NF2, but there are several promising therapies in active development.
Acknowledgments
None.
Conflicts of interest
J.B. is receiving nonsalary research support from GlaxoSmithKline, Lily, and Sanofi for ongoing research projects. There is ongoing grant support from Children’s Tumor Foundation, the Conquer Cancer Foundation of the American Society for Clinical Oncology, and the Cancer Therapy Evaluation Program of the National Cancer Institute.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 421–422).
- 1.Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26:93–97. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, Raymond FL, Barwell JG, Halliday D. Genetic testing and screening of individuals at risk of NF2. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01816.x. [DOI] [PubMed] [Google Scholar]
- 3.Dirks MS, Butman JA, Kim HJ, et al. Long-term natural history of neurofibromatosis Type 2-associated intracranial tumors. J Neurosurg. 2012;117:109–117. doi: 10.3171/2012.3.JNS111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otsuka G, Saito K, Nagatani T, Yoshida J. Age at symptom onset and long-term survival in patients with neurofibromatosis Type 2. J Neurosurg. 2003;99:480–483. doi: 10.3171/jns.2003.99.3.0480. [DOI] [PubMed] [Google Scholar]
- 5.Wilding A, Ingham SL, Lalloo F, et al. Life expectancy in hereditary cancer predisposing diseases: an observational study. J Med Genet. 2012;49:264–269. doi: 10.1136/jmedgenet-2011-100562. [DOI] [PubMed] [Google Scholar]
- 6.Acoustic neuroma. Consens Statement. 1991;9:1–24. [PubMed] [Google Scholar]
- 7.Baser ME, Friedman JM, Wallace AJ, et al. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59:1759–1765. doi: 10.1212/01.wnl.0000035638.74084.f4. [DOI] [PubMed] [Google Scholar]
- 8. Baser ME, Friedman JM, Joe H, et al. Empirical development of improved diagnostic criteria for neurofibromatosis 2. Genet Med. 2011;13:576–581. doi: 10.1097/GIM.0b013e318211faa9. Provides a review and analysis of the existing diagnostic criteria and presents the data to support a new diagnostic schema that incorporates age at presentation, mosaic forms of NF2, and genetic testing.
- 9.Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slattery WH, 3rd, Fisher LM, Hitselberger W, et al. Hearing preservation surgery for neurofibromatosis Type 2-related vestibular schwannoma in pediatric patients. J Neurosurg. 2007;106:255–260. doi: 10.3171/ped.2007.106.4.255. [DOI] [PubMed] [Google Scholar]
- 11.Evans DGR, Birch JM, Ramsden RT, et al. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43:289–294. doi: 10.1136/jmg.2005.036319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460–468. doi: 10.1227/01.NEU.0000255340.26027.53. [DOI] [PubMed] [Google Scholar]
- 13.Rowe J, Radatz M, Kemeny A. Radiosurgery for type II neurofibromatosis. Prog Neurol Surg. 2008;21:176–182. doi: 10.1159/000156907. [DOI] [PubMed] [Google Scholar]
- 14.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 15.McClatchey AI, Giovannini M. Membrane organization and tumorigenesis: the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- 16.Cooper J, Li W, You L, et al. Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to suppress oncogenic gene expression. Sci Signal. 2011;4:t6. doi: 10.1126/scisignal.2002314. [DOI] [PubMed] [Google Scholar]
- 17.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi C, Troutman S, Fera D, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–540. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. James MF, Stivison E, Beauchamp R, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10:649–659. doi: 10.1158/1541-7786.MCR-11-0425-T. Elegant presentation of data demonstrating the role of Merlin in regulating the mTORC1 and mTORC2 complexes and the implications for therapeutics for NF2- associated schwannomas and meningiomas.
- 20.Hennigan RF, Moon CA, Parysek LM, et al. The NF2 tumor suppressor regulates microtubule-based vesicle trafficking via a novel Rac, MLK and p38 (SAPK) pathway. Oncogene. 2012:1–9. doi: 10.1038/onc.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong HK, Lahdenranta J, Kamoun WS, et al. Antivascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis- related tumors. Cancer Res. 2010;70:3483–3493. doi: 10.1158/0008-5472.CAN-09-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair KJ, Kiang A, Wang-Rodriguez J, et al. EGF and bFGF promote invasion that is modulated by PI3/Akt kinase and Erk in vestibular schwannoma. Otol Neurotol. 2011;32:308–314. doi: 10.1097/MAO.0b013e318206fc3d. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Ercolano E, Ammoun S, et al. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–1112. doi: 10.1593/neo.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta-Jaquez HA, Keller JA, Foster KG, et al. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol. 2009;29:4308–4324. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curto M, Cole BK, Lallemand D, et al. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad ZK, Brown CM, Cueva RA, et al. ErbB expression, activation, and inhibition with lapatinib and tyrphostin (AG825) in human vestibular schwannomas. Otol Neurotol. 2011;32:841–847. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro Oncol. 2010;12:834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acevedo LM, Barillas S, Weis SM, et al. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–2680. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong HK, Shimizu A, Kirkpatrick ND, et al. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/semaphorin 3F-dependent mechanism. Neoplasia. 2012;14:84–94. doi: 10.1593/neo.111600. Important data further elucidating the mechanism by which Merlin dysfunction modulates abnormal angiogenesis and the therapeutic implications.
- 34.Harris GJ, Plotkin SR, Maccollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62:1314–1319. doi: 10.1227/01.neu.0000333303.79931.83. discussion 1319–1320. [DOI] [PubMed] [Google Scholar]
- 35.Mautner VF, Nguyen R, Knecht R, Bokemeyer C. Radiographic regression of vestibular schwannomas induced by bevacizumab treatment: sustain under continuous drug application and rebound after drug discontinuation. Ann Oncol. 2010;21:2294–2295. doi: 10.1093/annonc/mdq566. [DOI] [PubMed] [Google Scholar]
- 36.Riina HA, Burkhardt JK, Santillan A, et al. Short-term clinico-radiographic response to super-selective intra-arterial cerebral infusion of bevacizumab for the treatment of vestibular schwannomas in neurofibromatosis type 2. Interv Neuroradiol. 2012;18:127–132. doi: 10.1177/159101991201800201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Product Information. Product Information AVASTIN® IV infusion, bevacizumab IV infusion. South San Francisco, CA: Genentech, Inc; 2009. [Google Scholar]
- 38.Morrison H, Sperka T, Manent J, et al. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–527. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 39.Ammoun S, Schmid MC, Triner J, et al. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. 2011;13:759–766. doi: 10.1093/neuonc/nor056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angelo LS, Wu JY, Meng F, et al. Combining curcumin (diferuloylmethane) and heat shock protein inhibition for neurofibromatosis 2 treatment: analysis of response and resistance pathways. Mol Cancer Ther. 2011;10:2094–2103. doi: 10.1158/1535-7163.MCT-11-0243. Initial data about the possible mechanism of action of curcumin, used widely in the community as an herbal therapy, and the possibility of a therapeutic regimen with hsp90 inhibitors.
- 41.Bush ML, Oblinger J, Brendel V, et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro Oncol. 2011;13:983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subbiah V, Slopis J, Hong DS, et al. Treatment of patients with advanced neurofibromatosis type 2 with novel molecularly targeted therapies: from bench to bedside. J Clin Oncol. 2012;30:e64–e68. doi: 10.1200/JCO.2011.38.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]