We investigated the effect of stressors associated with offshore reef-based tourist platforms on the coral immune system. Our findings suggest that stressors associated with platform-related activities, in synergy with seasonal factors, compromise the coral immune system, contributing to the higher coral disease prevalence observed at these locations.
Keywords: coral, disease, GFP-like proteins, immunity, phenoloxidase, tourism
Abstract
Unravelling the contributions of local anthropogenic and seasonal environmental factors in suppressing the coral immune system is important for prioritizing management actions at reefs exposed to high levels of human activities. Here, we monitor health of the model coral Acropora millepora adjacent to a high-use and an unused reef-based tourist platform, plus a nearby control site without a platform, over 7 months spanning a typical austral summer. Comparisons of temporal patterns in a range of biochemical and genetic immune parameters (Toll-like receptor signalling pathway, lectin–complement system, prophenoloxidase-activating system and green fluorescent protein-like proteins) among healthy, injured and diseased corals revealed that corals exhibit a diverse array of immune responses to environmental and anthropogenic stressors. In healthy corals at the control site, expression of genes involved in the Toll-like receptor signalling pathway (MAPK p38, MEKK1, cFos and ATF4/5) and complement system (C3 and Bf) was modulated by seasonal environmental factors in summer months. Corals at reef platform sites experienced additional stressors over the summer, as evidenced by increased expression of various immune genes, including MAPK p38 and MEKK1. Despite increased expression of immune genes, signs of white syndromes were detected in 31% of study corals near tourist platforms in the warmest summer month. Evidence that colonies developing disease showed reduced expression of genes involved in the complement pathway prior to disease onset suggests that their immune systems may have been compromised. Responses to disease and physical damage primarily involved the melanization cascade and GFP-like proteins, and appeared to be sufficient for recovery when summer heat stress subsided. Overall, seasonal and anthropogenic factors may have interacted synergistically to overwhelm the immune systems of corals near reef platforms, leading to increased disease prevalence in summer at these sites.
Introduction
Increasing evidence that coral disease epizootics are causing significant declines in coral cover and degradation of coral reefs (Porter et al., 2001; Gardner et al., 2003; Osborne et al., 2011) suggests that coral immune systems are being overwhelmed by a combination of both anthropogenic and naturally occurring environmental disturbances at local and global scales. Teasing apart the roles of climate change-related environmental factors, such as warming and acidifying oceans, in disease causation from other anthropogenic disturbances, such as sedimentation, sewage disposal or eutrophication caused by agricultural run-off (Bruno et al., 2007; Harvell et al., 2007; Lesser et al., 2007; Haapkylä et al., 2011; Sutherland et al., 2011; Ruiz-Moreno et al., 2012; Redding et al., 2013; Lamb et al. 2014; Pollock et al. 2014), is key to understanding current challenges facing coral immune systems. A report of 15-fold greater prevalence of coral disease near permanent tourist reef platforms compared with adjacent reefs without such platforms (Lamb and Willis, 2011) suggests that such sites are ideal microcosms for characterizing responses of the coral innate immune system. With coral-reef based tourism becoming one of the fastest growing tourism sectors worldwide (Ong and Musa, 2011), determining whether differential coral immune responses can distinguish among anthropogenic and environmental drivers of reduced coral health would represent a significant step forward in the development of effective coral reef management and conservation strategies for the tourism industry.
Corals have a large repertoire of innate immune defence mechanisms available to maintain fitness and defend against biotic and abiotic stressors. Three of these have been relatively well documented, as described in further detail below: (i) the Toll-like receptor (TLR) pathway; (ii) the melanization cascade; and (iii) the complement system. However, the manner in which these immune mechanisms respond to different environmental and anthropogenic impacts is relatively unexplored.
Toll-like receptors are activated following the detection of microbial components (microbe-associated molecular patterns) and subsequently activate various signal transduction pathways [e.g. c-Jun N-terminal kinase (JNK), mitogen-activated protein kinase (MAPK) p38 and nuclear factor-κB (NF-κB) pathways], which regulate the expression of target genes involved in immunity and cell survival, thereby orchestrating the immune response. Recent molecular studies of the coral innate immune system have identified a large number of genes encoding TLRs and proteins involved in the downstream signalling pathways (Miller et al., 2007; Shinzato et al., 2011; Hamada et al., 2013); however, functional studies of the TLR signalling pathways in corals are limited.
The melanization cascade, or prophenoloxidase (proPO)-activating system (Mydlarz et al., 2008; Palmer et al., 2008), is a rapidly induced mechanism activated in response to microbe-associated molecular patterns (Cerenius et al., 2010). The capacity to activate this system within minutes, leading to the production of a hostile cellular environment and, ultimately, to the deposition of melanin that immobolizes microbes (Cerenius et al., 2010), suggests that this immune mechanism may be directed primarily at events requiring a rapid response, such as pathogen invasion and injury. Significant correlations between phenoloxidase (PO) activity levels and disease resistance in various invertebrates, including corals, plus evidence of the major role that the proPO-activating system plays in the disease response and wound-healing process (Palmer et al., 2010, 2011a, c), corroborates this interpretation. Although the biological function of the melanization cascade in corals has been studied extensively, the impacts of stressors other than elevated seawater temperatures are still unknown.
The complement system is another effector mechanism involved in the direct elimination of invading microbes, primarily via promoting phagocytosis and inducing the formation of the membrane attack complex. Key components of the complement system, including complement C3, Factor B (Bf), lectins and mannose-binding lectin-associated serine protease, are present in many invertebrates (Mydlarz et al., 2006; Cerenius et al., 2010). In corals, lectins and C3 have been implicated in the antibacterial and wounding response (Kvennefors et al., 2008, 2010; Brown et al., 2013), and membrane attack complex/perforin domain-containing genes have been identified (Miller et al., 2007). How this immune mechanism is affected by environmental and anthropogenic factors, however, remains to be elucidated in corals.
Elevated seawater temperatures are known to reduce immunocompetence, which is the ability of an organism to exhibit an immune response, in several coral species (Palmer et al., 2011a, b; Vidal-Dupiol et al., 2014). In other invertebrate systems, changes in salinity and elevated levels of nutrients or pollutants are also known to reduce immune system function, resulting in increased disease-related mortality (Coteur et al., 2001; Reid et al., 2003; Cheng et al., 2004a, b; Liu and Chen, 2004; Tseng and Chen, 2004; Danis et al., 2006; Li et al., 2010; Ellis et al., 2011). Higher prevalence of coral disease on reefs near permanent offshore tourist platforms than at reefs without such facilities (Lamb and Willis, 2011) suggests that platform-associated stressors also affect coral immunocompetence and, potentially, coral-associated microbial communities. As healthy bacterial communities are essential to the functioning of the coral holobiont, playing important roles in nutrient cycling (Raina et al., 2009; Lema et al., 2012) and protection from pathogens (Ritchie, 2006; Shnit-Orland and Kushmaro, 2009; Teplitski and Ritchie, 2009; Alagely et al., 2011), any changes in the structure of bacterial communities may signify that immune systems of corals living near tourist platforms are compromised.
In this study, we monitored colonies of the reef-building coral Acropora millepora near tourist platforms and control sites over a summer season to establish baseline levels of a suite of immune parameters in corals in undisturbed environments and to assess the effects of platforms on coral immunocompetence based on temporal patterns in immune protein levels and gene expression. The occurrence of both injury (associated with snorkelling and diving activities) and disease at platform sites, coupled with the recovery of injuries and lesions as warm summer temperatures subsided, enabled us to compare the functional responses of a range of immune parameters to both anthropogenic and environmental factors.
Materials and methods
Study site and sample collection
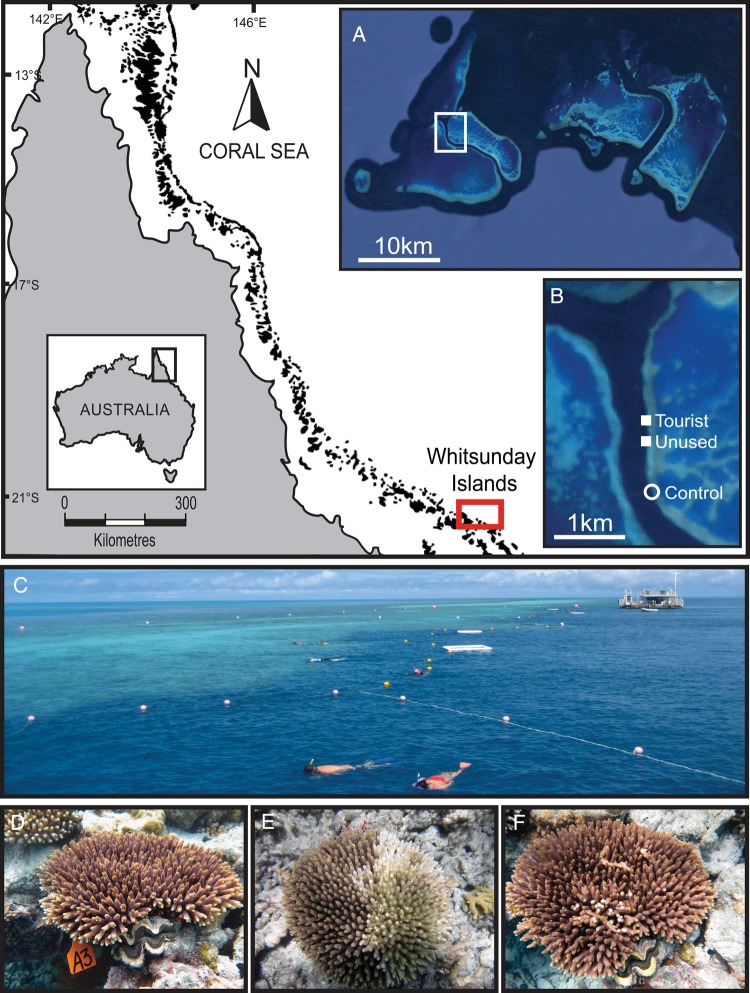
The study was conducted at Hardy Reef (19°44′33″S, 149°10′57″E) on the Great Barrier Reef of Australia, where two offshore platforms are permanently moored between November 2010 and June 2011 (Fig. 1A). The first platform (45 m × 12 m) was in use at the time of the study as the primary vessel mooring pontoon and could accommodate up to 400 visitors and associated recreational activities per day (study site 1; tourist platform), although such usage is significantly more than the recommended carrying capacity of 5000 recreational users per year at a single reef site (Hawkins and Roberts, 1997). A second platform (24 m × 10 m), which had not been used by tourists for a year prior to the study, was located 400 m south of the main tourist platform (study site 2; unused platform) (Fig. 1B and C). The platforms are located 5 m from the reef crest, and large numbers of seabirds rest on both platforms throughout the year. In addition, a control site (study site 3) was established 800 m south of the unused platform in similar reef habitat (Fig. 1B).
Figure 1:
Maps showing the location of Hardy Reef (A), located 75 km offshore within the central sector of the Great Barrier Reef, Australia, and three study sites (B): a high-use visitor platform, an unused visitor platform and a control site with no platform. The unused platform is situated ∼300 m south of the high-use platform (C), and the control site lies an additional 800 m south of the unused platform. Photographs showing representative images of healthy (D), diseased (white syndrome; E) and damaged colonies (F) of the coral Acropora millepora in January 2011.
Eight visually healthy colonies of similar size of A. millepora, a model coral species widely used in physiological and genomic studies, were tagged using plastic cattle tags and cable ties at 2–3 m depth at each of the three study sites. Colonies were sampled at the following six time points: November (late austral spring), December (early austral summer), January (austral summer), February (austral summer), March (late austral summer) and June (early austral winter). At each sampling time point, the health status of the tagged coral colonies was visually assessed and categorized as healthy, damaged (branches recently broken, with exposed skeleton) or diseased (signs of the coral disease white syndrome, as per Beeden et al. 2008; Fig. 1D–F). One branch (∼5 cm in length) was sampled from midway between the centre and the edge of each tagged colony, at each of the six time points during the study. For diseased and damaged colonies, an apparently healthy portion of each branch was sampled ∼1 cm from the lesion boundary or damaged area. In all instances, the disease lesion was radiating from the centre of the colony. A photograph of each tagged colony was taken before and after each sample was collected. Samples were collected in the same order for all time points, and the sampling took ∼2 h to complete. Samples were immediately snap-frozen in liquid nitrogen and stored at −80°C.
Messenger RNA isolation
Frozen samples of A. millepora were crushed in a liquid nitrogen-chilled, stainless-steel mortar and pestle using a hydraulic press. Messenger RNA was isolated from ∼100 mg of crushed coral using the Dynabeads mRNA DIRECT kit (Invitrogen Dynal AS, Oslo, Norway) according to a modified protocol based on the manufacturer’s recommendations (van de Water et al., 2015a). In short, crushed coral was added to 400 µl lysis buffer, incubated on a vortex at low speed for 7 min and centrifuged for 2 min at 12 000g. Supernatant was added to prewashed oligo(dT)-Dynabeads and incubated on the vortex at medium speed for 8 min to allow mRNA annealing. Tubes were placed on a DynaMag-2 magnetic particle concentrator for 5 min, and supernatant was removed. Using the DynaMag-2, oligo(dT) Dynabead–mRNA complexes were washed twice with 300 µl of Buffer A and subsequently twice with 400 µl of Buffer B. Complexes were resuspended in 27 µl ice-cold 10 mM Tris–HCl, incubated at 80°C for 2 min and rapidly cooled down on ice. Oligo(dT)-Dynabeads were concentrated on the DynaMag-2, and mRNA-containing supernatant was collected and stored at −80°C until use.
Gene expression analysis
Expression levels of 17 immune system-related genes and four reference genes (Seneca et al., 2010; Siboni et al., 2012) were analysed using the GenomeLab GeXP Start Kit and the CEQ-8800 Genetic Analysis System (Beckman-Coulter, Brea, CA, USA) following the protocol described by Siboni et al. (2012) and van de Water et al. (2015a) with some modifications. A description of all genes and primer sequences can be found in Supplementary Table S1. For each sample, cDNA was generated from 6.7 ng of mRNA. Forward primer concentrations were 200 nM. Reverse primer concentrations were optimized for the multiplex to ensure that signals in the electropherogram were within the CEQ-8800 detection range, as follows: 2 µM for CTL2; 1 µM for MEKK1, GAPDH, Bf, MAPK p38, ctg_1913, RPL9, CTL1, HL1 and Apextrin; 500 nM for TRAF6, TIR-1, C3/A2M-2, ERK-2, Millectin, HL2 and cJun; 62.5 nM for ATF4/5; 25 nM for cFos; 12.5 nM for NFκB; and 23 pM for RPS7. The PCR products were diluted 1:20 prior to loading on the CEQ-8800 Genetic Analysis System. Data were filtered and analysed using the GeXP Fragment Analysis and eXpress Profiler software packages (Beckman-Coulter). Gene expression levels were normalized to an internal control (KanR) and to the geometric mean of the expression levels of the three most stable reference genes (RPS7, RPL9 and ctg_1913) selected using geNorm (Vandesompele et al., 2002). Results were obtained for three independent technical replicates per sample.
Phenoloxidase activity
Phenoloxidase activity was assayed according to procedures outlined by Palmer et al. (2011a), with some modifications. Both total potential (trypsin-activated) phenoloxidase (tpPO; van de Water et al., 2015b) activity and PO activity were measured to analyse the total capacity and the active fraction of the proPO system, respectively, in each sample. To analyse tpPO activity, 20 µl of coral tissue lysate was loaded in triplicate into wells of a 96-well plate, to which Tris-buffered saline (50 mM, pH 7.8; 40 µl) and trypsin (25 µl 0.1 mg/ml) were added. Reaction mixtures were incubated for 20 min to allow for activation of proPO by trypsin, and then 30 µl of 10 mM dopamine hydrochloride (Sigma-Aldrich, St Louis, MO, USA) was added to each mixture. As a blank, 20 µl of extraction buffer was used. The same procedure was followed to analyse PO activity, except that 25 µl double distilled water was substituted for the trypsin solution. Absorbance was measured at 490 nm every 5 min for 45 min using the SpectraMax M2 (Molecular Devices, Sunnyvale, CA, USA). Data for each sample were independently obtained in triplicate. Phenoloxidase activity was calculated as the change in absorbance using the linear portion of the reaction curve over time and standardized to the total protein content of each sample.
Chromoprotein and fluorescent protein expression
Twenty microlitres of tissue lysate was added to each well of a black, clear-bottomed 384-well plate in triplicate for each sample. Expression of chromoprotein (CP) was analysed by measuring the absorbance at 588 nm using a SpectraMax M2. The fluorescence spectrum was analysed by measuring emission wavelengths between 400 and 700 nm, with a 5 nm resolution, emitted upon excitation of fluorescent proteins (FPs) at 280 nm. All data were normalized to total protein content. Fluorescence spectra and fluorescent protein expression levels were calculated in R using the method described by van de Water et al. (2015b). In summary, the exponentially decaying background scatter was subtracted from each spectrum between 445 and 645 nm, and multiple regression models based on purified FP spectra were fitted to the data to calculate the proportions of the individual fluorescent proteins [cyan (CFP), green (GFP) and red fluorescent protein (RFP)] present.
Environmental parameters
Daily water temperature, rainfall accumulation, light intensity and wind speed data were collected by the Australian Institute of Marine Science (AIMS) monitoring station located at the main tourist platform (data available from http://data.aims.gov.au/aimsrtds/datatool.xhtml). The means for each environmental parameter were calculated using daily values from a 14 day period including and immediately preceding each month’s sampling date (Supplementary Fig. S1).
Statistical analyses
Temporal patterns of individual gene and protein parameters in control site corals were analysed using a linear mixed effect model, with the random effect ‘colony’ as group variable, the response variable ‘immune parameter’ as a dependent variable, and time as an independent fixed effect. Outcomes were adjusted for multiple comparisons by a Tukey’s honest significant difference test. Analysis of variance (ANOVA) was used to test for differences in immune parameters between corals at the control site and corals with the following characteristics: (i) were healthy near the tourist platform; (ii) were healthy near the unused platform; (iii) sustained damage; and (iv) developed disease, followed by a Fisher’s least significant difference test. Because of low levels of gene expression resulting in a value of zero in a few cases, data sets with >20% zeros were tested for differences in the proportion of zero values across sites and health conditions using logistic regression models, and differences in expression of samples with expression values greater than zero between sites and health conditions using a linear mixed effect model. All analyses were conducted using the environment for statistical computing R (version 3.1.1). Data were considered significant when P < 0.05 or when the 95% confidence interval excluded zero.
Results
Coral health assessment
At the control site, all eight tagged colonies remained visually healthy and undamaged throughout the study period (from November to June; Fig. 1D). Colonies at the platform sites (i.e. the primary tourist platform and the unused platform) were visually healthy at the start of the study in November and December. In January, five colonies near the platforms (one at the tourist platform and four at the unused platform) developed macroscopic signs of white syndrome (WS), the collective name for a group of tissue-loss diseases (Willis et al., 2004), and sustained ∼40–50% partial colony mortality (Fig. 1E). In addition, three of the tagged colonies at the tourist platform sustained severe physical damage, with many broken branches on each colony, consistent with reef-based diving and snorkelling activities (Fig. 1C and F); corals at the unused platform remained undamaged. In February, lesions associated with the damaged corals recovered, and WS lesions ceased progressing in all but one colony (at the unused platform site). By March, lesions on all WS-affected colonies had healed, although areas of partial colony mortality remained. In June, all colonies were visually healthy.
Temporal changes in the immune system of corals at the control site
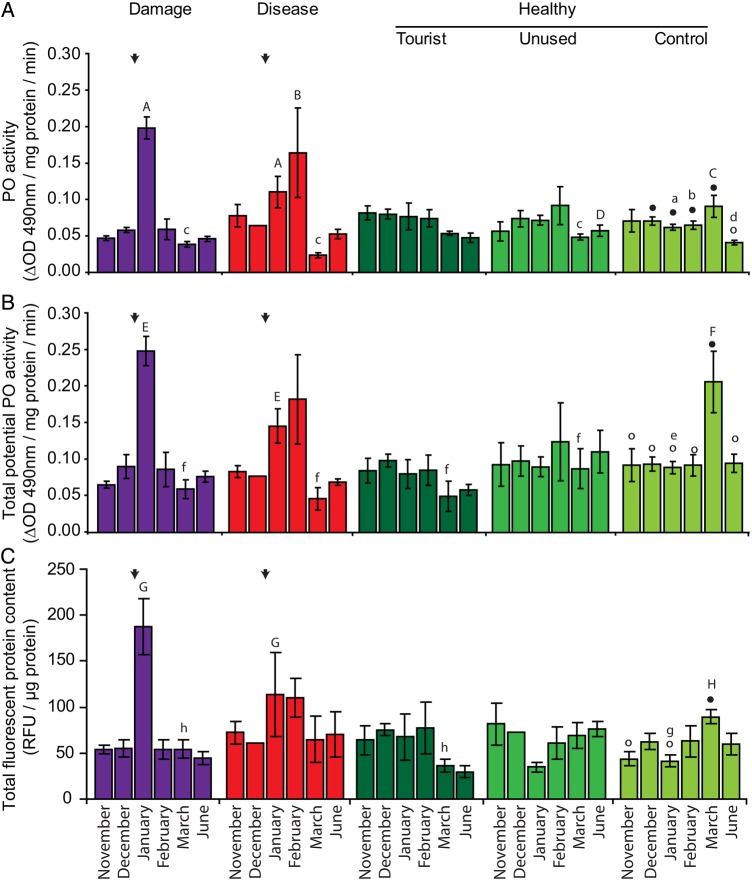
Although corals at the control site all remained healthy, expression levels of most immune parameters fluctuated significantly over the 7 months of the study. Four main patterns in the immune response of these healthy control corals were detected. First, mean PO and tpPO activity and total FP concentration (and all component FP classes, i.e. CFP, GFP and RFP) increased in March compared with levels in the previous summer months, although this increase was not significant for PO activity (Fig. 2A–C; Supplementary Fig. S2A–C). Second, we observed increased expression in the complement system’s Factor B (Bf ) gene in summer months (Fig. 3B) and a similar trend for C3 (Supplementary Fig. S3B; November to February, P = 0.0652), as well as ATF4/5, which is involved in the TLR pathway (Fig. 3A). The third pattern observed was that various genes involved in the TLR signalling pathway were expressed at lower levels in summer compared with other months, including MEKK1 and MAPK p38 (Fig. 3E and F), while similar trends were found for cFos (Fig. 3C; November to December, P = 0.0816; November to January, P = 0.0652; November to February, P = 0.0594) and TRAF6 (Supplementary Fig. S3K; November > December, P = 0.0697). Finally, several parameters showed a pattern of reduced expression or activity in winter (June) compared with all other months. These parameters were PO activity and HL-1 and NF-κB expression (Figs 2A and 3D; Supplementary Fig. S3I). All other parameters showed some temporal fluctuations in expression, but no clear patterns could be discerned. Details of the statistical significance of each comparison can be found in Supplementary File S1 and Supplementary Table S2.
Figure 2:
Comparative temporal patterns in phenoloxidase activities and total fluorescence levels in A. millepora at Hardy Reef, central Great Barrier Reef. Patterns are compared among corals that were healthy at three study sites (tourist platform, unused platform and control site) and those that were damaged or diseased at platform sites, for phenoloxidase (PO; A) and total potential phenoloxidase (tpPO) activity (B), and total fluorescence levels (C). Data are grouped by health status, with healthy corals split up by study location. Arrows indicate when disease and damage occurred. Letters indicate means that differ significantly from the corresponding mean at the control site, where upper case letters (A–H) denote the significantly higher mean in the comparison and lower case letters (a–h) denote the significantly lower mean. For temporal patterns in control corals, symbols (filled or open circles) denote means that differ significantly from means with the other symbol. Results were considered significant when P < 0.05 or 95% confidence interval excluded zero.
Figure 3:
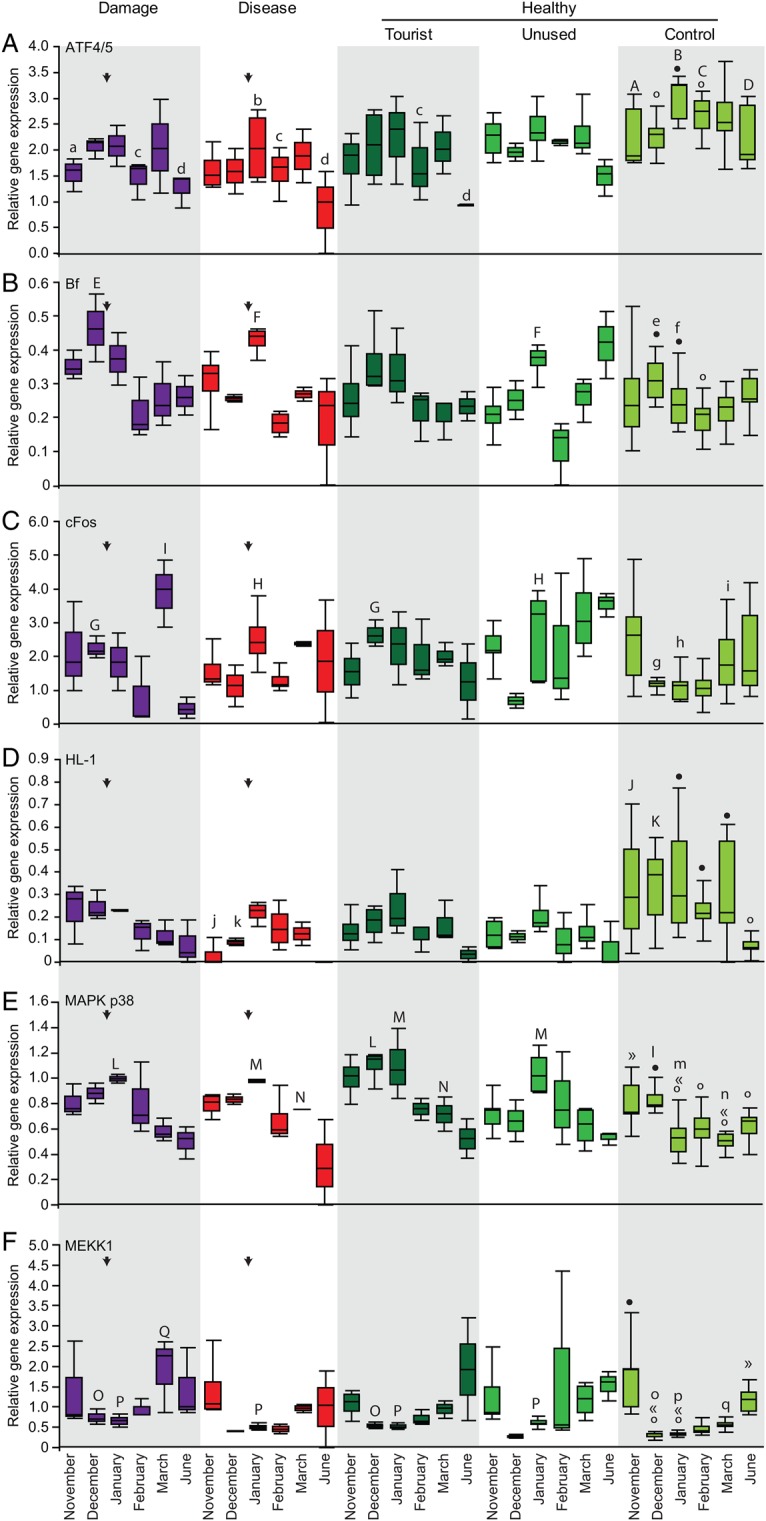
Comparative temporal patterns in immune gene expression levels in A. millepora at Hardy Reef, central Great Barrier Reef. Patterns are compared among corals that were healthy at three study sites (tourist platform, unused platform and control site) and those that were damaged or diseased at platform sites, for ATF4/5 (A), Bf (B), cFos (C), HL-1 (D), MAPK p38 (E) and MEKK1 (F). Data are grouped by health status, with healthy corals split up by study location. Arrows indicate when disease and damage occurred. Letters indicate means that differ significantly from the corresponding mean at the control site, where upper case letters (A–Q) denote the significantly higher mean in the comparison and lower case letters (a–q) denote the significantly lower mean. For temporal patterns in control corals, symbols (filled and open circles, or « and ») denote means that differ significantly from means with the other symbol. Results were considered significant when P < 0.05 or 95% confidence interval excluded zero.
The effect of anthropogenic disturbances associated with reef platforms on the immune system of corals
In general, temporal patterns in the immune parameters analysed for healthy corals near reef platforms followed profiles similar to those of healthy corals at the control site. In March, however, tpPO activity was reduced in corals at both platform sites in comparison to control corals (Fig. 2B). Total FP tissue concentrations were reduced only in corals at the tourist platform (Fig. 2C), which was attributed to reduced GFP and RFP expression at this site, although RFP expression was also reduced at the unused platform in March. In June, we observed an increase in PO activity at the unused platform (Fig. 2A), while the expression of GFP was reduced at the tourist platform (Supplementary Fig. S2B). Corals near platforms showed significant increases in the expression of genes involved in the TLR signalling pathway in summer. MEKK1 and MAPK p38 were upregulated at both platform sites (Fig. 3E and F), as well as Bf at the unused platform in January (Fig. 3B). In addition, these genes and the transcription factors cFos and cJun were upregulated at the tourist platform in December (Fig. 3C; Supplementary Fig. S3C), while these corals had lower ATF4/5 expression (Fig. 3A) and total fluorescence levels (Fig. 2C) in February and March, respectively.
The putative Toll-like receptor TIR-1 (Supplementary Fig. S3J) was present in a relatively small portion of the samples regardless of location and month of sampling. One exception occurred at the tourist platform, however, where all healthy corals expressed TIR-1 at detectable levels during the summer months of January and February. In addition, we found a significant difference in the number of corals with detectable levels of the C-type lectin CTL-2 between sites (P = 0.03; (Supplementary Fig. S3E). This effect was due to the presence of CTL-2 in 67.5 and 58.2% of the coral samples collected at the control site and at the tourist platform, respectively, while only 35.3% of the samples collected at the unused platform expressed CTL-2 at detectable levels.
The immune response of injured corals
In damaged corals, several immune parameters were significantly higher in January, the month when corals sustained injury. Both PO and tpPO activity were increased in injured corals relative to corals at the control site (Fig. 2A and B), as well as total relative fluorescent protein levels, due to increases in CFP, GFP and RFP expression (Fig. 2C; Supplementary Fig. S2A–C) and CP expression (Supplementary Fig. S2D). However, these parameters, except CFP and CP expression (Fig. 2A–C; Supplementary Fig. S2B and C), along with apextrin (Supplementary Fig. S3A), were all reduced in March, while TRAF6, MEKK1 and cFos were upregulated compared with controls (Supplementary Fig. S2K; Fig. 3C and E). The expression of GFP was also significantly reduced in June (Supplementary Fig. S2B). Signalling via the TLR pathway may also have been involved in the response, with upregulation of MEKK1 and MAPK p38 in January (Fig. 3E and F). In addition, elevated expression of the lectin CTL-1 was observed following physical damage in January (P = 0.04; Supplementary Fig. S3D). Surprisingly, we also found increased expression of Bf, cFos, and MEKK1 in December, prior to injury (Fig. 3B–E). ATF4/5 expression was downregulated in both January and February (Fig. 3A).
The immune response of white syndrome-affected corals
The immune system of corals that developed visual signs of disease showed significantly reduced expression of the lectin HL-1 and complement gene C3 prior to January, when signs of WS became apparent (Fig. 3D; Supplementary Fig. S3B). In January, PO and tpPO activities, as well as fluorescent protein levels (in particular CFP and GFP; Supplementary Fig. S2A and B), were increased relative to controls (Fig 2A–C), while expression levels of Bf, cFos, MEKK1 and MAPK p38 were upregulated (Fig. 3B, C, E and F). However, only PO activity was elevated in February (Fig. 2A), while ATF4/5 expression was downregulated in both January and February (Fig. 3A). In comparison with control site corals, various parameters were differentially expressed or activated in March, including reduced PO and tpPO activities (Fig. 2A and B), as well as apextrin levels and upregulation of ERK-2 (Supplementary Fig. S3A and F).
Corals that showed macroscopic signs of disease had significantly higher levels of TIR-1 expression (P < 0.01; Supplementary Fig. S3J). However, it should be noted that, of all diseased corals analysed in this study (n = 4), two consistently expressed TIR-1 at high levels in all 6 months (average relative expression 5.6 ± 0.88), in contrast to the other two colonies, which had low TIR-1 expression throughout the study (average relative expression 0.05 ± 0.035).
The C-type lectin, millectin
Millectin was found in the majority of samples, although there was a significant time effect (P = 0.01). This was probably due to the absence of expression in 31.6 and 30% of the samples from January and February, respectively; although in contrast, 4.3, 16.7 and 0% of corals lacked expression in November, December and March, respectively (Supplementary Fig. S3H).
Additional details listing the statistical significance of comparisons between corals at the control site and the following: (i) healthy corals near reef platforms; (ii) corals that sustained injury in January; and (iii) corals that developed visual signs of WS in January can be found in Supplementary Table S3.
Discussion
The presence of 15-fold higher levels of disease as well as injury associated with recreational activities at sites near permanently moored offshore platforms (Lamb and Willis, 2011) provided an important opportunity to compare immune responses of healthy corals with those of corals exposed to a number of anthropogenic and environmental stressors. The immune systems of all corals near reef platforms, including healthy corals, responded to platform-associated stressors, and corals further boosted their immune system in cases of disease or injury over the 7 month sampling period spanning the austral summer. The range of immune responses demonstrated by the coral A. millepora in this study highlights the complexity of the coral innate immune system, and such studies enhance understanding of the underlying mechanisms that are contributing to rising levels of coral disease globally (Willis et al., 2004; Sokolow, 2009; Ruiz-Moreno et al., 2012).
Temporal patterns in the immune system of healthy corals
Variation in the expression of immune genes and proteins in healthy control colonies of A. millepora suggests that healthy immune systems respond to seasonal variation in environmental parameters, notably in summer. Although no visual signs of disease were detected at the control site, four distinct temporal patterns in immune parameters were detected.
First, upregulation of several genes in summer (significant for ATF4/5 and Bf; trend for C3) suggests that visually healthy colonies of A. millepora boost components of their immune system in response to summer-related stressors, potentially including increased seawater temperature and/or solar radiation, or reduced salinity associated with the summer wet season. AP-1 transcription factors, such as ATFs, may regulate expression of complement C3, whose gene promoter contains binding sites for these transcription factors. In addition, C3 is activated by Bf in response to the detection of a microbe that requires elimination. Overall, this suggests that the coral complement system is under control of AP-1 transcription factors. Although ATF activity may be regulated by the kinase ERK-2, which itself is activated by stimuli like TLRs and pro-inflammatory cytokine signalling and is known to regulate C3 expression (Sugihara et al., 2010), our results cannot conclusively confirm the involvement of this pathway in corals. Development of proteomics approaches that investigate the activation status of proteins will undoubtedly reveal additional roles of signalling pathways in coral stress responses. Upregulation of several components of the complement system, potentially in an ERK-2-mediated manner, in summer is consistent with the presence of a seasonally related environmental stressor.
A second pattern detected in healthy control corals provides further support for our interpretation that corals boost their immune system in response to seasonally related environmental stressors. Sudden increases of up to 2.2-fold in both PO and tpPO activities, as well as 1.7-fold increases in total fluorescence levels (due to a rise in CFP, GFP and RFP expression) in late summer (March) strongly suggest that corals experienced a stressor at this time (Fig. 2). However, no anomalies were apparent in seawater temperature, light, rainfall or wind speed data at the sites in March (Supplementary Fig. S1) and, macroscopically, all corals remained visually healthy. Although we can only speculate, the occurrence of peaks in PO activities at the end of summer may represent a response to 3 months of accumulated summer heat stress (Fig. 1C; Supplementary Fig. S1C). Co-occurrence of peaks in PO and tpPO activity with the warmest summer month in a separate study of temporal patterns in the proPO-activating system at Orpheus Island (J. A. J. M. van de Water, unpublished data) lends support for the interpretation that warm summer temperatures are driving this response. Further studies of seasonal patterns in basal levels of immune parameters among populations on outer reefs, such as Hardy Reef, and inshore reefs are needed to test the generality of these patterns. They also highlight the need to investigate further the ecological immunity and basal immune status of corals on larger temporal and spatial scales.
A third pattern detected in healthy control corals was the reduced expression of four genes involved in the TLR signalling pathway (TRAF6, MEKK1, MAPK p38 and cFos) in summer. This arm of the TRAF6-mediated TLR pathway is crucial in immune responses against microbes and may be involved in AMP-mediated regulation of healthy coral-associated microbial communities, such as in Hydra (Fraune and Bosch, 2007; Fraune et al., 2010; Franzenburg et al., 2012, 2013). In addition, this signalling cascade is involved in the transcriptional regulation of immune genes, such as immunostimulatory cytokines. We also detected reduced expression of the lectin Millectin in summer, suggesting a compromised lectin–complement pathway and, possibly, a breakdown of the coral–Symbiodinium symbiosis, given the role of Millectin in the maintenance of this symbiosis (Kvennefors et al., 2008). Reduced function of these immune mechanisms may lead to a less resilient coral. The reduced expression levels found in summer in this study may also help to explain reported increases in disease prevalence in summer (Willis et al., 2004; Bruno et al., 2007; Harvell et al., 2007).
Finally, significant reductions in various immune parameters, including PO activity and the expression of HL-1 and NF-κB in winter (June), are consistent with a seasonally reduced need for these immune parameters. As these are primarily immune effector molecules, this may suggest that corals are exposed to significantly lower levels of microbial stress in winter, which would be consistent with previous observations of shifts in microbial communities (Bourne et al., 2008; Mouchka et al., 2010; Littman et al., 2011; Witt et al., 2011) and increased coral pathogen virulence (Sussman et al., 2008; Vidal-Dupiol et al., 2011) and coral disease (Willis et al., 2004; Bruno et al., 2007) when seawater temperatures are elevated. This would enable the coral to allocate fewer resources to the immune system and more to other life-history traits, such as growth and reproduction.
The effect of tourist reef platforms on coral immunocompetence
Significantly higher levels of TLR signalling pathway genes (MAPK p38 and MEKK1) in all corals (healthy, diseased and injured) near both platforms in January, as well as at the tourist platform in December (MAPK p38, MEKK1, cFos and cJun), in comparison to control corals, indicates that offshore platforms have an impact on the coral immune system. While physical damage was likely to be the result of reef-based activities, such as snorkelling (Lamb et al., 2014), consistent upregulation of this pathway suggests the presence of additional platform-associated stressors, which may have played a role in the development of WS. Potential platform-associated stressors include nutrient influxes derived from guano from sea birds that frequent these reef platforms (Bosman and Hockey, 1986; Bruno et al., 2003), as well as chemicals originating from human activities, such as cleaning products and sunscreen (Blais et al., 2005; Danovaro et al., 2008). Given that the TLR pathway may be involved in regulating the composition of coral-associated bacterial communities, the upregulation of various genes involved in the TLR pathway in corals near platforms in summer could indicate that these corals were responding to changes within their bacterial communities and attempting to re-establish a more favourable community via TLR-induced AMP production. While no cause can be pinpointed, there is a clear need to investigate further the potential anthropogenic disturbances near offshore reef platforms to enable development of management actions that could mitigate the effects of these stressors and prevent localized coral reef degradation.
The coral response to injury
The primary response to injury detected in this study was up to 3.2-fold increases in the proPO-activating system and up to 5-fold increases in fluorescent protein expression levels in January, when broken branches were first detected. Despite potentially additional, unidentified stressors at platform sites, corals near the tourist platform elicited a sufficient immune response following physical damage to enable recovery. While PO activity may have an antimicrobial function or form a physical barrier to seal the lesion (Mydlarz et al., 2008; Palmer et al., 2011a, c), GFP-like proteins probably protect regenerating tissues from light stress by dissipating high-energy-wavelength light (Baird et al., 2009). Activation of the melanization cascade and increased levels of GFP-like proteins in tissues at growth margins following physical damage found in an earlier study (D’Angelo et al., 2012; van de Water et al., 2015b) support this interpretation. The GFP-like proteins also have the capacity to scavenge reactive oxygen (Bou-Abdallah et al., 2006; Palmer et al., 2009) and may neutralize radicals produced by the melanization cascade; however, whether this is one of their primary functions is currently unclear. Furthermore, we found that the C-type lectin CTL-1 was upregulated following damage, suggesting activation of the lectin–complement pathway, which is consistent with studies that show wounding-induced upregulation in the expression of various lectins (Kvennefors et al., 2010; van de Water et al., 2015b). Another component of this pathway, Bf, was also upregulated, although this was observed a month prior to the significant damage event in January. While we have currently no functional explanation for the increased levels of Bf in corals that would sustain significant damage later on, it cannot be excluded that these corals were more prone to pressure from reef-based tourist activities, for example based on their proximity to the platform, and responded accordingly. Surprisingly, we did not observe a response via the TLR pathway following physical damage (as previously observed by van de Water et al., 2015b). However, the immune response following injury is very dynamic, and we may have missed the fully orchestrated response involving the complement and TLR pathways, which are typically upregulated within days (van de Water et al., 2015b), because of low sampling resolution.
Response of corals to white syndromes
Only corals near reef platforms developed signs consistent with the group of coral diseases known as white syndromes. When combined with the observation that lesions appeared during the warm summer month of January, our results suggest that seasonally related stressors, such as temperature, solar radiation or rainfall, were acting to compound local stressors associated with platforms and cause disease. For example, rainfall events prior to our January 2011 sampling time point may have caused a major influx of nutrients and bacteria associated with bird guano from the platforms onto the reef, thereby potentially affecting the microbial and physiological processes within the coral holobiont, contributing to disease development. Surprisingly, most instances of disease were observed on corals near the unused platform, which had significantly higher levels of bird guano than the tourist platform. While high bird guano influx into the ocean is a potential factor contributing to coral disease, other (still unknown) factors, such as cleaning or antifouling agents used on tourist platforms, cannot be excluded, and investigations into which factors are mainly responsible for disease development are warranted.
Interestingly, we found that prior to disease onset, these corals had significantly reduced levels of the lectin HL-1 and complement C3, suggesting that they may have been immunocompromised, which would have made them more susceptible to disease. However, these differences may also be attributed to biological variation on the genetic or epigenetic level within the coral population. As progression of WS lesions had ceased in all diseased corals by March, we conclude that the immune response was sufficient to halt the disease. The 1.8- to 2.5-fold upregulation of PO activity in January and February, respectively, indicates that the immune response to disease involved activation of the melanization cascade, as well as increased fluorescent protein expression, which was upregulated up to 3-fold compared with control corals in January. In addition, we observed an increase in the expression of the putative TLR, TIR-1. While this could indicate that these WS-affected corals regulated additional immune mechanisms in a TLR-dependent manner, it should be noted that expression was highly variable among colonies, and these results should therefore be interpreted with caution.
Conclusions
Our study of the immune responses of the model coral A. millepora to a range of anthropogenic and environmental disturbances reveals that corals have a complex array of immune responses that are differentially regulated according to the type of disturbance. Overall, in corals at the undisturbed control location, expression of a range of immune genes was significantly modulated by seasonal environmental factors, in particular during the summer months. In addition, corals near tourist platforms showed upregulation of genes involved in the TLR signalling pathway in summer, suggesting increased pressure on the coral immune system from reef platform-associated factors. As a result, the coral immune system may have been overwhelmed by the combined or synergistic effects of these stressors, leading to disease in corals near platforms. However, corals that developed disease or sustained injury responded effectively, primarily using the melanization cascade and enhanced GFP-like protein expression, and recovered when summer heat stress subsided. Further whole-transcriptome or proteome analyses may provide a more detailed understanding of the impacts that local anthropogenic stressors have on physiological and stress response processes in corals. Overall, our study shows that corals are able to cope with normal seasonal stressors in summer, but that additional anthropogenic stressors compromise their immune system, contributing to increased disease prevalence and thereby localized reef degradation at these sites. Identifying the anthropogenic stressors responsible will enable the implementation of management actions to reduce stress on corals and will aid conservation efforts.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by the Australian Research Council through funds allocated by the Centre of Excellence for Coral Reef Studies to B.L.W. (ARC CEO561435), and by AIMS@JCU through a grant awarded to J.B.L.
Supplementary Material
Acknowledgements
We thank L. Kelly, P. Cross, S. Beveridge, S. Harte, C. Heeres, V. Barry Dale, E. Smart, T. Heintz and the tourism operator for their assistance in sample collection and logistical support. Samples were collected on permit numbers G07/23617.1 in 2010 and G11/34003.1 in 2011, issued by the Great Barrier Reef Marine Park Authority.
References
- Alagely A, Krediet CJ, Ritchie KB, Teplitski M (2011) Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens . ISME J 5: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AH, Bhagooli R, Ralph PJ, Takahashi S (2009) Coral bleaching: the role of the host . Trends Ecol Evol 24: 16–20. [DOI] [PubMed] [Google Scholar]
- Beeden R, Willis BL, Raymundo LJ, Page CA, Weil E (2008) Underwater Cards for Assessing Coral Health on Indo-Pacific Reefs, Vol 22 Coral Reef Targeted Research and Capacity Building for Management Program. Currie Communications; , Melbourne. [Google Scholar]
- Blais JM, Kimpe LE, McMahon D, Keatley BE, Mallory ML, Douglas MS, Smol JP (2005) Arctic seabirds transport marine-derived contaminants . Science 309: 445. [DOI] [PubMed] [Google Scholar]
- Bosman A, Hockey P (1986) Seabird guano as a determinant of rocky intertidal community structure . Mar Ecol Prog Ser 32: 247–257. [Google Scholar]
- Bou-Abdallah F, Chasteen ND, Lesser MP (2006) Quenching of superoxide radicals by green fluorescent protein . Biochim Biophys Acta 1760: 1690–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne D, Iida Y, Uthicke S, Smith-Keune C (2008) Changes in coral-associated microbial communities during a bleaching event . ISME J 2: 350–363. [DOI] [PubMed] [Google Scholar]
- Brown T, Bourne D, Rodriguez-Lanetty M (2013) Transcriptional activation of c3 and hsp70 as part of the immune response of Acropora millepora to bacterial challenges . PLoS ONE 8: e67246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Petes LE, Drew Harvell C, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases . Ecol Lett 6: 1056–1061. [Google Scholar]
- Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal stress and coral cover as drivers of coral disease outbreaks . PLoS Biol 5: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L, Kawabata S, Lee BL, Nonaka M, Söderhäll K (2010) Proteolytic cascades and their involvement in invertebrate immunity . Trends Biochem Sci 35: 575–583. [DOI] [PubMed] [Google Scholar]
- Cheng W, Hsiao IS, Chen JC (2004. a) Effect of ammonia on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus . Fish Shellfish Immun 17: 193–202. [DOI] [PubMed] [Google Scholar]
- Cheng WT, Hsiao IS, Chen JC (2004. b) Effect of nitrite on immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus . Dis Aquat Organ 60: 157–164. [DOI] [PubMed] [Google Scholar]
- Coteur G, Danis B, Fowler SW, Teyssie JL, Dubois P, Warnau M (2001) Effects of pcbs on reactive oxygen species (ROS) production by the immune cells of Paracentrotus lividus (echinodermata) . Mar Pollut Bull 42: 667–672. [DOI] [PubMed] [Google Scholar]
- D’Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, Wiedenmann J (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments . Coral Reefs 31: 1045–1056. [Google Scholar]
- Danis B, Wantier P, Flammang R, Pernet Ph, Chambost-Manciet Y, Coteur G, Warnau M, Dubois P (2006) Bioaccumulation and effects of PCBs and heavy metals in sea stars (Asterias rubens, L.) from the North Sea: a small scale perspective . Sci Total Environ 356: 275–289. [DOI] [PubMed] [Google Scholar]
- Danovaro R, Bongiorni L, Corinaldesi C, Giovannelli D, Damiani E, Astolfi P, Greci L, Pusceddu A (2008) Sunscreens cause coral bleaching by promoting viral infections . Environ Health Perspect 116: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RP, Parry H, Spicer JI, Hutchinson TH, Pipe RK, Widdicombe S (2011) Immunological function in marine invertebrates: responses to environmental perturbation . Fish Shellfish Immun 30: 1209–1222. [DOI] [PubMed] [Google Scholar]
- Franzenburg S, Fraune S, Künzel S, Baines JF, Domazet-Loso T, Bosch TC (2012) Myd88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers . Proc Natl Acad Sci USA 109: 19374–19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzenburg S, Walter J, Künzel S, Wang J, Baines JF, Bosch TC, Fraune S (2013) Distinct antimicrobial peptide expression determines host species-specific bacterial associations . Proc Natl Acad Sci USA 110: E3730–E3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraune S, Bosch TCG (2007) Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra . Proc Natl Acad Sci USA 104: 13146–13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraune S, Augustin R, Anton-Erxleben F, Wittlieb J, Gelhaus C, Klimovich VB, Samoilovich MP, Bosch TC (2010) In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides . Proc Natl Acad Sci USA 107: 18067–18072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals . Science 301: 958–960. [DOI] [PubMed] [Google Scholar]
- Haapkylä J, Unsworth RKF, Flavell M, Bourne DG, Schaffelke B, Willis BL (2011) Seasonal rainfall and runoff promote coral disease on an inshore reef . PLoS ONE 6: e16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Shoguchi E, Shinzato C, Kawashima T, Miller DJ, Satoh N (2013) The complex nod-like receptor repertoire of the coral Acropora digitifera includes novel domain combinations . Mol Biol Evol 30: 167–176. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis BL (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates . Oceanography 20: 24. [Google Scholar]
- Hawkins JP, Roberts CM (1997) Estimating the carrying capacity of coral reefs for recreational scuba diving . In HA Lessios, IG Macintyre, eds, Proceedings of the 8th International Coral Reef Symposium, Vol 2. Smithsonian Tropical Research Institute, Panama. [Google Scholar]
- Kvennefors ECE, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC (2008) An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts . Dev Comp Immunol 32: 1582–1592. [DOI] [PubMed] [Google Scholar]
- Kvennefors ECE, Leggat W, Kerr CC, Ainsworth TD, Hoegh-Guldberg O, Barnes AC (2010) Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis . Dev Comp Immunol 34: 1219–1229. [DOI] [PubMed] [Google Scholar]
- Lamb JB, Willis BL (2011) Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park . Conserv Biol 25: 1044–1052. [DOI] [PubMed] [Google Scholar]
- Lamb JB, True JD, Piromvaragorn S, Willis BL (2014) Scuba diving damage and intensity of tourist activities increases coral disease prevalence . Biol Conserv 178: 88–96. [Google Scholar]
- Lema KA, Willis BL, Bourne DG (2012) Corals form characteristic associations with symbiotic nitrogen-fixing bacteria . Appl Environ Microbiol 78: 3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data . J Exp Mar Biol Ecol 346: 36–44. [Google Scholar]
- Li CC, Yeh ST, Chen JC (2010) Innate immunity of the white shrimp Litopenaeus vannamei weakened by the combination of a Vibrio alginolyticus injection and low-salinity stress . Fish Shellfish Immun 28: 121–127. [DOI] [PubMed] [Google Scholar]
- Littman R, Willis BL, Bourne DG (2011) Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef . Environ Microbiol Rep 3: 651–660. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chen JC (2004) Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus . Fish Shellfish Immun 16: 321–334. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K, Funayama N, Agata K, Bosch TCG (2007) The innate immune repertoire in cnidaria - ancestral complexity and stochastic gene loss . Genome Biol 8: R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchka ME, Hewson I, Harvell CD (2010) Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts . Integr Comp Biol 50: 662–674. [DOI] [PubMed] [Google Scholar]
- Mydlarz LD, Jones LE, Harvell CD (2006) Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates . Annu Rev Ecol Evol 37: 251–288. [Google Scholar]
- Mydlarz LD, Holthouse SF, Peters EC, Harvell CD (2008) Cellular responses in sea fan corals: granular amoebocytes react to pathogen and climate stressors . PLoS ONE 3: e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong TF, Musa G (2011) An examination of recreational divers’ underwater behaviour by attitude–behaviour theories . Curr Issues Tour 14: 779–795. [Google Scholar]
- Osborne K, Dolman AM, Burgess SC, Johns KA (2011) Disturbance and the dynamics of coral cover on the Great Barrier Reef (1995–2009) . PLoS ONE 6: e17516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CV, Mydlarz LD, Willis BL (2008) Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals . Proc R Soc B Biol Sci 275: 2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CV, Modi CK, Mydlarz LD (2009) Coral fluorescent proteins as antioxidants . PLoS ONE 4: e7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CV, Bythell JC, Willis BL (2010) Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals . FASEB J 24: 1935–1946. [DOI] [PubMed] [Google Scholar]
- Palmer CV, Bythell JC, Willis BL (2011. a) A comparative study of phenoloxidase activity in diseased and bleached colonies of the coral Acropora millepora . Dev Comp Immunol 35: 1098–1101. [DOI] [PubMed] [Google Scholar]
- Palmer CV, McGinty ES, Cummings DJ, Smith SM, Bartels E, Mydlarz LD (2011. b) Patterns of coral ecological immunology: variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor . J Exp Biol 214: 4240–4249. [DOI] [PubMed] [Google Scholar]
- Palmer CV, Traylor-Knowles NG, Willis BL, Bythell JC (2011. c) Corals use similar immune cells and wound-healing processes as those of higher organisms . PLoS ONE 6: e23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock FJ, Lamb JB, Field SN, Heron SF, Schaffelke B, Shedrawi G, Bourne DG, Willis BL (2014) Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs . PLoS ONE 9: e102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Dustan P, Jaap W, Patterson K, Kosmynin V, Meier O, Patterson M, Parsons M (2001) Patterns of spread of coral disease in the Florida Keys . Hydrobiologia 460: 1–24. [Google Scholar]
- Raina JB, Tapiolas D, Willis BL, Bourne DG (2009) Coral-associated bacteria and their role in the biogeochemical cycling of sulfur . Appl Environ Microbiol 75: 3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding JE, Myers-Miller RL, Baker DM, Fogel M, Raymundo LJ, Kim K (2013) Link between sewage-derived nitrogen pollution and coral disease severity in Guam . Mar Poll Bull 73: 57–63. [DOI] [PubMed] [Google Scholar]
- Reid HI, Soudant P, Lambert C, Paillard C, Birkbeck TH (2003) Salinity effects on immune parameters of Ruditapes philippinarum challenged with Vibrio tapetis . Dis Aquat Organ 56: 249–258. [DOI] [PubMed] [Google Scholar]
- Ritchie KB. (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria . Mar Ecol Prog Ser 322: 1–14. [Google Scholar]
- Ruiz-Moreno D, Willis BL, Page AC, Weil E, Cróquer A, Vargas-Angel B, Jordan-Garza AG, Jordán-Dahlgren E, Raymundo L, Harvell CD (2012) Global coral disease prevalence associated with sea temperature anomalies and local factors . Dis Aquat Organ 100: 249–261. [DOI] [PubMed] [Google Scholar]
- Seneca FO, Foret S, Ball EE, Smith-Keune C, Miller DJ, van Oppen MJH (2010) Patterns of gene expression in a scleractinian coral undergoing natural bleaching . Mar Biotechnol 12: 594–604. [DOI] [PubMed] [Google Scholar]
- Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T et al. (2011) Using the Acropora digitifera genome to understand coral responses to environmental change . Nature 476: 320–323. [DOI] [PubMed] [Google Scholar]
- Shnit-Orland M, Kushmaro A (2009) Coral mucus-associated bacteria: a possible first line of defense . FEMS Microbiol Ecol 67: 371–380. [DOI] [PubMed] [Google Scholar]
- Siboni N, Abrego D, Seneca F, Motti CA, Andreakis N, Tebben J, Blackall LL, Harder T (2012) Using bacterial extract along with differential gene expression in Acropora millepora larvae to decouple the processes of attachment and metamorphosis . PLoS ONE 7: e37774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolow S. (2009) Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence . Dis Aquat Organ 87: 5–18. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Kobori A, Imaeda H, Tsujikawa T, Amagase K, Takeuchi K, Fujiyama Y, Andoh A (2010) The increased mucosal mRNA expressions of complement c3 and interleukin-17 in inflammatory bowel disease . Clin Exp Immunol 160: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M, Willis BL, Victor S, Bourne DG (2008) Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific . PLoS ONE 3: e2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland KP, Shaban S, Joyner JL, Porter JW, Lipp EK (2011) Human pathogen shown to cause disease in the threatened eklhorn coral Acropora palmata . PLoS ONE 6: e23468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M, Ritchie K (2009) How feasible is the biological control of coral diseases? Trends Ecol Evol 24: 378–385. [DOI] [PubMed] [Google Scholar]
- Tseng IT, Chen JC (2004) The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress . Fish Shellfish Immun 17: 325–333. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F(2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water JAJM, Leggat W, Bourne DG, van Oppen MJH, Willis BL, Ainsworth TD (2015. a) Elevated seawater temperatures have a limited impact on the coral immune response following physical damage . Hydrobiologia doi:10.1007/s10750-015-2243-z. [Google Scholar]
- Van de Water JAJM, Ainsworth TD, Leggat W, Bourne DG, Willis BL, van Oppen MJH (2015. b) The coral immune response facilitates protection against microbes during tissue regeneration . Mol Ecol 24: 3390–3404. [DOI] [PubMed] [Google Scholar]
- Vidal-Dupiol J, Ladrière O, Meistertzheim AL, Fouré L, Adjeroud M, Mitta G (2011) Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus . J Exp Biol 214: 1533–1545. [DOI] [PubMed] [Google Scholar]
- Vidal-Dupiol J, Dheilly NM, Rondon R, Grunau C, Cosseau C, Smith KM, Freitag M, Adjeroud M, Mitta G (2014) Thermal stress triggers broad Pocillopora damicornis transcriptomic remodeling, while Vibrio coralliilyticus infection induces a more targeted immuno-suppression response . PLoS ONE 9: e107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis B, Page C, Dinsdale E (2004) Coral disease on the Great Barrier Reef . In Rosenberg E, Loya Y, eds, Coral Health and Disease. Springer; , Berlin, Heidelberg, pp 69–104. [Google Scholar]
- Witt V, Wild C, Anthony KR, Diaz-Pulido G, Uthicke S (2011) Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef . Environ Microbiol 13: 2976–2989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.