Migratory movements are dynamic interaction of intrinsic and extrinsic factors. To improve our understanding of the mechanisms underlying movement decisions, we review and provide a novel framework for integrating observed movement behaviors and extrinsic conditions with recent technical advances in the physiology-related fields of energetics, nutrition, endocrinology, immunology and ecotoxicology.
Keywords: Endocrinology, energetics, immunology, migration, movement, nutrition
Abstract
Movements are a consequence of an individual's motion and navigational capacity, internal state variables and the influence of external environmental conditions. Although substantial advancements have been made in methods of measuring and quantifying variation in motion capacity, navigational capacity and external environmental parameters in recent decades, the role of internal state in animal migration (and in movement in general) is comparatively little studied. Recent studies of animal movement in the wild illustrate how direct physiological measurements can improve our understanding of the mechanisms underlying movement decisions. In this review, we synthesize and provide examples of how recent technical advances in the physiology-related fields of energetics, nutrition, endocrinology, immunology and ecotoxicology provide opportunities for direct measurements of physiological state in the study of animal movement. We then propose a framework for practitioners to enable better integration of studies of physiological state into animal movement ecology by assessing the mechanistic role played by physiology as both a driver and a modulator of movement. Finally, we highlight the current limitations and research priorities for better integration of direct measurements of animal physiological state into the study of animal movement.
Introduction
Animals move to fulfil their basic biological goals of gaining energy, increasing survival and reproductive advantage (Nathan et al., 2008). However, their motivation to move is determined by a combination of internal state and external environmental factors, such as climate, predation risk, competition and food availability (Fryxell and Sinclair, 1988). The ongoing, rapid global change is shifting patterns of species movements, their interactions and adaptive abilities, thereby affecting their motivation to move (Lundberg and Moberg, 2003; Pulido, 2007; Singh et al., 2010). These changes are challenging our abilities to understand and predict animal movements, particularly as they relate to the process of migration, which can be defined broadly as the seasonal movement toward non-overlapping areas (Wilcove and Wikelski, 2008). Therefore, to provide better prediction of how migratory animals will respond to global change, it is vital to develop a more complete understanding of the relative role of internal vs. external drivers of animal movement (Bowlin et al., 2010; Lennox et al., 2015).
The internal state of an animal is recognized as a central component of movement ecology in addressing the question of why animals move (Nathan et al., 2008). However, only recently have movement ecologists begun to clarify and emphasize the role of internal state in observed movement behaviours (Patterson et al., 2008). Recent rapid technical and analytical advances in the monitoring of moving animals and quantification of their movements have resulted in a variety of movement models inferring behavioural responses to internal state based on the location of successive locational fixes (Morales et al., 2004; Schick et al., 2008). However, such inferential models are indirect, and the causal mechanisms behind these observed movement paths remain relatively unknown (Getz and Saltz, 2008; Holyoak et al., 2008). To date, a majority of the work describing how directly observed changes in the internal state of animals influence movements is restricted to captive animals exposed to different controlled, experimental manipulations (Meier et al., 1965; Wingfield et al., 1990; Ward et al., 2002).
Recent studies that have attempted to directly measure the internal state of moving, free-ranging vertebrate animals in the wild have revealed intricate linkages between an individual's internal physiological state and movement. For example, the use of biologging techniques has greatly advanced our understanding of animal movement over the last two decades by providing the opportunity to link expenditure and conservation of energy with movement behaviour (Urban et al., 2007; Shepard et al., 2009; Bogard et al., 2010; Louzao et al., 2014). Likewise, integration of endocrine system function with animal tracking through non-invasive stress hormone measurements has resulted in an improved understanding of complex movement behaviours, such as refuge use and corridor streaking (Jachowski et al., 2012, 2013a). Collectively, there is a growing body of evidence illustrating how internal state interacts with, and sometimes overrides, the more commonly assessed role of external factors used to explain observed movement behaviours. Perhaps most importantly, through measurements of internal state, we are beginning to access the mechanisms underlying animal movement (Cooke et al., 2012).
As technical developments progress and more studies attempt to address questions about internal state and its role in animal movement, it becomes important to review the status of the field and identify future directions. In this special issue, Lennox et al., 2015 discuss how physiology has improved our understanding of animal migration and can inform conservation. In our review, we focus on providing a synthetic summary of the tools and techniques that can be used to measure the physiological state of moving animals. We also provide a conceptual framework for how measures of physiological state can be better integrated into studies of animal movement. Finally, we describe limitations of the physiological data currently being obtained and used to answer questions on animal movements, and identify future research opportunities. Throughout our review, because migration is composed of a diversity of movement behaviours, such as nomadic movements within seasonal ranges or restricted movement at a specific feeding patch (Sawyer and Kauffman, 2011; Singh and Ericsson, 2014), we use the terms movements and migration simultaneously because most of our arguments apply to all types of movements in general and not only migration.
Review of techniques for measurement of the physiological state of moving animals
When attempted, the incorporation of multiple subdisciplines of physiology has informed our understanding of animal movement ecology. These subdisciplines primarily include studies of animal energetics, nutrition, endocrinology, immunology and ecotoxicology. Each of these fields has been extensively investigated in a laboratory setting, and concepts and methods are increasingly being applied to free-living animals in a field setting. Our purpose here is briefly to review how measures derived from each of these fields have informed our study of animal movement.
Energetics
The study of energetics is fundamental to understanding processes such as reproduction, behavioural interactions and movement behaviour (Schmidt-Nielsen, 1997; Isaac et al., 2012). The rapidly advancing field of ecological energetics is of particular relevance to movement ecologists because of its focus on free-ranging organisms and its aim to integrate physiological limits of an organism with the external, environmental constraints surrounding it (Tomlinson et al., 2014). Within a movement ecology context, attention is typically focused on the energetic costs or benefits of various movement decisions; for example, tracking studies of invertebrate and avian movement have revealed correlations between migratory movements and periods of favourable wind speed and direction that are probably based on derived energetic benefits (Wikelski et al., 2006). Also, a number of direct measures are available to assess metabolic rates during movement, including changes in isotope levels and direct measurements of body temperature and heart rate through biotelemetry (Nagy and Costa, 1980; Speakman, 1997), both of which are discussed below in further detail.
Isotopes
The isotope method measures energy expenditure and water turnover rate through the technique of doubly labelled water (DLW). The technique relies on the extent to which an organism loses heavy oxygen isotopes (18O) in the form of carbon dioxide and water, in relationship to the loss of heavy hydrogen isotopes (2H or 3H) in the form of water. The resulting difference between the hydrogen and oxygen isotope turnover rate indicates CO2 production and therefore metabolic rate and energetic requirements (Speakman, 1997). However, there are several assumptions behind the DLW technique that are not always met, thus restricting its use in some taxa, such as amphibians, diving birds and mammals, and organisms of small body size (Speakman, 2005; Hambly and Voigt, 2011; Shaffer, 2011). Furthermore, the 18O isotope is expensive to procure, and the cost of this analysis has proved prohibitive for widespread general application in ecological studies. Other radioactive alternatives besides water, such as rubidium, have also been tested, but their use has been contested due to ethical reasons (Tomlinson et al., 2014). Regardless of the technique used, isotope measurements require repeated capture of individuals, which is a factor that risks disturbing movement behaviours of targeted individuals.
Biologgers and biotelemetry
With the advent of internal temperature and heart rate loggers, it is possible to correlate changes in observed movement behaviours with an animal's metabolic rate (Cooke et al., 2004; Green et al., 2009). By inserting a sensor device within an animal at a location close to the heart or a blood vessel, both body temperature and heart rate can be recorded continuously at high frequencies (Bolen et al., 2005; Ropert-Coudert and Wilson, 2005; Wilson et al., 2006; Signer et al., 2010). The data are recorded continuously by an on-board logger (biologger) or transmitted (biotelemetry), without a need for short-term recapture of the individual.
The rapid growth of biologger and biotelemetry technology over the past several decades has improved our ability to evaluate the physiological cost of different movement behaviours. Progress in biotelemetry and the development of biologgers began mostly in marine systems, where it has been difficult to obtain information about the animals in other ways (Ropert-Coudert and Wilson, 2005). For example, Bevan et al. (1995), in a study of black-browed albatrosses (Diomedea melanophris), measured both abdominal temperature and heart rate and reported significant variations in energy expenditure during different behaviours and stages of the reproductive cycle. Likewise, biotelemetry tags implanted into the swimming musculature of migratory Fraser River sockeye salmon (Oncorhynchus nerka) cannot only provide a measure of locomotor activity, but once calibrated, can be used to estimate the energetic cost of migration (Cooke et al., 2008; Wilson et al., 2015).
Biologgers have also proved particularly useful for monitoring energetic costs of behaviours that were otherwise difficult to detect through movement data alone. For example, although Cocherell et al. (2011) observed minimal upstream or downstream movements by rainbow trout (Oncorhynchus mykiss) in response to pulsed water flows from a hydroelectric dam, use of biosensor tags revealed that they generally exhibit increased energetic output and decreased foraging opportunities. While additional investigations into the potential longer-term consequences and conservation implications are needed, this study illustrates the value of accounting for physiology when assessing the movement ecology of species. Overall, with the development of smaller and longer-lasting biologger and biotelemetry devices (allowing for their implementation across a wide range of taxa) that can record multiple variables simultaneously and transmit remotely, this area offers one of the most exciting and rapidly growing techniques in the study of animal movement physiology (Cooke et al., 2004; Bolen et al., 2005; Bogard et al., 2010; Wilson et al., 2015).
Nutrition
Meeting dietary and nutritional needs can also be an internal physiological factor driving animal movement behaviours. Water is a key limiting compound for almost all terrestrial vertebrates, influencing their movement path decisions and longer-term patterns in animal spatial ecology (Redfern et al., 2003). In addition, demand for other key limiting nutrients often drives movement decisions in complex and interactive ways. For example, Ortiz-Maciel et al. (2010) observed that variation in movement and space use patterns by maroon-fronted parrots (Rhynchopsitta terrisi) was likely to be a complex interaction of reproductive state, food availability and the location of clay licks that contain salt and other key nutrients (Emmons and Stark, 1979; Powell et al., 2009). Furthermore, once key limiting nutrients are identified, resource managers can manipulate their availability to modify animal movement and migration (Sahlsten et al., 2010; Jones et al., 2014).
Investigation of the relationship between animal nutrition and movement is most easily accomplished by simultaneous monitoring of foraging and movement behaviour or by retrospective analyses of nutrition combined with tracking data (Giroux et al., 2012). At a fine spatial scale, focal monitoring of movement and foraging activity can be combined to relate movement to nutritional intake. Likewise, forage intake and behaviour can be estimated retroactively from evidence at a kill or foraging site (Sand et al., 2005), analysis of faecal sample content (Munro et al., 2006) or measurement of stable isotopes in blood or faecal samples (Vogel, 1978). Once these types of data are collected, they can be linked retrospectively with tracking data to provide information on the likely energy expenditure and nutritional status of tracked individuals during specific time periods (Hebblewhite et al., 2008). Furthermore, stable isotopes can be used to monitor for potentially important carry-over effects of diet and nutrition between life-history periods that are likely to influence various processes, including movement behaviour (O'Connor et al., 2014).
Measurements of body fat provide another important indication of an individual's nutritional status. Body fat indices offer a method of quantifying past energetic accumulation as well as energy reserve potential (Robbins, 1983). For invertebrates, this is most easily achieved through molecular techniques, such as the extraction and quantification of lipids from migratory individuals sampled along their route (Brower et al., 2006). For vertebrates, in addition to molecular tools, ultrasonography and manual palpation can be used to estimate percentage body fat (Cooke et al., 2004; Cooke and O'Connor, 2010), which can then be related to different life-history parameters and to individual- and population-level performance. These methods resemble the isotope method in that the same individual needs to be recaptured to estimate the relative gain or loss of body fat in a given time interval. For example, Monteith et al. (2011) related the tendency to migrate in mule deer to the nutritional condition of the females by measuring the ingesta-free body fat, which showed that females in poorer condition were more likely to migrate. Studies of birds also demonstrated distinct patterns, where individuals in better condition after migration, as indicated by fat reserves, were more likely to survive harsh weather periods (Morrison et al., 2007). Finally, in addition to simultaneous monitoring of movement behaviour and nutritional metrics, the most promising area of research is in integrating these metrics with monitoring of extrinsic or environmental conditions. Findings that integrate across these data streams (e.g. McWilliams et al., 2004; Brower et al., 2006; Middleton et al., 2013) have transformed our understanding of a population or species′ movement behaviour and will continue to guide conservation.
Endocrinology
The endocrine system and its associated hormones control or modulate physiological functions within the body. The rapidly growing subject of field endocrinology is likely to be of particular interest to movement ecologists because of its focus on the hormonal drivers that often serve as mechanisms underlying observed behavioural responses to environmental challenges (Walker et al., 2005). For instance, it is widely appreciated that release of stress hormones is the physiological mechanism that facilitates an adaptive response by an individual following exposure to a stressor. Resultant movement behaviours include long-distance dispersal or restricted movements that are indicative of refuge behaviour (Wingfield and Romenofsky, 1997; Jachowski et al., 2012, 2013a). Thus, knowledge of hormonal control mechanisms is important not only for correct identification of the baseline physiological state of an organism, but also for understanding how it responds to variation in its environment.
Three specific types of hormones and their associated functions are currently of most interest to movement ecologists. First, as mentioned above, measurement of glucocorticoid stress hormones (cortisol and corticosterone) is often used to quantify the effect of chronic or acute stressors on an individual or population (Millspaugh and Washburn, 2004). Glucocorticoids released by the hypothalamic–pituitary–adrenal axis after exposure to a stressor interact with internal receptors to facilitate the direction and prioritization of energy to different processes within the organism (McEwen and Wingfield, 2003; Romero and Butler, 2007). Concentrations of these hormones can be monitored by invasive sampling (by blood, saliva or urine collection) or by non-invasive extraction of hormones from hair, feathers or faeces (Millspaugh and Washburn, 2004; Walker et al., 2005). Regardless of the sampling technique, all approaches must be validated, and some sort of physiological baseline or basal physiological state must be established with which to compare subsequent samples (Millspaugh and Washburn, 2004). Finally, in order to integrate data on circulating stress hormone concentrations with movement data effectively, it is important to determine what is causing the stress response so that appropriate external factors are considered in building models of animal movement.
A second important use of hormonal monitoring in animal movement is to discern the reproductive state of an individual. At the population level, many animal species undertake seasonal migrations to areas that serve as breeding or natal areas, the timing of which is known to vary based on physiological conditions (Newton, 2010). Even for species that do not exhibit such distinct migration periods, differences in movement exist based on reproductive state. For example, in male African elephants (Loxodonta africana), the sampling of testosterone provides valuable insight into when individuals are in musth and can be expected to vary in their movement behaviours (Poole, 1987; Whitehouse and Schoeman, 2003). Movement behaviours can also vary in females based on their reproductive state (Singh and Ericsson, 2014). For example, female moose exhibit restricted movements and select areas of dense cover during parturition (Bowyer et al., 1999). Unfortunately, there are few direct studies addressing the role of oestrogen hormone concentrations on animal movement in a field setting and those that do exist show less clear correlative trends with movement behaviour in comparison to testosterone (Cooke et al., 2006). However, limited information from observational field studies suggests that elevated concentrations of oestrogens are likely to play a mechanistic role in animal behaviour and spatial ecology. For example, Wasserman et al. (2012) found that the consumption of plants that produce oestrogen-mimicking compounds was correlated with increased copulation and territoriality in red colobus monkeys (Procolobus rufomitratus). The potential hormonal mechanisms underlying dispersal decisions by individual animals, which can be highly variable and not based solely on external factors, are in need of further research (Paradis et al., 1998). Thus, while further research into the role of androgens and oestrogens in movement behaviour of free-ranging animals is needed, it is clear that these hormones are not only associated with reproductive state, but that they are also likely to have a mechanistic role in movement behaviour.
Melatonin, produced by the pineal gland, represents a third major type of hormone of potential importance to movement ecologists owing to its role in control of circadian rhythms (Cassone and Menaker, 1984). A majority of research on the role of melatonin in movement decisions has focused on passerine birds, in which melatonin concentrations have been shown to differ seasonally based on changes in day length (Gwinner et al., 1993; Fusani and Gwinner, 2005). Increases in melatonin concentrations during migration have been linked to the timing of migration (Schneider et al., 1994; Fusani and Gwinner, 2004), as well as the increased ability to orient during migration (Cooke et al., 2008). There has been little further work on the role played by melatonin in movement decisions by other migratory species.
Immunology and ecotoxicology
Disease and toxic agents can have direct impacts on the fitness and movement behaviour of animals. Diseases are known not only to impact overall fitness of an individual and limit its ability to move, but also to alter the movements and behaviour of infected individuals (Moore, 1995; Poulin, 2000, 2010; Klein et al., 2004). One of the more interesting examples comes from the protozoan parasite, Toxoplasma gondii, which reverses the innate aversion of rats (Rattus spp.) to cat (Felis catus) odour, causing cat odour to be an attractant to rats and allowing the parasite to reach its definitive host, the cat (Berdoy et al., 2000). Furthermore, even the risk of encountering parasites and infectious agents can influence animal movement behaviours, such as the bunching of herd animals and selection of habitats based on avoidance of insect pests (Rutberg, 1987; Mooring and Hart, 1992).
Exposure to toxicants also can contribute to observed variation in movement patterns in obvious and subtle ways. The exposure to external contaminants, such as oils and detergents, can impact thermoregulation and movement capacity of birds and terrestrial animals (Stephenson, 1997). Internally, contaminants not only provide a potential limit to fitness, leading to disease-related concerns, but also can influence physiological processes important to animal movement. For example, elevated concentrations of mercury in fish and wildlife can impair neurological and endocrine functions (Eisler, 2006; Franceschini et al., 2008; Wada et al., 2009), probably influencing hormone-modulated movement behaviours as well as cognitive processes, such as navigational capacity.
While some indices of epidemiological state and toxicological burden can be detected by external features (e.g. facial tumours, oiling, etc.), physiological measures provide a more robust assessment that can often be integrated simultaneously with other physiological measures. Disease surveillance is primarily accomplished through blood-based assays for blood-borne pathogens or titres produced as part of the immune response (Artois et al., 2009). Likewise, toxicological burdens can often be quantified through the blood, and in some cases non-invasively through faeces, saliva and epidermis (skin, nails, etc.; Elliott et al., 2011). Beyond what can be inferred by measuring only the presence or absence of a disease or amount of a toxicological burden, we encourage researchers to evaluate energetic and endocrine system functions simultaneously in order to maximize the potential inference gained from invasive and non-invasive sampling. For example, the use of biotelemetry simultaneously to assess energetic state, disease status and movement behaviour offers particularly exciting opportunities to gain new insights into observed movement behaviour and disease ecology (Adelman et al., 2014).
A framework for better linking animal movement and physiology
As discussed in the preceding sections, there are multiple examples that illustrate how researchers have successfully integrated physiology into studies of animal movement. Inclusion of physiological measurements not only complements field studies of animal movement, but in some cases reveals hidden costs or consequences not apparent by assessment of spatial movement data alone (e.g. Walker et al., 2005; Cocherell et al., 2011). Furthermore, as discussed by Lennox et al., 2015, such findings have greatly advanced our understanding of migration. However, we feel that broader integration of physiological metrics to develop a greater understanding of animal movement has been limited up to this point, owing in part to the lack of a conceptual framework governing how to integrate these measures in practice.
Nathan et al. (2008), in their initial description of a conceptual framework for the field of movement ecology (which they describe as being composed of external factors, internal state, navigation capacity and motion capacity), define internal state as a term that accounts for the physiological (and perhaps psychological) state ‘driving the organism to fulfill one or more goals’. They go on to describe various goals of gaining energy, seeking safety, reproduction and other phenomena as examples of how, as a field, ‘internal state consists of a multidimensional vector of many states’. Clearly, ‘internal state’ has been used as an umbrella term for a number of internal characteristics. However, we feel that internal state requires a more nuanced redefinition based on recent advances and the need for more pragmatic guidance on how to quantify and integrate measurements of internal state into movement ecology.
The mechanisms that drive the movement of an individual or population are best viewed in an evolutionary context, in which species have evolved life-history strategies that include physiological adjustments that are a key element of animal plasticity. Physiological control mechanisms dictate how an animal exhibits sufficient phenotypic plasticity during its life to maintain fitness (Ricklefs and Wikelski, 2002). Thus, in order to understand variation in movement patterns and address the question of why animals move the way they do, we need to understand how physiological control mechanisms constrain and dictate variation in how an individual responds to its surrounding environment (Cooke et al., 2008, 2012).
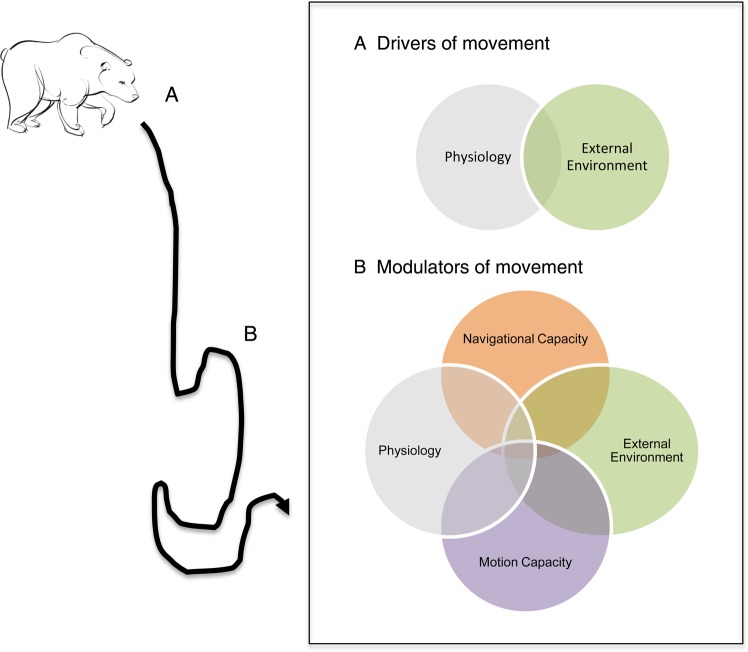
Accordingly, within the conceptual framework of movement ecology, we suggest that the term ‘internal state’ be abandoned in favour of the more accurate term ‘physiological state’. Similar to the movement ecology paradigm put forth by Nathan et al. (2008) involving internal state, we propose that physiological state should be one of the primary fields of investigation in movement ecology, where observed movement behaviours operate at the intersection of physiological state, navigational capacity, motion capacity and external factors (Fig. 1). However, in contrast to previous frameworks, we propose that physiological state plays an important mechanistic role in movement behaviour that can best be investigated in two phases or stages. First, we propose that physiological measures be investigated and considered along with external cues as proximate drivers of an individual's decision to move (Fig. 1). The driving role played by physiology and external conditions in the decision to move is a relatively well-established relationship in ectothermic animals for thermoregulation (Angilletta et al., 2009; Sears and Angilletta, 2015). Similar patterns are evident in some endotherms, where Signer et al. (2010) have demonstrated that alpine ibex (Capra ibex) migrate across an elevational gradient to facilitate digestion by increasing their rumen temperature (i.e. hypermetabolism). Furthermore, across a range of vertebrate and invertebrate species, physiology is more broadly documented as a key driver in the timing of migratory movements (Lennox et al., 2015).
Figure 1:
A proposed revised conceptual framework for investigations into animal movement ecology (adapted from Nathan et al. 2008), which depicts how movement behaviour of an individual is a function of two distinct phases. First (A), we propose that an animal is motivated or driven to move as a result of its internal physiological state as well as proximate external cues. Second (B), the movement path taken by an individual is modulated or influenced by a complex interaction of physiological state and external environment, as well as by motion and navigational capacity.
Second, in addition to driving the decision to move, physiological state clearly interacts in complex ways with external environmental conditions to modulate animal movement behaviour along a movement track (Fig. 1). While less commonly studied in comparison to the driving role played by physiology in movement, this area of work has great potential to inform our understanding of movement behaviour. For example, real-time GPS tracking of African elephants has been combined with non-invasive sampling of stress hormones to reveal complex movement path decisions related to glucocorticoid concentrations and refugia or stopover use, corridor use and streaking behaviour (i.e. rapid unidirectional movement along a corridor; Jachowski et al., 2012, 2013a, b). Thus, the extent to which an animal revisits or avoids a particular site along a movement track is based on its past experiences with external factors at that site and its ability to navigate toward or away from it and is modulated by physiological condition.
Better integration of physiological measures within our framework can also improve our understanding of complex phenomena that modulate movement patterns, such as memory and learning. For example, toxic concentrations of mercury have been shown to impair hormone-modulated movement behaviours and navigational capacity (Stephenson, 1997; Eisler, 2006). Thus, while the concepts of memory and learning in movement ecology have been difficult to quantify and somewhat controversial (such as the topic of cognitive maps; Bennett, 1996; Mueller and Fagan, 2008), under our framework we encourage further research on how memory in free-ranging animals can be investigated as a separate cognitive process based on the intersection of physiological state, external factors and navigational capacity.
Current limitations and recommendations for future research
Despite progress and developments in the measurement of the physiological state of free-ranging animals, and a framework for incorporating those measures as summarized above, it is important to acknowledge current limitations and areas in need of further research. A number of concerns remain about the assumptions, applicability, scale, technical capability, animal welfare and cost issues associated with current methods used to measure physiological state in animals. Furthermore, in addition to the need for methodological advancements, theoretical advancements will be needed to provide better integration of measures of physiological state into studies of animal movement. In this section, we discuss these issues and attempt to lay the path of future progress in the field.
Limitations and assumptions of current techniques
One of the first issues that should be addressed when attempting to monitor physiological state is the reliability of these methods in what they measure. Each technique comes with unique assumptions, costs and benefits and is subject to criticism. For example, Butler et al. (2004) reviewed the advantages and disadvantages of DLW and heart rate methods and found that the biotelemetry methods have a great potential, especially due to their accuracy and versatility. Furthermore, DLW techniques typically make several common assumptions that deserve additional evaluation, such as the following: (i) rate of flow of materials and size of the body are constant throughout the temporal scale of measurement; (ii) all materials leaving the body take isotopes with them at a similar rate to body water; and (iii) hydrogen and oxygen atoms only take part in reactions that involve water and CO2, and spent isotopes do not re-enter the body (Nagy and Costa, 1980; Speakman, 1997; Tomlinson et al., 2014). Measures of heart rate, respiratory rate and body temperature also involve assumptions because they assess only some of the many physiological factors that influence metabolic rate (Cooke et al., 2004; Green, 2011). Thus, while logistical constraints often drive sampling methodologies in field-based studies, it is also important to be aware of the assumptions and biases inherent in each technique.
A second critical issue is to establish what the physiological metrics being collected genuinely represent. Physiological state metrics can vary greatly among techniques, as well as among individual animals, populations and species (Sheriff et al., 2011). Thus, it is necessary to consider not only the logistical constraints and the validity of measurements, but also what those values mean to the individual or population. It is for this reason that most physiological studies of wildlife are based on comparisons of measurements collected before and after experimental treatment or exposure to a discrete environmental stressor (Wingfield et al., 1997). In this way, researchers can establish a baseline with which to compare the physiological response of an individual or set of individuals post-treatment. Likewise, in the study of wild animals, where experimental manipulation might not be possible, it is critical to establish a physiological baseline with which to compare subsequent measurements. This can be accomplished by comparisons with captive populations or other wild populations, but it is ideally based on individual physiological metrics prior to a movement activity or exposure to a stressor, taking into account seasonal and daily rhythms that are often inherent in physiological state metrics (Millspaugh and Washburn, 2004).
Following on the issue of what a specific physiological metric represents, there is the question of how representative it is of the individual, population or species. Similar to other fields of ecological inquiry, researchers must think critically about issues of spatial and temporal scale when sampling physiological state. A spatially balanced sampling design should be devised to enable accounting for individual, population or species-specific variability. Temporally, because most direct measures of physiological state require repeated capturing of individuals, most physiological measures are limited to periods of time between or immediately preceding sampling events. For many free-ranging animals, the difficulty of reliably capturing them and the limitation of inference to a specific window of time might make direct measurement of physiological state unsuitable as a method. While repeated captures or attached biologgers can assist in expanding the temporal period of inference, the benefits of such techniques should be balanced with concerns about the resulting disturbance to the animal (White et al., 2013).
Animal welfare and non-invasive sampling
The rapid proliferation of techniques to monitor the physiological state of animals involves both invasive and non-invasive techniques that bring up important animal welfare concerns. Historically, most techniques required repeated capture and handling that, at a minimum, momentarily influence animal movement and, in some cases, can have prolonged negative impacts. For example, the recent use of radioactive isotopes, such as rubidium, has been shown to track field metabolic rate reliably, but it has clear animal welfare and toxicological implications (Tomlinson et al., 2013). Biologgers offer a tremendous tool for intensively monitoring the physiological state of an organism over an extended period of time, but similar to other attached tracking devices, they must be evaluated carefully so as not to impact movement behaviours or otherwise negatively affect marked individuals (Authier et al., 2013; Thomson and Heithaus, 2014). These concerns highlight the value of non-invasive sampling of faeces, hair and other materials for certain direct and indirect physiological measures. Rapid advances in such techniques make them increasingly valuable tools for gathering reliable metrics of animal physiology with little or no disturbance to the animal. However, this benefit must be weighed against the difficulty of discerning the period of time represented by the sample, as well as the identity of the individual sampled, both of which can be critical to interpretation of results and subsequent inference (Goyman, 2012; Jachowski et al., 2013a). Thus, while certain measures of animal physiology will continue to require animal capture, less invasive (such as self-detaching collars) and non-invasive technologies are rapidly advancing that minimize animal welfare concerns.
Establishment of indirect measures of physiological state
In general, the monitoring of physiological state in a field setting is likely to take one of the following two forms: (i) fine-scale simultaneous tracking of individual animal movements and physiological state that allows for comparisons in movement and physiological correlates over time and across geographical gradients; or (ii) comparative studies of movement behaviours between animals or populations categorized as being in different physiological states. Across both approaches, as mentioned above, a key limitation of many techniques is the need to capture individuals repeatedly in order to measure and assess physiological state. However, once a basic understanding of physiological state is developed, more easily detected indirect measures of physiological state can be used in some scenarios. For example, once a fundamental understanding of the hormonal rhythms of males and females during reproductive periods is understood, physiological state can sometimes be generalized for specific individuals, populations or species based on time of year or visual observation (Jainudeen et al., 1972). Likewise, visual observation of diseased individuals or those carrying a toxic burden can be integrated into studies of movement ecology once direct physiological monitoring is undertaken to validate such gross categorizations.
Inference of physiological state based on movement path or body accelerometry is increasingly common in movement ecology (Brown et al., 2013). In the emerging field of dynamic accelerometry, the assumption that movement requires energy serves as a basis for the modelling of energy expenditure based on movement behaviours (Green et al., 2009; Wilson et al., 2011). Developing a more nuanced and broader understanding of energy flow across space and time allows for the development of ‘energy landscape’ models in the study of animal movement (Shepard et al., 2013). However, we again caution that such assumptions must first be evaluated through rigorous direct sampling of physiological state to obtain corroborative evidence that such an energetic relationship exists for a given species and to identify other factors that are likely to contribute to energetic state (Halsey et al., 2011). Likewise, a ripe area for further research is the correlation between movement path characteristics or body accelerometry and endocrine system functions (Jachowski et al., 2013a). Once hormone sampling and assay techniques (such as faecal radioimmunoassays) are validated and measured for a species in a field setting, it might be possible to discern physiological state indirectly through movement behaviours.
Conclusion
Collectively, it is evident that studies of animal movement can be advanced greatly by better integration of measures of physiological state. Rapid advances over the past several decades have made a wide variety of tools available to measure the physiological state of free-ranging animals. Conceptually, we propose that these physiological measures should be investigated as both drivers and modulators of movement (Fig. 1). To achieve this in practice, researchers must proceed on two key fronts. First, research should be directed at discerning basic, predictable biorhythms of a species (e.g. energetic needs, reproductive timing). This can be viewed as a type of physiological baseline or envelope within which an individual typically operates. Second, research is needed to evaluate how physiological control mechanisms can influence an individual's response to disturbances or change and to enable a distinction to be made between the responses generated by different extrinsic conditions.
A major theme from our review is the need to integrate multiple measures or streams of data (e.g. reproductive status, energy expenditure, ambient temperature) to improve our understanding of relatively complex movement behaviours, such as migration. Undertaking such interdisciplinary investigations will not only help lead to a more mechanistic understanding of animal movement behaviour, but also allow us to improve our ability to predict how species are likely to respond to perturbations in a rapidly changing world and to develop appropriate conservation strategies.
Acknowledgements
We thank R. Jachowski, R. Nathan, S. Cooke and two anonymous reviewers for helpful comments that improved this manuscript.
References
- Adelman JS, Moyers SC, Hawley DM. (2014) Using remote biomonitoring to understand heterogeneity in immune-responses and disease-dynamics in small, free-living animals. Integr Comp Biol 54: 377–386. [DOI] [PubMed] [Google Scholar]
- Angilletta MJ, Sears MW, Pringle RM. (2009) Spatial dynamics of nesting behavior: lizards shift microhabitats to construct nests with beneficial thermal properties. Ecology 90: 2933–2939. [DOI] [PubMed] [Google Scholar]
- Artois M, Bengis R, Delahay RJ, Duchêne MJ, Duff JP, Ferroglio E, Gortaxar C, Hutchings MR, Kock RA, Leighton FA, et al. (2009) Wildlife disease surveillance and monitoring. In Delehay RJ, Smith GC, Hutchings MR, eds, Management of Disease in Wild Mammals. Springer, Japan, pp 187–213. [Google Scholar]
- Authier M, Péron C, Mante A, Vidal P, Grémille D. (2013) Designing observational biologging studies to assess the causal effect of instrumentation. Methods Ecol Evol 4: 802–810. [Google Scholar]
- Bennett AT. (1996) Do animals have cognitive maps? J Exp Biol 199: 219–224. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Webster JP, Macdonald DW. (2000) Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci 267: 1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan RM, Butler PJ, Woakes AJ, Prince PA. (1995) The energy expenditure of free-ranging black-browed albatrosses. Philos Trans R Soc B 350: 119–131. [Google Scholar]
- Bogard SJ, Block BA, Costa DP, Godley BJ. (2010) Biologging technologies: new tools for conservation. Endangered Species Res 10: 1–7. [Google Scholar]
- Bolen MS, Cochran WW, Wikelski MC. (2005) Biotelemetry of new world thrushes during migration: physiology, energetics and orientation in the wild. Integr Comp Biol 45: 295–304. [DOI] [PubMed] [Google Scholar]
- Bowlin MS, Bisson IA, Shamoun-Baranes J, Reichard JD, Sapir N, Marra PP, Kunz TH, Wilcove DS, Hedenström A, Guglielmo CG, et al. (2010) Grand challenges in migration biology. Integr Comp Biol 50: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer RT, Van Ballenberghe V, Kie JG, Maier JA. (1999) Birth-site selection by Alaskan moose: maternal strategies for coping with a risky environment. J Mammal 80: 1070–1083. [Google Scholar]
- Brower LP, Fink LS, Walford P. (2006) Fueling the fall migration of the monarch butterfly. Integr Comp Biol 46: 1123–1142. [DOI] [PubMed] [Google Scholar]
- Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. (2013) Observing the unwatchable through acceleration logging of animal behavior. Anim Biotelem 1: 20. [Google Scholar]
- Butler PJ, Green JA, Boyd IL, Speakman JR. (2004) Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct Ecol 18: 168–183. [Google Scholar]
- Cassone VM, Menaker M. (1984) Is the avian circadian system a neuroendocrine loop? J Exp Zool 232: 539–549. [DOI] [PubMed] [Google Scholar]
- Cocherell SA, Cocherell DE, Jones GJ, Miranda JB, Thompson LC, Cech JJ, Klimley AP. (2011) Rainbow trout Oncorhynchus mykiss energetic responses to pulsed flows in the American River, California, assessed by electromyogram telemetry. Environ Biol Fish 90: 29–41. [Google Scholar]
- Cooke SJ, O'Connor CM. (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. [Google Scholar]
- Cooke SJ, Hinch SG, Wikelski M, Andreas RD, Kuchel LJ, Wolcott TG, Butler PJ. (2004) Biotelemetry: a mechanistic approach to ecology. Trends Ecol Evol 19: 334–343. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Hinch SG, Crossin GT, Patterson DA, English KK, Healey MC, Shrimpton JM, Van Der Kraak G, Farrell AP. (2006) Mechanistic basis of individual mortality in Pacific salmon during spawning migrations. Ecology 87: 1575–1586. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Hinch SG, Farrell AP, Patterson DA, Miller-Saunders K, Welch DW, Donaldson MR, Hanson KC, Crossin GT, Mathes MT, et al. (2008) Developing a mechanistic understanding of fish migrations by linking telemetry with physiology, behavior, genomics and experimental biology: an interdisciplinary case study on adult Fraser River sockeye salmon. Fisheries 33: 321–338. [Google Scholar]
- Cooke SJ, Hinch SG, Donaldson MR, Clark TD, Eliason EJ, Crossin GT, Raby GD, Jeffries KM, Lapointe M, Miller K, et al. (2012) Conservation physiology in practice: how physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Philos Trans R Soc Lond B Biol Sci 367: 1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisler R. (2006) Mercury Hazards to Living Organisms. Taylor and Francis Publishers, London. [Google Scholar]
- Elliott JE, Bishop CA, Morrissey CA. (2011) Wildlife ecotoxicology: forensic approaches. In Elliott JE, Bishop CA, Morrissey CA, eds, Wildlife Ecotoxicology: Forensic Approaches. Springer, New York, pp 1–9. [Google Scholar]
- Emmons LH, Stark NM. (1979) Elemental composition of a natural mineral lick in Amazonia. Biotropica 11: 311–313. [Google Scholar]
- Franceschini MD, Custer CM, Custer TW, Reed JM, Romero LM. (2008) Corticosterone stress response in tree swallows nesting near polychlorinated biphenyl- and dioxin-contaminated rivers. Environ Toxicol Chem 11: 2326–2331. [DOI] [PubMed] [Google Scholar]
- Fryxell JM, Sinclair ARE. (1988) Causes and consequences of migration by large herbivores. Trends Ecol Evol 3: 237–241. [DOI] [PubMed] [Google Scholar]
- Fusani L, Gwinner E. (2004) Simulation of migratory flight and stopover affects night levels of melatonin in a nocturnal migrant. Proc Biol Sci 271: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, Gwinner E. (2005) Melatonin and nocturnal migration. Annu Rev NY Acad Sci 1046: 264–270. [DOI] [PubMed] [Google Scholar]
- Getz WM, Saltz D. (2008) A framework for generating and analyzing movement paths on ecological landscapes. Proc Natl Acad Sci USA 105: 19066–19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux MA, Berteaux D, Lecomte N, Gauthier G, Szor G, Bêty J. (2012) Benefiting from a migratory prey: spatio-temporal patterns in allochthonous subsidization of an arctic predator. J Anim Ecol 81: 533–542. [DOI] [PubMed] [Google Scholar]
- Goyman W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- Green JA. (2011) The heart rate method for estimating metabolic rate: review and recommendations. Comp Biochem Physiol A Mol Integr Physiol 158: 287–304. [DOI] [PubMed] [Google Scholar]
- Green JA, Halsey LG, Wilson RP, Frappell PB. (2009) Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart rate technique. J Exp Biol 212: 471–482. [DOI] [PubMed] [Google Scholar]
- Gwinner E, Schwabl-Benzinger I, Schwabl H, Dittami J. (1993) Twenty-four hour melatonin profiles in a nocturnally migrating bird during and between migratory seasons. Gen Comp Endocrinol 90: 119–124. [DOI] [PubMed] [Google Scholar]
- Halsey LG, Shepard ELC, Wilson RP. (2011) Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp Biochem Physiol A Mol Integr Physiol 158: 305–314. [DOI] [PubMed] [Google Scholar]
- Hambly C, Voigt C. (2011) Measuring energy expenditure in birds using bolus injections of 13C-labelled Na-bicarbonate. Comp Biochem Physiol A Mol Integr Physiol 258: 323–328. [DOI] [PubMed] [Google Scholar]
- Hebblewhite M, Merrill E, McDermid G. (2008) A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol Monogr 78: 141–166. [Google Scholar]
- Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. (2008) Trends and missing parts in the study of movement ecology. Proc Natl Acad Sci USA 105: 19060–19065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac NJB, Carbone C, McGill B. (2012) Population and community ecology. In Sibly RM, Browh JH, Kodric-Brown A, eds, Metabolic Ecology: A Scaling Approach. Wiley-Blackwell, London, pp 77–85. [Google Scholar]
- Jachowski DS, Slotow R, Millspaugh JJ. (2012) Physiological stress and refuge behavior by African elephants. PLoS ONE 7: e31818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jachowski DS, Slotow R, Montgomery RA, Millspaugh JJ. (2013a) Unravelling complex associations between physiological state and movement in African elephants. Funct Ecol 27: 1166–1175. [Google Scholar]
- Jachowski DS, Slotow R, Millspaugh JJ. (2013b) Corridor use and streaking behavior by African elephants in relation to physiological state. Biol Conserv 167: 276–282. [Google Scholar]
- Jainudeen MR, Katongole CB, Short RV. (1972) Plasma testosterone levels in relation to musth and sexual activity in the male Asiatic elephant, Elephas maximus. J Reprod Fertil 29: 99–103. [DOI] [PubMed] [Google Scholar]
- Jones JD, Kauffman MJ, Monteith KL, Scurlock BM, Albeke SE, Cross PC. (2014) Supplemental feeding alters migration of a temperate ungulate. Ecol Appl 24: 1769–1779. [DOI] [PubMed] [Google Scholar]
- Klein SL, Zink MC, Glass GE. (2004) Seoul virus infection increases aggressive behaviour in male Norway rats. Anim Behav 67: 421–429. [Google Scholar]
- Lennox RJ, Chapman J, Souliére C, Tudorache C, Wikelski M, Metcalfe J, Cooke SJ. (2015) Conservation physiology in animal migration. Conserv Physiol, in press. [DOI] [PMC free article] [PubMed]
- Louzao M, Wiegand T, Bartumeus F, Weimerskirch H. (2014) Coupling instantaneous energy-budget models and behavioural mode analysis to estimate optimal foraging strategy: an example with wandering albatrosses. Mov Ecol 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J, Moberg F. (2003) Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6: 87–98. [Google Scholar]
- McEwen BS, Wingfield JC. (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- McWilliams SR, Guglielmo C, Pierce B, Klaassen M. (2004) Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35: 377–393. [Google Scholar]
- Meier AH, Farner DS, King JR. (1965) A possible endocrine basis for migratory behaviour in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Anim Behav 13: 453–465. [DOI] [PubMed] [Google Scholar]
- Middleton AD, Kauffman MJ, McWhirter DE, Cook JG, Cook RC, Nelson AA, Jimenez MD, Klaver RW. (2013) Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology 94: 1245–1256. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE. (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–199. [DOI] [PubMed] [Google Scholar]
- Monteith KL, Bleich VC, Stephenson TR, Pierce BM, Conner MM, Klaver RW, Bowyer RT. (2011) Timing of seasonal migration in mule deer: effects of climate, plant phenology, and life-history characteristics. Ecosphere 2: art47. [Google Scholar]
- Moore J. (1995) The behavior of parasitized animals. BioScience 45: 89–96. [Google Scholar]
- Mooring MS, Hart BL. (1992) Animal grouping for protection from parasites: selfish herd and encounter-dilution effects. Behaviour 123: 173–193. [Google Scholar]
- Morales JM, Haydon DT, Friar J, Holsinger KE, Fryxell JM. (2004) Extracting more out of relocation data: building movement models as mixtures of random walks. Ecology 85: 2436–2445. [Google Scholar]
- Morrison GRI, Davidson NC, Wilson JR. (2007) Survival of the fattest: body stores on migration and survival in red knots Calidris canutus islandica. J Avian Biol 38: 479–487. [Google Scholar]
- Mueller T, Fagan WF. (2008) Search and navigation in dynamic environments – from individual behaviors to population distributions. Oikos 117: 654–664. [Google Scholar]
- Munro RHM, Nielsen SE, Price MH, Stenhouse GB, Boyce MS. (2006) Seasonal and diel patterns of grizzly bear diet and activity in west-central Alberta. J Mammal 87: 1112–1121. [Google Scholar]
- Nagy KA, Costa DP. (1980) Water flux in animals: analysis of potential errors in the tritiated water method. Am J Physiol Regul Integr Comp Physiol 238: R454–R465. [DOI] [PubMed] [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. (2008) A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA 105: 19052–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton I. (2010) The Migration Ecology of Birds. Academic Press, New York. [Google Scholar]
- O'Connor CM, Norris DR, Crossin GT, Cooke SJ. (2014) Biological carryover effects: linking common concepts and mechanisms in ecology and evolution. Ecosphere 5: art28. [Google Scholar]
- Ortiz-Maciel SG, Hori-Ochoa C, Enkerlin-Hoeflich E. (2010) Maroon-fronted parrot (Rhynchopsitta terrisi) breeding home range and habitat selection in the northern Sierra Madre Oriental, Mexico. Wilson J Ornithol 122: 513–517. [Google Scholar]
- Paradis E, Baillie SR, Sutherland WJ, Gregory RD. (1998) Patterns of natal and breeding dispersal in birds. J Anim Ecol 67: 518–536. [Google Scholar]
- Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulo J. (2008) State–space models of individual animal movement. Trends Ecol Evol 23: 87–94. [DOI] [PubMed] [Google Scholar]
- Poole JH. (1987) Rutting behavior in African elephants: the phenomenon of musth. Behaviour 102: 283–316. [Google Scholar]
- Poulin R. (2000) Manipulation of host behaviour by parasites: a weakening paradigm? Proc Biol Sci 267: 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. (2010) Parasite manipulation of host behavior: an update and frequently asked questions. Adv Study Behav 41: 151–186. [Google Scholar]
- Powell LL, Powell TU, Powell GV, Brightsmith DJ. (2009) Parrots take it with a grain of salt: available sodium content may drive collpa (clay lick) selection in southeastern Peru. Biotropica 41: 279–282. [Google Scholar]
- Pulido F. (2007) Phenotypic changes in spring arrival: evolution, phenotypic plasticity, effects of weather and condition. Clim Res 35: 5–23. [Google Scholar]
- Redfern JV, Grant R, Biggs H, Getz WM. (2003) Surface-water constraints on herbivore foraging in the Kruger National Park, South Africa. Ecology 84: 2092–2107. [Google Scholar]
- Ricklefs RE, Wikelski M. (2002) The physiology/life-history nexus. Trends Ecol Evol 17: 462–468. [Google Scholar]
- Robbins C. (1983) Wildlife Feeding and Nutrition. Academic Press, London, UK. [Google Scholar]
- Romero LM, Butler LK. (2007) Endocrinology of stress. Int J Comp Psychol 20: 85–95. [Google Scholar]
- Ropert-Coudert Y, Wilson RP. (2005) Trends and perspectives in animal-attached remote sensing. Front Ecol Environ 3: 437–444. [Google Scholar]
- Rutberg AT. (1987) Horse fly harassment and the social behavior of feral ponies. Ethology 75: 145–154. [Google Scholar]
- Sahlsten J, Bunnefeld N, Månsson J, Ericsson G, Bergström R, Dettki H. (2010) Can supplementary feeding be used to redistribute moose? Wildl Biol 16: 85–92. [Google Scholar]
- Sand H, Zimmermann B, Wabakken P, Andrèn H, Pedersen HC. (2005) Using GPS technology and GIS cluster analyses to estimate kill rates in wolf-ungulate ecosystems. Wildl Soc Bull 33: 914–925. [Google Scholar]
- Sawyer H, Kauffman MJ. (2011) Stopover ecology of a migratory ungulate. J Anim Ecol 80: 1078–1087. [DOI] [PubMed] [Google Scholar]
- Schick RS, Loarie SR, Colchero F, Best BD, Boustany A, Conde DA, Halpin PN, Joppa LN, McClellan CM, Clark JS. (2008) Understanding movement data and movement processes: current and emerging directions. Ecol Lett 11: 1338–1350. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. (1997) Animal Physiology: Adaptation and Environment. Cambridge University Press, Cambridge. [Google Scholar]
- Schneider T, Thalau HP, Semm P, Wiltschko W. (1994) Melatonin is crucial for the migratory orientation of pied flycatchers (Ficedula hypoleuca Pallas). J Exp Biol 194: 255–262. [DOI] [PubMed] [Google Scholar]
- Sears MW, Angilletta MJ. (2015) Costs and benefits of thermoregulation revisited: both the heterogeneity and spatial structure of temperature drive energetic costs. Am Nat 185: E94–E102. [DOI] [PubMed] [Google Scholar]
- Shaffer SA. (2011) A review of seabird energetics using doubly labeled water method. Comp Biochem Physiol A Mol Integr Physiol 258: 315–322. [DOI] [PubMed] [Google Scholar]
- Shepard E, Wilson R, Quintana F, Gomez Laich A, Forman D. (2009) Pushed for time or saving on fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proc Biol Sci 276: 3149–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard EL, Wilson RP, Rees WG, Grundy E, Lambertucci SA, Vosper SB. (2013) Energy landscapes shape animal movement ecology. Am Nat 182: 298–312. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166: 869–887. [DOI] [PubMed] [Google Scholar]
- Signer C, Ruf T, Schober F, Fluch G, Paumann T, Arnold W. (2010) A versatile telemetry system for continuous measurement of heart rate, body temperature and locomotor activity in free-ranging ruminants. Methods Ecol Evol 1: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Ericsson G. (2014) Changing motivations during migration: linking movement speeds to reproductive status in a migratory large mammal. Biol Lett 10: 20140379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Grachev IA, Bekenov AB, Milner-Gulland EJ. (2010) Saiga antelope calving site selection is increasingly driven by human disturbance. Biol Conserv 143: 1770–1779. [Google Scholar]
- Speakman J. (1997) Doubly Labeled Water: Theory and Practice. Chapman and Hall, London, UK. [Google Scholar]
- Speakman JR. (2005) The role of technology in the past and future development of the doubly labelled water method. Isotopes Environ Health Stud 41: 335–343. [DOI] [PubMed] [Google Scholar]
- Stephenson R. (1997) Effects of oil and other surface-active organic pollutants on aquatic birds. Environ Conserv 2: 121–129. [Google Scholar]
- Thomson JA, Heithaus MR. (2014) Animal-borne video reveals seasonal activity patterns of green sea turtles and the importance of accounting for capture stress in short-term biologging. J Exp Mar Biol Ecol 450: 15–20. [Google Scholar]
- Tomlinson S, Maloney SK, Withers PC, Voigt CC, Cruz-Neto AP. (2013) From doubly labelled water to half-life; validating radio-isotopic rubidium turnover to measure metabolism in small vertebrates. Methods Ecol Evol 4: 619–628. [Google Scholar]
- Tomlinson S, Arnall SG, Munn A, Bradshaw SD, Maloney SK, Dixon KW, Didham RK. (2014) Applications and implications of ecological energetics. Trends Ecol Evol 29: 280–290. [DOI] [PubMed] [Google Scholar]
- Urban M, Phillips BL, Skelly D, Shine R. (2007) The cane toad's (Chaunus [Bufo] marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proc Biol Sci 274: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JC. (1978) Isotopic assessment of the dietary habits of ungulates. S Afr J Sci 74: 298–301. [Google Scholar]
- Wada H, Cristol DA, McNabb FA, Hopkins WA. (2009) Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 43: 6031–6038. [DOI] [PubMed] [Google Scholar]
- Walker BG, Boersma PD, Wingfield JC. (2005) Field endocrinology and conservation biology. Integr Comp Biol 45: 12–18. [DOI] [PubMed] [Google Scholar]
- Ward S, Bishop CM, Woakes AJ, Butler PJ. (2002) Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J Exp Biol 205: 3347–3356. [DOI] [PubMed] [Google Scholar]
- Wasserman MD, Chapman CA, Milton K, Gogarten JF, Wittwer DJ, Ziegler TE. (2012) Estrogenic plant consumption predicts red colobus monkey (Procolobus rufomitratus) hormonal state and behavior. Horm Behav 62: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Cassey P, Schimpf N, Halsey L, Green J, Portugal S. (2013) Implantation reduces the negative effects of bio-logging devices on birds. J Exp Biol 216: 537–542. [DOI] [PubMed] [Google Scholar]
- Whitehouse AM, Schoeman DS. (2003) Ranging behaviour of elephants within a small, fenced area in Addo Elephant National Park, South Africa. Afr Zool 38: 95–108. [Google Scholar]
- Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML. (2006) Simple rules guide dragonfly migration. Biol Lett 2: 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove DS, Wikelski M. (2008) Going, going, gone: is animal migration disappearing? PLoS Biol 6: e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ADM, Wikelski M, Wilson RP, Cooke SJ. (2015) Utility of biological sensor tags in animal conservation. Conserv Biol 29: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. (2006) Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol 75: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Griffiths IW, Legg PA, Friswell MI, Bidder OR, Halsey LG, Shepart ELC. (2011) Turn costs change the value of animal search paths. Ecol Lett 16: 1145–1150. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Romenofsky M. (1997) Corticosterone and facultative dispersal in response to unpredictable events. Ardea 85: 155–166. [Google Scholar]
- Wingfield JC, Schwabl H, Mattocks PW. (1990) Endocrine mechanisms of migration. In Winner E, ed., Bird Migration. Springer, Berlin, pp 232–256. [Google Scholar]
- Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J. (1997) Environmental stress, field endocrinology, and conservation biology. In Clemmons JR, Buchholz R, eds, Behavioral Approaches to Conservation in the Wild. Cambridge University Press, Cambridge, pp 95–131. [Google Scholar]