Artificial light at night (ALAN) is known to disrupt the nocturnal dispersal behaviour of Atlantic salmon fry, yet the underlying physiological mechanism is unknown. Here novel methodologies are utilised to examine whether behavioural disruption seen in dispersing fry is mediated via a cortisol stress response. Fry showed the capacity to mount a stress response, however, the two methods for sampling cortisol provide conflicting results as to whether ALAN acts as a stressor to dispersing fry.
Keywords: Artificial light at night, Atlantic salmon, cortisol
Abstract
Artificial light at night (ALAN) is gaining recognition as having an important anthropogenic impact on the environment, yet the behavioural and physiological impacts of this stressor are largely unknown. This dearth of information is particularly true for freshwater ecosystems, which are already heavily impacted by anthropogenic pressures. Atlantic salmon (Salmo salar L.) is a species of conservation and economic importance whose ecology and behaviour is well studied, making it an ideal model species. Recent investigations have demonstrated that salmon show disrupted behaviour in response to artificial light; however, it is not yet clear which physiological processes are behind the observed behavioural modifications. Here, two novel non-invasive sampling methods were used to examine the cortisol stress response of dispersing salmon fry under different artificial lighting intensities. Fish egg and embryos were reared under differing ALAN intensities and individual measures of stress were subsequently taken from dispersing fry using static sampling, whereas population-level measures were achieved using deployed passive samplers. Dispersing fry exposed to experimental confinement showed elevated cortisol levels, indicating the capacity to mount a stress response at this early stage in ontogenesis. However, only one of the two methods for sampling cortisol used in this study indicated that ALAN may act as a stressor to dispersing salmon fry. As such, a cortisol-mediated response to light was not strongly supported. Furthermore, the efficacy of the two non-invasive methodologies used in this study is, subject to further validation, indicative of them proving useful in future ecological studies.
Introduction
Since the Industrial Revolution, and particularly over the last 60 years, the number of outdoor lights has increased rapidly across the UK. Globally, the number of artificial lights is increasing by 6% each year (Hölker et al., 2010) and 3% annually in the UK (Royal Commission Report, 2009). Despite growing concerns (Royal Commission Report, 2009), there are few systematic data demonstrating the ecological effects of artificial light at night (ALAN; Rich and Longcore, 2006). Artificial light at night is primarily, but not entirely, as a result of streetlights in public areas, along roads and highways (Gaston and Bennie, 2014). Artificial light at night is increasingly thought to alter the behaviour and/or physiology of a broad range of species and taxa (Rich and Longcore, 2006). Under ALAN, disruptions have been documented in the daily rhythms of nocturnal primates (LeTallec et al., 2013), bird singing behaviour (Miller, 2006) and the community composition of terrestrial invertebrates (Davies et al., 2012). There is, however, a notable paucity of information on the impacts of ALAN on aquatic systems (Perkin et al., 2011; Kronfeld-Schor et al., 2013). There has been a recent upsurge in interest in determining whether light may be having a detrimental impact on the health and functioning of organisms (Gaston et al., 2014), but successful conservation and mitigation requires that the impact of ALAN is better understood across a range of taxa and ecosystems.
While the physiological effects of artificial lights used in aquaculture systems are well known and often intended (Boeuf and LeBail, 1999), there is evidence that ALAN can cause physiological stress (increased plasma cortisol and glucose) in farmed Atlantic salmon (Salmo salar L.; Migaud et al., 2007). Artificial light at night may, therefore, impact upon the physiology of wild fish species (McConnell et al., 2010). Artificial lighting can be over a million times brighter than natural nocturnal illumination and, as such, changes to the lighting regimen to which animals are adapted will be likely to result in large-scale behavioural changes (Perry et al., 2008). Artificial light at night has been shown to have a negative effect on the behaviour of a number of important stages in the life cycle of Atlantic salmon, such as the delayed dispersal of fry (Riley et al., 2013, 2015) and the migration of smolts (Riley et al., 2012a), because these occur primarily at night (Riley and Moore, 2000; Riley et al., 2012b). However, it is not known how these behaviours are mediated physiologically, despite suggestions from previous studies that cortisol stress responses are mounted by teleost fish exposed to daytime aquarium light of different type, colour and intensity (Migaud et al., 2007). On the contrary, a recent study found no effect of ALAN on the cortisol response of European perch (Perca fluviatilis; Brüning et al., 2015).
Anthropogenic impacts on the environment have increased in number and diversified greatly to include a number of chemical, biological and physical factors (Fair and Becker, 2000) and should often be considered as stressors to the species they impact upon. Freshwater ecosystems are the most heavily debased ecosystem globally and are subject to many stressors, both anthropogenic (pollution, habitat alteration and invasive species) and as a result of climate change. For this reason, it is of the utmost importance to gain a full understanding of the influence of any anthropogenic impacts on juvenile Atlantic salmon. This study sought to apply novel methodology to test whether the behavioural delay seen in dispersing Atlantic salmon fry under ALAN (Riley et al., 2013, 2015) is mediated by a cortisol stress response. Previous research suggests strong species specificity regarding the ontogeny, magnitude and duration of the cortisol response (Feist and Schreck, 2002; Fanouraki et al., 2011). Specifically, the developmental stage at which fish are able to mount a response to stressors is dependent upon both species and environment (De Jesus and Hirano, 1992; Barry et al., 1995; Stephens et al., 1997; Stouthart et al., 1998; Feist and Schreck, 2002; Jentoft et al., 2002; Auperin and Geslin, 2008). Two methodologies were applied to this ecological concern: firstly, deployable passive samplers were used to determine the population-level response; and secondly, individual measures of stress were assessed using static water samples. If these studies provide viable methods for non-invasively determining the stress response of Atlantic salmon, and other vulnerable freshwater fish species, to a given external stimulus, their use in conservation research will be an extremely interesting subject for further validation.
Materials and methods
Experimental design
Experimental work was conducted at the Centre for Environment Fisheries and Aquatic Science (Cefas) laboratory aquarium, Lowestoft, UK (52°27′33″N, 1°44′22″E). For full details of the aquarium set-up see Riley et al. (2015). Daytime lighting was representative of natural daylight (1177–728 lux, 14 h light–10 h dark), provided by daylight-mimicking, low-pressure mercury discharge fluorescent lamps. Nocturnal illumination was provided by a metal halide streetlight (Philips Master Cosmo White) mounted in a luminaire (Philips ‘iridium series’ opti-C unit). Neutral density filters were attached to the streetlight to reduce the intensity to levels measured in the field without altering the spectrum. The incubators (test tanks) were 1.7 m below the streetlight, positioned to create different nocturnal light intensities at the surface of the water, as follows: 8, 4, 2, 1 and 0.1 lux, with two replicates at each light level treatment. The 0.1 lux level is representative of moonlight (Riley et al., 2013) and was considered a control treatment.
On 22 February 2012, 500 Atlantic salmon fertilized eggs (development ∼260 deg days) sourced from wild-caught broodstock (Kielder Hatchery, Northumberland, UK) were implanted into each of ten 75 l black plastic deep substrate incubators. Eggs developed in the gravel (as in the wild) under the different nocturnal light treatments until they hatched, emerged from the gravel and swam up into the water column to disperse. Fish dispersing through the outflow of the incubators were retained in mesh collecting boxes over a 9 day period of 24 h monitoring (10–19 April 2012; see Riley et al., 2015).
The stress status of these fry from different light treatments was assessed by non-invasive measurement of cortisol released into the surrounding water via the gills, a technique previously applied to salmon, although not at this early life-history stage (Ellis et al., 2007, 2012; Kittilsen et al., 2009). The surrounding water, into which fish release free steroids (including cortisol) via the gills, is the most common matrix used for non-invasive monitoring of fish hormones (see Ellis et al., 2013). The following two independent sampling methods were used to collect free cortisol: (i) water from the flow-through incubators; and (ii) static water from separate containers.
Population cortisol sampling from the incubators
Owing to the low expected concentration of cortisol in the water of the incubator, direct point sampling was not attempted; instead, a novel method was used, in which cortisol is absorbed by a passive sampler to provide an integrated hormonal history of a fish population over time (Scott and Ellis, 2007). Although it is assumed that passive samplers absorb steroids at a rate dependent upon their concentration in the water, their use for cortisol has not yet been validated. However, as all samplers were treated identically in all experimental incubators, for the purpose of this study, any differences in the recovery rates of the individual Polar Organic Chemical Integrated Samplers (POCIS) will not have influenced the overall results. It must also be recognized that tank-specific factors, such as water flow (mean 252.54 l h−1, minimum 200 l h−1 and maximum 300 l h−1) and biofilm growth on samplers, could affect uptake rate.
The POCIS were prepared by a standard method (see Alvarez et al., 2004), and one was deployed in each incubator, in similar positions on the surface of the gravel. Each incubator was implanted with 500 fertilized eggs at the start of the experiment. The POCIS were deployed when it was calculated that the embryos had absorbed the majority of their yolk sac and were close to dispersal (based on predicted development using degree-days) to limit uptake of any residual maternal cortisol and reflect any cortisol response to treatment. The POCIS were placed in each of the incubators on 25 March 2012, removed on 21 April 2012 and stored at −20°C. Cortisol was extracted from the sorbent by methanol elution, followed by solid-phase extraction (see Alvarez et al., 2004) with ethyl acetate as the final eluate. Extracts were stored at −20°C until evaporation (under nitrogen) and reconstitution in 1 ml of radioimmunoassay (RIA) buffer for assay.
Individual cortisol sampling using separate static containers
In this method, individual fish were removed from the mesh collecting boxes and placed in a small container of clean water for a standard time. Sampling of water from static containers has been used to assess the release of a variety of steroids in a diverse range of fish species (Ellis et al., 2013). This technique, however, has not previously been applied to fish <0.5 g in weight, at an early ontogenetic stage. As such, the static sampling technique suffers from the potential disadvantage that the procedure itself (handling and confinement) may affect the amount of cortisol released into the water; this impact can be limited by restricting the duration of the collection period.
Immediately after entry into the mesh collecting box, individual fry were netted and placed in a beaker containing 10 ml of clean inflow water. After 30 min, the fry were removed and weighed. Water samples were placed temporarily on ice (maximum 2 h) before storage at −20°C. Thawed water samples were passed through solid-phase extraction cartridges [Sep-pak® Plus, C18 (360 mg), Waters Ltd., UK], the cortisol was retrieved by ethyl-acetate elution, and this eluate was evaporated under nitrogen before reconstitution in 0.5 ml of RIA buffer for assay (Ellis et al., 2004). Additional quality-control samples (blank samples of clean inflow water and cortisol-spiked inflow water samples) were prepared and processed contemporaneously. The spiked samples were prepared by addition of cortisol (Sigma Aldrich, UK) dissolved in ethanol; the genuine (rather than nominal) spike was determined by RIA of equivalent 50 µl aliquots of the spiking solution (after evaporation under nitrogen and reconstitution in RIA buffer).
As this method has not previously been applied to small fish at an early ontogenetic stage, three individual fry were placed in beakers of clean inflow water for 1 h (ascribed as positive control, PC). This was to determine whether fry at the dispersal stage mount a cortisol response to a known stressor of older fish (i.e. handling and confinement). Although a number of species appear able to synthesize cortisol at the time of hatching, the development of a cortisol response to stressors appears later in development (Jentoft et al., 2002). It has previously been demonstrated that Atlantic salmon are not capable of mounting a stress response until 72 days post-hatching at 6°C (432 deg days; Neachev et al., 2006).
Radioimmunoassay
Amounts of cortisol in extracts were measured with an in-house radioimmunoassay (Ellis et al., 2004). A 100 µl aliquot of extract (or dilution of extract) was added to duplicate glass tubes, and nine standards [2, 4, 8, 16, 32, 64, 125, 250 and 500 pg (100 µl)−1] were made up by serial dilution. To all tubes was then added 100 µl of assay buffer containing ∼5700 d.p.m. of tritiated cortisol and sufficient antibody to bind 48% of the radiolabel in the absence of radioinert steroid. Tubes were left to equilibrate overnight (≥16 h, 4°C), and unbound steroid was removed by addition of dextran-coated charcoal (30 min on ice). After centrifugation (12 min at 1000g), the liquid was decanted and the remaining tritiated cortisol counted (Beckman LS6500 scintillation counter). Amounts of cortisol in extracts were determined from the standard curve. The intra-assay coefficient of variation was 6%, and the inter-assay coefficient of variation has previously been assessed at 11% (Ellis et al., 2004).
Data analysis
The cortisol RIA provides estimates of the amount of cortisol (in picograms) in an extract. For statistical comparisons, individual water cortisol sample values were converted to a release rate (in picograms per gram per hour) to standardize for fish size (Ellis et al., 2013); population water cortisol values (from POCIS samples) were not converted because it is not known how sampler uptake relates to the amount in the water. Differences in fish biomass and exposure time between tanks and treatments may therefore affect the population water cortisol values. Statistical analyses were conducted in R (version 2.13.2; R Development Core Team, 2012). Factors (light, fish mass, day and incubator) influencing the cortisol release rate of the dispersing fry were evaluated using generalized linear models, refined using Akaike information criterion comparisons between candidate model structures, combined with deletions of non-significant terms to identify a minimal adequate model containing only significant factors.
Results
A total of 297 fish were sampled individually during the dispersal period of 9 days. A generalized linear model revealed no significant differences in the body mass of sampled fish over time (F1,31 = 1.722, P = 0.199) or between individual experimental incubators (F9,31 = 0.942, P = 0.504) or light intensities (F1,36 = , P = 0.538). A representative subset of 48 treatment samples from across the sampling period and experimental incubators was initially assayed, plus three PC and six quality-control samples. The remaining samples were not processed owing to the lack of significant treatment effects.
Population cortisol sampling from the incubators
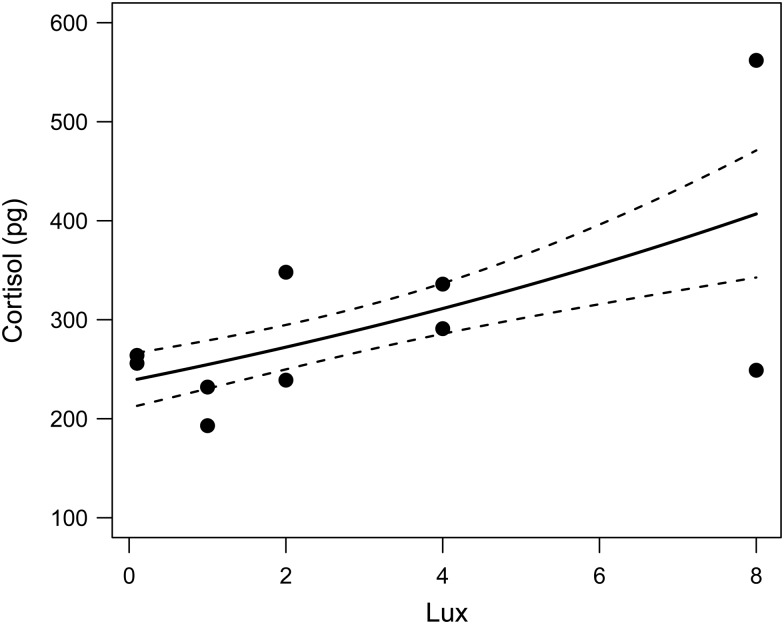
Cortisol was readily measurable in all 10 POCIS samples, falling in the middle of the RIA standard curve (median 26 pg). Light intensity had a marginally significant influence on the cortisol content of the POCIS samples, with cortisol content in the POCIS sample shown to increase as light intensity increased (Fig. 1).
Figure 1:
Amounts of cortisol (in picograms) retrieved from the Polar Organic Chemical Integrated Samplers at each of the experimental light intensities (in lux), with a line of best fit (±1 SEM) generated from a generalized linear model of cortisol (dependent variable) in relationship to light intensity (independent variable) using a γ error family and a log-link function (F1,8 = 5.979, P = 0.0415).
Individual cortisol sampling using separate static containers
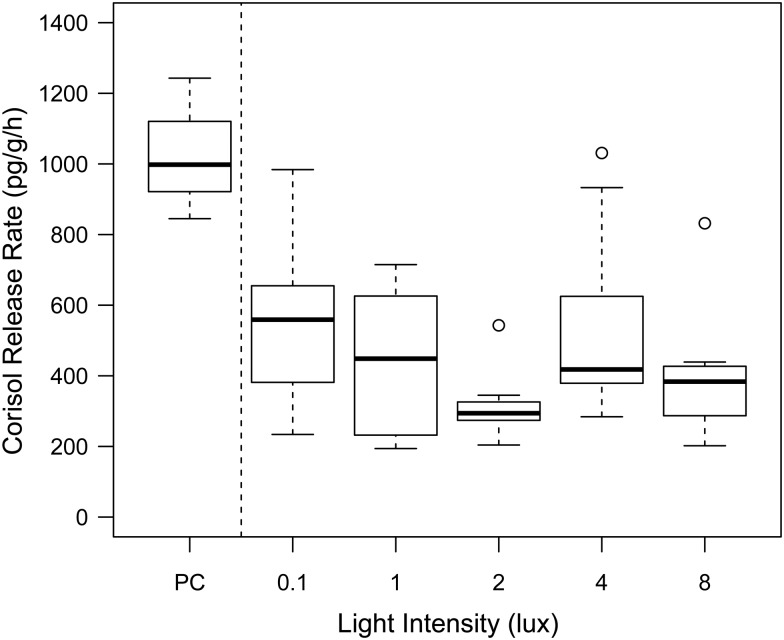
Cortisol was readily measurable in the three PC samples (confined for 1 h), with samples falling in the middle of the RIA standard curve (median 34 pg). The amounts of cortisol in the 48 treatment samples (confined for 30 min) were lower, largely falling within the upper third of the RIA standard curve (median 15 pg). The three quality-control blank samples returned zero or negligible cortisol values. The three spiked quality-control samples indicated highly variable recoveries of 125–350% of the cortisol spike (21 pg) added.
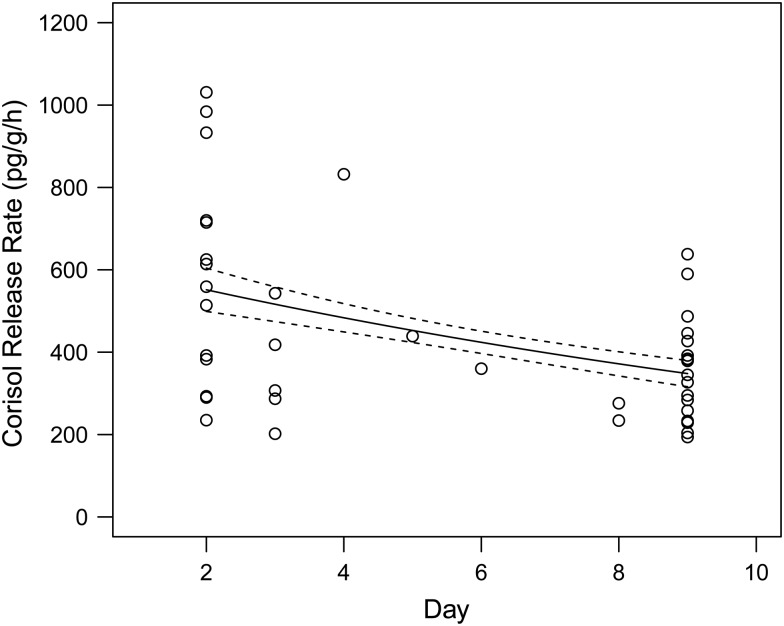
Cortisol release rates (i.e. adjusted for duration) were significantly greater in the PC, actively stressed, fry than treatment fry (F1,43 = 12.37, P = 0.001), indicating that the PC fry mounted a cortisol response to handling and/or confinement (Fig. 2). Within the light treatment groups, there was no significant difference in cortisol release rate between the control (0.1 lux) and elevated light groups (1, 2, 4 and 8 lux, F4,36 = 2.006, P = 0.114). There was a significant effect of sampling day on cortisol release rates (F1,40 = 11.351, P = 0.002), with cortisol release rates decreasing in fish sampled later in the study period (Fig. 3).
Figure 2:
Cortisol release rate of individual fry in relationship to nocturnal light level (0.1–8 lux) in each of the experimental treatments (n = 48; number of samples from each treatment: 0.1 lux n = 9, 1 lux n = 8, 2 lux n = 8, 4 lux n = 9 and 8 lux n = 10) and in the positive control (PC, n = 3). Significantly different cortisol release rates in the actively stressed fry (F1,43 = 12.37, P = 0.001) are indicated (*).
Figure 3:
Cortisol release rate declined significantly across the nine sampling days. The line of best fit (±1 SEM) was generated from the generalized linear model with a γ error family and a log-link function (F1,36 = 9.793, P = 0.003).
Discussion
The work described here demonstrates two important points. Firstly, the results of this study showed no significant effect of ALAN, at varying intensities, on the cortisol stress response of individually sampled fry. As such, the results do not clearly support the hypothesis that the previously observed delay in dispersal behaviour caused by ALAN (Riley et al., 2013, 2015) is associated with a cortisol stress response. The results show that ALAN did not significantly affect the levels of cortisol sampled in individual fish, although there was a marginally significant effect in the population data as sampled using the POCIS method. The lack of a significant result seen here suggests that although the dispersal behaviour of Atlantic salmon fry is disrupted by ALAN, this behaviour does not appear to be mediated by a cortisol stress response. However, an alternative explanation for the lack of response seen to dispersing under ALAN is that the very act of dispersing may itself produce a stress response in the fry, which could have masked any treatment effect. There was, however, a significant effect of sampling day on the cortisol release rate of the dispersing fry, with fish dispersing later in the sampling period having a lower cortisol release rate than those dispersing earlier. The finding that fry dispersing under ALAN had a delayed dispersal compared with the control fry (Riley et al., 2015), but with no increase in cortisol seen, could possibly suggest acclimation to ALAN; as such, the cortisol response of an individual fry may be negatively correlated with the length of time they are exposed to the light. The relatively small sample size limits the power of these tests, and further samples would clarify the result.
Secondly, we have demonstrated that non-invasive sampling of cortisol status is possible at this early stage in salmon development. Previous studies examining the ontogeny or presence of a cortisol response have measured total body cortisol or plasma cortisol levels. Passive sampling and separate static container sampling proved to be viable non-invasive techniques for investigating the cortisol status of fry at the dispersal stage. Nevertheless, additional refinement and validation is required (see Ellis et al., 2013) to ensure that methodological issues are not masking or confounding experimental conclusions.
For the passive sampling, it remains to be seen that cortisol uptake is related to the concentration in the water, and corrections for changes in biomass during the exposure period must be incorporated. For the separate static container method, an appropriate duration of confinement must be determined. The duration needs to be short enough to minimize any impact of handling and confinement on cortisol release, but long enough to ensure that sufficient cortisol is collected for assay. The observed difference in release rate between the 30 and 60 min periods of confinement indicates that this initial selection (based upon previous studies) was appropriate. Nevertheless, the unexplained high and variable recovery of the spiked samples within this study needs to be addressed because it may be associated with the low water volume and amounts of cortisol.
A final point to note is that through the positive controls used, this is the first study to reveal a cortisol stress response to an external stimulus in Atlantic salmon at this early stage of development. Juvenile Atlantic salmon were previously thought to be unable to mount a stress response except in response to an induced stimulus, such as injection of adrenaline (Neachev et al., 2006). Here we demonstrate that, in line with other salmonid species (Oncorhynchus mykiss, Barry et al., 1995, Karakatsouli et al., 2008; Oncorhynchus tshawytscha, Feist and Schreck, 2002), Atlantic salmon can mount a cortisol response to an external stimulus at the dispersal stage.
In summary, further work is required to elucidate whether the physiology of dispersing Atlantic salmon fry is influenced by ALAN; however, the techniques described here provide valuable non-destructive methodologies to assess the hormonal status of fish at this early ontological stage. The methodologies have proved capable of detecting cortisol in dispersing salmon and will no doubt prove useful in future ecological studies subject to subsequent validation, as outlined previously. Artificial light at night is only one of many anthropogenic pressures that impact upon freshwater ecosystems, and a full understanding of the physiological effect that these pressures have on freshwater species is required for conservation and management purposes. Furthermore, the methodologies provided in the present study allow for the response to anthropogenic stressors to be characterized over time and could, for example, determine whether acclimatization occurs when fish are exposed to these stressors for a sustained period. In the case of ALAN, an understanding of whether acclimatization or a reduced response with age occurs in salmon would be of use when attempting to mitigate its impact.
Funding
The study was funded by the Department for the Environment, Food and Rural Affairs (Defra), UK Government, under contract SF0258. R.C.N. was funded by a KESS PhD scholarship.
Acknowledgements
The authors would like to thank the aquarium staff and Jan Brant at Cefas, Lowestoft for their assistance during the planning/experimental stages and Richard Bond and David Kirkland, UK Environment Agency Kielder Hatchery, for supplying the eyed salmon ova.
References
- Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan JE. (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23: 1640–1648. [DOI] [PubMed] [Google Scholar]
- Auperin B, Geslin M. (2008) Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen Comp Endocrinol 158: 234–239. [DOI] [PubMed] [Google Scholar]
- Barry TP, Malison JA, Held JA, Parrish JJ. (1995) Ontogeny of the cortisol stress response in larval rainbow trout. Gen Comp Endocrinol 97: 57–65. [DOI] [PubMed] [Google Scholar]
- Boeuf G, Le Bail P. (1999) Does light have an influence on fish growth? Aquaculture 177: 129–152. [Google Scholar]
- Brüning A, Hölker F, Franke S, Preuer T, Kloas W. (2015) Spotlight on fish: light pollution affects circadian rhythms of European perch but does not cause stress. Sci Total Environ 511: 516–522. [DOI] [PubMed] [Google Scholar]
- Davies TW, Bennie J, Gaston KJ. (2012) Street lighting changes the composition of invertebrate communities. Biol Lett 8: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus EGT, Hirano T. (1992) Changes in whole body concentrations of cortisol, thyroid hormones, and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen Comp Endocrinol 85: 55–61. [DOI] [PubMed] [Google Scholar]
- Ellis T, James JD, Stewart JC, Scott AP. (2004) A non-invasive stress assay based upon measuring cortisol release into the water by rainbow trout. J Fish Biol 65: 1233–1252. [Google Scholar]
- Ellis T, James JD, Fridell F, Sundh H, Sundell K, Scott AP. (2007) Non-invasive measurement of cortisol and melatonin in seawater Atlantic salmon tanks. Aquaculture 272: 698–706. [Google Scholar]
- Ellis T, Yavuzcan H, López-Olmeda J, Spedicato MT, Tort L, Øverli Ø, Martins C. (2012) Cortisol and finfish welfare. Fish Physiol Biochem 38: 163–188. [DOI] [PubMed] [Google Scholar]
- Ellis T, Sanders MB, Scott AP. (2013) Non-invasive monitoring of steroids in fishes. Vet Med Austria 100: 255–269. [Google Scholar]
- Fair PA, Becker PR. (2000) Review of stress in marine mammals. J Aquat Ecosys Stress Recov 7: 335–354. [Google Scholar]
- Fanouraki E, Mylonas CC, Papandroulakis N, Pavlidis M. (2011) Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen Comp Endocrinol 173: 313–322. [DOI] [PubMed] [Google Scholar]
- Feist G, Schreck CB. (2002) Ontogeny of the stess response in chinook salmon, Oncorhynchus tshawytscha. Fish Physiol Biochem 25: 31–40. [Google Scholar]
- Gaston KJ, Bennie J. (2014) Demographic effects of artificial nighttime lighting on animal populations. Environ Rev 22: 323–330. [Google Scholar]
- Gaston KJ, Duffy JP, Gaston S, Bennie J, Davies TW. (2014) Human alteration of natural light cycles: causes and ecological consequences. Oecologia 176: 917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölker F, Wolter C, Perkin EK, Tockner K. (2010) Light pollution as a biodiversity threat. Trends Ecol Evol 25: 681–682. [DOI] [PubMed] [Google Scholar]
- Jentoft S, Held JA, Malison JA, Barry TP. (2002) Ontogeny of the cortisol stress response in yellow perch (Perca flavescens). Fish Physiol Biochem 26: 371–378. [Google Scholar]
- Karakatsouli N, Papoutsoglou SE, Panopoulos G, Papoutsoglou ES, Chadio S, Kalogiannis D. (2008) Effects of light spectrum on growth and stress response of rainbow trout Oncorhynchus mykiss reared under recirculating system conditions. Aquacul Eng 38: 36–42. [Google Scholar]
- Kittilsen S, Ellis T, Schjolden J, Braastad BO, Øverli Ø. (2009) Determining stress-responsiveness in family groups of Atlantic salmon (Salmo salar) using non-invasive measures. Aquaculture 298: 146–152. [Google Scholar]
- Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. (2013) Chronobiology by moonlight. Proc R Soc B 280: 20123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeTallec T, Perret M, Thery M. (2013) Light pollution modifies the expression of daily rhythms and behavior patterns in a nocturnal primate. PLoS ONE 8: e79250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A, Routledge R, Connors BM. (2010) Effect of artificial light on marine invertebrate and fish abundance in an area of salmon farming. Mar Ecol Prog Ser 419: 147–156. [Google Scholar]
- Migaud H, Cowan M, Taylor J, Ferguson HW. (2007) The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar . Mar Environ Res 270: 390–404. [Google Scholar]
- Miller MW. (2006) Apparent effects of artificial light on singing behaviour of American robins. Condor 108: 130–139. [Google Scholar]
- Neachev IV, Dikhnich AV, Kostin VV, Romanenko VO. (2006) Dynamics of cortisol and the development of the glucocorticoid function in the early ontogenesis of Atlantic salmon Salmo salar. J Ichthyol 46: 328–341. [Google Scholar]
- Perkin EK, Hölker F, Richardson JS, Sadler JP, Wolter C, Tockner K. (2011) The influence of artificial light on stream and riparian ecosystems: questions, challenges, and perspectives. Ecosphere 2: 1–16. [Google Scholar]
- Perry G, Buchanan BW, Fisher RN, Salmon M, Wise SE. (2008) Effects of artificial night lighting on amphibians and reptiles in urban environments. In Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, UT. [Google Scholar]
- Rich C, Longcore T. (2006) Ecological Consequences of Artificial Night Lighting. Island Press, Washington, DC. [Google Scholar]
- Riley WD, Moore A. (2000) Emergence of Atlantic salmon, Salmo salar L., fry in a chalk stream. Fisher Manag Ecol 7: 465–468. [Google Scholar]
- Riley WD, Bendall B, Ives MJ, Edmonds NJ, Maxwell DL. (2012a) Street lighting disrupts the diel migratory pattern of wild Atlantic salmon, Salmo salar L., smolts leaving their natal stream. Aquaculture 330–333: 74–81. [Google Scholar]
- Riley WD, Maxwell DL, Ives MJ, Bendall B. (2012b) Some observations on the impact of temperature and low flow on the onset of downstream movement of wild Atlantic salmon, Salmo salar L., smolts. Aquaculture 362–363: 216–223. [Google Scholar]
- Riley WD, Davison PI, Maxwell DL, Bendall B. (2013) Street lighting delays and disrupts the dispersal of Atlantic salmon (Salmo salar) fry. Biol Conserv 158: 140–146. [Google Scholar]
- Riley WD, Davison PI, Maxwell DL, Newman RC, Ives MJ. (2015) A laboratory experiment to determine the dispersal response of Atlantic salmon (Salmo salar) fry to street light intensity. Freshwater Biol 60: 1016–1028. [Google Scholar]
- Royal Commission on Environmental Pollution (2009) Artificial Light in the Environment. Royal Commission on Environmental Pollution. The Stationary Office Limited, London, UK. [Google Scholar]
- Scott AP, Ellis T. (2007) Measurement of fish steroids in water—a review. Gen Comp Endocrinol 153: 392–400. [DOI] [PubMed] [Google Scholar]
- Stephens SM, Brown JA, Frankling SC. (1997) Stress responses of larval turbot, Scophthalmus maximus L., exposed to sub-lethal concentrations of petroleum hydrocarbons. Fish Physiol Biochem 17: 433–439. [Google Scholar]
- Stouthart AJHX, Lucassen ECHET, van Strien FJC, Balm PHM, Lock RAC, Wendelaar Bonga SE. (1998) Stress responsiveness of the pituitary–interrenal axis during early life stages of common carp (Cyprinus carpio). J Endocrinol 157: 127–137. [DOI] [PubMed] [Google Scholar]