We tested the impacts of food availability and water temperature on Striped Marsh Frog survivorship, growth and development. Tadpole size was largest, and survivorship lowest, at warmer temperatures. Food availability mediated the effects of temperature, with smaller tadpole size and higher survivorship in stochastic food availability treatments.
Keywords: Development, food availability, growth, metamorphosis, phenotypic plasticity, survival
Abstract
Food availability and temperature are known to trigger phenotypic change, but the interactive effects between these factors are only beginning to be considered. The aim of this study was to examine the independent and interactive effects of long-term stochastic food availability and water temperature on larval survivorship, growth and development of the striped marsh frog, Limnodynastes peronii. Larval L. peronii were reared in conditions of either constant or stochastic food availability and in water at three different temperatures (18, 22 and 26°C), and effects on survival, growth and development were quantified. Over the experimental period, larval growth rate was highest and survivorship lowest at the warmest temperature. However, changes in food availability mediated the effects of temperature, with slower larval growth and higher survivorship in stochastic food availability treatments. Tadpoles in the stochastic food availability treatments did not reach metamorphosis during the experimental period, suggesting that developmental stasis may have been induced by food restriction. Overall, these results demonstrate that changes in food availability alter the effects of water temperature on survival, growth and development. From an applied perspective, understanding how environmental factors interact to cause phenotypic change may assist with amphibian conservation by improving the number of tadpoles generated in captive breeding programmes.
Introduction
Phenotypic plasticity is the ability of an organism to change its phenotype in response to varying abiotic and biotic environmental factors (Miner et al., 2005). When faced with dynamic environmental conditions, some organisms can readily respond by changing phenotypes, allowing for a range of optimal phenotypes to be produced in response to multiple environments (DeWitt et al., 1998). If the optimal phenotype can change with environmental conditions, this presents an adaptive advantage that can improve organismal fitness (De Jong, 2005; Reed et al., 2010). Phenotypic plasticity has evolved in an array of organismal traits, but two traits that appear to be particularly plastic are growth (somatic growth) and development (ontogenic change; Relyea, 2001; Pfennig et al., 2010). Plastic responses in growth and development can be triggered by various environmental factors. One environmental factor known to trigger plastic responses across a variety of taxa is food availability (see Monaghan, 2008; Munn et al., 2010; Enriquez-Urzelai et al., 2013; Rosen et al., 2014). Empirical studies suggest that changes in food availability have long-term consequences for various life-history traits due to a reduction in the amount of energy that can be allocated to somatic growth (Yoneda and Wright, 2005; Inatsuchi et al., 2010; Enriquez-Urzelai et al., 2013).
Several theoretical models have considered how insufficient energy intake in conditions of reduced food availability might influence organismal growth and development and, ultimately, the probability of surviving and reproducing. The ‘metabolic down-regulation model’ predicts that food deprivation induces an overall metabolic depression that may occur as a physiological adaptation to reduce metabolic costs, via the down-regulation of metabolic rates, limiting processes such as growth and development (Keys et al., 1950; Fischer and Lindermayer, 2000; Rosen et al., 2014). There is some empirical evidence to support this prediction. For example, in periods of stochastic food availability, coral reef fish exhibit a longer time to metamorphosis and smaller size at maturity (McLeod et al., 2013; see also Nicieza and Metcalfe, 1997; Yoneda and Wright, 2005; Inatsuchi, Yamato and Yusa, 2010; Enriquez-Urzelai et al., 2013). The ‘general optimization model’, a mathematical formalization of the ‘Wilbur–Collins model’, also predicts slower growth rate and longer developmental periods in response to poor growth conditions. However, this model proposes that developmental thresholds (such as minimum size) need to be attained prior to life-history transition (Day and Rowe, 2002). This model predicts that to meet minimum size thresholds for metamorphosis, individuals should extend the larval period (Day and Rowe, 2002).
The Wilbur–Collins model, developed explicitly for species that experience metamorphosis, also proposes that there should be a minimum threshold size at which developmental transitions occur (Wilbur and Collins, 1973; Day and Rowe, 2002). However, this model hypothesizes a trade-off between growth and development. The model predicts that in conditions of stochastic food availability, larval development is increased to evade the resource-poor environment, and growth rate is slowed, resulting in a smaller size at metamorphosis (Wilbur and Collins, 1973). This negative relationship between growth and metamorphosis has been reported in three spadefoot toad species (genus: Scaphiopus; Morey and Reznick, 2000). In low food availability conditions, larvae underwent earlier development to evade the resource-poor environment; however, a minimal threshold size had to be met before development could be expedited. Alternatively, if the minimal threshold size was not met, larvae entered a developmental stasis (Morey and Reznick, 2000).
The effect of food availability on growth and development has also been explored using the stochastic dynamic programming (SDP) approach, which has been developed to determine an optimal strategy to maximize a particular fitness trait (Tenhumberg et al., 2000). Using the SDP approach, Tenhumberg et al. (2000) developed an SDP model for a syrphid fly system to determine optimal size and age at maturity when exposed to stochastic food availability, but considered the timing of food availability during development, a novel inclusion largely ignored in other models of growth and development. In this SDP model, it is predicted that exposure to stochastic food availability throughout development would result in larvae pupating earlier and at a smaller size. Exposure to stochastic food availability in the early phase of development would result in syrphid larvae pupating later without altering size at pupation. In contrast, exposure to stochastic food availability conditions during the late phase of development would alter weight at pupation, not developmental time (Tenhumberg et al., 2000), providing support for the notion that the timing of changes in food availability can control growth and development (see Nicieza and Metcalfe, 1997; Morey and Reznick, 2000, 2004; Yoneda and Wright, 2005; Inatsuchi et al., 2010; Enriquez-Urzelai et al., 2013).
Importantly, while the aforementioned models examine the effects of food availability on growth and development, multiple environmental factors can affect these life-history traits, and the effects of these factors are rarely independent. For instance, in temporal water bodies, plastic responses in growth and development may be triggered not only by food availability, but also by temperature (Leips and Travis, 1994; Sanuy et al., 2008). The ‘temperature-size rule’ predicts that ectothermic species reared at cold temperatures display slow growth rates and a prolonged larval period, resulting in a larger size at metamorphosis (Kozłowski et al., 2004). The temperature–size rule has been widely tested, and there is now considerable empirical evidence to suggest that this rule applies to the vast majority of ectothermic animals (Angilletta et al., 2004; Walters and Hassall, 2006).
While the independent effects of food availability and temperature on growth and development in larval species are well established (Inatsuchi et al., 2010), the interactive effects of these factors on growth, development and survival to maturity are only beginning to be considered (Álvarez and Nicieza, 2002a). One of the few models considering the interaction between environmental factors is the ‘fixed-rate model’ (Travis, 1984). The model postulates that while food availability may regulate specific life-history traits, such as larval growth and size at metamorphosis, the developmental rate becomes fixed at a certain point (Travis, 1984; Rose, 2005). However, the length of larval period can be regulated by other environmental factors, such as temperature (Álvarez and Nicieza, 2002b). There is some experimental evidence for interactive effects. For example, in a study investigating the interactive effects of diet type and temperature on larvae of the Iberian painted frog (Discoglossus galganoi), it was found that larval period was extended with cooler temperatures; however, size at metamorphosis was regulated by the interaction between temperature and diet type (Álvarez and Nicieza, 2002b). More specifically, when exposed to plant- or animal-based diets, size at metamorphosis varied inversely to temperature, and although diet did not influence size at 12°C, exposure to the animal-based diet resulted in bigger metamorphs at 17 and 22°C (Álvarez and Nicieza, 2002b).
To date, few studies have investigated how food availability, and interactions between food availability and temperature, influence growth and development in ectotherms. Nevertheless, evidence is emerging to suggest that such interactions can alter developmental trajectories. Newman (1998) conducted a dietary experiment using spadefoot toad tadpoles (Scaphiopus couchii) and demonstrated that an abrupt change in food level during development had significant effects on an individual’s age and size at metamorphosis. However, the magnitude and direction of these effects differed depending on the environmental temperature and tadpole density. More recently, in a study of coral reef fish McLeod et al. (2013) manipulated food availability by increasing time lags between feeding, at increasing temperatures. Overall, lower feeding regimens decreased survivorship to adulthood, and longer time to metamorphosis was observed. However, in their study McLeod et al. (2013) noted that predictable time lags between food supply may not be symptomatic of natural food supplies, indicating the importance of investigating the influence of stochastic food availability. Furthermore, changes in food availability occurring throughout the entire developmental period have received limited empirical attention (see Leips and Travis, 1994). Using an SDP model approach, Tenhumberg et al. (2000) considered the effects of timing of changes in food availability on the optimal size at maturity in the syrphid fly system; however, further empirical evidence of the effects of timing of changes on growth and development in other species remains limited (see Bull et al., 1996; Tenhumberg et al., 2000). Empirical testing of the interaction between long-term changes in food availability and water temperature is now needed to broaden our understanding of how interactions between environmental conditions shape plastic growth and development responses in ectotherms.
Implications for amphibian conservation
Knowledge of how interactions between food availability and temperature influence larval growth, development and survivorship may also be of value to amphibian conservation. Globally, amphibians are declining faster than any other vertebrate group and, for threatened species, the recommended recovery action is captive breeding and reintroduction (Stuart et al., 2004; Gascon et al., 2007). While captive breeding programmes have been established for various amphibian species (Stuart et al., 2004; Gascon et al., 2007), many programmes have been constrained by an inability to generate large numbers of healthy individuals consistently. The ability to generate large numbers of individuals is critical for three main reasons. First, it allows the captive population to be maintained at a size that avoids problems associated with inbreeding and/or natural attrition. Second, it supplies large numbers of individuals for release, which in various species is a predictor of reintroduction success (Armstrong and Seddon, 2008; Tarszisz et al., 2014). Third, it reduces the cost of captive breeding, making recovery programmes more financially viable (Canessa et al., 2014; Tarszisz et al., 2014). In recognition of the need to improve the productivity of amphibian captive breeding programmes, empirical studies have begun to investigate how anuran growth, development and survivorship are influenced by various abiotic factors, including nutrition (Ogilvy et al., 2012; Dugas et al., 2013; Cothran et al., 2015), pH (Mantellato et al., 2013), salinity (Christy and Dickman, 2002), food availability (Gillespie, 2002) and temperature (Browne and Edwards, 2003). Surprising, however, there remains a limited understanding of how interactive effects between abiotic factors influence anuran life-history traits. Testing for such effects in common model species can be a valuable first step towards identifying optimal rearing environments for threatened species with analogous life histories. For example, by studying the growth and development of the common frog, Geocrinia rosea, Mantellato et al. (2013) expedited the establishment of ex situ breeding programmes for two rare and threatened species, Geocrinia alba and Geocrinia vitellina.
The aim of the present study was to investigate the independent and interactive effects of long-term exposure to stochastic food availability and water temperature on larval survivorship, growth and development of the striped marsh frog, Limnodynastes peronii. To evaluate the effects of food availability and temperature, a 2 × 3 factorial experiment was performed. The ‘food availability’ factor had two levels, ad libitum food supply (constant availability) and stochastic food supply (stochastic availability; Tenhumberg et al., 2000), while the ‘temperature’ factor had three levels, i.e. 18, 22 and 26°C. The following hypotheses were tested: (i) stochastic food availability would decrease larval survivorship, growth and development; (ii) increased water temperature would increase larval survivorship, growth and development; and (iii) indicative of an interaction between these environmental factors, water temperature would mediate the effects of food availability on survival, growth and development, with decreased food availability having less effect at lower water temperatures due to a lowered metabolic rate, thus requiring less energy to be extracted from the external environment.
Methods
Ethics information
This study was conducted under approval from the University of Wollongong Animal Ethics Committee (Permit Number AE12/23) and the NSW Office of Environment and Heritage (Parks Permit SL101104).
Study species
The striped marsh frog (L. peronii) is a common Australian frog species with a wide distribution along the east coast, extending from cool temperate regions in Victoria to the tropical regions of northern Queensland (Wilson, 2001). Larval L. peronii are found in various aquatic environments that experience a broad range of nutritional and temperature conditions, making L. peronii an ideal model species in which to examine the effects of food availability in combination with temperature variation on larval survivorship, growth and development (Niehaus et al., 2006). The breeding season of L. peronii varies depending on geographical location. Within cool-temperate zones, including the Greater Illawarra where the present study was conducted, the breeding season is predominantly late winter through till early summer (Wilson, 2001). Eggs clutches are laid in an aquatic foam nest, and the number of eggs per clutch ranges between 150 and 2000 (Schell and Burgin, 2002).
Clutch collection and tadpole acclimation
Six egg clutches were collected from 25 to 30 January 2013 from a breeding site in the Greater Illawarra region of south-eastern New South Wales (34°26′S 150°51′E). Clutches were collected by hand and stored in separate polyethylene tubs (600 mm × 350 mm × 250 mm) filled with 20 l of Reverse Osmosis water (RO water) and transported to the Ecological Research Centre at the University of Wollongong, Wollongong (34°24′24″S 150°52′46″E). Clutches were maintained in these tubs in natural light conditions at ∼25 ± 2°C for a 10 day acclimation period. This period was imposed to ensure that tadpoles were viable before being entered into the experiment. To ensure no build-up of nitrogenous waste in tubs during the acclimation period, one-third of the water was replaced every fifth day, resulting in two water changes during the acclimation period. Tadpoles hatched from eggs 2–3 days after collection, and once tadpoles had hatched the egg jelly was removed from the tubs and tadpoles were fed ad libitum every second day with fish flakes (75% Flora, 25% San; sera GmbH, Heinsberg, Germany).
Approximately 10 days after hatching, tadpoles were entered into experimental housing. Tadpoles were acclimated to the experimental housing for a period of 24 h (see Experimental Design), and any individuals that died during the 24 h were replaced with individuals maintained in identical conditions in order to maintain sample sizes. Tadpoles were fasted during this acclimation period and were provided with food only at the time when they were entered into experimental treatments. Upon entry into the experimental treatments, tadpoles (n = 48; split between two replicate rearing tanks per clutch per treatment) were photographed, so that baseline body size measurements could be made at a later date (see Experimental Design). Measurements were not made on back-up replicates, which were euthanased after use. Once tadpoles were entered into the experimental treatments, no further replacement of individual tadpoles occurred.
Experimental design
To examine the effect of temperature and food availability on larval survival, growth and development, a 3 × 2 factorial design was used. The experiment involved three rearing temperatures (18, 22 and 26°C) and two feeding regimens (constant and stochastic food availabilities), resulting in six experimental treatments referred to as follows: (i) constant 18°C; (ii) constant 22°C; (iii) constant 26°C; (iv) stochastic 18°C; (v) stochastic 22°C; and (vi) stochastic 26°C. A split-clutch design was used, with tadpoles from each clutch being randomly allocated to an experimental treatment (i.e. 48 tadpoles per clutch in each treatment split between two replicate plastic rearing tanks, and a total of 288 tadpoles per treatment). Split-clutch designs provide effect controls for both clutch effects and parental genetic effects. The experimental period lasted 14 weeks, and during this time the tadpoles were monitored daily. This experimental period was selected because the larval period in populations of L. peronii in southern NSW typically lasts 2–3 months (Anstis, 2013). Furthermore, a past experimental study in L. peronii reported that time to metamorphosis in conditions of constant food (ad libitum lettuce) and temperature (24°C) ranged between 36 and 55 days (Kraft et al., 2005). The experiment commenced on 12 February 2013 and was terminated on 24 May 2013. During the experimental period, tadpoles were housed in plastic rearing tanks (250 mm × 150 mm × 110 mm). The plastic rearing tanks were rafted within the polyethylene tubs (600 mm × 350 mm × 250 mm), and a Jäger 100 W aquarium water heater (Eheim, Germany) was placed in the polyethylene tub to set the experimental treatment temperature.
A total of 24 tadpoles were housed in each plastic rearing tank at any one time (two replicates of n = 24 tadpoles per clutch per treatment); each plastic rearing tank had 2.5 l of RO water, resulting in one tadpole per 105 ml of water. To account for changes to tadpoles per volume of water as a result of tadpole mortality or metamorphosis and to reduce any potential density-dependent effects on growth and development (Miner et al., 2005), 105 ml of water per tadpole was removed to maintain a fixed number of tadpoles per volume of water. These water volume adjustments were carried out on a weekly basis. Experimental tubs were kept in a temperature- and light-controlled room maintained at 12 ± 2°C ambient temperature and a 12 h–12 h light–dark regimen. To control water salinity, which can have a significant impact on tadpole growth, development and survivorship (see Chinathamby et al., 2006; Kearney et al., 2012), Aquasonic Ocean Nature sea salt was added to the RO water (0.14 g/l). To prevent water fouling, partial water changes (∼40%) were made once per week.
The three water temperature treatments (18, 22 and 26°C) in which tadpoles were reared were selected because they reflected the average lower and upper estimates of temperatures that L. peronii tadpoles experience in NSW systems during the period between December and April, which is when peak development and metamorphosis typically occurs in this region (Wilson, 2001). It is of note that L. peronii tadpoles in NSW have the capacity to overwinter, and metamorphose from October to November (Anstis, 2013). However, we did not simulate temperatures experienced during this period because, in an effort to make our findings relevant to amphibian captive breeding programmes, we were focused on identifying conditions that promoted rapid larval development without compromising tadpole survival. To ensure that temperatures were maintained at treatment temperatures throughout the entire experimental period, water temperatures were monitored on a weekly basis using a calibrated digital thermometer probe (traceable snap-in module with probe; Thomas Scientific). All treatment temperatures remained within a range of ±2°C. To minimize any room effects or tub effects, the temperature of polyethylene tubs was randomly assigned, and plastic tanks were rotated within the polyethylene tub on a daily basis.
Animals were exposed to one of two feeding regimens: constant food availability or stochastic food availability. Constant food availability treatments supplied food ad libitum (i.e. no food restrictions applied) throughout the entire experimental period. The stochastic food availability treatment had randomly allocated fasting periods of up to 3 days during which no fresh food was provided. At the start of the fasting period, any uneaten food was removed using a siphon, leaving tadpoles with access to faecal material only. On days when tadpoles had access to food, food was provided ad libitum (i.e. no food restrictions applied). Ad libitum quantities of food were adjusted throughout the experimental period to account for increased tadpole body size (increased quantities of food) and changes in tadpole density (reduced quantities of food). Food consisted of a mixture of frozen endive (Cichorium endivia) and commercial Algae sinking fish pellets (Australian Pet Supplies Feedwell Fishfood, Smithfield, NSW, Australia). To ensure that each plastic tank was treated in the same way, the plastic tanks used for constant food availability treatments also had water siphoned and replaced, and this occurred simultaneous to the beginning of fasting periods for stochastic food availability treatments. This process also assisted in aerating the water.
Effects of food availability and water temperature on survival, development and growth
Survivorship of individual tadpoles in each experimental treatment was monitored on a weekly basis throughout the 14 week experimental period. In addition, for each experimental treatment, the number of tadpoles reaching metamorphosis and the time taken to reach metamorphosis were recorded. Metamorphosis was defined as the time taken for the emergence of at least one forelimb (Gosner Stage 42; Gosner, 1960).
The effects of food availability and water temperature on tadpole size were determined by measuring individual snout-to-vent length (in millimetres). Measurements were made from digital images acquired on a weekly basis using a standardized overhead digital camera (Canon Powershot D20 12.1 MP CMOS waterproof digital camera). To measure snout-to-vent length, each plastic tank had ∼40% of the water removed (coinciding with the partial water change), allowing for enough water to cover the tadpoles but restrict movement within the water column. Snout-to-vent length measurements were made using Image J Image Processing Software (Open Source, version 1.42q), calibrated using a standardized scale present in each photograph. As the tadpoles were housed in groups during the experimental period, tank means, using eight randomly selected tadpoles per tank, were used to preserve data independence. A subsample of eight randomly selected tadpoles was assumed to account for any size variation occurring within each plastic replicate tank (Capellan and Nicieza, 2007).
Within 12 h of the emergence of at least one forelimb (Gosner Stage 42; Gosner, 1960), metamorphs were removed from the experimental treatment container, photographed and maintained in a separate plastic container with an RO water-soaked sponge (3.0 cm2) until the time of tail reabsorption. Prior to tail reabsorption, commercial fish pellets were provided ad libitum, and after tail reabsorption pinhead crickets (Acheta domestica) were provided ad libitum. Containers housing metamorphs were kept in a temperature- and light-controlled room at 22 ± 2°C ambient temperature under a 12 h–12 h light–dark regimen. Metamorphs were measured within 2 days of tail reabsorption. Vernier callipers were used to measure snout-to-vent length (in millimetres).
Statistical analysis
Effects of food availability and water temperature on tadpole survivorship
To examine the effects of food availability and water temperature on tadpole survivorship over the 14 week experimental period, a Cox proportional hazard model (Andersen and Gill, 1982) was used to determine differences in survivorship distribution; this was displayed as a Kaplan–Meier survival curve. For survival analysis, censorship was applied to death occurring as a result of handling, tadpoles that survived (without metamorphosing) over the experimental period and tadpoles that metamorphosed before the conclusion of the experimental period. To account for any potential clutch effects, clutch identity was included in the model as a random factor. Survival analysis was conducted in the statistical package R v3.1.0 (R Developer Core Team, 2014) in conjunction with the survival package (Therneau, 2014).
Survivorship at week 14 was examined further using a generalized linear mixed-effects model (GLMM). In this model, food availability and water temperature were fixed effects and clutch identity was a random effect. The model also included an interaction term between food availability and temperature. Data were analysed in the statistical package R v3.1.0 (R Developer Core Team, 2014) in conjunction with the survival package (Therneau, 2014).
Effects of food availability and water temperature on tadpole size
To examine the effects of food availability and water temperature on tadpole size over the 14 week experimental period, a general additive mixed model was used (Lin and Zhang, 1999). The additive model was used because it allows a non-linear growth trajectory in response to experimental treatment (Zuur et al., 2009). Comparisons were made on a weekly basis (weeks 0–9) to examine the additive and interactive effects of food availability and water temperature on tadpole size. To account for any potential clutch effects, clutch identity was included in the model as a random intercept. Tadpole size was measured as snout-to-vent length. Week 0 was used to provide the baseline snout-to-vent measurements, while weeks 1–9 provided the size measurements in response to the experimental treatments. Weeks 10–14 were not included in the general additive mixed model because complete tadpole mortality occurred in several replicates during this period. Data were analysed in the statistical package R v3.1.0 (R Developer Core Team, 2014) using statistical package gamm4 (Wood, 2014).
Effects of food availability and water temperature on development
Over the experimental period, only tadpoles from constant food availability treatments metamorphosed; therefore, all measures relating to metamorphosis were restricted to the constant feeding treatments. To determine the effect of water temperature on the time to metamorphosis and post-metamorphic size, GLMMs were used. In these models, water temperature was the fixed effect and clutch identity was a random effect to account for potential clutch effects. All post-metamorphic data were analysed using the statistical package R v3.1.0 (R Developer Core Team, 2014) using statistical package nlme (Pinheiro et al., 2014).
Results
Effects of food availability and water temperature on tadpole survivorship
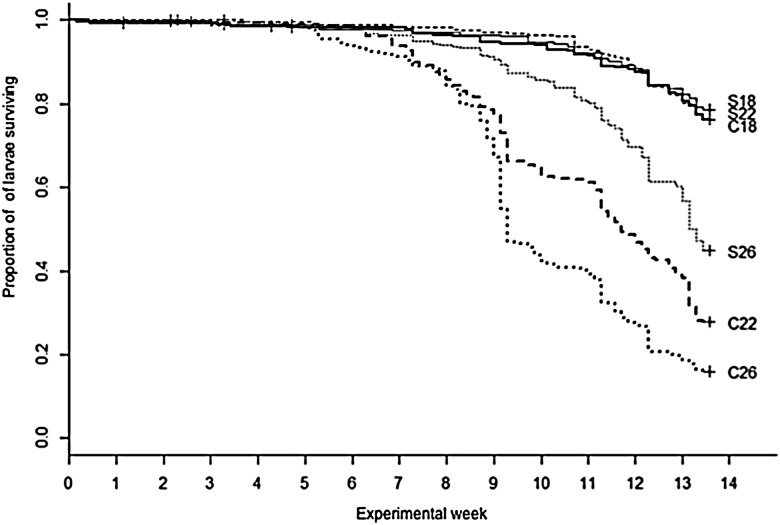
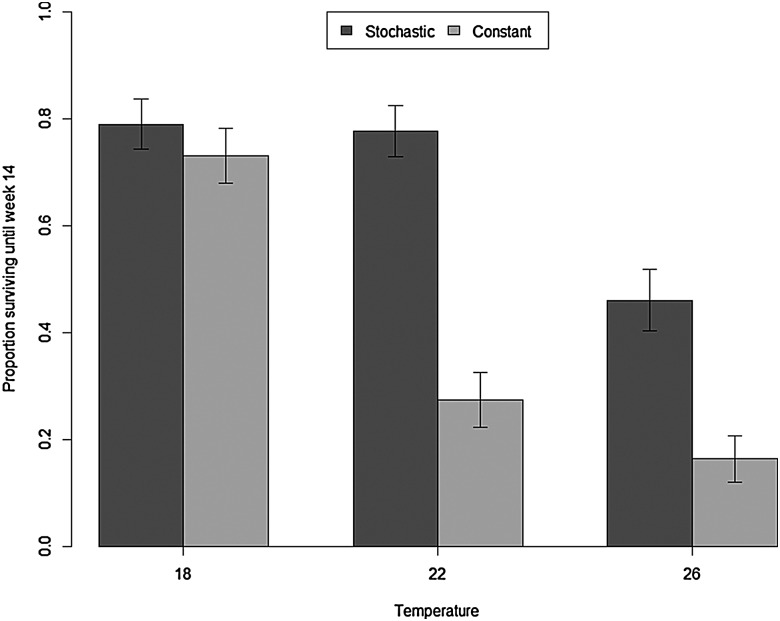
Survival of L. peronii tadpoles over the 14 week experimental period was significantly different between water temperatures (Cox proportional hazard test: z = 6.105, P < 0.0001), but not between food availability treatment groups (Cox proportional hazard test: z = 0.311, P = 0.760). There was no significant interaction between food availability and water temperature (Cox proportional hazard test: z = −0.843, P = 0.400; Table 1 and Fig. 1). Survival in all treatment groups was high (>90%) until week 5. After this time, tadpoles began dying in all treatment groups. By experimental week 14, survivorship was lowest in the two warmest constant food treatments (22 and 26°C treatments) and highest in the two coolest stochastic food treatments (18 and 22°C treatments). Survivorship was intermediate in the constant 18°C and stochastic 26°C treatments. Clutch had a significant effect on survival (GLMM: z = 7.531, P < 0.001; Tables 2 and 3 and Fig. 2). Whilst there was no overall significant difference between constant and stochastic food availability treatments (GLMM: z = 1.664, P = 0.0961; Tables 2 and 3 and Fig. 2), there were significant differences in survival between water temperature treatments, with higher survivorship in the 18°C treatment than in the 22 and 26°C treatments (GLMM: z = −10.758, P < 0.001 and z = −12.943, P < 0.001, respectively; Tables 2 and 3 and Fig. 2).
Table 1:
Output from Cox proportional hazard model testing the effects of food availability and water temperature on proportion of tadpoles surviving a 14 week experimental period in the striped marsh frog (Limnodynastes peronii)
| Coefficient | Exponential (coefficient) | Standard error (coefficient) | Robust SE | z-value | P-value | |
|---|---|---|---|---|---|---|
| Stochastic vs. constant | 0.5937 | 1.811 | 0.5798 | 1.9113 | 0.311 | 0.760 |
| Temperature | 0.2392 | 1.27 | 0.0146 | 0.0392 | 6.105 | <0.001 |
| Stochastic: temperature | −0.0742 | 0.929 | 0.0246 | 0.088 | −0.843 | 0.400 |
Figure 1:
Proportion of Limnodynastes peronii tadpoles surviving over a 14 week experimental period in the following six experimental treatments: constant food at 18°C (C18); stochastic food at 18°C (S18); constant food at 22°C (C22); stochastic food at 22°C (S22); constant food at 26°C (C26); and stochastic food at 26°C (S26). ‘+’ indicates a censored event.
Table 2:
Effect of food availability and water temperature on percentage of tadpoles surviving to week 14 in the striped marsh frog (L. peronii)
| Treatment | |||
|---|---|---|---|
| Food availability | Temperature (°C) | Sample size | Survival at week 14 (%) |
| Constant | 18 | 288 | 73.2 ± 5.2 |
| 22 | 289 | 27.5 ± 6.9 | |
| 26 | 289 | 16.1 ± 5.7 | |
| Stochastic | 18 | 287 | 78.9 ± 5.9 |
| 22 | 288 | 77.8 ± 4.7 | |
| 26 | 289 | 46.1 ± 9.1 | |
Values are means ± SEM. Statistical outputs are from a generalized linear mixed-effects model (see Table 3).
Table 3:
Output from general linear mixed-effects model testing the effects of food availability and water temperature on proportion of L. peronii tadpoles surviving to week 14
| Estimate | Standard error | z-value | P-value | |
|---|---|---|---|---|
| (Intercept) | 1.0547 | 0.2439 | 4.324 | <0.001 |
| Stochastic vs. constant | 0.3362 | 0.202 | 1.664 | 0.0961 |
| Temperature 22 vs. 18°C | −2.0841 | 0.1937 | −10.758 | <0.001 |
| Temperature 26 vs. 18°C | −2.7671 | 0.2138 | −12.943 | <0.001 |
| Stochastic diet: temperature 22 vs. 18°C | 2.0122 | 0.284 | 7.085 | <0.001 |
| Stochastic diet: temperature 26 vs. 18°C | 1.1995 | 0.2858 | 4.196 | <0.001 |
Figure 2:
Effect of food availability and water temperature on proportion of striped marsh frog (L. peronii) tadpoles surviving to week 14. Stochastic food availability treatments are represented by dark grey bars and constant food availability treatments by light grey bars. Values are shown as means ± SEM.
Effects of food availability and water temperature on tadpole size
In week 0, there were no significant differences in baseline body size (Table 4). In weeks 1–9, there were significant differences in body size between treatment groups. In week 1, body size was largest in tadpoles from treatments with the warmest water temperatures (22 and 26°C), irrespective of whether food availability was constant or stochastic. In week 2, however tadpole body size was larger in warmest water temperatures (22 and 26°C) and constant food availability treatments (Table 4). In weeks 2–9, body size was largest in treatments with constant food availability, irrespective of the treatment temperature (Table 4). At week 9, a decrease in size with increasing water temperature was evident. An interaction between food availability and water temperature occurred at weeks 3 and 9. Between weeks 0 and 9, there was no effect of clutch identity on tadpole size.
Table 4:
Effect of food availability and water temperature on tadpole size across experimental period weeks 0–9
| Week | Water temperature | Food availability | Food availability × Water temperature |
|---|---|---|---|
| 0 | |||
| 1 | + | ||
| 2 | + | − | |
| 3 | − | x | |
| 4 | − | ||
| 5 | − | ||
| 6 | − | ||
| 7 | − | ||
| 8 | − | ||
| 9 | − | − | x |
Data from weeks 10–14 were excluded because of incomplete sample sizes due to mortality. Positive values (+) indicate a significant (P < 0.05) increase in size with increasing water temperature and negative (−) a significant (P < 0.05) increase in size with decreasing water temperature. In the case of diet, (−) indicates that the tadpoles with the stochastic diet were significantly (P < 0.05) smaller than those with the constant diet. In the interaction term, x indicates a significant interaction between food availability and water temperature occurring. Significance values were derived from the general additive mixed model analysis.
Effects of food availability and water temperature on development
Over the 14 week experimental period, only 2.02% of tadpoles (35 of 1730) reached metamorphosis, and all were from treatments with constant food availability. Of the 35 individuals that successfully metamorphosed, there was no significant effect of temperature or clutch identity on mean time to metamorphosis (in days; Tables 5 and 6) and no effect on post-metamorphic body size (Tables 5 and 6).
Table 5:
Effect of food availability and temperature on percentage of tadpoles reaching metamorphosis, time to metamorphosis and post-metamorphic size (snout-to-vent length), in the Striped Marsh frog (L. peronii)
| Treatment |
Percentage metamorphoseda |
Time to metamorphosis (days) | Post-metamorphic size:snout-to-vent length (mm) | ||
|---|---|---|---|---|---|
| Food availability | Water temperature (°C) | ||||
| Constant | 18 | 5.2% | (15/288) | 62.1 ± 4.9 | 15.3 ± 1.9 |
| 22 | 2.8% | (8/289) | 76.4 ± 4.6 | 15.7 ± 1.5 | |
| 26 | 4.2% | (12/289) | 59.4 ± 3.5 | 14.5 ± 0.7 | |
| Stochastic | 18 | (0/287) | |||
| 22 | (0/289) | ||||
| 26 | (0/289) | ||||
Values are means ± SEM. Note that there are no data presented for the stochastic treatments because no tadpoles in these treatments reached metamorphosis. Sample sizes for post-metamorphic size are n = 32. aTotal number of tadpoles reaching metamorphosis in each treatment is reported in parentheses.
Table 6:
Output from generalized linear mixed effects models testing the effect of water temperature on time to metamorphosis and post-metamorphic size in the striped marsh frog (L. peronii)
| Time to metamorphosis | ||||
|---|---|---|---|---|
| Estimate | Standard error | t-value | P-value | |
| (Intercept) | 4.08631 | 0.06403 | 63.82 | <2 × 10−16 |
| factor(Temperature)22 | 0.18496 | 0.11979 | 1.544 | 0.133 |
| factor(Temperature)26 | −0.03484 | 0.09844 | −0.354 | 0.726 |
| Post-metamorphic size | ||||
| (Intercept) | 2.73954 | 0.03124 | 87.687 | <2 × 10−16 |
| 22 vs. 18°C | 0.02102 | 0.05069 | 0.415 | 0.681 |
| 26 vs. 18°C | −0.05044 | 0.03899 | −1.294 | 0.206 |
Discussion
The aim of this study was to investigate the independent and interactive effects of food availability and water temperature on larval growth, development and survival in the Striped Marsh frog, L. peronii. Variation in food availability was found to impact larval size and development, with smaller larval size and slower developmental rates in stochastic food availability treatments. Furthermore, changes in food availability mediated the effects of increasing water temperature on survivorship. Specifically, there was smaller larval size and higher survivorship in conditions of stochastic food availability, rejecting our first hypothesis that stochastic food availability would decrease larval survivorship, growth and development. Interestingly, clutch identity did not have a significant effect on any of our measures of tadpole growth and development, but clutch identity did have a significant effect on tadpole survivorship. Given that our clutches were collected over a period of 5 days, it is possible that embryos from different clutches were exposed to different environmental conditions (e.g. pre-treatment temperatures) that subsequently affected their probability of survival. Alternatively, survivorship may have been affected by variable maternal provisioning (Dziminski and Roberts, 2006), differences in parental compatibility (Dziminski et al., 2008) or differences in parental genetic quality (Sheldon et al., 2003). Such clutch effects have previously been reported in anurans (Sheldon et al., 2003; Dziminski and Roberts, 2006; Dziminski et al., 2008) and underscore the importance of considering the effects of clutch identity in experimental studies aimed at investigating the influence of rearing environment on anuran life-history traits.
The reported effects of food availability on larval growth and development support the predictions of the ‘general optimization model’ (Day and Rowe, 2002), which predicts slower growth and longer developmental periods in stochastic food availability conditions. However, the prediction of a trade-off between growth and development, as described in the Wilbur–Collins model, was not possible to test because the tadpoles did not reach metamorphosis in stochastic food availability conditions (Wilbur and Collins, 1973). Such trade-offs may not have been observed in the present study due to the long-term exposure to stochastic food availability conditions, whereby the threshold sizes or developmental stages were unable to be met. It is probable that the smaller larval size in stochastic food availability conditions resulted in tadpoles being unable to reach this threshold size (to increase developmental rate) within the experimental period. When the experiment was terminated, tadpoles in the stochastic food treatment were still displaying positive growth. Therefore, if the experiment had continued it is possible that tadpoles in these conditions would have reached the minimal size required for metamorphosis, and these individuals would have metamorphosed later and at a smaller size (see Lind et al., 2008). Such a result would have provided support for the Wilbur–Collins model.
Exposure to stochastic food availability conditions throughout development impeded the ability of tadpoles to reach metamorphosis, contrary to the predictions of the ‘stochastic dynamic programming model’, which predicts that larvae respond by pupating earlier and at a smaller size when exposed to changes in food availability throughout development (Tenhumberg et al., 2000). This inability of L. peronii to reach metamorphosis in stochastic food availability conditions suggests that a lack of constant food supply may induce developmental stasis. Developmental stasis may occur due to the inability to reach the threshold size or developmental stage required to increase developmental rates (Wilbur and Collins, 1973). Induced developmental stasis due to changes in food supply has previously been observed in spadefoot toad species (Morey and Reznick, 2000). Spadefoot toad larvae accelerated development in response to restricted food supply, but individuals that had not met the minimal threshold size for development entered developmental stasis (Denver et al., 1998; Morey and Reznick, 2000). An alternative reason why tadpoles did not reach metamorphosis is that tadpoles did not have enough time to reach the developmental threshold required to metamorphose. Tadpoles were still growing when the experiment was terminated, so metamorphosis might have been reached if the experimental period was extended. For this reason, future studies investigating the effect of stochastic food availability on larval growth and development should maintain tadpoles in treatment conditions until either growth rates plateau or tadpoles reach metamorphosis.
According to the temperature-size rule (Kozłowski et al., 2004), growth rate is expected to increase with increasing water temperature because temperature regulates metabolism, growth and differentiation in ectothermic species (Álvarez and Nicieza, 2002b; McLeod et al., 2013). In support of the temperature-size rule and the second hypothesis, the present study shows that L. peronii display increased growth with increased water temperature in constant food availability conditions. However, in conditions of stochastic food availability, the effects of water temperature were reduced, suggesting that food availability may restrict the overall energy available for somatic growth processes, thereby preventing significant differences in growth at different water temperatures (Yoneda and Wright, 2005; Inatsuchi et al., 2010; Enriquez-Urzelai et al., 2013).
The slowed growth and developmental rates in conditions of stochastic food availability may have resulted from an overall metabolic downregulation, as predicted by the ‘metabolic downregulation model’ (Keys et al., 1950). Whilst the metabolic rate of larvae was not quantified in this study, lack of food (food restriction) is expected to decrease metabolic costs by limiting growth and development (Wang et al., 2006; Hulbert et al., 2007). The ‘fixed-rate model’ predicts a slower growth rate in conditions of stochastic food availability, with temperature regulating the developmental rate, and cooler temperatures extending the length of the larval period (Travis, 1984). Based on the ‘fixed-rate model’, it would be expected that larvae reared in warmer waters would reach metamorphosis earlier but exhibit a slower growth rate in stochastic food availability conditions. However, long-term exposure to stochastic food availability may reduce energy available for development, preventing metamorphosis, regardless of increased water temperature. Interestingly, there were no differences in time to metamorphosis or post-metamorphic size between water temperature treatments in constant food availability conditions, which may suggest that the extremes in water temperatures used in the present study may not have differed enough to cause differences in developmental rate. However, this does not seem likely because temperature differences similar to those imposed in this study (18–26°C) have been shown to have drastic effects on the growth and development of various temperate-breeding anuran species (see Blouin and Brown, 2000; Álvarez and Nicieza, 2002a, b; Browne and Edwards, 2003; Walsh et al., 2008).
As mortality in the juvenile life stages of amphibians is typically high (Canessa et al., 2014), it can be difficult to make generalizations about the effects of experimental treatments on growth, development and survivorship. However, experimental studies are still useful for making inferences about treatment effects (e.g. Kearney et al., 2012, 2014). It was found that survivorship decreased in the warmest temperature treatments and stochastic food availability conditions buffered against mortality losses at higher temperatures. Warmer waters may have compromised survival because of decreased oxygen availability (O’Connor et al., 2007; Blaustein et al., 2010), oxidative stress due to higher metabolic rate (Hulbert et al., 2007), the build-up of microbes from decomposing food (McWilliams, 2008) or nitrogenous waste products (Morey and Reznick, 2004) or changes in the intensity of competition (Álvarez and Nicieza, 2002b; Blaustein et al., 2010; Enriquez-Urzelai et al., 2013; McLeod et al., 2013). A previous study examining the effects of long-term changes to food availability and water temperature on coral fish species also reported low survivorship in warmer waters and suggested that survival may have been compromised due to starvation. Consequently, when exposed to high food availability conditions, coral fish survivorship increased (McLeod et al., 2013). Conversely, in the present study, the long-term stochastic food availability treatment reduced the effects of the warmest water temperatures, with higher survivorship in stochastic food availability treatments. Slower growth rate as a result of stochastic food availability conditions may reduce the effects of water temperature on survivorship in larval L. peronii. As a result, growth conditions may influence the risk of mortality (Enriquez-Urzelai et al., 2013).
A number of studies have investigated the impacts of changes in food availability (or quality) interacting with other environmental factors and have documented induced changes in growth and development; these include interactions between food quality and temperature (Álvarez and Nicieza, 2002b), between predation risk and food availability (Nicieza, 2000) and between pond desiccation and food availability (Enriquez-Urzelai et al., 2013). The interactions between environmental factors can be varied, with the interactive effects also being difficult to predict (Álvarez and Nicieza, 2002b). For example, in a study investigating the effects of water temperature and food quality on growth and development in Iberian painted frogs (D. galganoi), it was found that water temperature had persistent effects on development and metamorphic traits, with larvae metamorphosing later and at larger body size when reared at lower temperatures. However, the effects of food quality on growth and development were largely dependent on water temperature, with larvae fed carbohydrate-rich diets being smaller at metamorphosis compared with larvae fed protein-rich diets, but not at all water temperatures (Álvarez and Nicieza, 2002b). Conversely, Enriquez-Urzelai et al. (2013) investigated the interactive effects between food availability and desiccation on the painted frog (Discoglossus pictus), observing that size and weight at metamorphosis were determined by food availability but not by the water desiccation regimen. The results of the present study strongly suggest that environmental differences in food availability and water temperature, and their interaction, cause differences in growth, development and survivorship. In this experiment, it was determined that food availability was more important than water temperature for survivorship, growth and development; a pattern that has also has been described in larval coral fish species (McLeod et al., 2013).
Implications for amphibian conservation
Our finding that changes in food availability mediate the effects of temperature on L. peronii growth, development and survivorship has implications for amphibian conservation. Similar to L. peronii, many threatened anurans are temperate-zone pond-breeding species, in which larvae experience marked fluctuations in temperature and food availability over extended developmental periods (i.e. >2 months). Captive breeding programmes attempting to breed such species generally rear tadpoles in constant environmental conditions, but our findings suggest that managers might benefit from manipulating both food availability and temperature. Specifically, providing individuals with stochastic food availability at warmer temperatures may improve individual survivorship and the likelihood of generating large numbers of tadpoles. This could benefit the recovery of a target species by improving the sustainability of a captive ‘insurance’ population, whilst minimizing expenses associated with establishing and maintaining colonies (Canessa et al., 2014). Furthermore, generating large numbers of individuals for release could improve the success of reintroduction programmes by ensuring the release of large groups, which could overcome problems associated with high dispersal, demographic stochasticity or low reproduction and/or survival at low population densities (Allee effects; Fischer and Lindermayer, 2000; Armstrong and Seddon, 2008). For these reasons, we propose that broadening our knowledge of the effects of interactions between environmental factors on anuran growth, development and survivorship might improve the success of management of threatened amphibian species (Carey, 2005; Muths et al., 2014).
Conclusions
In conclusion, the aim of this study was to use a manipulative laboratory experiment to examine the independent and interactive effects of long-term stochastic food availability and water temperature on larval L. peronii survivorship, growth and development. Larval growth rate was highest and survivorship lowest at the warmest temperature. However, changes in food availability mediated the effects of temperature, with slower larval growth and higher survivorship in stochastic food availability treatments. These findings contribute to a small but growing body of evidence that interactions between environmental factors can influence anuran growth, development and survivorship. Such advances have the potential to improve the output of amphibian captive breeding programmes and aid amphibian conservation.
Funding
The research was funded by a grant from the New South Wales Environmental Trust (grant no. 2012/RD/0105) and an Australian Research Council Linkage Grant (LP140100808). This project was also made possible by the generous support of the University of Wollongong and the University of Wollongong’s Institute for Conservation Biology and Environmental Management.
Acknowledgements
We are very grateful to Peter Harlow, Michael McFadden and Adam Skidmore for all their technical assistance with experimental set-up. We thank Aimee Bullock, Alexandra Leslie, James Lidsey, Alexandra May, Emma McInerney, Elizabeth Price, Sarah Simmonds and Mathew Stewart for all their animal husbandry assistance. We also thank Aidan Johnson, Urtzi Enriquez-Urzelai and the anonymous reviewer for discussion and helpful comments on the manuscript.
References
- Álvarez D, Nicieza A. (2002a) Effects of induced variation in anuran larval development on postmetamorphic energy reserves and locomotion . Oecologia 131: 186–195. [DOI] [PubMed] [Google Scholar]
- Álvarez D, Nicieza AG. (2002b) Effects of temperature and food quality on anuran larval growth and metamorphosis . Funct Ecol 16: 640–648. [Google Scholar]
- Andersen PK, Gill RD. (1982) Cox’s regression model for counting processes: a large sample study . Ann Stat 10: 1100–1120. [Google Scholar]
- Angilletta MJ, Steury TD, Sears MW. (2004) Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle . Integr Comp Biol 44: 498–509. [DOI] [PubMed] [Google Scholar]
- Anstis M. (2013) Tadpoles and Frogs of Australia. New Holland Publishers; , Chatswood, NSW. [Google Scholar]
- Armstrong DP, Seddon PJ. (2008) Directions in reintroduction biology . Trends Ecol Evol 23: 20–25. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Walls SC, Bancroft BA, Lawler JJ, Searle CL, Gervasi SS. (2010) Direct and indirect effects of climate change on amphibian populations . Diversity 2: 281–313. [Google Scholar]
- Blouin MS, Brown ST. (2000) Effects of temperature-induced variation in anuran larval growth rate on head width and leg length at metamorphosis . Oecologia 125: 358–361. [DOI] [PubMed] [Google Scholar]
- Browne R, Edwards D. (2003) The effect of temperature on the growth and development of the endangered green and golden bell frog (Litoria aurea) . J Therm Biol 28: 295–299. [Google Scholar]
- Bull CD, Metcalfe NB, Mangel M. (1996) Seasonal matching of foraging to anticipated energy requirements in anorexic juvenile salmon . Proc R Soc Lond B Biol Sci 263: 13–18. [Google Scholar]
- Canessa S, Hunter D, McFadden M, Marantelli G, McCarthy MA. (2014) Optimal release strategies for cost-effective reintroductions . J Appl Ecol 51: 1107–1115. [Google Scholar]
- Capellan E, Nicieza A. (2007) Non-equivalence of growth arrest induced by predation risk or food limitation: context-dependent compensatory growth in anuran tadpoles . J Anim Ecol 76: 1026–1035. [DOI] [PubMed] [Google Scholar]
- Carey C. (2005) How physiological methods and concepts can be useful in conservation biology . Integr Comp Biol 45: 4–11. [DOI] [PubMed] [Google Scholar]
- Chinathamby K, Reina RD, Bailey PCE, Lees BK. (2006) Effects of salinity on the survival, growth and development of tadpoles of the brown tree frog, Litoria ewingii . Aust J Zool 54: 97–105. [Google Scholar]
- Christy MT, Dickman CR. (2002) Effects of salinity on tadpoles of the Green and Golden Bell Frog (Litoria aurea) . Amphibia-Reptilia 23: 1–11. [Google Scholar]
- Cothran RD, Gervasi SS, Murray C, French BJ, Bradley PW, Urbina J, Blaustein AR, Relyea RA. (2015) Carotenoids and amphibians: effects on life history and susceptibility to the infectious pathogen, Batrachochytrium dendrobatidis . Conserv Physiol 3: doi:10.1093/conphys/cov005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T, Rowe L. (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions . Am Nat 159: 338–350. [DOI] [PubMed] [Google Scholar]
- De Jong G. (2005) Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes . New Phytol 166: 101–118. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Mirhadi N, Phillips M. (1998) Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation . Ecology 79: 1859–1872. [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS. (1998) Costs and limits of phenotypic plasticity . Trends Ecol Evol 13: 77–81. [DOI] [PubMed] [Google Scholar]
- Dugas MB, Yeager J, Richards-Zawacki CL. (2013) Carotenoid supplementation enhances reproductive success in captive strawberry poison frogs (Oophaga pumilio) . Zoo Biol 32: 655–658. [DOI] [PubMed] [Google Scholar]
- Dziminski MA, Roberts JD. (2006) Fitness consequences of variable maternal provisioning in Quacking Frogs (Crinia georgiana) . J Evol Biol 19: 144–155. [DOI] [PubMed] [Google Scholar]
- Dziminski MA, Roberts JD, Simmons LW. (2008) Fitness consequences of parental compability in the frog Crinia georgiana . Evolution 62: 879–886. [DOI] [PubMed] [Google Scholar]
- Enriquez-Urzelai U, San Sebastián O, Garriga N, Llorente G. (2013) Food availability determines the response to pond desiccation in anuran tadpoles . Oecologia 173: 117–127. [DOI] [PubMed] [Google Scholar]
- Fischer J, Lindenmayer DB. (2000) An assessment of the published results of animal relocations . Biol Conserv 96: 1–11. [Google Scholar]
- Gascon C, Collins JP, Moore RD, Church DR, McKay JE, Mendelson JR., III (eds) (2007) Amphibian Conservation Action Plan. IUCN/SSC Amphibian Specialist Group. Gland, Switzerland and Cambridge, UK, 64 pp. [Google Scholar]
- Gillespie GR. (2002) Impacts of sediment loads, tadpole density, and food type on the growth and development of tadpoles of the spotted tree frog Litoria spenceri: an in-stream experiment . Biol Conserv 106: 141–150. [Google Scholar]
- Gosner KL. (1960) A simplified table for staging anuran embryos and larvae with notes on identification . Herpetologica 16: 183–190. [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. (2007) Life and death: metabolic rate, membrane composition, and life span of animals . Physiol Rev 87: 1175–1213. [DOI] [PubMed] [Google Scholar]
- Inatsuchi A, Yamato S, Yusa Y. (2010) Effects of temperature and food availability on growth and reproduction in the neustonic pedunculate barnacle Lepas anserifera . Mar Biol 157: 899–905. [Google Scholar]
- Kearney BD, Byrne PG, Reina RD. (2012) Larval tolerance to salinity in three species of Australian anuran: an indication of saline specialisation in Litoria aurea . PLoS ONE 7: e43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney BD, Pell RJ, Byrne PG, Reina RD. (2014) Anuran larval developmental plasticity and survival in response to variable salinity of ecologically relevant timing and magnitude . J Exp Zool A Ecol Genet Physiol 321: 541–549. [DOI] [PubMed] [Google Scholar]
- Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL. (1950) The Biology of Human Starvation. University of Minnesota Press; , Minneapolis, MN. [Google Scholar]
- Kozłowski J, Czarnołęski M, Dańko M. (2004) Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr Comp Biol 44: 480–493. [DOI] [PubMed] [Google Scholar]
- Kraft PG, Wilson RS, Franklin CE. (2005) Predator-mediated phenotypic plasticity in tadpoles of the striped marsh frog, Limnodynastes peronii . Austral Ecol 30: 558–563. [Google Scholar]
- Leips J, Travis J. (1994) Metamorphic responses to changing food levels in two species of Hylid frogs . Ecology 75: 1345–1356. [Google Scholar]
- Lin X, Zhang D. (1999) Inference in generalized additive mixed models by using smoothing splines . J R Stat Soc Series B Stat Methodol 61: 381–400. [Google Scholar]
- Lind MI, Persbo F, Johansson F. (2008) Pool desiccation and developmental thresholds in the common frog, Rana temporaria . Proc Biol Sci 275: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod IM, Rummer JL, Clark TD, Jones GP, McCormick MI, Wenger AS, Munday PL. (2013) Climate change and the performance of larval coral reef fishes: the interaction between temperature and food availability . Conserv Physiol 1: doi:10.1093/conphys/cot024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams DA. (2008) Nutrition recommendations for some captive amphibian species (Anura and Caudata) . Can Assoc Zoo Aquariums Nutr Advis Res Group, http://www.caza-narg.ca/ref/amphibian%20nutrition%20report%20CAZA%202008.pdf. [Google Scholar]
- Mantellato L, Gaikhorst G, Kruger R, Vitali S, Robertson H. (2013) Growth and development of captive Geocrinia rosea (Myobatrachidae): a rare species analogue . Zoo Biol 32: 374–380. [DOI] [PubMed] [Google Scholar]
- Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. (2005) Ecological consequences of phenotypic plasticity . Trends Ecol Evol 20: 685–692. [DOI] [PubMed] [Google Scholar]
- Monaghan P. (2008) Early growth conditions, phenotypic development and environmental change . Philos Trans R Soc B Biol Sci 363: 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey SR, Reznick DN. (2000) A comparative analysis of plasticity in larval development in three species of spadefoot toads . Ecology 81: 1736–1749. [Google Scholar]
- Morey SR, Reznick DN. (2004) The relationship between habitat permanence and larval development in California spadefoot toads: field and laboratory comparisons of developmental plasticity . Oikos 104: 172–190. [Google Scholar]
- Munn A, Kern P, McAllan B. (2010) Coping with chaos: unpredictable food supplies intensify torpor use in an arid-zone marsupial, the fat-tailed dunnart (Sminthopsis crassicaudata) . Naturwissenschaften 97: 601–605. [DOI] [PubMed] [Google Scholar]
- Muths E, Bailey LL, Watry MK. (2014) Animal reintroductions: an innovative assessment of survival . Biol Conserv 172: 200–208. [Google Scholar]
- Newman RA. (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level . Oecologia 115: 9–16. [DOI] [PubMed] [Google Scholar]
- Nicieza AG. (2000) Interacting effects of predation risk and food availability on larval anuran behaviour and development . Oecologia 123: 497–505. [DOI] [PubMed] [Google Scholar]
- Nicieza AG, Metcalfe NB. (1997) Growth compensation in juvenile Atlantic salmon: responses to depressed temperature and food availability . Ecology 78: 2385–2400. [Google Scholar]
- Niehaus AC, Wilson RS, Franklin CE. (2006) Short- and long-term consequences of thermal variation in the larval environment of anurans . J Anim Ecol 75: 686–692. [DOI] [PubMed] [Google Scholar]
- O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM. (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation . Proc Natl Acad Sci USA 104: 1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy V, Preziosi RF, Fidgett AL. (2012) A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red-eye tree frog . Anim Conserv 15: 480–488. [Google Scholar]
- Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. (2010) Phenotypic plasticity’s impacts on diversification and speciation . Trends Ecol Evol 25: 459–467. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. (2014) R core team (2014). Nlme: Linear and nonlinear mixed effects models. R package version 3.1-117, http://cran.r-project.org/web/packages/nlme/index.html.
- R Developer Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; , Vienna, Austria. [Google Scholar]
- Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. (2010) Phenotypic plasticity and population viability: the importance of environmental predictability . Proc Biol Sci 277: 3391–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyea RA. (2001) Morphological and behavioral plasticity of larval anurans in response to different predators . Ecology 82: 523–540. [Google Scholar]
- Rose CS. (2005) Integrating ecology and developmental biology to explain the timing of frog metamorphosis . Trends Ecol Evol 20: 129–135. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Volpov BL, Trites AW. (2014) Short-term episodes of imposed fasting have a greater effect on young northern fur seals (Callorhinus ursinus) in summer than in winter . Conserv Physiol 2: doi:10.1093/conphys/cou021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanuy D, Oromí N, Galofré A. (2008) Effects of temperature on embryonic and larval development and growth in the Natterjack Toad (Bufo calamita) in a semi-arid zone . Anim Biodiv Conserv 31: 41–46. [Google Scholar]
- Schell C, Burgin S. (2002) Swimming against the current: the brown striped marsh frog Limnodynastes peronii success story . Aust Zool 32: 401–405. [Google Scholar]
- Sheldon BC, Arponen H, Laurila A, Crochet PA, Merilä J. (2003) Sire coloration influences offspring survival under predation risk in the moorfrog . J Evol Biol 16: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, Waller RW. (2004) Status and trends of amphibian declines and extinctions worldwide . Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Tarszisz E, Dickman CR, Munn AJ. (2014) Physiology in conservation translocations . Conserv Physiol 2: doi:10.1093/conphys/cou054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhumberg B, Tyre AJ, Roitberg B. (2000) Stochastic variation in food availability influences weight and age at maturity . J Theor Biol 202: 257–272. [DOI] [PubMed] [Google Scholar]
- Therneau T. (2014) A package for survival analysis in s. 2014, r package version 2.37-7, http://CRAN.R-project.org/package=survival.
- Travis J. (1984) Anuran size at metamorphosis: experimental test of a model based on intraspecific competition . Ecology 65: 1155–1160. [Google Scholar]
- Walsh PT, Downie JR, Monaghan P. (2008) Temperature-mediated morphology changes during metamorphic climax in the African clawed frog, Xenopus laevis . J Therm Biol 33: 244–249. [Google Scholar]
- Walters RJ, Hassall M. (2006) The temperature-size rule in ectotherms: may a general explanation exist after all? Am Nat 167: 510–523. [DOI] [PubMed] [Google Scholar]
- Wang T, Hung CCY, Randall DJ. (2006) The comparative physiology of food deprivation: from feast to famine . Annu Rev Physiol 68: 223–251. [DOI] [PubMed] [Google Scholar]
- Wilbur HM, Collins JP. (1973) Ecological aspects of amphibian metamorphosis: nonnormal distributions of competitive ability reflect selection for facultative metamorphosis . Science 182: 1305–1314. [DOI] [PubMed] [Google Scholar]
- Wilson RS. (2001) Geographic variation in thermal sensitivity of jumping performance in the frog Limnodynastes peronii . J Exp Biol 204: 4227–4236. [DOI] [PubMed] [Google Scholar]
- Wood S. (2014). Gamm4: Generalized additive mixed models using mgcv and lme4. Version 0.2-2. R package.
- Yoneda M, Wright PJ. (2005) Effect of temperature and food availability on reproductive investment of first-time spawning male Atlantic Cod, Gadus morhua . ICES J Mar Sci 62: 1387–1393. [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. (2009) Mixed Effects Models and Extensions in Ecology with R. Springer Science & Business Media; , New York, USA. [Google Scholar]