We compare cortisol levels in monkeys at two sites with varying habitat disturbance within Kibale National Park, Uganda. Both species have higher cortisol levels at the less disturbed of the two sites. Factors such as social dynamics or predation may be responsible, illustrating the subtleties of wild primate ecophysiology.
Keywords: Ecophysiology, fragmentation, Kibale, predation, primates, stress
Abstract
Non-invasive measurement of urinary cortisol is a proven method of evaluating the impact of environmental stressors on wild primates. Variation in cortisol concentrations can reflect physiological stress, and prolonged elevation of circulating cortisol can significantly affect individual and population-level health. In a previous study, we found that urinary cortisol concentrations in grey-cheeked mangabeys (Lophocebus albigena) were higher at a highly disturbed site (Mainaro) in Kibale National Park, Uganda compared with a minimally disturbed site (Ngogo) in the same habitat. Here, we expand on this research, reporting on cortisol concentrations in two other cercopithecid monkeys (Cercopithecus ascanius and Piliocolobus rufomitratus) at the same two sites. We hypothesized that C. ascanius would show no difference between sites, given its preference for secondary forests, while P. rufomitratus would have higher cortisol concentrations at the disturbed site. Contrary to expectations, both species exhibited significantly higher cortisol concentrations at Ngogo (minimally disturbed) compared with Mainaro (disturbed). We suggest that these results may be caused by inter- or intragroup social dynamics, intersite differences in predation rate, fruit/food availability and chemistry, or a combination of these factors. These initial evaluations of urinary cortisol concentrations provide testable hypotheses on habitat disturbance and Kibale primate ecophysiology.
Introduction
Deterioration of environmental quality due to factors such as drought, increased predation and habitat disturbance can affect wild animal population health. Social factors, including competition for food and mating opportunities and instability in social relationships, can also influence well-being. Ecological or social perturbations may disrupt homeostasis and force immediate physiological adjustments, changing the hormonal milieu that regulates growth, immune function and reproduction (Reeder and Kramer, 2005). The development of methods to analyse stress biomarkers via non-invasive collection of urine and faeces from wild populations provides insights into ecophysiology in general (Wikelski and Cooke, 2006; Romano et al., 2010; Cooke et al., 2013b). Primate habitat disturbance varies greatly along a spectrum from large, relatively undisturbed swathes of suitable habitat (e.g. closed canopy forests) minimally affected by human activity to small, threatened fragments in landscapes extensively altered by humans (Johns and Skorupa, 1987; Bicca-Marques, 2003; Marsh, 2003). Comparing primate communities in undisturbed vs. disturbed habitats can illustrate the influence of environmental conditions on physiological stress.
Cortisol is a glucocorticoid produced by the adrenal cortex under stimulation by adrenocorticotrophic hormone. It is responsible for classic stress responses to environmental challenges. It acts as an anti-inflammatory agent, facilitates the cellular uptake of glucose and acts as a catabolic agent. Its molecular structure and function are conserved across most vertebrates and all mammals, making it a viable and robust agent of investigation for conservation biology (Griffin and Ojeda, 2004; Norris and Carr, 2013). Multiple stressors can stimulate increases in cortisol, such as food shortages, social instability, infection and injury, predation threat and habitat disturbance (Sapolsky, 2005; Honess and Marin, 2006; Wikelski and Cooke, 2006; Anestis, 2010). While the functional utility of short-term increases in cortisol are well known, chronically elevated cortisol can disrupt reproductive function, immunocompetence, growth and neurological function (Sapolsky et al., 1990; Chrousos et al., 1998; Habib et al., 2000; Boonstra, 2013a,b). Cortisol concentrations in primate urine reflect the amount of the hormone circulating in both conjugated and unconjugated forms (Bahr et al., 2000) and can be assessed using samples collected non-invasively in the field (Whitten et al., 1998). As analytical methods improve, primatologists are providing more nuanced analyses of wild primate physiology and habitat-associated stressors such as habitat disturbance (Cavigelli, 1999; Muller and Wrangham, 2004; Strier and Ziegler, 2005; Chapman et al., 2006; Martínez-Mota et al., 2007; Dittami et al., 2008; Arlet et al., 2009; Arlet and Isbell, 2009; Shutt et al., 2012; Rimbach et al., 2013; Balestri et al., 2014).
We previously found that urinary cortisol concentrations were higher in grey-cheeked mangabeys (Lophocebus albigena johnstoni) at a highly disturbed site than in conspecifics at a relatively undisturbed site in Kibale National Park, Uganda, presumably due to the physiological stress of living in the disturbed habitat (Jaimez et al., 2012). Here, we expand on this research to determine whether two other cercopithecid primates (redtail monkeys, Cercopithecus ascanius schmidti, and red colobus monkeys, Piliocolobus rufomitratus tephrosceles), sympatric with mangabeys at both sites, show similar disturbance-related differences in cortisol concentrations. We predicted that red colobus monkeys would show such differences because, like mangabeys, they preferentially use old-growth forest. In contrast, we predict that between-site differences would not occur in redtail monkeys, because they prefer young successional forest, like that covering much of the disturbed site.
Materials and methods
Study site
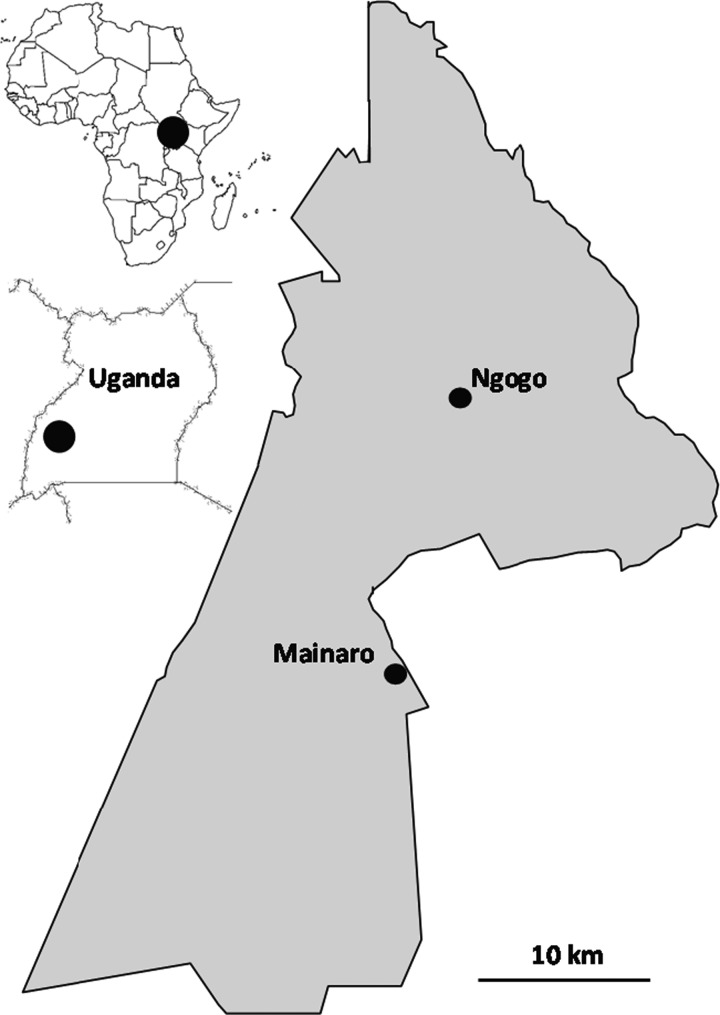
Kibale National Park, Uganda (766 km2) is a moist, evergreen medium-altitude forest with a mosaic of old-growth forest, swamp, grassland and regenerating forest of varying ages (Struhsaker, 1997). Mainaro, on the southeastern edge of the park (Fig. 1), experienced severe encroachment by local farmers between the 1960s and the early 1990s despite Kibale's protected status (Van Orsdol, 1986; Aronsen, 2010). Replanting of endemic tree species was initiated in 1994, and the site now has a mosaic of old-growth forest patches interspersed with patches of previously disturbed vegetation at various stages of succession and a large area of replanted, young forest (Verweij and Emmer, 1998; Mucunguzi et al., 2007). Ngogo, in the centre of Kibale (Fig. 1), was not subjected to commercial logging while Kibale was a forest reserve (Struhsaker, 1997), nor did it experience recent conversion of forest to farmland. It contains large areas of old-growth forest plus areas of regenerating forest, swamp forest and grassland (Lwanga et al., 2000). Human presence is more conspicuous at Mainaro because it is on the edge of the park and immediately accessible via public road. Ngogo, at the centre of the park, is accessible only by a long, difficult dirt road and footpaths. Average diameter at breast height (dbh) for trees >10 cm dbh is similar at both sites [for Mainaro, 24.2 ± 16.31 cm (SD), n = 3447 trees, G. P. Aronsen and S. Teelen, unpublished data; and for Ngogo, 25.0 ± 24.0 cm, n = 2600 trees, C. A. Chapman, unpublished data]. However, Ngogo has more large trees (>50 cm dbh), reflecting its lack of recent human disturbance (Kolmogorov–Smirnov Z = 2.02, P = 0.001; G. P. Aronsen and S. Teelen, unpublished data; C. A. Chapman, unpublished data).
Figure 1:
Map of Kibale National Park, Uganda.
Study taxa
Red colobus monkeys are colobines whose diet comprises mostly young leaves and petioles but also includes fruits and mature leaves from a variety of species (Struhsaker, 1975; Chapman and Chapman, 1999). They use a variety of habitat types and occupy some forest fragments outside the park (Lwanga, 2006), but they form relatively smaller groups and have higher parasite loads in forest fragments than within the park (Gillespie et al., 2003; Chapman et al., 2006). Red colobus at Ngogo are under severe predation pressure from the large chimpanzee community there; hunting offtakes have been as much as 188 monkeys within 7 months (Teelen, 2008). As a result, the local red colobus population has declined steeply in the last decade (Teelen, 2008; Lwanga et al., 2011; Watts and Amsler, 2013). Red colobus abundance is higher at Mainaro than at Ngogo; although limited data indicate a slightly higher mean group size at Ngogo (34–45 individuals per group for four groups; Teelen, 2007a,b, 2008) than at Mainaro (mean = 30 individuals per group; Chapman and Chapman, 2000), census data give much higher group abundance counts at Mainaro (mean = 0.29 groups/km; G. P. Aronsen and S. Teelen, unpublished data) than at Ngogo (mean = of 0.06 groups/km; Lwanga et al., 2011).
Redtail monkeys, which are common at both sites, are smaller than mangabeys and red colobus. They use a broad mix of vegetation types, including old-growth forest, but apparently prefer young, regenerating forest (Lambert, 1995; Sheppard, 1999; Baranga, 2004). Redtails in unprotected forest fragments also have higher parasite loads than those within the park (Gillespie et al., 2005), but they use areas where even colobines are uncommon (Chapman et al., 2003), indicating better ability to persist in suboptimal habitats. Redtail abundance may be slightly higher at Ngogo than at Mainaro; the mean number of groups encountered per kilometre during censuses there was 0.70 in 2007 (Lwanga et al., 2011), whereas the mean for Mainaro was 0.57 during 2007–2010 (G. P. Aronsen and S. Teelen, unpublished data). Published group size estimates are slightly higher for Ngogo (n = 32 individuals; Mitani et al., 2001) than for Mainaro (n = 25; Chapman and Chapman, 2000; Mitani et al., 2001), although the variation in group size is considerable (Struhsaker and Leland, 1988; Windfelder and Lwanga, 2004; Lwanga et al., 2011).
Collection of urine and analysis of urinary cortisol
Animal encounter and urine collection followed the methods of Jaimez et al. (2012). Between June and August 2010, G.P.A. and field assistants walked along existing forest transects to find primate groups; on encountering one group, they estimated its size and followed it until they lost it or until the end of the day. In Kibale, redtail day ranges average about 1 km (Lambert, 1999); red colobus day ranges average ∼570 m (Isbell, 1983). Redtails sampled at Ngogo were habituated, but those at Mainaro were not, and red colobus were poorly habituated at both sites. To avoid generating additional stress via human presence, we attempted to avoid resampling the same group on consecutive days by spacing out collection locations by distances >1 km. This method was deployed successfully by Jaimez et al. (2012). In related species, urinary cortisol values reflect circulating concentrations 4–8 h in the past (Whitten et al., 1998), thereby reducing the potential impact of observational contact with researchers.
The study period occurred during what is typically a dry season, but note that neither energy availability nor reproduction shows strong seasonal variation in red colobus or redtails (Struhsaker, 1997). At each site, two different groups were sampled from each species (four groups from each site; eight groups in total). While following groups, we used plastic pipettes to collect urine from vegetation at the ground layer immediately after we saw an animal void. We immediately placed the urine into 1.5 ml snap tubes that were labelled with sample identification numbers and information on individual sex and age (if known), date and time of day. To minimize the risk of sample cross-contamination, urine was collected only when fresh and when it was clear that multiple individuals had not urinated in the same area. The GPS location was noted for each sample. No samples were collected on days when it rained. Collections were made over 15 day periods at each site. Monkeys at Mainaro were assigned to the disturbed forest (DF) group category, while those at Ngogo were assigned to the undisturbed forest (UF) group category. No monkeys are individually tagged or identifiable at these sites. We made every effort to avoid repeated sampling of single individuals by systematically moving through the group's spatial distribution and collecting samples during feeding, travel and resting. We did not resample individuals in an area where a team member had already walked through. We sampled across age and sex classes and pooled data for analysis. There is a risk of repeated sampling when studying any primate group, but our use of multiple groups and attempts to move exhaustively across the group over the course of a day provided our best effort to limit pseudoreplication.
Field handling and storage followed previously deployed and validated field protocols (Knott, 1997; Jaimez et al., 2012). At camp, 100–200 μl of urine (depending on amount collected) from each sample was pipetted onto an Atago PAL-10S Portable Digital Clinical Refractometer to record specific gravity to supplement creatinine measurements to correct for urine concentration. The refractometer was cleaned between samples. Each urine sample was then transferred onto Whatman filter paper. Each piece of filter paper was placed on aluminum foil and labelled with a sample identification number and with information on the sex and age of the individual (when known), date and time of day. Between 100 and 200 μl of urine was evenly pipetted onto the filter paper, in duplicate when the volume was sufficient. To protect samples against mould contamination, each piece of filter paper was immediately placed into a small ziplock bag that was in turn placed on aluminum foil resting over a layer of silica. These ziplock bags were placed in the shade and left undisturbed for 2 days (Campbell, 1994; Shideler et al., 1995). Once dried, samples were wrapped in foil, labelled with the sample number and placed in plastic slide sheets for storage and transport back to the Yale University Reproductive Ecology Laboratory.
Urinary cortisol was assayed as follows. Elution of the filter paper was conducted by insertion into labelled 16 mm × 100 mm borosilicate tubes. Five millilitres of 100% methanol was added to each test tube, and tubes were sealed with parafilm and refrigerated overnight. The following morning, the filter paper was squeezed against the side of the tube using sterilized forceps. Sample tubes were dried under compressed air or nitrogen, reconstituted with 1 ml of distilled water, vortexed for 2 min and sealed with parafilm until they were assayed (Knott, 2005; Marshall and Hohmann, 2005; Dittami et al., 2008). Creatinine values were also assessed via Jaffe's reaction (Taussky, 1954).
Creatinine correction was calculated by dividing the cortisol value by the creatinine value. Immediately after creatinine assays were completed, samples were assayed in duplicate using an unmodified high-sensitivity salivary cortisol enzyme immunoassay suitable for detecting the range of reconstituted urine hormone values (catalogue 1-3102; Salimetrics, State College, PA, USA). This assay has previously been deployed successfully in non-human primates (Jaimez et al., 2012). Coefficients of variation for internal high- and low-quality control were 0.45 and 5.16%. Blanks read below detection levels. Samples that were excluded either had concentrations below detection limits of the assay or had insufficient volume.
Statistical analysis
Results showed heteroscedastic distribution, so we logarithmically transformed raw data. Runs tests and residual plotting confirmed normality, with all results having a P-value > 0.05. Associations between cortisol and collection times were determined using standard linear regression. We used Student's unpaired t-tests to assess differences in mean urinary cortisol values. Statistical analyses were conducted using Prism 6.02 for Windows (GraphPad, Inc., San Diego, CA, USA). The value of α was set at 0.05.
This research adhered to the legal requirements of Uganda and to the American Society of Primatologists Principles for the Ethical treatment of Nonhuman Primates. The Yale University Institutional Animal Care and Use Committee issued a waiver for the research because of its strictly non-invasive methods.
Results
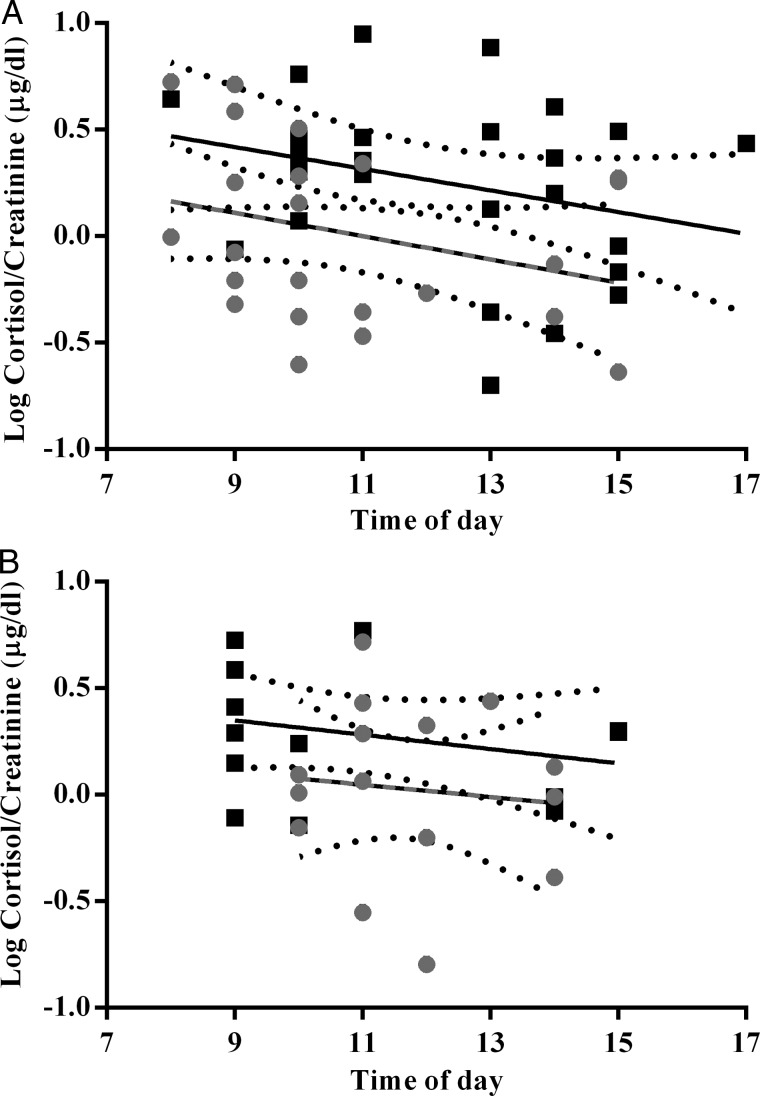
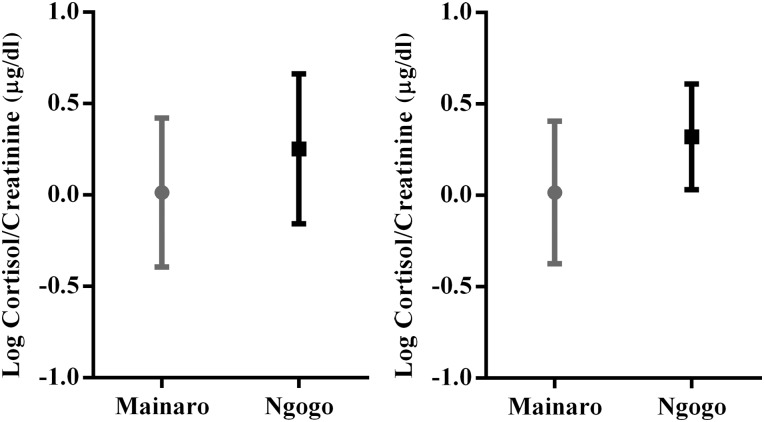
We analysed 50 redtail samples (Mainaro, n = 26; and Ngogo, n = 24) and 30 red colobus samples (Mainaro, n = 17; and Ngogo, n = 13). Redtails and red colobus at both sites followed the expected circadian pattern of decreasing cortisol from morning to evening. For redtails, cortisol concentrations did not decline significantly in relationship to time of day at either site (Mainaro, β = −0.05, r2 = 0.09, P = 0.16; and Ngogo, β = −0.05, r2 = 0.08, P = 0.16; Fig. 2), but slopes for the two sites differed significantly (F1,47 = 7.26, P = 0.01). Diurnal variation in cortisol concentrations was not significant for red colobus at either site, nor did the slopes differ significantly (Mainaro, β = −0.03, r2 = 0.01, P = 0.71; and Ngogo, β = −0.03, r2 = 0.07, P = 0.34; slope comparison, F1,26 = 2.89, P = 0.10, Fig. 2). Contrary to our predictions, both red colobus and redtails had significantly higher cortisol concentrations at Ngogo than at Mainaro (redtails, t48 = 2.063, P = 0.045; and red colobus, t28 = 2.536, P = 0.017; Fig. 3).
Figure 2:
Daily variation in cortisol in redtail (left) and red colobus monkeys (right). Mainaro data are grey circles and dashed lines; Ngogo data are black squares and solid lines. The 95% confidence intervals are plotted.
Figure 3:
Logarithmically transformed cortisol concentrations for redtail (left) and red colobus monkeys (right). Means and standard deviations are shown. Mainaro (disturbed forest) is grey, and Ngogo (undisturbed forest) is black. Intersite variation is significant for both species (for redtails, t = 2.063, d.f. = 48, P = 0.045; and for red colobus, t = 2.536, d.f. = 28, P = 0.017).
Discussion
Cortisol can serve as an indicator of physiological stress, and habitat disturbance can lead to increases in cortisol secretion. The hypothalamic–pituitary–adrenal feedback response is conservative across primates and largely consistent across the family Cercopithecidae (Coe et al., 1992). Our previous research showed that mangabeys had elevated cortisol at Mainaro relative to Ngogo, suggesting physiological stress due to habitat disturbance (Jaimez et al., 2012). In northern Kibale, mangabeys and red colobus in disturbed habitats show longer travel duration, lower body mass and (for red colobus) higher faecal cortisol concentrations relative to conspecifics in less disturbed areas (Olupot et al., 1994; Olupot, 2000; Gillespie et al., 2005; Chapman et al., 2006, 2007).
Given these results for mangabeys, we predicted similar cortisol elevations for Mainaro red colobus, but no difference between Mainaro and Ngogo for redtails. Instead, both species showed higher cortisol concentrations at Ngogo, the less disturbed of our two study sites. Groups at both sites also showed the expected diurnal decrease in cortisol concentrations, but the decrease was non-significant, suggesting physiological stress as the reason for the between-site differences. We therefore need an explanation for why these results differ from those reported earlier for mangabeys. Factors that may contribute independently or in combination to cortisol variation are described below.
Environmental stressors
Plasma cortisol concentrations are influenced by environmental perturbations and subsequent changes to food and/or activity levels (Rhynes and Ewing, 1973; Follenius et al., 1982; Martínez-Mota et al., 2007; Beehner and McCann, 2008; Foerster et al., 2012; Rangel-Negrín et al., 2014). Fragmented habitat structure may allow for increased insolation (and therefore ambient temperature) and increased travel time, but these variables are expected to affect Mainaro (disturbed) populations more than Ngogo (undisturbed). The study was conducted during the dry season at Kibale, but temperature and rainfall data for the study period were not markedly different from previous years (J. S. Lwanga, unpublished data).
Intragroup competition
Interindividual agonism influences primate cortisol concentrations. Factors such as male–male competition, female dominance, resource access, reproductive status and increased group size can all lead to increased social conflict (Alberts et al., 1992; Strier et al., 1999; Creel, 2001; Muller and Wrangham, 2004; Fichtel et al., 2007; Arlet et al., 2009; Mendonça-Furtado et al., 2014). During our study period, group sizes were consistent with previously reported data (Chapman and Chapman, 2000; Struhsaker, 1975, 1980). Ngogo redtail group fission (and associated agonism) has been described (Windfelder and Lwanga, 2004), and thus, could be a source of intersite cortisol variation. The red colobus group distribution was relatively small at Ngogo during the study period (see Predation section below), making this explanation less viable. It is possible that we sampled Ngogo groups during a period of social instability, which could be tested via more long-term sampling of specific groups.
Intergroup competition
Spatiotemporal variation in food availability can also lead to increased encounter rates and conflict between groups or species, thus altering primate cortisol concentrations (Pride, 2005; Schoof and Jack, 2013). While Ngogo has an old-growth forest, access to preferred fruiting trees/resources could lead to higher competition and associated increases in cortisol concentrations. During the study period, no interspecific/intergroup aggression or encounters were observed, and the fruits eaten by each species are neither high demand nor uncommon. At Mainaro, both redtails and red colobus fed on Chrysophyllum albidum and Uvariopsis congensis fruits most frequently. These resources are neither rare nor uncommon within Kibale. At Ngogo, redtails fed from these tree species, while red colobus were feeding most heavily on Ptyerygota mildbraedii fruits. This tree species is rare at Mainaro, but very common at Ngogo.
Phytohormone contamination
The influence of exogenous sources on endocrine function has been described (Colborn et al., 1993; Tyler et al., 1998; Hotchkiss et al., 2008), but many aspects remain unclear (Retana-Márquez et al., 2012; Zhabinskii et al., 2014). Research on Ugandan red colobus has shown that consumption of Milettia dura can have an effect on cortisol concentrations due to phytoestrogen concentrations (Wasserman et al., 2012a,b). This specific plant species was not eaten during the study period, and we are unaware of phytohormone studies of the plant species consumed during our study. This is an additional confounding factor, requiring both sampling of plants consumed by primates and analysis of these species' pharmacokinetic influence on endocrine function (Rode et al., 2006; Lu et al., 2011; Rothman et al., 2012; Wasserman et al., 2013b).
Predation
Effects of predation pressure on cortisol concentrations are well documented in other animals (Tilbrook et al., 2000; Engh et al., 2006; Arlet and Isbell, 2009; Sheriff et al., 2011; Wang et al., 2011; Soares et al., 2012; Michelena et al., 2012; Cooke et al., 2013a; Fischer et al., 2014; but see Baker et al., 2013). Variation in predation rates may explain the cortisol concentration variation between Mainaro and Ngogo, especially for red colobus. Red colobus are the preferred prey of chimpanzees wherever the two species are sympatric (Boesch, 1994; Stanford, 1995; Watts and Mitani, 2002). The Ngogo chimpanzee community is the largest yet recorded and exerts unusually high predation pressure on the local red colobus population. While Ngogo chimpanzees hunt all diurnal primate species, effort and offtake is comparatively low for those other than red colobus. This is especially so for redtails and mangabeys, the most abundant primate species there (Lwanga et al., 2011). Predation by Ngogo chimpanzees has strongly affected red colobus demography and distribution (Mitani and Watts, 1999; Watts and Mitani, 2002; Pobiner et al., 2007; Teelen, 2008; Watts and Amsler, 2013). Census data show a steady decline of red colobus density within the chimpanzees' home range, with encounter rates for red colobus declining steeply since 1998, almost certainly because of predation by chimpanzees (Mitani et al., 2010; Lwanga et al., 2011; Watts and Amsler, 2013). Chimpanzees sometimes wait under or stalk red colobus groups for more than an hour before hunting them or leaving and sometimes attack the same group repeatedly over several hours (Watts and Mitani, 2002; D. Watts, personal observation). Besides their much lower frequency, hunts of redtails have much shorter durations (Watts and Mitani, 2002). By implication, exposure to chimpanzees should have much greater physiological effects on red colobus than on redtails or mangabeys, and these effects could easily outweigh any direct effects of variation in energy availability. Following a chimpanzee predation event, Kanyawara red colobus faecal samples (n = 6) showed elevated cortisol concentrations 2–5 days afterwards (Wasserman et al., 2013a). Given this, the pattern of elevated urinary cortisol in Ngogo red colobus may be associated with an unobserved chimpanzee predation event, constant vigilance for chimpanzee presence and/or variation in urinary/faecal cortisol concentrations following predation events (Bahr et al., 2000; Voellmy et al., 2014).
Crowned hawk-eagles (Stephanoaetus coronatus) are also major primate predators in Kibale (Skorupa, 1989; Struhsaker and Leakey, 1990), and redtails are their most common prey at Ngogo (Mitani et al., 2001; Sanders et al., 2003). Polyspecific associations between redtails and red colobus are more frequent at Ngogo than at Kanyawara, and redtails appear to initiate and maintain associations to limit eagle predation (Teelen, 2007a). Habitat disturbance strongly restricts crowned hawk-eagle distribution in the Kibale region, where the species occurs only inside the park (Seavy and Apodaca, 2002; Sekercioglu, 2002). How much its distribution within Kibale varies is unknown. Crowned hawk-eagle home range size is estimated at 10 km2, which implies that few pairs could use the Mainaro study area given its narrow east–west width (Fig. 1). Crowned hawk-eagles and other raptors may have higher densities or higher predation success at Ngogo than at Mainaro; if so, this could explain why redtail cortisol concentrations were lower at Mainaro.
Our previous study described higher concentrations of cortisol in mangabeys at Mainaro than at Ngogo, the inverse of the patterns seen in redtails and red colobus (Jaimez et al., 2012). Why does this contrast exist? Available data indicate that successful predation of mangabeys by chimpanzees and crowned hawk-eagles is relatively low (Ham, 1994; Waser, 1985; Watts and Mitani, 2002; D. P. Watts, unpublished data). Adult male mangabeys often chase crowned hawk-eagles; they show elevated faecal cortisol concentrations for days afterwards (Arlet and Isbell, 2009). At Ngogo, mangabeys usually flee from hunting attempts by chimpanzees, and, as for redtails, average hunt duration is short (Watts and Mitani, 2002). Consequently, chimpanzee predation attempts probably have limited physiological effects, and any such effects are likely to be outweighed by those of variation in food availability and variation in foraging effort.
Our preliminary results illustrate the complexity of associating endocrine variation with single variables such as habitat fragmentation. The increased cortisol concentrations recorded in Ngogo redtails and red colobus relative to their Mainaro conspecifics may be associated with one or several variables, such as social group instability, fruit and leaf phytochemistry and/or predation pressure. The downstream effects of increased cortisol concentrations on individual fitness are under debate (Sapolsky, 1985; Rabin et al., 1988; Goymann, 2012; Liu et al., 2012; Boonstra, 2013a,b; Crespi et al., 2013; Dantzer et al., 2014). If increased cortisol has a detrimental impact on aspects of senescence, immune function and fertility, the Ngogo red colobus population, already under severe predation pressure, may dwindle further due to the lethal combination of high predation loss and severe psychological/physiological stress. For Ngogo redtails, increased cortisol concentrations may be an indicator of impending social group fission, hierarchy instability or other stressful events. At Mainaro, which is currently undergoing restoration, the relatively lower cortisol concentrations suggest that this habitat currently provides a suitable environment for redtail and red colobus monkeys. The Mainaro mangabeys, with their relatively higher requirements for mature, undisturbed forests, may still be struggling with environmental stressors.
Our study provides insights on the evaluation of primate condition and concerns within a national park, and the results are useful for conservation biology and park/site management protocols. The present results indicate that the Mainaro restoration efforts are providing suitable habitat for some, but not all, primate species, and continued efforts to bring Mainaro back into a more old-growth state will enhance and improve primate diversity. The results also indicate that primate population declines (red colobus) may result from biogenic (intra- or interspecific factors) rather than anthropogenic causes. Future studies at Kibale and for other primate species must combine physiology, socioecology, habitat structure, ecological community and nutritional data collection. Monitoring these variables will provide a more nuanced and cohesive evaluation of glucocorticoid responses in wild primates and provide measures of individual/community health and potential stressors.
Funding
We received funding support from the Yale Institute for Biospheric Studies Program in Reproductive Ecology, the L.S.B. Leakey Foundation, the Great Ape Trust of Iowa and the Yale University Department of Anthropology.
Acknowledgements
We thank the Ugandan President's Office, the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for their permission to work in Kibale National Park. We thank the UWA/FACE Project Director (W. Chemutai), the Ngogo Chimpanzee Project Manager (J. Lwanga) and the camp staff of Ngogo and Mainaro for logistics in the field. G.P.A. especially thanks P. Birungi, D. Kamekune, P. Mwesigwa, F. Kamekune and J. Turyasingura for their field assistance during this project, and Stephanie Anestis for laboratory support. We appreciate the comments of two anonymous reviewers in improving this manuscript.
References
- Alberts SC, Sapolsky RM, Altmann J. (1992) Behavioral, endocrine, and immunological correlates of immigration by an aggressive male into a natural primate group. Horm Behav 26: 167–178. [DOI] [PubMed] [Google Scholar]
- Anestis SF. (2010) Hormones and social behavior in primates. Evol Anthropol 19: 66–78. [Google Scholar]
- Arlet ME, Isbell L. (2009) Variation in behavioral and hormonal responses of adult male gray-cheeked mangabeys (Lophocebus albigena) to crowned eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behav Ecol Sociobiol 63: 491–499. [Google Scholar]
- Arlet ME, Grote MN, Molleman F, Isbell LA, Carey JR. (2009) Reproductive tactics influence cortisol levels in individual male gray-cheeked mangabeys (Lophocebus albigena). Horm Behav 55: 210–216. [DOI] [PubMed] [Google Scholar]
- Aronsen GP. (2010) New photographic evidence of the African golden cat (Profelis aurata Temminck) at Mainaro, Kibale National Park, Uganda. Afr J Ecol 48: 541–545. [Google Scholar]
- Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. (2000) Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen Comp Endocrinol 117: 427–438. [DOI] [PubMed] [Google Scholar]
- Baker MR, Gobush KS, Vynne CH. (2013) Review of factors influencing stress hormones in fish and wildlife. J Nat Conserv 21: 309–318. [Google Scholar]
- Balestri M, Barresi M, Campera M, Serra V, Ramanamanjato JB, Heistermann M, Donati G. (2014) Habitat degradation and seasonality affect physiological stress levels of Eulemur collaris in littoral forest fragments. PLoS ONE 9: e107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranga D. (2004) Red-tail monkey groups in forest patches outside the protected area system in the Kampala area. Afr J Ecol 42: 78–83. [Google Scholar]
- Beehner JC, McCann C. (2008) Seasonal and altitudinal effects on glucocorticoid metabolites in a wild primate (Theropithecus gelada). Physiol Behav 95: 508–514. [DOI] [PubMed] [Google Scholar]
- Bicca-Marques JC. (2003) How do howler monkeys cope with habitat fragmentation. In Marsh LK, eds, Primates in Fragments: Ecology and Conservation. Springer, Chicago, pp 283–303. [Google Scholar]
- Boesch C. (1994) Chimpanzees–red colobus monkeys: a predator–prey system. Anim Behav 47: 1135–1148. [Google Scholar]
- Boonstra R. (2013a) The ecology of stress: a marriage of disciplines. Funct Ecol 27: 7–10. [Google Scholar]
- Boonstra R. (2013b) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–23. [Google Scholar]
- Campbell KL. (1994) Blood, urine, saliva and dip-sticks: experiences in Africa, New Guinea, and Boston. In Campbell KL, Wood JW, eds, Human Reproductive Ecology: Interactions of Environment, Fertility, and Behavior. New York Academy of Sciences, New York, pp 312–330. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA. (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav 57: 935–944. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ. (1999) Implications of small scale variation in ecological conditions for the diet and density of red colobus monkeys. Primates 40: 215–231. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ. (2000) Constraints on group size in red colobus and red-tailed guenons: examining the generality of the ecological constraints model. Int J Primatol 21: 565–585. [Google Scholar]
- Chapman CA, Chapman LJ, Lambert JE, Struhsaker TT. (2003) Thirty years of research in Kibale National Park, Uganda reveals a complex picture for conservation. Am J Primatol 60: 85–86.12874840 [Google Scholar]
- Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TL, Ziegler TE. (2006) Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am J Phys Anthropol 131: 525–534. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Naughton-Treves L, Lawes MJ, Wasserman MD, Gillespie TR. (2007) Population declines of colobus in Western Uganda and conservation value of forest fragments. Int J Primatol 28: “513–528. [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. (1998) Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 129: 229–240. [DOI] [PubMed] [Google Scholar]
- Coe CL, Savage A, Bromley LJ. (1992) Phylogenetic influences on hormone levels across the primate order. Am J Primatol 28: 81–100. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RF, Bohnert DW, Reis MM, Cappellozza BI. (2013a) Wolf presence in the ranch of origin: impacts on temperament and physiological responses of beef cattle following a simulated wolf encounter. J Anim Sci 91: 5905–5911. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013b) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel S. (2001) Social dominance and stress hormones. Trends Ecol Evol 16: 491–497. [Google Scholar]
- Crespi EJ, Williams TD, Jessop TS, Delehanty B. (2013) Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct Ecol 27: 93–106. [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ. (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2: doi:10.1093/conphys/cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittami J, Katina S, Möstl E, Eriksson J, Machatschke IH, Hohmann G. (2008) Urinary androgens and cortisol metabolites in field-sampled bonobos (Pan paniscus). Gen Comp Endocrinol 155: 552–557. [DOI] [PubMed] [Google Scholar]
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. (2006) Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc Biol Sci 273: 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel C, Kraus C, Ganswindt A, Heistermann M. (2007) Influence of reproductive season and rank on fecal glucocorticoid levels in free-ranging male Verreaux's sifakas (Propithecus verreauxi). Horm Behav 51: 640–648. [DOI] [PubMed] [Google Scholar]
- Fischer EK, Harris RM, Hofmann HA, Hoke KL. (2014) Predator exposure alters stress physiology in guppies across timescales. Horm Behav 65: 165–172. [DOI] [PubMed] [Google Scholar]
- Foerster S, Cords M, Monfort SL. (2012) Seasonal energetic stress in a tropical forest primate: proximate causes and evolutionary implications. PLoS ONE 7: e50108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Oyono S, Candas V. (1982) Cortisol as a sensitive index of heat-intolerance. Physiol Behav 29: 509–513. [DOI] [PubMed] [Google Scholar]
- Gillespie T, Chapman CA, Greiner EC. (2003) Impact of logging-induced ecological change on disease dynamics in African forest primates. Am J Primatol 60: 148. [Google Scholar]
- Gillespie TR, Chapman CA, Greiner EC. (2005) Effects of logging on gastrointestinal parasite infections and infection risk in African primates. J Appl Ecol 42: 699–707. [Google Scholar]
- Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- Griffin JE, Ojeda SR. (2004) Textbook of Endocrine Physiology. Oxford, New York. [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, et al. (2000) Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci USA 97: 6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham RM. (1994) Behaviour and ecology of grey-cheeked mangabeys (Cercocebus albigena) in the Lopé Reserve, Gabon. PhD Thesis. University of Stirling, UK. [Google Scholar]
- Honess PE, Marin CM. (2006) Behavioural and physiological aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev 30: 390–412. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. (2008) Fifteen years after ‘Wingspread’—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci 105: 235–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell LA. (1983) Daily ranging behavior of red colobus (Colobus badius tephrosceles) in Kibale Forest, Uganda. Folia Primatol 41: 34–48. [Google Scholar]
- Jaimez NA, Bribiescas RG, Aronsen GP, Anestis SA, Watts DP. (2012) Urinary cortisol levels of gray-cheeked mangabeys are higher in disturbed compared to undisturbed forest areas in Kibale National Park, Uganda. Anim Conserv 15: 242–247. [Google Scholar]
- Johns A, Skorupa J. (1987) Responses of rain-forest primates to habitat disturbance: a review. Int J Primatol 8: 157–191. [Google Scholar]
- Knott CD. (1997) Field collection and preservation of urine in orangutans and chimpanzees. Trop Biodivers 4: 95–102. [Google Scholar]
- Knott CD. (2005) Radioimmunoassay of estrone conjugates from urine dried on filter paper. Am J Primatol 67: 121–135. [DOI] [PubMed] [Google Scholar]
- Lambert JE. (1995) Feeding behavior of common chimpanzees and redtail monkeys: seed dispersal in the Kibale Forest, Uganda. Am J Phys Anthropol 20: 128. [Google Scholar]
- Lambert JE. (1999) Seed handling in chimpanzees (Pan troglodytes) and redtail monkeys (Cercopithecus ascanius): implications for understanding hominoid and cercopithecine fruit-processing strategies and seed dispersal. Am J Phys Anthropol 109: 365–386. [DOI] [PubMed] [Google Scholar]
- Liu YX, Cheng YN, Miao YL, Wei DL, Zhao LH, Luo MJ, Tan JH. (2012) Psychological stress on female mice diminishes the developmental potential of oocytes: a study using the predatory stress model. PLoS ONE 7: e48083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Beehner JC, Czekala NM, Koenig A, Larney E, Borries C. (2011) Phytochemicals and reproductive function in wild female Phayre's leaf monkeys (Trachypithecus phayrei crepusculus). Horm Behav 59: 28–36. [DOI] [PubMed] [Google Scholar]
- Lwanga JS. (2006) Spatial distribution of primates in a mosaic of colonizing and old growth forest at Ngogo, Kibale National Park, Uganda. Primates 47: 230–238. [DOI] [PubMed] [Google Scholar]
- Lwanga JS, Butynski TM, Struhsaker TT. (2000) Tree population dynamics in Kibale National Park, Uganda 1975–1998. Afr J Ecol 38: 238–247. [Google Scholar]
- Lwanga JS, Struhsaker TT, Struhsaker PJ, Butynski TM, Mitani JC. (2011) Primate population dynamics over 32.9 years at Ngogo, Kibale National Park, Uganda. Am J Primatol 73: 997–1011. [DOI] [PubMed] [Google Scholar]
- Marsh LK. (ed) (2003) Primates in Fragments: Ecology and Conservation. Kluwer, The Netherlands. [Google Scholar]
- Marshall AJ, Hohmann G. (2005) Urinary testosterone levels of wild male bonobos (Pan paniscus) in the Lomako Forest, Democratic Republic of Congo. Am J Primatol 65: 87–92. [DOI] [PubMed] [Google Scholar]
- Martínez-Mota R, Valdespino C, Sánchez-Ramos MA, Serio-Silva JC. (2007) Effects of forest fragmentation on the physiological stress response of black howler monkeys. Anim Conserv 10: 374–379. [Google Scholar]
- Mendonça-Furtado O, Edaes M, Palme R, Rodrigues A, Siqueira J, Izar P. (2014) Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behav Processes 109 Part A: 79–88. [DOI] [PubMed] [Google Scholar]
- Michelena P, Pillot MH, Henrion C, Toulet S, Boissy A, Bon R. (2012) Group size elicits specific physiological response in herbivores. Biol Lett 8: 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC, Watts DP. (1999) Demographic influences on the hunting behavior of chimpanzees. Am J Phys Anthropol 109: 439–454. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Sanders WJ, Lwanga JS, Windfelder TL. (2001) Predatory behavior of crowned hawk-eagles (Stephanoaetus coronatus) in Kibale National Park, Uganda. Behav Ecol Sociobiol 49: 187–195. [Google Scholar]
- Mitani JC, Watts DP, Amsler SJ. (2010) Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol 20: R507–R508. [DOI] [PubMed] [Google Scholar]
- Mucunguzi P, Kasenene J, Midgley J, Ssegawa P, Tabuti JRS. (2007) Distinguishing forest tree communities in Kibale National Park, western Uganda using ordination and classification methods. Afr J Ecol 45: 99–108. [Google Scholar]
- Muller MN, Wrangham RW. (2004) Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DO, Carr JA. (2013) Vertebrate Endocrinology. Academic Press, Amsterdam. [Google Scholar]
- Olupot W. (2000) Mass differences among male mangabey monkeys inhabiting logged and unlogged forest compartments. Conserv Biol 14: 833–843. [Google Scholar]
- Olupot W, Chapman CA, Brown CH, Waser PM. (1994) Mangabey (Cercocebus albigena) population density, group size, and ranging: a twenty-year comparison. Am J Primatol 32: 197–205. [DOI] [PubMed] [Google Scholar]
- Pobiner BL, DeSilva J, Sanders WJ, Mitani JC. (2007) Taphonomic analysis of skeletal remains from chimpanzee hunts at Ngogo, Kibale National Park, Uganda. J Hum Evol 52: 614–636. [DOI] [PubMed] [Google Scholar]
- Pride R. (2005) Foraging success, agonism, and predator alarms: behavioral predictors of cortisol in Lemur catta. Int J Primatol 26: 295–319. [Google Scholar]
- Rabin D, Gold P, Margioris A, Chrousos G. (1988) Stress and reproduction: physiologic and pathophysiologic interactions between the stress and reproductive axes. In Chrousos G, Loriaux DL, Gold P, eds, Mechanisms of Physical and Emotional Stress. Springer, New York, 377–387. [DOI] [PubMed] [Google Scholar]
- Rangel-Negrín A, Coyohua-Fuentes A, Chavira R, Canales-Espinosa D, Dias PAD. (2014) Primates living outside protected habitats are more stressed: the case of black howler monkeys in the Yucatán Peninsula. PLoS ONE 9: e112329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder DM, Kramer KM. (2005) Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86: 225–235. [Google Scholar]
- Retana-Márquez S, Hernández H, Flores J, Muñoz-Gutiérrez M, Duarte G, Vielma J, Fitz-Rodríguez G, Fernández I., Keller M, Delgadillo J. (2012) Effects of phytoestrogens on mammalian reproductive physiology. Trop Subtrop Agroecosyst 15: S129–S145. [Google Scholar]
- Rhynes WE, Ewing LL. (1973) Plasma corticosteroids in Hereford bulls exposed to high ambient temperature. J Anim Sci 36: 369–373. [DOI] [PubMed] [Google Scholar]
- Rimbach R, Link A, Heistermann M, Gómez-Posada C, Galvis N, Heymann EW. (2013) Effects of logging, hunting, and forest fragment size on physiological stress levels of two sympatric ateline primates in Colombia. Conserv Physiol 1: doi:10.1093/conphys/cot031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode KD, Chapman CA, McDowell LR, Stickler C. (2006) Nutritional correlates of population density across habitats and logging intensities in redtail monkeys (Cercopithecus ascanius). Biotropica 38: 625–634. [Google Scholar]
- Romano MC, Rodas AZ, Valdez RA, Hernández SE, Galindo F, Canales D, Brousset DM. (2010) Stress in wildlife species: noninvasive monitoring of glucocorticoids. Neuroimmunomodulation 17: 209–212. [DOI] [PubMed] [Google Scholar]
- Rothman J, Chapman C, Van Soest P. (2012) Methods in primate nutritional ecology: a user's guide. Int J Primatol 33: 542–566. [Google Scholar]
- Sanders W, Trapani J, Mitani JC. (2003) Taphonomic aspects of crowned-hawk eagle predation on monkeys. J Hum Evol 44: 87–105. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (1985) Stress-induced suppression of testicular function in the wild baboon: role of glucocorticoids. Endocrinology 116: “2273–2278. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. (2005) The influence of social hierarchy on primate health. Science 308: 648–652. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert C, Finch C. (1990) Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci 10: 2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof VAM, Jack KM. (2013) The association of intergroup encounters, dominance status, and fecal androgen and glucocorticoid profiles in wild male white-faced capuchins (Cebus capucinus). Am J Primatol 75: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seavy NE, Apodaca CK. (2002) Raptor abundance and habitat use in a highly-disturbed-forest landscape in western Uganda. J Raptor Res 36: 51–57. [Google Scholar]
- Sekercioglu CH. (2002) Effects of forestry practices on vegetation structure and bird community of Kibale National Park, Uganda. Biol Conserv 107: 229–240. [Google Scholar]
- Sheppard DJ. (1999) Comparative ecology of redtail monkeys (Cercopithecus ascanius schmidti) in logged and unlogged forest compartments of the Budongo Forest Reserve, Uganda. Am J Primatol 49: 101. [Google Scholar]
- Sheriff M, Krebs C, Boonstra R. (2011) From process to pattern: how fluctuating predation risk impacts the stress axis of snowshoe hares during the 10-year cycle. Oecologia 166: 593–605. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Munro CJ, Johl HK, Taylor HW, Lasley BL. (1995) Urine and fecal sample collection on filter paper for ovarian hormone evaluations. Am J Primatol 37: 305–315. [DOI] [PubMed] [Google Scholar]
- Shutt K, Setchell JM, Heistermann M. (2012) Non-invasive monitoring of physiological stress in the Western lowland gorilla (Gorilla gorilla gorilla): validation of a fecal glucocorticoid assay and methods for practical application in the field. Gen Comp Endocrinol 179: 167–177. [DOI] [PubMed] [Google Scholar]
- Skorupa JP. (1989) Crowned eagles (Stephanoaetus coronatus) in rainforest: observations on breeding chronology and diet at a nest in Uganda. Ibis 131: 294–298. [Google Scholar]
- Soares MC, Bshary R, Cardoso SC, Côté IM, Oliveira RF. (2012) Face your fears: cleaning gobies inspect predators despite being stressed by them. PLoS ONE 7: e39781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford CB. (1995) The influence of chimpanzee predation on group size and anti-predator behaviour in red colobus monkeys. Anim Behav 49: 577–587. [Google Scholar]
- Strier KB, Ziegler TE. (2005) Advances in field-based studies of primate behavioral endocrinology. Am J Primatol 67: 1–4. [DOI] [PubMed] [Google Scholar]
- Strier KB, Ziegler TE, Wittwer DJ. (1999) Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Horm Behav 35: 125–134. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. (1975) The Red Colobus Monkey. University of Chicago Press, Chicago. [Google Scholar]
- Struhsaker TT. (1980) Comparison of the behavior and ecology of red colobus and redtail monkeys in the Kibale Forest, Uganda. Afr J Ecol 18: 33–51. [Google Scholar]
- Struhsaker TT. (1997) Ecology of an African Rain Forest: logging in Kibale and the conflict between conservation and exploitation. University Press of Florida, Gainesville, FL, USA. [Google Scholar]
- Struhsaker TT, Leakey M. (1990) Prey selectivity by crowned hawk-eagles on monkeys in the Kibale Forest, Uganda. Behav Ecol Sociobiol 26: 435–443. [Google Scholar]
- Struhsaker TT, Leland L. (1988) Group fission in redtail monkeys (Cercopithecus ascanius) in the Kibale Forest, Uganda. In Gautier-Hion A, Bourliere F, Gautier JP, Kingdon J, eds, A Primate Radiation: Evolutionary Biology of the African Guenons. Cambridge University Press, New York, pp 364–388. [Google Scholar]
- Taussky HH. (1954) A microcalorimetric determination of creatinine in urine by the Jaffe reaction. J Biol Chem 208: 853–861. [PubMed] [Google Scholar]
- Teelen S. (2007a) Influence of chimpanzee predation on associations between red colobus and red-tailed monkeys at Ngogo, Kibale National Park, Uganda. Int J Primatol 28: 593–606. [Google Scholar]
- Teelen S. (2007b) Primate abundance along five transect lines at Ngogo, Kibale National Park, Uganda. Am J Primatol 69: 1030–1044. [DOI] [PubMed] [Google Scholar]
- Teelen S. (2008) Influence of chimpanzee predation on the red colobus population at Ngogo, Kibale National Park, Uganda. Primates 49: 41–49. [DOI] [PubMed] [Google Scholar]
- Tilbrook A, Turner A, Clarke I. (2000) Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 5: 105–113. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Jobling S, Sumpter JP. (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28: 319–361. [DOI] [PubMed] [Google Scholar]
- Van Orsdol KG. (1986) Agricultural encroachment in Uganda's Kibale Forest. Oryx 20: 115–117. [Google Scholar]
- Verweij HJA, Emmer IM. (1998) Implementing carbon sequestration projects in two contrasting areas: the Czech Republic and Uganda. Commonw Forest Rev 77: 203–208. [Google Scholar]
- Voellmy IK, Goncalves IB, Barrette M-F, Monfort SL, Manser MB. (2014) Mean fecal glucocorticoid metabolites are associated with vigilance, whereas immediate cortisol levels better reflect acute anti-predator responses in meerkats. Horm Behav 66: 759–765. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang B, Lu J. (2011) Behavioral and physiological responses of striped field mice (Apodemus agrarius) to predator odor. Integr Zool 6: 334–340. [DOI] [PubMed] [Google Scholar]
- Waser P. (1985) Spatial structure in mangabey groups. Int J Primatol 6: 569–580. [Google Scholar]
- Wasserman MD, Chapman CA, Milton K, Gogarten JF, Wittwer DJ, Ziegler TE. (2012a) Estrogenic plant consumption predicts red colobus monkey (Procolobus rufomitratus) hormonal state and behavior. Horm Behav 62: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman MD, Taylor-Gutt A, Rothman JM, Chapman CA, Milton K, Leitman DC. (2012b) Estrogenic plant foods of red colobus monkeys and mountain gorillas in Uganda. Am J Phys Anthrop 148: 88–97. [DOI] [PubMed] [Google Scholar]
- Wasserman M, Chapman C, Milton K, Goldberg T, Ziegler T. (2013a) Physiological and behavioral effects of capture darting on red colobus monkeys (Procolobus rufomitratus) with a comparison to chimpanzee (Pan troglodytes) predation. Int J Primatol 34: 1020–1031. [Google Scholar]
- Wasserman M, Milton K, Chapman C. (2013b) The roles of phytoestrogens in primate ecology and evolution. Int J Primatol 34: 861–878. [Google Scholar]
- Watts DP, Amsler SJ. (2013) Chimpanzee-red colobus encounter rates show a red colobus population decline associated with predation by chimpanzees at Ngogo. Am J Primatol 75: 927–937. [DOI] [PubMed] [Google Scholar]
- Watts DP, Mitani JC. (2002) Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. Int J Primatol 23: 1–28. [Google Scholar]
- Whitten PL, Brockman DK, Stavisky RC. (1998) Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Am J Phys Anthropol Suppl 27: 1–23. [DOI] [PubMed] [Google Scholar]
- Wikelski M, Cooke SJ. (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Windfelder T, Lwanga JS. (2004) Group fission in red-tailed monkeys (Cercopithecus ascanius) in Kibale National Park, Uganda. In Glenn ME,, Cords M, eds, The Guenons: Diversity and Adaptation in African Monkeys. Kluwer Academic Publishers, New York, pp 147–159. [Google Scholar]
- Zhabinskii VN, Khripach NB, Khripach VA. (2014) Steroid plant hormones: effects outside plant kingdom. Steroids doi:10.1016/j.steroids.2014.08.025. [DOI] [PubMed]