Long-term elevation of glucocorticoid hormones associated with chronic stress may be detrimental. Chronic treatment of White's treefrogs with exogenous corticosterone reduced eosinophil numbers but did not alter sperm count or sperm viability. We suggest that corticosterone inhibits immune but not reproductive function in this species in response to chronic stress.
Keywords: Eosinophil, frog, glucocorticoid, leucocyte, sperm, stress
Abstract
Amphibian populations are declining globally. The potential contribution of glucocorticoid hormones to these declines has received little attention, but chronic elevation of glucocorticoids has been linked to a suite of negative outcomes across vertebrate taxa. Recently, chronic environmental stress has been associated with precipitous declines in sperm count and sperm viability in White’s treefrogs (Litoria caerulea), but the mechanism remains unknown. In order to determine whether corticosterone is responsible for suppressing reproductive and immune function in this species, we elevated circulating concentrations of corticosterone in 10 male captive-bred frogs via transdermal application for 7 days. We compared sperm count, sperm viability, splenic cell count and circulating leucocyte counts in corticosterone-treated frogs with those in untreated control frogs. Chronic application of exogenous corticosterone led to supraphysiological circulating concentrations of corticosterone, but had no effect on sperm count or viability. However, corticosterone-treated frogs demonstrated a significant decrease in circulating eosinophils, which are immune cells implicated in fighting a variety of pathogens, including extracellular parasites. These findings suggest that although chronic elevation of circulating corticosterone is not necessarily associated with reproductive suppression in this species, it may cause immunosuppression. Thus, chronic glucocorticoid elevations in amphibians might enhance susceptibility to infection with pathogens and parasites, and their potential contributions to global population declines warrant further study.
Introduction
Stress elicits many neural and endocrine changes, including activation of the hypothalamic–pituitary–adrenal (HPA) axis. Activation of this axis, in turn, stimulates secretion of glucocorticoid hormones (corticosterone and/or cortisol), which have numerous physiological consequences. Although acute HPA activation stimulates processes that increase the likelihood of survival, chronic stress is generally associated with negative outcomes (Munck and Náray-Fejes-Tóth, 1992; Irwin, 1994; McEwen et al., 1997; Dhabhar, 2002, 2008; Viswanathan and Dhabhar, 2005; Martin et al., 2010).
Adverse consequences of chronic HPA activation can include effects on immune function. While acute glucocorticoid elevations may stimulate immune cell production, chronically elevated glucocorticoid concentrations often suppress cytokine synthesis, leucocyte mobilization and other immune-promoting reactions (reviewed by Irwin, 1994; McEwen et al., 1997; Sapolsky et al., 2000). Consequently, chronic HPA activation may lead to immunosuppression. In amphibians, for example, studies have linked long-term exposure to environmental stressors, such as agrochemicals (Hayes et al., 2006; McMahon et al., 2011), and extreme environments, such as arid climates (Jessop et al., 2013), to parasitic (e.g. trematode) infection and fitness (Gendron et al., 2003; Hayes et al., 2006; Rohr et al., 2008a; Shutler and Marcogliese, 2011).
Prolonged stimulation of the HPA axis can also lead to numerous other adverse outcomes for animals. For example, chronically increased glucocorticoid concentrations suppress reproductive behaviour in a variety of taxa (Moore and Miller, 1984; Moore and Mason, 2001) and may also mediate suppression of reproductive physiology (Moore and Zoeller, 1985; Biswas et al., 2000; Tsai et al., 2003; Gore et al., 2006; Schoech et al., 2009).
In light of the current status of declining amphibian populations (Blaustein and Kiesecker, 2002; Stuart et al., 2004; Hayes et al., 2010), an understanding of the effects of chronic environmental stress on the physiology of these animals is of particular interest. Despite the well-established detrimental effects in many other taxa (McEwen, 2000; Sapolsky et al., 2000; Wingfield and Sapolsky, 2003), however, the potential adverse effects of chronic stress on amphibians have been largely overlooked. Some studies have examined the correlation between amphibian stress and the effects of human encroachment on habitat (e.g. the introduction of pesticides or pollution and the spread of diseases; Newcomb-Homan et al., 2003; Gabor et al., 2013; Kindermann et al., 2013), and recently, Janin et al. (2011, 2012) have examined the effect of substrate (as a proxy for matrix habitat) and habitat availability on stress hormones. Tennessen et al. (2014) reported that noise led to increased circulating corticosterone and altered mate choice. Recently, Kaiser et al. (2015) found that exposing frogs to an anthropogenic stressor (traffic noise) over a 7 day period led to increases in circulating corticosterone concentrations as well as significant decreases in sperm count and sperm viability. However, the mechanism for this suppression was not investigated. Models from other vertebrates suggest corticosterone as a likely candidate to mediate reproductive suppression. In the present study, therefore, we measured the effects of chronic treatment with exogenous corticosterone on the amphibian reproductive system using male White’s treefrogs (Litoria caerulea). Given that a chronic increase in corticosterone is frequently associated with immunosuppresion, we also tested the effects of chronic exogenous corticosterone application on immune cell counts. We predicted that animals exposed to high concentrations of exogenous corticosterone for 1 week would show decreases in sperm count and sperm viability, similar to our previous findings (Kaiser et al., 2015), and would also show decreases in circulating lymphocyte counts and splenic cell counts.
Materials and methods
Animals and experimental design
Twenty captive-bred adult male White’s treefrogs were acquired from a North American breeder and individually housed in plastic tanks measuring approximately 40 cm × 24 cm × 32 cm (Kritter Keepers, size XL, San Marcos, CA, USA). This species has been used widely in physiological and ecological studies (Buttemer, 1990; Baker and Waights, 1994; Coddington and Cree, 1995; Warburg et al., 2000; Woodhams et al., 2007; Voyles et al., 2009; Kaiser et al., 2015). Frogs were given dechlorinated tap water that was changed daily and were fed three or four medium-sized crickets twice weekly. All crickets were removed 24 h prior to blood collection. Lighting and temperature were controlled on a 12 h–12 h light–dark cycle (lights on at 09.00 h) and at 21–23°C, respectively. Tanks included a 10 cm length of PVC pipe for enrichment. The study was conducted during the North American breeding season for this species (from 6 August to 2 September 2012). All procedures were approved by the University of California, Riverside Institutional Animal Care and Use Committee Protocol A-20100040. The University of California, Riverside is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Frogs were divided into three treatment groups: two control groups [undisturbed control (UC) and blood-sampled control (BC)] and a corticosterone-treated experimental group (CORT). The two control groups allowed us to obtain data from animals that were not manipulated during the period of data collection as well as from animals that did not undergo treatment but for which we obtained measures of plasma corticosterone concentrations at all time points for which we had comparable measures from CORT animals.
Blood sampling and exogenous ’corticosterone application
To elevate circulating corticosterone concentrations, we used the dermal patch method developed for use in amphibians (Wack et al., 2010). Based on preliminary data (K. Kaiser, unpublished data), we prepared a 20 mg/ml solution of corticosterone (92% pure, C2505; Sigma Aldrich, St Louis, MO, USA), which was suspended in sesame oil (unrefined expeller pressed; Whole Foods Market, Austin, TX, USA). Dermal patches were filter-paper pieces ∼5 mm in diameter (FisherBrand Filter Paper, P8, Pittsburgh, PA, USA). We applied 7 µl of the corticosterone solution to patches applied to the dorsal trunk region of CORT frogs for a dose of 140 µg per corticosterone treatment. Patches remained in place for 1 h, and treatment was repeated every 8 h for 7 days. We chose a chronic exposure of 7 days to replicate the exposure duration of the study by Kaiser et al. (2015), in which significant reproductive effects were observed. During lights-off periods, applications were performed under red lights to avoid interference with circadian rhythms. Frogs were monitored periodically during the hour that patches were in place. In a few cases, frogs removed patches; when this occurred, we immediately reapplied the patch.
We collected blood from CORT and BC animals at three time points: day −5 (5 days before CORT treatment began in CORT animals), day 4 (4 days after CORT application began in CORT animals on day 0) and day 7 (the end of the study). Blood was collected from UC animals only on days −5 and 7.
On days −5 and 4, blood was collected via cardiac puncture as in the study by Kaiser et al. (2015). Frogs were treated with a topical antibiotic, and blood was collected using a sterile 27-gauge needle and a 1 ml syringe. On day 7, animals were killed by decapitation followed by double pithing, and blood and organs (see below) were harvested. At all time points, blood was collected within 3 min of initial disturbance to the animal. At each time point, we were unable to collect samples from some individuals, so these data are missing. Samples were taken at 12.30–13.30 h, ∼4.5 h after the most recent application of exogenous corticosterone. This time frame was chosen to allow animals time to metabolize the exogenous corticosterone from the most recent experimental application and to minimize differences due to diel rhythms in baseline corticosterone. Blood was centrifuged for 12 min at 13.3 g at 4°C, and plasma was extracted and stored at −80°C until assayed for corticosterone.
Corticosterone assay
We measured plasma corticosterone concentrations using a double-antibody 125I-radioimmunoassay kit (MP Biomedicals, Costa Mesa, CA, USA) previously validated in our laboratory for this species (Kaiser et al., 2015). Samples were extracted and assayed in duplicate in two assays. Assay sensitivity was 50.4 pg/ml. The intra-assay coefficient of variation (CV) of an internal control plasma pool at the low end of the standard curve (83% bound) sampled in duplicate was 1.6%; interassay CV of this pool was 1.4%. The intra-assay CV of an internal pool at the high end of the standard curve (26% bound) was 8.0%; interassay CV of this pool was 5.0%.
Reproductive measures
The right testis was collected from each frog, and sperm were collected and labelled as described by Kaiser et al. (2015). Briefly, we minced the testis in 5 ml of 0.1 M phosphate-buffered saline and incubated it at room temperature for 1 h to allow sperm to separate from the testicular tissue. Total sperm count was measured as the average of duplicate counts for each testis on a haemocytometer loaded with sample in a 10% solution of Trypan blue (Thermo Scientific, Logan, UT, USA).
For analysis of sperm viability, we used the same protocol as Kaiser et al. (2015). Sperm were centrifuged at 1000g for 5 min at 4°C. Cells were washed with FACS buffer (phosphate-buffered saline containing 0.5% bovine serum albumin and 2 mM EDTA) and labelled using CellTrace™ CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions (2 µl/ml cells from 5 mM stock solution for 10 min). Cells were further treated with Fixable Viability Dye eFluor® 660 (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions (1 µl of undiluted dye/ml cells for 30 min). Cells were then fixed with 500 µl of 4% paraformaldehyde in FACS buffer and stored at 4°C for later analysis with a BD FACSCantoII flow cytometer (BD Biosciences, San Jose, CA, USA).
Immune measures
Blood smears and white blood cell count
We collected two blood smears from each frog immediately after they had been killed on day 7. Smears were stained with haematoxylin and eosin, photographed at ×40 magnification, and counted using the ImageJ Cell Counter plugin (ImageJ v. 1.46r; NIH, available at http://imagej.nih.gov/ij). A minimum of three investigators blinded to sample treatments counted each smear. Prior to counting images for the experiment, all investigators were trained and tested on a set of images not included in this study. Cell types were distinguished based on characters described and illustrated by Claver and Quaglia (2009) and Hadji-Azimi et al. (1987). Each investigator counted the number of each type of white blood cell (WBC: eosinophils, neutrophils and lymphocytes) from among at least 500 erythrocytes (RBCs). Other WBCs were encountered infrequently and not counted. We used the mean counts from all three scorers for analysis; any outlier counts were rescored. We calculated the ratio of each type of WBC to RBCs and the ratio of neutrophils to lymphocytes (Woodhams et al., 2007; Davis et al., 2008).
Spleen cell count
Spleens were harvested immediately after animals had been killed and placed in cRPMI media (RPMI media; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, l-glutamine, penicillin–streptomycin antibiotic, Hepes, sodium pyruvate, non-essential amino acids and 2-β-mercaptoethanol) on ice. Splenic cell suspensions were prepared by straining cells through 40 μm nylon filters (Fisher Scientific, Pittsburgh, PA, USA) and rinsing with cRPMI media. Cells were centrifuged at 300 g for 5 min at 4°C; supernatant was discarded, and each cell pellet was resuspended in ACK Lysing Buffer (Lonza, Walkersville, MD, USA) for 5 min on ice. Cells were washed three times with cRPMI media. The final cell pellet was resuspended in 1 ml of cRPMI media. A subsample of the cell suspension was labelled with a 10% solution of Trypan blue (Thermo Scientific) and counted in duplicate on a haemocytometer.
Data analysis
We used FlowJo (v. 8.7.3; TreeStar, Ashland, OR, USA) for analysis of flow cytometry data, SPSS 18.0.0 (PASW Inc., Hong Kong) for analysis of corticosterone data, and StataIC (v. 10; StataCorp, College Station, TX, USA) for all other statistical analyses. All data were tested for normality using Shapiro–Wilk tests. Corticosterone concentrations were logarithmically transformed and analysed using general linear models that allowed for repeated measures. We first tested for differences in plasma concentrations of corticosterone between UC and BC frogs at days −5 and 7 to ensure that blood sampling on day 4 did not affect hormone concentrations in the BC frogs. We then compared circulating corticosterone concentrations in BC and CORT animals at all three time points (days −5, 4 and 7); to interpret a significant interaction term, we used the EMMEANS command in SPSS and a Bonferroni correction for multiple comparisons. Given that we did not sample UC animals on day 4, we omitted them from this analysis. Finally, because we found no significant differences in circulating concentrations of corticosterone between UC and BC animals, we combined these two treatments to increase statistical power and conducted a general linear model (GLM) for the combined control groups vs. the CORT group for days −5 and 7 to test the effect of exogenous corticosterone application on circulating corticosterone concentrations.
Sperm and spleen cell counts were logarithmically transformed prior to analysis to meet the assumption of normality. Sperm-viability data met normality assumptions and were not transformed. Blood-smear data were converted to a ratio of WBCs to RBCs or neutrophils to lymphocytes, square-root transformed, and analysed with Student’s unpaired t-tests. ANCOVAs were used for all sperm and splenic cell count analyses, with body mass as a covariate. As no significant differences were detected between the UC and BC groups in any of these measures, data from the two control groups were combined for each ANCOVA, and subsequent comparisons were made between control and CORT animals. Data were analysed using SPSS v.21. All tests were two tailed, with a critical P-value of 0.05.
Results
Plasma corticosterone concentrations
As expected, the blood-sampled control (BC) and undisturbed control (UC) groups did not differ in plasma corticosterone concentrations on days −5 and 7 of the experiment (F1,5 = 4.4, P = 0.091), nor was a significant interaction between day and treatment found (F1,5 = 1.6, P = 0.25). However, because UC frogs were not sampled on day 4, we did not pool the two control groups for analysis of the effect of corticosterone treatment on circulating corticosterone concentrations.
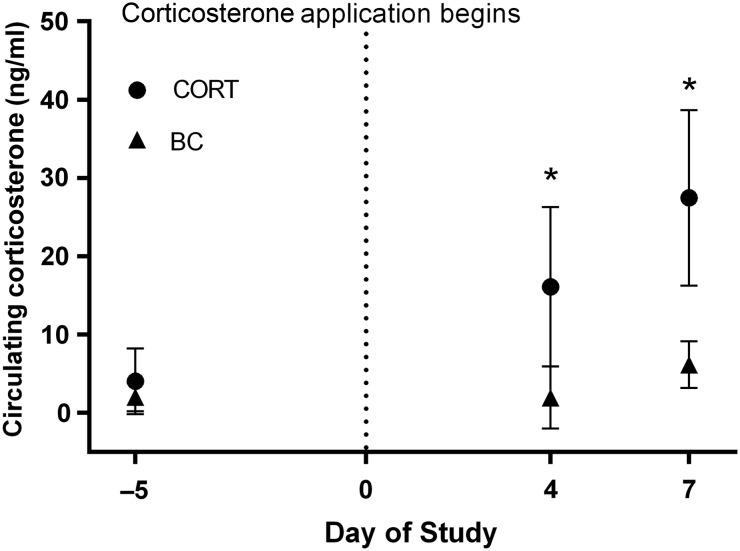
A repeated-measures GLM using logarithmically transformed circulating corticosterone concentrations, comparing CORT and BC frogs, confirmed that transdermal treatment with exogenous corticosterone every 8 h significantly elevated circulating corticosterone concentrations in male White’s treefrogs, as reflected in significant effects of treatment, day of study and day × treatment interaction (treatment, F1,5 = 12, P = 0.016; day, F2,10 = 18, P < 0.001; and interaction, F2,10 = 6.9, P = 0.013). Within-group planned pairwise comparisons revealed that BC animals had statistically indistinguishable levels of circulating corticosterone across all three time points, whereas plasma corticosterone concentrations in CORT frogs increased significantly across the study (day −5 vs. day 4, P = 0.026; day −5 vs. day 7, P = 0.003; and day 4 vs. day 7, P = 0.004). This divergence is evident when the treatments are compared at each time point; plasma corticosterone concentrations did not differ between groups on day −5 (F1,5 = 0.10, P = 0.76), but were significantly higher in CORT frogs than in control frogs on day 4 (F1,5 = 12, P = 0.017) and day 7 (F1,5 = 180, P < 0.001; Fig. 1 and Table 1).
Figure 1:
Circulating concentrations of corticosterone after transdermal treatment. Transdermal treatment with corticosterone every 8 h increased plasma corticosterone concentrations in White’s treefrogs over the course of the study. Shown are back-transformed means ± 95% confidence intervals of circulating corticosterone in treated (CORT) and blood-sampled control (BC) frogs. Asterisks mark time points at which control frogs were significantly different from CORT frogs.
Table 1:
Mean, SEM and sample size for corticosterone, sperm and splenic cell measures as well as average body mass at the end of the study, for each treatment group
| Parameter | Exogenous corticosterone (CORT) group | Undisturbed control (UC) group | Blood-sampled control (BC) group | Combined UC and BC groups |
|---|---|---|---|---|
| Plasma corticosterone (ng/ml), day −5 | 4.0 ± 1.8 (n = 8) | 2.7 ± 1.4 (n = 4) | 2.0 ± 0.6 (n = 4) | 2.3 ± 0.6 (n = 8) |
| Plasma corticosterone (ng/ml), day 4 | 16 ± 4.2 (n = 7) | n.d. | 2.0 ± 0.92 (n = 5) | n.d. |
| Plasma corticosterone (ng/ml), day 7 | 27 ± 4.8 (n = 9) | 3.2 ± 0.92 (n = 5) | 6.2 ± 1.1 (n = 5) | 3.2 ± 0.61 (n = 10) |
| Sperm count (millions) | 8.2 ± 1.4 (n = 10) | 7.6 ± 2.3 (n = 5) | 7.3 ± 3.4 (n = 5) | 7.6 ± 2.2 (n = 10) |
| Sperm viability (% viable) | 88.3 ± 1.2 (n = 8) | 88.4 ± 2.1 (n = 3) | 89.5 ± 2.1 (n = 5) | 89.1 ± 1.4 (n = 8) |
| Splenic cell count (millions) | 1.2 ± 0.36 (n = 10) | 3.2 ± 1.6 (n = 5) | 3.1 ± 1.3 (n = 5) | 3.2 ± 1.0 (n = 10) |
| Mass (g) | 34.40 ± 2.4 | 36.00 ± 2.3 | 34.40 ± 0.24 | 35.20 ± 1.1 |
Undisturbed control animals were not sampled on day 4 and thus no corticosterone data are reported (n.d.). Although most data were transformed for analyses (see main text), raw data are presented for ease of interpretation, and data are presented separately for each group. For analyses, the two control groups were pooled because there were no statistically significant differences between them; pooled values are also shown.
Sperm count and viability
To determine whether treatment with exogenous corticosterone led to a decrease in reproductive function, we measured sperm count and the proportion of sperm that were viable in the right testis of each frog. As previously described, the BC and UC control groups did not differ in any measures of reproductive function (sperm count, t = 0.11, d.f. = 8, P = 0.91; and sperm viability, t = −0.34, d.f. = 6, P = 0.75); therefore, data from the two control groups were combined for subsequent analyses. Frogs in the CORT group did not show a difference in sperm count (F3,16 = 0.73, P = 0.55; Table 1) or sperm viability (F3,16 = 1.6, P = 0.23; Table 1), compared with control frogs. Body mass was not a significant predictor of either sperm count or of sperm viability.
Immune measures
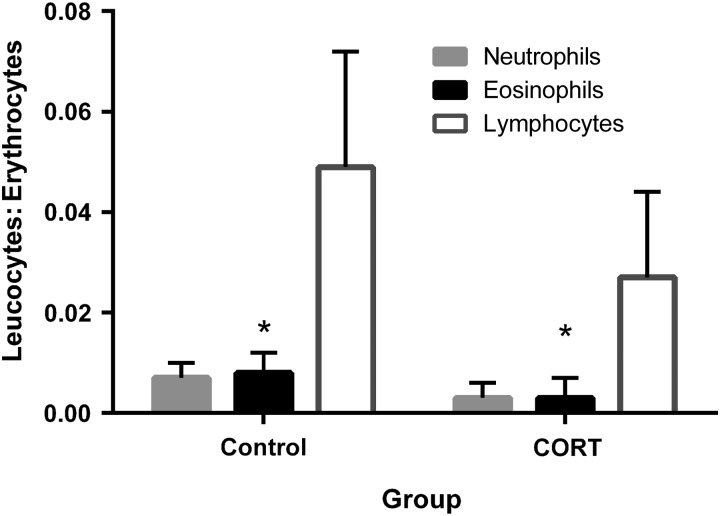
To determine whether chronically elevated corticosterone concentrations led to suppressed immune function, we phenotyped circulating leucocytes and compared splenic cell counts in frogs that did and did not receive corticosterone treatment. Hormone treatment reduced the ratio of circulating WBCs to RBCs; CORT frogs had significantly lower ratios of eosinophils to RBCs compared with control frogs (BC and UC groups combined; t = 3.3, d.f. = 17, P = 0.004; Fig. 2 and Table 1). Although lymphocyte-to-RBC and neutrophil-to-RBC ratios did not differ significantly between groups, the lymphocyte ratio tended to be lower in CORT animals (t = 1.9, d.f. = 17, P = 0.078; Fig. 2). We found no effect of treatment on the neutrophil-to-lymphocyte ratio (t = 0.41, d.f. = 17, P = 0.69).
Figure 2:
Ratios of white blood cell counts to red blood cells after corticosterone treatment. Back-transformed means and 95% confidence intervals are presented. Asterisks indicate measures that differed significantly between CORT and control (BC and UC combined) frogs.
The BC and UC control groups did not differ in splenic cell count (t = 0.39, d.f. = 8, P = 0.97); therefore, data from the two control groups were combined for analysis. Splenic cell count was not influenced by corticsterone treatment (F2,17 = 2.5, P = 0.12). Body mass was included in the ANCOVA model but was not a significant predictor of splenic cell count.
Discussion
We previously found that chronic (7 day) exposure to an anthropogenic noise stressor elevated circulating corticosterone concentrations and decreased sperm count and sperm viability in captive White’s treefrogs (Kaiser et al., 2015). In the present experiment, therefore, we treated treefrogs with exogenous corticosterone for the same duration to determine whether increased corticosterone concentrations might have mediated this stress-induced reproductive suppression and to evaluate possible effects on immune function. Here, we found that despite chronically elevated plasma corticosterone concentrations, neither sperm count nor sperm viability was affected. Indeed, in this study, plasma corticosterone concentrations were elevated to levels that were likely to be supraphysiological (K. Kaiser, unpublished data) and were an order of magnitude above those seen in our previous study (Kaiser et al., 2015). The exogenous corticosterone treatment in the present experiment yielded decreases in ratios of circulating eosinophils to red blood cells, relative to control frogs. No other immune measures differed among treatments.
Studies in many vertebrate taxa have demonstrated a link between chronically elevated corticosterone or cortisol concentrations and reproductive suppression in both males and females (Carragher et al., 1989; Brann and Mahesh, 1991; Salvante and Williams, 2003). However, chronic increases in corticosterone do not always lead to such changes, and some organisms, potentially including male L. caerulea, may be able to decouple the HPA and hypothalamic–pituitary–gonadal axes (Astheimer et al., 2000; reviewed by Wingfield and Sapolsky, 2003). Alternatively, it is possible that another hormone(s) in the HPA axis, such as corticotrophin-releasing hormone (CRH), endogenous opioids (Moore and Miller, 1984) or adrenocorticotrophic hormone, rather than corticosterone, is responsible for stress-induced reproductive suppression in male L. caerulea. The use of exogenous corticosterone in this experiment is likely to have downregulated the HPA axis through negative feedback. Therefore, while it is unlikely that these hypothalamic and pituitary hormones directly suppressed immune measures in this study, we cannot rule out the possibility that reductions in these hormones, rather than or in addition to increases in corticosterone, contributed to our findings. Another caveat to our results is that because of logistical constraints, we did not incorporate a vehicle-treated control group. However, pilot studies suggested that patch application alone did not elicit a stress response (K. Kaiser, unpublished data).
The use of blood smears in the present study allowed us to quantify changes in circulating leucocyte numbers. Only eosinophils, which have been implicated in fighting parasitic infections, such as trematodes (Mitchell, 1982; Claver and Quaglia, 2009), showed significant reduction in CORT animals, though lymphocytes also showed a slight, non-significant decrease. This reduction in eosinophils should be interpreted with caution, because it might have resulted from supraphysiological corticosterone concentrations (Kaiser et al., 2015). In addition, we did not evaluate the functional effects, if any, of chronic corticosterone elevation on the immune system. In multiple species, however, increased glucocorticoid concentrations have been found to be correlated with increased infection rates, suggesting that chronic elevations of corticosterone concentrations may contribute to functional immunosuppression in amphibians as well (Gendron et al., 2003; Hayes et al., 2006, 2010; Rohr et al., 2008a, b).
In conclusion, although stress-induced reproductive suppression is frequently attributed to elevated circulating concentrations of glucocorticoids (Brann and Mahesh, 1991; Rivier and Rivest, 1991; Moore and Jessop, 2003; Wingfield and Sapolsky, 2003; Schoech et al., 2009), we found no evidence for such an effect in male treefrogs. Thus, our findings add to the growing literature suggesting that the interactions of glucocorticoids with reproduction are species specific and complex; effects of these hormones are likely to be not only concentration dependent but also context dependent (Schoech et al., 1997; Moore and Jessop, 2003; Wingfield and Sapolsky, 2003). Understanding other mechanisms for reproductive suppression may be important in allowing for conservation of threatened species. This is particularly applicable to anurans: although laboratory and wild animals often exhibit different physiological traits (Calisi and Bentley, 2009), amphibians are increasingly being captive bred in assurance colonies due to global declines.
Nevertheless, chronic increases of circulating corticosterone concentrations in this experiment led to decreased circulating eosinophils, suggesting a possible role for corticosterone and stress in disease- or parasite-related amphibian declines. Because of the potential for such population-level effects due to chronic stress, we suggest that future work should focus on functional assays, such as experimentally determining the effect of corticosterone concentrations on WBCs and infection rates in animals affected by parasites, and understanding the levels of pesticides and/or corticosterone necessary to cause immunosuppression. These results could provide insight into the endocrine correlates of amphibian population dynamics and could prove useful in advancing amphibian conservation.
Funding
This work was supported by the National Science Foundation [Grant DBI–1003283] to K.K. and a University of California, Riverside Undergraduate Research Grant to A.M. L.U. was supported by a National Institutes of Health Minority Access to Research Careers Undergraduate Student Training in Academic Research fellowship [Grant 2T34GM062756-09].
Acknowledgements
B. N. Harris assisted with experimental design. S. Noor assisted with assay validation and logistics. R. A. Cardullo provided guidance in the early planning of the project. A. P. Pessier gave helpful feedback throughout the study. R. Mailloux provided frogs. Comments from two anonymous reviewers improved the manuscript; we gratefully acknowledge their contribution.
References
- Astheimer LB, Buttemer WA, Wingfield JC. (2000) Corticosterone treatment has no effect on reproductive hormones or aggressive behavior in free-living male tree sparrows, Spizella arborea . Horm Behav 37: 31–39. [DOI] [PubMed] [Google Scholar]
- Baker JMR, Waights V. (1994) The effects of nitrate on tadpoles of the tree frog (Litoria caerulea) . Herpetol J 4: 106–108. [Google Scholar]
- Biswas NM, Chaudhuri GR, Sarkar M, Sengupta R. (2000) Influence of adrenal cortex on testicular activity in the toad during the breeding season . Life Sci 66: 1253–1260. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Kiesecker JM. (2002) Complexity in conservation: lessons from the global decline of amphibian populations . Ecol Lett 5: 597–608. [Google Scholar]
- Brann DW, Mahesh VB. (1991) Role of corticosteroids in female reproduction . FASEB J 5: 2691–2698. [DOI] [PubMed] [Google Scholar]
- Buttemer WA. (1990) Effect of temperature on evaporative water loss of the Australian tree frogs Litoria caerulea and Litoria chloris . Physiol Zool 63: 1043–1057. [Google Scholar]
- Calisi RM, Bentley GE. (2009) Lab and field experiments: are they the same animal? Horm Behav 56: 1–10. [DOI] [PubMed] [Google Scholar]
- Carragher JF, Sumpter JP, Pottinger TG, Pickering AD. (1989) The deleterious effects of cortisol implantation on reproductive function in two species of trout, Salmo trutta L. and Salmo gairdneri Richardson . Gen Comp Endocrinol 76: 310–321. [DOI] [PubMed] [Google Scholar]
- Claver JA, Quaglia AI. (2009) Comparative morphology, development, and function of blood cells in nonmammalian vertebrates . J Exot Pet Med 18: 87–97. [Google Scholar]
- Coddington EJ, Cree A. (1995) Effect of acute captivity stress on plasma concentrations of corticosterone and sex steroids in female whistling frogs, Litoria ewingi . Gen Comp Endocrinol 100: 33–38. [DOI] [PubMed] [Google Scholar]
- Davis AK, Maney DL, Maerz JC. (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists . Funct Ecol 22: 760–772. [Google Scholar]
- Dhabhar FS. (2002) Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines . Brain Behav Immun 16: 785–798. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. (2008) Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology . Allergy Asthma Clin Immunol 4: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor CR, Fisher MC, Bosch J. (2013) A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels . PloS One 8: e56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron AD, Marcogliese D, Barbeau S, Christin M-S, Brousseau P, Ruby S, Cyr D, Fournier M. (2003) Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae . Oecologia 135: 469–476. [DOI] [PubMed] [Google Scholar]
- Gore AC, Attardi B, DeFranco DB. (2006) Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels . Mol Cell Endocrinol 256: 40–48. [DOI] [PubMed] [Google Scholar]
- Hadji-Azimi I, Coosemans V, Canicatti C. (1987) Atlas of adult Xenopus laevis laevis hematology . Dev Comp Immunol 11: 807–874. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Case P, Chui S, Chung D, Haeffele C, Haston K, Lee M, Mai VP, Marjuoa Y, Parker J, et al. (2006) Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect 114: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Falso P, Gallipeau S, Stice M. (2010) The cause of global amphibian declines: a developmental endocrinologist’s perspective . J Exp Biol 213: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. (1994) Stress-induced immune suppression: role of brain corticotropin releasing hormone and autonomic nervous system mechanisms . Adv Neuroimmunol 4: 29–47. [DOI] [PubMed] [Google Scholar]
- Janin A, Léna J-P, Joly P. (2011) Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad . Biol Conserv 144: 1008–1016. [Google Scholar]
- Janin A, Léna JP, Deblois S, Joly P. (2012) Use of stress-hormone levels and habitat selection to assess functional connectivity of a landscape for an amphibian . Conserv Biol 26: 923–931. [DOI] [PubMed] [Google Scholar]
- Jessop TS, Letnic M, Webb JK, Dempster T. (2013) Adrenocortical stress responses influence an invasive vertebrate’s fitness in an extreme environment . Proc Biol Sci 280: 20131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K, Devito J, Jones CG, Marentes A, Perez R, Umeh L, Weickum RM, McGovern KE, Wilson EH, Saltzman W. (2015) Effects of anthropogenic noise on endocrine and reproductive function in White’s treefrog, Litoria caerulea . Conserv Physiol 3: doi:10.1093/conphys/cou061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann C, Narayan EJ, Wild F, Wild CH, Hero J-M. (2013) The effect of stress and stress hormones on dynamic colour-change in a sexually dichromatic Australian frog . Comp Biochem Physiol A Mol Integr Physiol 165: 223–227. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2000) The neurobiology of stress: from serendipity to clinical relevance . Brain Res 886: 172–189. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, et al. (1997) The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions . Brain Res Rev 23: 79–133. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Boughton RK, Martin LB, Rohr JR. (2011) The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians . Environ Health Perspect 119: 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. (2010) The effects of anthropogenic global changes on immune functions and disease resistance . Ann N Y Acad Sci 1195: 129–148. [DOI] [PubMed] [Google Scholar]
- Mitchell J. (1982) The effect of host age on Rana temporaria and Gorgoderina vitelliloba interactions . Int J Parasitol 12: 601–604. [Google Scholar]
- Moore FL, Miller LJ. (1984) Stress-induced inhibition of sexual behavior: corticosterone inhibits courtship behaviors of a male amphibian (Taricha granulosa) . Horm Behav 18: 400–410. [DOI] [PubMed] [Google Scholar]
- Moore FL, Zoeller RT. (1985) Stress-induced inhibition of reproduction: evidence of suppressed secretion of LH-RH in an amphibian . Gen Comp Endocrinol 60: 252–258. [DOI] [PubMed] [Google Scholar]
- Moore IT, Jessop TS. (2003) Stress, reproduction, and adrenocortical modulation in amphibians and reptiles . Horm Behav 43: 39–47. [DOI] [PubMed] [Google Scholar]
- Moore IT, Mason RT. (2001) Behavioral and hormonal responses to corticosterone in the male red-sided garter snake, Thamnophis sirtalis parietalis . Physiol Behav 72: 669–674. [DOI] [PubMed] [Google Scholar]
- Munck A, Náray-Fejes-Tóth A. (1992) The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited . Mol Cell Endocrinol 90: C1. [DOI] [PubMed] [Google Scholar]
- Newcomb-Homan R, Regosin JV, Rodrigues DM, Reed JM, Windmiller BS, Romero LM. (2003) Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum) . Anim Conserv 6: 11–18. [Google Scholar]
- Rivier C, Rivest S. (1991) Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms . Biol Reprod 45: 523–532. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK, Hudson PJ. (2008a) Understanding the net effects of pesticides on amphibian trematode infections . Ecol Appl 18: 1743–1753. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD. (2008b) Agrochemicals increase trematode infections in a declining amphibian species . Nature 455: 1235–1239. [DOI] [PubMed] [Google Scholar]
- Salvante KG, Williams TD. (2003) Effects of corticosterone on the proportion of breeding females, reproductive output and yolk precursor levels . Gen Comp Endocrinol 130: 205–214. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions . Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Mumme RL, Wingfield JC. (1997) Corticosterone, reproductive status, and body mass in a cooperative breeder, the Florida scrub-jay (Aphelocoma coerulescens) . Physiol Zool 70: ’68–73. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Bridge ES, Boughton RK, Wilcoxen TE. (2009) Environment, glucocorticoids, and the timing of reproduction . Gen Comp Endocrinol 163: 201–207. [DOI] [PubMed] [Google Scholar]
- Shutler D, Marcogliese DJ. (2011) Leukocyte profiles of northern leopard frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands . Copeia 2011: 301–307. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. (2004) Status and trends of amphibian declines and extinctions worldwide . Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Tennessen JB, Parks SE, Langkilde T. (2014) Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs . Conserv Physiol 2: doi:10.1093/conphys/cou032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P-S, Lunden JB, Jones JT. (2003) Effects of steroid hormones on spermatogenesis and GnRH release in male leopard frogs, Rana pipiens . Gen Comp Endocrinol 134: 330–338. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Dhabhar FS. (2005) Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation . Proc Natl Acad Sci USA 102: 5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines . Science 326: 582–585. [DOI] [PubMed] [Google Scholar]
- Wack CL, Lovern MB, Woodley SK. (2010) Transdermal delivery of corticosterone in terrestrial amphibians . Gen Comp Endocrinol 169: 269–275. [DOI] [PubMed] [Google Scholar]
- Warburg MR, Rosenberg M, Roberts JR, Heatwole H. (2000) Cutaneous glands in the Australian hylid Litoria caerulea (Amphibia, Hylidae) . Anat Embryol 201: 341–348. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM. (2003) Reproduction and resistance to stress: when and how? J Neuroendocrinol 15: 711–724. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA. (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses . Anim Conserv 10: 409–417. [Google Scholar]