In this paper we present a new analytical method that allows the extraction of the stress hormone cortisol from naturally sloughed cetacean skin cells. Skin cortisol is unaffected by short-term cortisol fluctuations and could thus be used to assess chronic stress levels in cetaceans.
Keywords: Cetacean, cortisol, glucocorticoid, minimally invasive, skin, stress
Abstract
We developed a chemical analytical procedure for sampling, extracting and determining epidermal skin cortisol concentrations (SCCs) in the harbour porpoise (Phocoena phocoena) using gas chromatography–tandem mass spectrometry. In brief, this involved a pressurized liquid extraction with a two-step solid-phase clean-up. A derivatization step was conducted prior to detection. To evaluate the new assay, cortisol was analysed in three different sample types obtained from four harbour porpoises: skin plates, dorsal fin skin plugs (with and without lidocaine) and epidermal scrapes. Skin cortisol concentrations could be measured using the new assay in the majority of the tested skin samples down to a minimal sample size of 49 mg dry weight (dw). Water content ranged from 10 to 46% in the plug samples, which had SCCs from 2.1 to 77.7 ng/g dw. Epidermal scrape samples had the highest water content (83–87%) and lower SCCs (0.6–15 ng/g dw), while the skin plates had intermediate water contents (60–66%) and SCCs of 2.6–13.0 ng/g dw. SCC was slightly higher in plugs with lidocaine than without (average values of 41 and 33 ng/g dw, respectively). Substantial within-individual variations in cortisol concentrations are also common in other matrices such as blood and hair. Some important factors behind this variation could be e.g. the animal's sex, age, body condition, reproductive stage, and the body region sampled, as well as season, moulting cycles and water temperature. Clearly, more research into SCCs is required. The findings described here represent the first critical steps towards using epidermal skin cell samples to assess chronic stress levels in cetaceans and the development of a widely applicable health-assessment tool in these species.
Introduction
Marine mammals face numerous anthropogenic stressors, including chemical pollution, underwater noise and climate change, the combined consequences of which are often difficult to assess (Durner et al., 2009; MMC, 2009; Wright, 2009; Sonne, 2010). Chronically stressful situations have been shown to affect the general state of marine mammal health in various ways; these include, but are not limited to, impaired immune function, increased mortality and reduced production of offspring (Fair and Becker, 2000; St Aubin and Dierauf, 2001; Sonne, 2010). In most mammals, cortisol is the dominant circulating glucocorticoid associated with a stress response and is typically measured in blood samples (St Aubin, 2001). However, baseline blood concentrations can be difficult to ascertain owing to short-term natural diurnal and seasonal fluctuations (Barrell and Montgomery, 1989; Suzuki et al., 2003; Oki and Atkinson, 2004). Furthermore, the acute stress response associated with capture, handling and sampling, especially in wild animals, is itself reflected in the blood within minutes (Cook et al., 2000; Eskesen et al., 2009; Fair et al., 2014). The cortisol peak caused by such an acute stressor may potentially obscure baseline values or overshadow any effects on cortisol concentrations resulting from exposure to other (longer-term) stressors. These difficulties are enhanced in free-ranging marine mammals, where many species are simply too difficult or too large to capture or handle. Consequently, several forums have identified the pressing need for more suitable methods of assessing stress responses, and chronic stress in particular, in marine mammals (e.g. Wright et al., 2007; ONR, 2009).
This has led to a growing interest in several promising new cortisol matrices in marine mammals, including vibrissae, hair, urine, saliva, baleen and faeces (Constable et al., 2006; Pedernera-Romano et al., 2006; Bechshøft et al., 2012; Rolland et al., 2012; Fair et al. 2014; Hunt et al. 2014). Whale blow may also be a potential candidate (Hogg et al., 2009; Tizzi et al., 2010; Dunstan et al., 2012). Keratinous materials, such as vibrissae and hair, have been shown to be feasible indicators of chronic stress responses (Cook, 2012). Unfortunately, these two matrices are of little use when assessing chronic stress in cetaceans, because these species have little (if any) hair as adults. However, all other currently available minimally invasive matrices, such as faeces, may only be used to assess short-term cortisol fluctuations over extended time periods (Terio et al., 1999; Suzuki et al., 2003; Pedernera-Romano et al., 2006; Desportes et al., 2007; Sheriff et al., 2009, 2010; Schmitt et al., 2010; Funasaka et al., 2011; Cook, 2012). Unfortunately, the collection of such samples from animals in the wild is opportunistic at best (e.g. Rolland et al., 2012). Thus, no reliable methods currently exist for assessing cortisol at baseline levels or over the longer term in free-ranging cetaceans. Such methods would preferably involve matrices that reflect chronic, not acute, cortisol concentrations and, ideally, also be minimally invasive to avoid handling/sampling artifacts.
Considering this, expert discussions have highlighted skin (epidermis) as a potential alternative hormone matrix (e.g. Wright et al., 2007; ONR, 2009). Cetacean epidermal cells have previously been used for genetic, diet and contaminant studies (e.g. heavy metals and trace elements; Hunt et al., 2013; Savery et al., 2013; Browning et al., 2014), but never for assessment of hormones. Hormones have, however, been found in cetacean blubber (Trego et al., 2013; Trana et al., 2015), a matrix closely associated with skin.
Cortisol released into the bloodstream following an acute stressor (e.g. capture) would not be anticipated to enter the skin matrix for days or even weeks (Hicks et al., 1985; Browning et al., 2014). Thus, cortisol in cetacean skin is expected to reflect levels of chronic stress. Validation of this statement is still required, but if found to be true, skin samples could provide information on long-term physiological status in cetaceans in terms of chronic stress. Furthermore, cetacean skin samples can be obtained in non- or at least minimally invasive ways (Noren and Mocklin, 2012). In fact, cetacean skin samples for DNA analyses have already been collected non-invasively and without direct handling of the animal using green kitchen scourers, although with mixed success (Harlin et al., 1999; but see also Farro et al., 2008).
Accordingly, the main aim of this study was to develop a chemical analytical procedure for sampling, extracting and determining epidermal skin cortisol concentrations (SCCs) in the harbour porpoise (Phocoena phocoena) by use of gas chromatography–tandem mass spectrometry (GC-MS/MS).
Materials and methods
Samples
Bycaught, dead animals
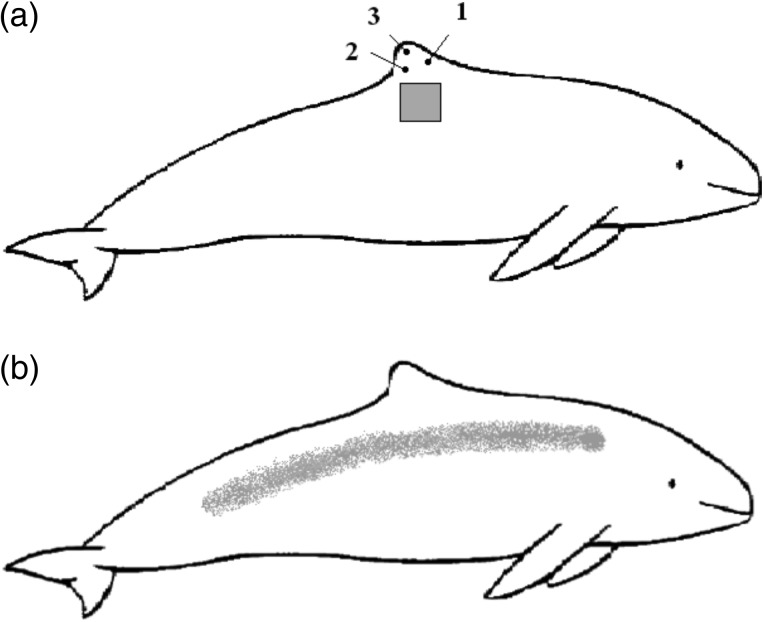
Skin samples (epidermis) were collected from two dead harbour porpoises (ID 43720, a subadult female, and ID 43721, an adult female; Table 1) that were accidentally bycaught in gill nets in Danish waters, frozen within a day of death and kept frozen at −20°C for 12 or 18 months (for IDs 43720 and 43721, respectively) until 24 h before sampling. After collection, the skin samples were returned to −20°C for 13 months until analysis. The samples collected were a 10 cm × 10 cm skin plate, taken from the right side of each animal just below the dorsal fin, and three 6 mm dorsal fin biopsies (Fig. 1a), taken as if the animal was to be pinned for tagging in the wild (Teilmann et al., 2007; Sonne et al., 2012). Published methods were followed for the pin biopsies (Teilmann et al., 2007; Sonne et al., 2012), except that the analgesic lidocaine (5%) was applied only to the left side of the dorsal fin before cork boring to allow for comparison with wild tagged animals as well as a preliminary assessment of any possible effects of the application. The skin plate was primarily used for assay development, while the plugs were used to assess the potential value of such biopsies to future SCC studies.
Table 1:
Skin cortisol concentration (in nanograms per gram dry weight) as measured in skin samples from harbour porpoises (Phocoena phocoena)
| ID no. | Sex | Standard length (cm) | Date (circumstance) | Sample type | Sample location | Sample wet weight (mg) | Sample dry weight (mg) | Water content (%) | Cortisol concentration (ng/g dw) |
|---|---|---|---|---|---|---|---|---|---|
| 43720 | Female | 117 | February 2011 (bycaught in gill net, dead) | Skin plate | Right | 900 | 306 | 66 | 13.0 ± 1.5a |
| Plugs (clean) | Right 1 | 91 | 49 | 46 | 2.1 | ||||
| Right 2 + 3 | 164 | 111 | 32 | 2.2 | |||||
| Plugs (with lidocaine) | Left 1 | 87 | 66 | 24 | LODb | ||||
| Left 2 + 3 | 174 | 97 | 44 | 4.7 | |||||
| 43721 | Female | 154 | August 2010 (bycaught in gill net, dead) | Skin plate | Right | 670 | 268 | 60 | 2.6 |
| Plugs (clean) | Right 1 | 71 | 64 | 10 | 70.1 | ||||
| Right 2 + 3 | 162 | 106 | 35 | 57.7 | |||||
| Plugs (with lidocaine) | Left 1 | 50 | 34 | 32 | LODb | ||||
| Left 2 + 3 | 132 | 99 | 25 | 77.7 | |||||
| 47501 | Male | 151 | April 2012 (bycaught in pound net, alive) | Scraper | Right | 701 | 89 | 87 | 0.6 |
| Left | 869 | 112 | 87 | NAc | |||||
| 2012/60022 | Male | 144 | August 2012 (bycaught in pound net, alive) | Scraper | Right | 414 | 72 | 83 | 15 |
| Left | 629 | 96 | 85 | 2 |
Abbreviations: dw, dry weight; LOD, limit of detection; NA, not available. a±1.5 corresponds to 11.6% (relative standard deviation). bSignal-to-noise ratio <3. cMissing internal standard.
Figure 1:
(a) Outline of a harbour porpoise (Phocoena phocoena) with indications of sampling locations of skin plates and biopsy plugs taken from dead bycaught animals in Danish waters and used in the development of the skin cortisol analysis method presented in this study. The shaded square area indicates the location of the skin plate, 10 cm × 10 cm in size. Points 1–3 indicate the approximate locations of the 6 mm biopsies. (b) Outline of a harbour porpoise (P. phocoena) indicating where alongside the animal's left and right side non-invasive skin sampling devices were used to collect samples of sloughed skin cells. (Porpoise outline adapted from the Wikimedia Commons file ‘Harbor_porpoise_size.svg’.)
Bycaught, live animals
Prior to commencing this project, several non-invasive skin sampling techniques had been tested on nine free-ranging harbour porpoises caught in pound nets in Danish waters and handled in the ongoing tagging project there (Teilmann et al., 2007). Sampling involved simply running one of the various sterilized collection devices along each side of each animal once using single, not overly forceful strokes that were easy to replicate (Fig. 1b). The devices tested were as follows: (i) a green kitchen sccourer; (ii) a plastic laboratory tube; (iii) P40 and P80 grit emery cloth; and (iv) a rubberized ice scraper (Supplementary material Fig. 1). For reasons discussed in detail in the results section of the paper, the ice scraper was the only one of these tested devices to yield sufficient epidermal skin samples. The collected samples ultimately used here were transported on dry ice (−70°C) and stored in a −20°C freezer for 8 or 11 months (for IDs 2012/60022 and 47501, respectively) before analysis. No plugs taken for the purpose of tagging were used in this study.
Application
To evaluate the new assay, cortisol was analysed in three different skin samples: those obtained by the minimally invasive use of a rubberized ice scraper; the dorsal fin plugs taken during simulated tagging procedures on lethally bycaught animals (with or without prior application of lidocaine); and the skin plates, also from the lethally bycaught animals. Sample size [dry weight (dw)] and water content of these samples are shown in Table 1. Plug 1 was analysed on its own, while plugs 2 and 3 were pooled to test for potential effects of an increased sample size (lidocaine-covered samples and clean samples were analysed separately).
Cortisol analysis
The method developed was initially based on the work of Hansen et al. (2011a), in which three steroid hormone classes were extracted and analysed from environmental matrices (i.e. soil and animal manure). We believed this method to be adaptable because we expected that cortisol would have similar physicochemical properties to those steroid hormone classes and that cetacean skin would have a high lipid content, much like the environmental matrices analysed.
Chemicals
Cortisol with an analytical purity >96% was used in calibration, optimization and method development studies. As a part of our quality assurance and quality control scheme, a derivatization quality control standard (DCS), 17β-estradiol-17-acetate (purity >99%), and an instrument control standard (ICS), estrone-3-methyl ether (purity >99%), were added to each sample. All three compounds were obtained from Sigma-Aldrich (Glostrup, Denmark). The deuterated analogue of cortisol, d4-cortisol (purity >98%; Toronto Research Chemicals, Ontario, Canada), was applied to all samples as an internal standard (IS). All organic solvents were of high-performance liquid chromatography grade, with a purity >99.5%. The derivatization reagents N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA), N-trimethylsilylimidazole (TMSI) and 1,4-dithioerythritol (DTE) were obtained from Sigma-Aldrich (Glostrup, Denmark). The derivatization mixture consisted of 1000 μl MSTFA, 50 μl of a 40 µg µl−1 DTE pyridine dilution and 2 μl TMSI. Cortisol, IS, DCS and ICS were all dissolved in methanol in separate volumetric glass flasks to produce stock solutions of ∼100 ng µl−1. Moreover, working dilutions in methanol were prepared from these stock solutions. Helium was used as the carrier gas and argon as the collision gas in the GC-MS/MS process.
Sample preparation
Method development was performed using 10 cm × 10 cm skin plates, a size chosen to ensure sufficient material for the exploratory use. In skin plate and plug samples, only the 2 mm top layer of epidermal cells was used to ensure that no blubber was included in the analysis. For the scrape samples, the epidermal cells were used as they were collected. Water content and dry weight were then determined by weighing samples before and after 24 h of lyophilization using a freeze dryer (FD3; Heto Lab equipment, Allerød, Denmark) operating at −48°C and 1 Pa (10−5 bar).
Pressurized liquid extraction procedure
As mentioned above, the pressurized liquid extraction (PLE) extraction procedure used was adapted from a method for measuring cortisol in animal manure and agricultural soil (Hansen et al., 2011a), using the ASE 200 PLE system (Dionex, Sunnyvale, CA, USA). Prior to extraction, 22 ml PLE stainless-steel cells were packed with 1 g activated silica gel (particle size 0.063–0.100 mm mesh; Merck, Darmstadt, Germany) and 1 g diatomaceous earth (DE; Dionex). Furthermore, sample tissue was mixed with 1 g activated DE and ground in a mortar before being applied to the cell. Silica gel and DE were activated for 24 h at 105°C before use. A single cellulose filter (size 19.8 mm; Dionex) was applied at the bottom and at the top of the cell. Cortisol was extracted using a methanol and acetone mixture (1:1, v/v) at 1500 psi and 80°C with a 5 min preheat followed by two 5 min static extraction cycles with 60% flush volume and 120 s purge period.
Cleaning up of extracts
The crude PLE extracts were subjected to a two-step clean-up process to reduce matrix components that may interfere with the sensitive GC-MS/MS system (Hansen et al., 2011b).The first step used an amino-propyl column (NH2, 500 mg amino-propyl SPE cartridge; Waters Sep-pak, Dublin, Ireland), which had been preconditioned twice, each time using 2 ml n-heptane, to remove fatty acids and phospholipids (Kaluzny et al., 1985). The PLE extracts were evaporated close to dryness (∼1–2 ml left in the vials) at 60°C under a gentle stream of N2 with circular motions. Two millilitres of isopropanol was added to the vial and evaporated close to dryness a second time. After this, a second aliquot of 2 ml of isopropanol was added, and the mix once again evaporated close to dryness, before 100 μl CHCl3 was added to the vials and the extracts transferred to the preconditioned NH2 column. The vials were then washed with 200 μl CHCl3:isopropanol (2:1,v/v), to ensure a quantitative transfer of the analyte from the vials to the column. Finally, the steroid hormones were re-extracted from the column using 5 ml CHCl3: isopropanol (2:1, v/v).
The second clean-up step for separating the steroid hormones from lipids (such as mono-, di- and triglycerides, sterols, stanols and cholesterols) required a silica gel SPE column consisting of a 3 ml glass column (LiChrolut; Merck, Darmstadt, Germany), a cellulose filter and 1 g activated silica gel soaked in n-heptane (Hansen et al., 2011a, b). The extracts from the first step were evaporated to dryness and reconstituted in 50 μl CHCl3 and 450 μl n-heptane before being loaded into the silica gel column. The vials were washed with 500 μl n-heptane to ensure quantitative transfer. The gel columns were then flushed with 5 ml n-heptane followed by 10 ml n-heptane:acetone (90:10, v/v). Hereafter, androgens, estrogens and progestagens were eluted with 5 ml n-heptane:acetone (65:35, v/v; Hansen et al., 2011a). One final flush in 5 ml methanol was used to extract the more polar hormones, such as the corticosteroids, including cortisol.
Derivatization
The purified extract was evaporated to dryness and transferred to a GC-vial with a 300 μl insert using two times 100 μl methanol. Next, 100 μl 0.2 ppm DCS solution was added, and the purified extract was evaporated to dryness using N2 as described in the previous subsection. The steroid hormones were derivatized using 50 μl derivatization reagent mixture (1000 μl MSTFA, 50 μl DTE dilution and 2 μl TMSI) at 60°C for 1 h. The extract was then reconstituted in 200 μl 0.1 ppm ICS n-heptane solution (Hansen et al., 2011a).
Gas chromatography–tandem mass spectrometry
A Bruker Scion™ TQ hyphenated system (EI interface; Bruker Daltonik, Bremen, Germany) with a GC PTV-injector and a Zebron-5HT Inferno column (30 m × 0.25 mm, 0.25 μm; Phenomenex Inc., Torrance, CA, USA) was used to determine SCC. A sample size of 10 μl was injected with a solvent-split/splitless PTV-program starting at 120°C and kept in the injector for 0.30 min (glass split liner with a glass frit; Varian Inc., Palo Alto, CA, USA) before it was subjected to a temperature ramp of 200°C/min to 325°C, where the temperature was held for 11.50 min. The column oven temperature programme was set at 150°C for the first 2 min, before increasing the temperature to 230°C (25°C/min); this temperature was maintained for 3.7 min. The total run time was 30 min, during which a constant carrier gas flow of 1.0 ml/min helium was applied. The triple-quadrupole mass spectrometer was operated in positive electron ionization mode using selected reaction monitoring mode with argon as the collision gas. The optimized ion transitions and collision energies were 578.4 > 331.1 (collision energy 10 V), 578.4 > 374.2 (collision energy 10 V) and 578.4 > 432.2 (collision energy 30 V) for cortisol. The IS, d4-cortisol, yielded two transitions: 582.4 > 311.1 (collision energy 18 V) and 582.4 > 378.4 (collision energy 5 V). A reference chromatogram is displayed in Supplementary material Fig. 3.
The assay described above was then subjected to a PLE optimization and recovery experiment, to determine absolute recovery and relative recovery rates, as well as a standard addition experiment (see Supplementary material). The primary reason for using GC-MS/MS instead of a radioimmunoassay when measuring SCCs was the hope that the newly developed method can later be expanded to cover more glucocorticoid hormones (which would not all be measurable by using radioimmunoassay). In addition, this method should avoid the cross-reactivity that is frequently observed when using radioimmunoassays.
Method validation
Initially, the absolute recovery of each step in the method was found, followed by a determination of full assay absolute and relative recovery using a pre- and postspike approach. Full assay and instrument variation were based on the d4-cortisol peak area. Experimental details of this as well as of the GC-MS and derivatization procedure, the PLE-optimization and recovery experiment and the standard addition tests can be found in the Supplementary material.
Results
Method validation
We aimed to obtain an optimal 100% absolute recovery when analysing skin samples, but were able to achieve only 20.6% for cortisol and 20.1% for the internal standard (d4-cortisol) when spiked at 100 ng per sample. While not ideal, this is a sufficient recovery rate for use in a new analysis method with no other contemporaries. In any case, and more importantly, we found an impressive relative recovery rate of 102.4 ± 10.5% when using d4-cortisol as an internal correction standard for cortisol (Supplementary material).
In the standard addition experiment, the native cortisol concentration was determined in triplicate from a homogenized sample, subdivided into three aliquots (sample sizes of 306 ± 3 mg dw). The endogenous concentration of cortisol was 13.0 ng/g, with an assay repeatability of 11.6% (relative standard deviation, n = 3). Furthermore, one sample was injected three times to determine instrument repeatability for cortisol and d4-cortisol. The peak area variations (relative standard deviation) were 2.7 and 1.7% for cortisol and d4-cortisol, respectively, in the skin samples containing 306 ± 3 mg dw. Given that no skin reference material was available, the limits of detection (LODs) were evaluated visually based on signal-to-noise ratios. The instrument LOD and limit of quantification (LOQ) in neat standards were calculated using a cortisol calibration curve with a linier dynamic range of a cortisol standard curve from 2.5 ppb to 2.5 ppm and a r2 of 0.9978. The calculated instrument LOD and LOQ were 37 and 113 ppb, respectively (Araujo, 2009).
Of the devices tested, only the ice scraper consistently collected enough material (sample size of ≥49 mg dw, n = 4, two each from two tagged male porpoises, ID 47501 and ID 2012/60022; Table 1) that could be easily extracted for the ensuing analysis. The samples collected via the other techniques were more variable in size (and would thus require multiple strokes to be assured of reaching the critical sample size of ≥49 mg dw) and, with the exception of the plastic tube, harder to extract from the collection devices.
Analysis results
Cortisol could be measured using the new assay in 79% of the tested skin samples, reliably down to the limit seen in this study of 49 mg dw in one clean plug sample (14 samples in total, out of which two were below the LOD and one was not available owing to missing internal standard; see Table 1). The instrument control standard deviated <10% within batch, and the derivatization reaction was to completion (99% or higher) in all skin samples. The signal-to-noise ratio of d4-cortisol was >30 in every sample except for one (47501 left), where d4-cortisol could not be detected. Water content in the plug samples ranged from 10 to 46% and the SCCs from 2.1 and 77.7 ng/g dw. The skin scrape samples had the highest water content (83–87%) and lower concentrations of cortisol (0.6–15 ng/g dw), while the two skin plates had water contents and SCCs of 60 and 66% and 2.6 and 13.0 ng/g dw, respectively. Measured SCCs were slightly higher in plugs with lidocaine than without, with average values of 41 and 33 ng/g dw, respectively.
Discussion
Cortisol concentration could be determined in all plug samples of sufficient size (≥49 mg dw), including those treated with lidocaine. Use of the rubberized ice scraper provided sufficient sample material for the assay as described here. Accordingly, it is clearly possible to obtain samples from any smaller cetacean already in hand, for example in connection with tagging, disentanglement efforts or health assessments (although it should be noted that collection of skin prior to direct handling at the sample site is advised because direct handling may result in loss of sloughed skin cells and thus a considerably smaller scraper sample). Although remote skin collection was not attempted here, it may also be possible to collect sufficient samples of skin naturally sloughed by larger cetaceans at sea (Harlin et al., 1999; Engelhaupt, 2004). Skin biopsy punches from tissue banks could also be used as source material, which would enable retrospective temporal studies. Cortisol has proved highly stable in other keratinous matrices (Bortolotti et al., 2009; Warnock et al., 2010; Webb et al., 2010), so it is entirely possible that even relatively old frozen skin samples (even if lipokeratinocytic in nature; Menon et al., 1986; Pfeiffer and Rowntree, 1996; Reeb et al., 2007) could provide useful analysis material.
Considerable differences in measured SCCs were observed both between and within sample types (Table 1). However, substantial differences in inter-individual cortisol concentrations are commonly observed in other matrices, such as blood and hair (Tryland et al., 2002; Haave et al., 2003; Davenport et al., 2006; Suavé et al., 2007; Eskesen et al., 2009). Although we do not know whether this is the case in the present study, such differences can be dependent on factors such as the animal's sex, age, body condition, reproductive stage and which body region is sampled, as well as season, moulting cycles and water temperature (St Aubin et al., 1990; Suzuki et al., 2003; Macbeth et al., 2010; Funasaka et al., 2011).
Water content in the samples may also play a role. Differences between the scrape samples were already visible to the naked eye during the clean-up process of the analysis (Supplementary materialFig. 2), with the colour of these samples ranging from clear to yellow and green. This difference in colour was not observed between the plug samples. It is also possible that the presence of algae (or other interfering compounds) may potentially be increasing the weight of the sample and thus influencing the concentration calculation. Although the low sample size should again be kept in mind here, this could be another potential factor in the observed variation in scrape SCCs.
As evident in Table 1, a single clean plug from a sampled animal yielded enough material to provide a cortisol reading. However, this was not the case for the single lidocaine-treated plugs. Whether this was due to the lidocaine or to the lower sample weight of the lidocaine-treated plugs remains to be resolved. It was not possible to fully determine whether lidocaine application compromised the assay. However, the finding that the SCC values obtained from the various plug samples for any given animal were much closer to each other than to the SCC value of that animal's skin plate suggests that the analgesic had little, if any, effect.
Another possible cause of some of the observed differences is that the plugs were from dead animals, while the scrapes were from live animals. However, given that the concentrations in the skin plate were closer to the flank scrapes (Table 1), we believe that it is more likely that the differences observed are due to natural variation between body regions (keeping in mind that there could also potentially be variations in cortisol over the length of the flank scrape itself). Such intra-individual differences between body regions have also been reported elsewhere and are likely to relate to diversity in physiological parameters, such as epidermal cell growth rates, degree of vascularization and variations in epidermal thickness (Colbert et al., 1998; Bryan et al., 2010; Miller et al., 2011). Choice of cetacean body region(s) to be sampled in further SCC studies should thus be considered carefully.
The present study describes an analytical method for quantifying cortisol in harbour porpoise epidermal skin cells. In addition, it documents that cortisol concentrations can be assessed in a range of different harbour porpoise skin samples. Based on the methodology described here, ensuing studies should investigate questions such as whether other hormones can be measured in cetacean skin and whether SCC is related to other hormones, such as aldosterone, which also appears to be of importance in the cetacean stress response (Thomson and Geraci, 1986; St Aubin and Geraci, 1990; Fair et al., 2014). Another question is whether SCC reflects only adrenal activity or whether some of the cortisol is locally derived (as has been shown in other mammals; Slominski et al., 2013). This will require, at least in part, an exploration of the relationship between blood cortisol concentration and SCC. A study by Trego et al. (2013) lends credibility to the existence of biologically relevant concentrations of hormones in skin as their study linked progesterone in blubber, a matrix very closely associated with skin, with pregnancy in four different species of cetaceans.
Besides these questions, the essential next step in the use of SCCs is a physiological validation to determine the biological relevance of the SCC values obtained and how long the hormonal response to an acute stressor takes to get into, and persist within, the skin matrix. Importantly, there is also a need to understand how hormones carried in the bloodstream are incorporated into the skin matrix; by diffusion or by simple cell renewal. Browning et al. (2014) found that isotope signatures took 14–23 days before their signal was detectable in the epidermis of bottlenose dolphins, while Hicks et al. (1985) found the skin cell renewal rate in bottlenose dolphins to be somewhat slower than that, with 99% of the [6-3H]-thymidine-labelled skin cells reaching the epidermis by day 22 after injection.
In addition, the relationship between blood cortisol concentrations and cortisol concentrations measured in the skin samples must be assessed, and basal cortisol concentrations and any inter- or intra-individual fluctuations must be evaluated. Finally, as discussed above, studies of the potential influence on SCC of basic variables, such as body region, sex, age, reproductive status and season, are also required in order to assess the further uses of cetacean skin in stress-related research. The present study provides a basis for these further investigations. Despite the outstanding questions, the findings described here represent the first critical steps towards the use of epidermal skin to assess chronic stress levels in cetaceans. The potential use of cortisol obtained from cetacean skin samples as a measure for chronic stress in these species has widespread management implications. As managers gain greater appreciation of the role of stress responses in mediating cumulative impacts, the potential exists for SCC to become a powerful tool for monitoring the general health of various cetacean populations.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
The analyses presented here were funded in part by the Office of Naval Research [grant no. N000141210896], the Aage V. Jensen Foundation, Humane Society International and OceanCare. The authors thank each organization for their interest and support.
Supplementary Material
Acknowledgements
The authors would like to thank everyone at Fjord & Bælt for their advice and suggestions regarding the selection of sampling methods and techniques. We would also like to thank all those involved in the collection of samples, which made this project possible. Specifically, we thank the various fishermen and scientists involved in the Danish tagging programme. All samples included in this project were obtained during the ongoing harbour porpoise studies under authorization from Danish Forest and Nature Agency (SNS 342-00042) and the Animal Welfare Committee (Ministry of Justice, 2010/561-1801).
References
- Araujo P. (2009) Key aspects of analytical method validation and linearity evaluation. J Chromatogr B Analyt Technol Biomed Life Sci 87: 2224–2234. [DOI] [PubMed] [Google Scholar]
- Barrell GK, Montgomery GW. (1989) Absence of circadian patterns of secretion of melatonin or cortisol in Weddell seals under continuous natural daylight. J Endocrinol 122: 445–449. [DOI] [PubMed] [Google Scholar]
- Bechshøft TØ, Rigét FF, Sonne C, Letcher RJ, Muir DCG, Novak MA, Henchey E, Meyer JS, Eulaers I, Jaspers V, et al. (2012) Measuring environmental stress in East Greenland polar bears, 1892–1927 and 1988–2009: what does hair cortisol tell us? Environ Int 45: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti GR, Marchant T, Blas J, Cabezas S. (2009) Tracking stress: localization and stability of corticosterone in feathers. J Exp Biol 212: 1477–1482. [DOI] [PubMed] [Google Scholar]
- Browning NE, Dold C, I-Fan J, Worthy GAJ. (2014) Isotope turnover rates and diet–tissue discrimination in skin of ex situ bottlenose dolphins (Tursiops truncatus). J Exp Biol 217: 214–221. [DOI] [PubMed] [Google Scholar]
- Bryan CE, Christopher SJ, McLellan WA, McFee WE, Schwacke LH, Wells RS. (2010) Application of ICP-MS to examining the utility of skin as a monitoring tissue for trace elements in bottlenose dolphin, Tursiops truncatus. Open Chem Biomed Methods J 3: 169–178. [Google Scholar]
- Colbert A, Stoskopf M, Brownie C, Scott GI, Levine J. (1998) Anatomic site and interanimal variability in morphologic characteristics of bottlenose dolphin (Tursiops truncatus) skin likely to affect dermal absorption studies. Am J Vet Res 59: 1398–1403. [PubMed] [Google Scholar]
- Constable S, Parslow A, Dutton G, Rogers T, Hogg C. (2006) Urinary cortisol sampling: a non-invasive technique for examining cortisol concentrations in the Weddell seal, Leptonychotes weddellii. Zoo Biol 25: 137–144. [Google Scholar]
- Cook CJ, Mellor DJ, Harris PJ, Ingram JR, Matthews LR. (2000) Hands-on and hands-off measurement of stress. In Moberg GP, Mench JA, eds, The Biology of Animal Stress. CABI Publishing, Wallingford, Oxfordshire, UK, pp 123–146. [Google Scholar]
- Cook NJ. (2012) Review: Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can J Anim Sci 92: 227–259. [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. (2006) Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147: 255–261. [DOI] [PubMed] [Google Scholar]
- Desportes G, Buholzer L, Anderson-Hansen K, Blanchet M, Acquarone M, Shephard G, Brando S, Vossen A, Siebert U. (2007) Decrease stress; train your animals: the effect of handling methods on cortisol levels in harbor porpoises (Phocoena phocoena) under human care. Aquat Mamm 33: 286–292. [Google Scholar]
- Dunstan J, Gledhill A, Hall A, Miller P, Ramp C. (2012) Quantification of the hormones progesterone and cortisol in whale breath samples using novel, non-invasive sampling and analysis with highly-sensitive ACQUITY UPLC and Xevo TQ-S. Application note, Waters Corporation, Milford, MA, USA, http://www.waters.com/webassets/cms/library/docs/720004277en.pdf. [Google Scholar]
- Durner GM, Douglas DC, Nielson RM, Amstrup SC, McDonald TL, Stirling I, Mauritzen M, Born EW, Wiig Ø, DeWeaver E, et al. (2009) Predicting 21st-century polar bear habitat distribution from global climate models. Ecol Monogr 79: 25–58. [Google Scholar]
- Engelhaupt DT. (2004) Phylogeography, kinship and molecular ecology of sperm whales (Physeter macrocephalus). Doctoral thesis Durham University; http://etheses.dur.ac.uk/3059/. [Google Scholar]
- Eskesen IG, Teilmann J, Geertsen BM, Desportes G, Rigét F, Dietz R, Larsen F, Siebert U. (2009) Stress level in wild harbor porpoises (Phocoena phocoena) during satellite tagging measured by respiration, heart rate and cortisol. J Mar Biol Assoc UK 89: 885–892. [Google Scholar]
- Fair PA, Becker PR. (2000) Review of stress in marine mammals. J Aquat Ecosyst Stress Recovery 7: 335–354. [Google Scholar]
- Fair PA, Schaefer AM, Romano TA, Bossart GD, Lamb SV, Reif JS. (2014) Stress response of wild bottlenose dolphins (Tursiops truncatus) during capture–release health assessment studies. Gen Comp Endocrinol 206: 203–212. [DOI] [PubMed] [Google Scholar]
- Farro APC, Rollo MM, Jr, Silva JM, Jr, Marino CL. (2008) Isolation and characterization of microsatellite DNA markers for spinner dolphin (Stenella longirostris). Conserv Genet 9: 1319–1321. [Google Scholar]
- Funasaka N, Yoshioka M, Suzuki M, Ueda K, Miyahara H, Uchida S. (2011) Seasonal difference of diurnal variations in serum melatonin, cortisol, testosterone, and rectal temperature in Indo-Pacific bottlenose dolphins (Tursiops aduncus). Aquat Mamm 37: 433–442. [Google Scholar]
- Haave M, Ropstad E, Derocher AE, Lie E, Dahl E, Wiig Ø, Skaare JU, Jenssen BM. (2003) Polychlorinated biphenyls and reproductive hormones in female polar bears at Svalbard. Environ Health Perspect 111: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Krogh KA, Halling-Sørensen B, Bjørklund E. (2011. a) Determination of ten steroid hormones in animal waste manure and agricultural soil using inverse and integrated clean-up pressurized liquid extraction and gas chromatography-tandem mass spectrometry. Anal Methods 3: 1087–1095. [Google Scholar]
- Hansen M, Jacobsen NW, Nielsen KN, Björklund E, Styrishave B, Halling-Sørensen B. (2011. b) Determination of steroid hormones in blood by GCMS/MS. Anal Bioanal Chem 400: 3409–3417. [DOI] [PubMed] [Google Scholar]
- Harlin AD, Würsig B, Baker CS, Markowitz TM. (1999) Skin swabbing for genetic analysis, application on dusky dolphins (Lagenorhynchus obscurus). Mar Mammal Sci 15: 409–425. [Google Scholar]
- Hicks BD, St Aubin DJ, Geraci JR, Brown WR. (1985) Epidermal growth in the bottlenose dolphin, Tursiops truncatus. J Invest Dermatol 85: 60–63. [DOI] [PubMed] [Google Scholar]
- Hogg CJ, Rogers TL, Shorter A, Barton K, Miller PJO, Nowacek D. (2009) Determination of steroid hormones in whale blow: it is possible. Mar Mammal Sci 25: 605–618. [Google Scholar]
- Hunt KE, Moore MJ, Rolland RM, Kellar NM, Hall AJ, Kershaw J, Raverty SA, Davis CE, Yeates LC, Fauquier DA, et al. (2013) Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv Physiol 1: doi:10.1093/conphys/cot006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KE, Stimmelmayr R, George C, Hanns C, Suydam R, Brower H, Jr, Rolland RM. (2014) Baleen hormones: a novel tool for retrospective assessment of stress and reproduction in bowhead whales (Balaena mysticetus). Conserv Physiol 2: doi:10.1093/conphys/cou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny MA, Duncan LA, Merritt MV, Epps DE. (1985) Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res 26: 135–140. [PubMed] [Google Scholar]
- Macbeth BJ, Cattet MRL, Stenhouse GB, Gibeau ML, Janz DM. (2010) Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): considerations with implications for other wildlife. Can J Zool 88: 935–949. [Google Scholar]
- Menon GK, Grayson S, Brown BE, Elias PM. (1986) Lipokeratinocytes of the epidermis of a cetacean (Phocoena phocoena). Cell Tissue Res 244: 385–394. [DOI] [PubMed] [Google Scholar]
- Miller DL, Woshner V, Styer EL, Ferguson S, Knott KK, Gray MJ, Wells RS, O'Hara TM. (2011) Histologic findings in free-ranging Sarasota Bay bottlenose dolphin (Tursiops truncatus) skin: mercury, selenium, and seasonal factors. J Wildlife Dis 47: 1012–1018. [DOI] [PubMed] [Google Scholar]
- MMC (The Marine Mammal Commission) (2009) Annual report to Congress. Marine Mammal Commission, Bethesda, MD, USA http://mmc.gov/reports/annual/welcome.shtml.
- Noren DP, Mocklin JA. (2012) Review of cetacean biopsy techniques: factors contributing to successful sample collection and physiological and behavioral impacts. Mar Mammal Sci 28: 154–199. [Google Scholar]
- Oki C, Atkinson S. (2004) Diurnal patterns of cortisol and thyroid hormones in the Harbor seal (Phoca vitulina) during summer and winter seasons. Gen Comp Endocrinol 136: 289–297. [DOI] [PubMed] [Google Scholar]
- ONR (Office of Naval Research) (2009) Final Workshop Proceedings for Effects of Stress on Marine Mammals Exposed to Sound, Arlington, VA, 4–5 November 2009. Office of Naval Research, Arlington, VA, USA, 59 pp.
- Pedernera-Romano C, Valdez RA, Singh S, Chiappa X, Romano MC, Galindo F. (2006) Salivary cortisol in captive dolphins (Tursiops truncatus): a non-invasive technique. Anim Welfare 15: 359–362. [Google Scholar]
- Pfeiffer CJ, Rowntree VL. (1996) Epidermal ultrastructure of the southern right whale calf (Eubalaena australis). J Submicr Cytol Pathol 28: 277–286. [PubMed] [Google Scholar]
- Reeb D, Best PB, Kidson SH. (2007) Structure of the integument of southern right whales Eubalaena australis. Anat Rec 290: 596–613. [DOI] [PubMed] [Google Scholar]
- Rolland RM, Parks SE, Hunt KE, Castellote M, Corkeron PJ, Nowacek DP, Wasser SK, Kraus SD. (2012) Evidence that ship noise increases stress in right whales. Proc Biol Sci 279: 2363–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savery LC, Evers DC, Wise SS, Falank C, Wise J, Gianios C, Kerr I, Payne R, Thompson WD, Perkins C, et al. (2013) Global mercury and selenium concentrations in skin from free-ranging sperm whales (Physeter macrocephalus). Sci Total Environ 450–451: 59–71. [DOI] [PubMed] [Google Scholar]
- Schmitt TL, St Aubin DJ, Schaefer AM, Dunn JL. (2010) Baseline, diurnal variations, and stress-induced changes of stress hormones in three captive beluga whales, Delphinapterus leucas. Mar Mammal Sci 26: 635–647. [Google Scholar]
- Sheriff MJ, Bosson CO, Krebs CJ, Boonstra R. (2009) A non-invasive technique for analyzing fecal cortisol metabolites in snowshoe hares (Lepus americanus). J Comp Physiol B 179: 305–313. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. (2010) Assessing stress in animal populations: do fecal and plasma glucocorticoids tell the same story? Gen Comp Endocrinol 166: 614–619. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbyteka B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, et al. (2013) Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem 137: 107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Aubin (2001) Endocrinology. In Dierauf LA, Gulland FMD, eds, CRC Handbook of Marine Mammal Medicine, Ed 2 CRC Press, New York, pp 165–192. [Google Scholar]
- St Aubin DJ, Dierauf LA. (2001) Stress and marine mammals. In Dierauf LA, Gulland FMD, eds, CRC Handbook of Marine Mammal Medicine, Ed 2 CRC Press, New York, pp 253–269. [Google Scholar]
- St Aubin DJ, Geraci JR. (1990) Adrenal responsiveness to stimulation by adrenocorticotropic hormone (ACTH) in captive beluga whales, Delphinapterus leucas. Can Bull Fish Aquat Sci 224: 149–157. [Google Scholar]
- St Aubin DJ, Smith TG, Geraci JR. (1990) Seasonal epidermal moult in beluga whales, Delphinapterus leucas. Can J Zool 68: 359–367. [Google Scholar]
- Sonne C. (2010) Health effects from long-range transported contaminants in Arctic top predators: an integrated review based on studies of polar bears and relevant model species. Environ Int 36: 461–491. [DOI] [PubMed] [Google Scholar]
- Sonne C, Teilmann J, Wright AJ, Dietz R, Leifsson P. (2012) Tissue healing in two harbor porpoises (Phocoena phocoena) following long-term satellite transmitter attachment. Mar Mammal Sci 28: E316–E324. [Google Scholar]
- Suavé B, Koren G, Grace W, Tokmakejian S, Van Uum SHM. (2007) Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30: E183–E191. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Uchida S, Ueda K, Tobayama T, Katsumata E, Yoshioka M, Aida K. (2003) Diurnal and annual changes in serum cortisol concentrations in Indo-Pacific bottlenose dolphins Tursiops aduncus and killer whales Orcinus orca. Gen Comp Endocrinol 132: 427–433. [DOI] [PubMed] [Google Scholar]
- Teilmann J, Larsen F, Desportes G. (2007) Time allocation and diving behavior of harbor porpoises (Phocoena phocoena) in Danish and adjacent waters. J Cetac Res Manage 9: 201–210. [Google Scholar]
- Terio KA, Citino SB, Brown JL. (1999) Fecal cortisol metabolite analysis for noninvasive monitoring of adrenocortical function in the cheetah (Acinonyx jubatus). J Zoo Wildlife Med 30: 484–491. [PubMed] [Google Scholar]
- Thomson CA, Geraci JR. (1986) Cortisol, aldosterone, and leucocytes in the stress response sf bottlenose dolphins, Tursiops truncatus. Can J Fish Aquat Sci 43: 1010–1016. [Google Scholar]
- Tizzi R, Accorsi PA, Azzali M. (2010) Non-invasive multidisciplinary approach to the study of reproduction and calf development in bottlenose dolphin (Tursiops truncatus): the Rimini Delfinario experience. Int J Comp Psychol 23: 734–776. [Google Scholar]
- Trana MR, Roth JD, Tomy GT, Anderson G, Ferguson SH. (2015) Influence of sample degradation and tissue depth on blubber cortisol in beluga whales. J Exp Mar Biol Ecol 462: 8–13. [Google Scholar]
- Trego ML, Kellar NM, Danil K. (2013) Validation of blubber progesterone concentrations for pregnancy determination in three dolphin species and a porpoise. PLoS ONE 8: e69709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryland M, Brun E, Derocher AE, Arnemo JM, Kierulf P, Ølberg RA, Wiig Ø. (2002) Plasma biochemical values from apparently healthy free-ranging polar bears from Svalbard. J Wildlife Dis 38: 566–575. [DOI] [PubMed] [Google Scholar]
- Warnock F, McElwee K, Seo RJ, McIsaac S, Seim D, Ramirez-Aponte T, Macritchie KAN, Young AH. (2010) Measuring cortisol and DHEA in fingernails: a pilot study. Neuropsychiatr Dis Treat 6: 1–7. [PMC free article] [PubMed] [Google Scholar]
- Webb E, Thomson S, Nelson A, White C, Koren G, Rieder M, Van Uum S. (2010) Assessing individual systemic stress through cortisol analysis of archaeological hair. J Archaeol Sci 37: 807–812. [Google Scholar]
- Wright AJ. (ed). (2009) Report of the Workshop on Assessing the Cumulative Impacts of Underwater Noise with Other Anthropogenic Stressors on Marine Mammals: From Ideas to Action. Monterey, CA, USA, 26–29 August 2009. Okeanos – Foundation for the Sea, Auf der Marienhöhe 15, D-64297 Darmstadt. 67 + iv pp http://www.sound-in-the-sea.org/download/CIA2009_en.pdf.
- Wright AJ, Aguilar Soto N, Baldwin AL, Bateson M, Beale C, Clark C, Deak T, Edwards EF, Fernández A, Godinho A, et al. (2007) Do marine mammals experience stress related to anthropogenic noise? Int J Comp Psychol 20: 274–316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.