Behavioural changes in the stickleback caused by near-future CO2 levels can be restored by treatment with gabazine, revealing an involvement of the GABAA receptor ion. Importantly, these effects can be conveniently studied in the stickleback, with a sequenced and annotated genome and exceptionally well-characterized behaviour and physiology
Keywords: γ-Aminobutyric acid, brain, global change, hypercapnia, lateralization, temperate fish
Abstract
Studies on the consequences of ocean acidification for the marine ecosystem have revealed behavioural changes in coral reef fishes exposed to sustained near-future CO2 levels. The changes have been linked to altered function of GABAergic neurotransmitter systems, because the behavioural alterations can be reversed rapidly by treatment with the GABAA receptor antagonist gabazine. Characterization of the molecular mechanisms involved would be greatly aided if these can be examined in a well-characterized model organism with a sequenced genome. It was recently shown that CO2-induced behavioural alterations are not confined to tropical species, but also affect the three-spined stickleback, although an involvement of the GABAA receptor was not examined. Here, we show that loss of lateralization in the stickleback can be restored rapidly and completely by gabazine treatment. This points towards a worrying universality of disturbed GABAA function after high-CO2 exposure in fishes from tropical to temperate marine habitats. Importantly, the stickleback is a model species with a sequenced and annotated genome, which greatly facilitates future studies on underlying molecular mechanisms.
Introduction
Burning of fossil fuel continues to increase the atmospheric CO2 level, widely affecting the ocean chemistry in a process commonly referred to as ocean acidification. According to the fifth assessment report from the Intergovernmental Panel on Climate Change in 2013, the atmospheric CO2 level may reach 800–1150 µatm within this century (Collins et al., 2013), and the resultant increase in oceanic CO2 levels is now recognized as a serious threat to the marine ecosystem.
Initial experimental studies on the effects of these near-future CO2 levels on fish behaviour have focused on tropical species, suggested to be more sensitive to carbon chemistry changes owing to their high metabolic rate and therefore high CO2 exchange with water (Nilsson et al., 2012). These studies show that sustained exposure to such near-future CO2 levels causes an array of sensory and behavioural alterations in coral reef damselfishes. The alterations observed include reversed olfactory and auditory preferences (Munday et al., 2009; Dixson et al., 2010; Simpson et al., 2011), loss of behavioural lateralization (Domenici et al., 2012), loss of learning (Ferrari et al., 2012), increased boldness and activity (Munday et al., 2010) and reduced temporal resolution in the retinal response to light (Chung et al., 2014).
It has recently become clear that the behaviour of temperate fish also may be affected by elevated CO2. It was found that the three-spined stickleback (Gasterosteus aculeatus) displays decreased behavioural lateralization, learning, boldness and curiosity when exposed to 990 µatm CO2 (Jutfelt et al., 2013). Moreover, the Californian rockfish (Sebastes diploproa) shows increased anxiety when exposed to 1125 µatm CO2 (Hamilton et al., 2014).
For the coral reef fish, the neural mechanisms causing behavioural abnormalities have been found to involve altered function of the γ-aminobutyric acid (GABA) neurotransmitter, because normal behaviour can be restored effectively by a moderate dose of the specific GABAA-receptor antagonist gabazine (SR-95531; Nilsson et al., 2012; Chivers et al., 2014; Chung et al., 2014). Furthermore, Hamilton et al. (2014) suggested that the increase of anxiety in the Californian rockfish is linked to altered function of the GABAA receptor.
γ-Aminobutyric acid is the major inhibitory neurotransmitter in vertebrate brains. The GABAA receptor is an ion channel with conductance for Cl− and HCO3− (Bormann et al., 1987). A net inflow of these negatively charged ions into the postsynaptic neuron will cause hyperpolarization of the neuronal membrane that counteracts depolarizing excitatory input. When exposed to elevated CO2, fish regulate their acid–base balance to avoid acidosis by accumulating HCO3−, accompanied by a release of H+ and Cl− to the water (Ishimatsu et al., 2008; Brauner and Baker, 2009). It is likely that such ion-regulatory mechanisms lead to altered transmembrane Cl− and HCO3− gradients in the brain during high-CO2 exposure, which in turn cause a reversal of GABAA-receptor function (Nilsson et al., 2012). It is also likely that the ion-regulatory changes and/or changes in GABAA-receptor function involve complex long-term perturbations, such as altered gene expression, because the behavioural dysfunctions are manifested only after several days of high-CO2 exposure and persist for several days after normal CO2 levels have been restored (Munday et al., 2012).
Our ability to characterize the physiological and molecular mechanisms linking elevated CO2 to altered GABAA-receptor function would be greatly enhanced if studies can be done in a model species with a sequenced genome and well-characterized behaviour, life history and physiology. Clearly, the three-spined stickleback is such a species if it can be shown that the behavioural effects of high-CO2 displayed by this fish are linked to the GABAA receptor. Consequently, the aim of this study was to test whether the loss of behavioural lateralization seen in high-CO2-exposed stickleback can be reversed by treatment with the specific GABAA-receptor antagonist, gabazine.
Materials and methods
Ethics statement
The experiment was conducted in accordance with Swedish law and regulations and approved by the Ethical Committee on Animal Experiments in Gothenburg, Sweden (ethical permit numbers 100-2010 and 151-2011).
Experimental animals
Adult marine three-spined stickleback (G. aculeatus) were collected using a hand-trawl in the Gullmars Fjord (58° 15.781′ N, 11° 29.815′ E) on the Swedish west coast and kept at the nearby Sven Lovén Centre for Marine Sciences (Kristineberg), University of Gothenburg (Sweden). After being sedated using 2-phenoxyethanol in seawater (0.5 ml l−1), fish were tagged using subcutaneous implants of fluorescent elastomer tags (Northwest Marine Tech. Inc., Shaw Island, WA, USA), allowing us to keep track of individual variables measured during the experiment.
Fish were divided into eight groups and transferred to eight 80 l aquaria with either control or high-CO2 water. Initially, four fish were put into each aquarium. During experiments, the fish were kept with a 14 h–10 h light–dark cycle and fed twice a day ad libitum with frozen Artemia sp. nauplii (Kordon Golden Gate, Hayward, CA, USA). At the beginning of the experiment, fish weight was 1.30 ± 0.230 g and standard length 46.50 ± 2.219 mm (mean ± SD). Subsequent weight and length measures are given in Supplementary Figs S1 and S2.
Manipulation of CO2
The eight aquaria (four for each treatment group) were continuously supplied with water from four header tanks, two providing aerated control water and two providing high-CO2 water. The header tanks were supplied with surface seawater pumped from the fjord. Water from the header tanks was gravity fed through silicone tubing to each holding aquarium at a rate of ∼1 l min−1. In the high-CO2 tanks, the level of CO2 was regulated by keeping the pH stable near 7.7 with a pH stat (Aqua Medic, Bissendorf, Germany) connected to a solenoid valve controlling the bubbling of the water with CO2. Temperature, pH and partial pressure of CO2 (pCO2) were measured daily from a randomly chosen aquarium from each exposure group. The pCO2 was measured with an infra-red CO2 probe connected to a submerged CO2-permeable membrane (GM70 CARBOCAP; Vaisala, Vantaa, Finland) equipped with an aspiration pump, as described by Munday et al. (2012). Alkalinity was measured every third day by taking a sample of 25 ml from one treatment and one control aquarium. These samples were filtered through a 2 µm filter and analysed in a titration machine (SI Analytics, Mainz, Germany) by stepwise addition of hydrogen chloride. Water salinity measures were provided by the field station's monitoring of surface water. The measured pCO2 in the treatment aquaria was 992.1 ± 119.3 μatm CO2 and the pH was 7.69 ± 0.057. The control aquaria had a pCO2 of 442.4 ± 71.0 μatm CO2 and a pH of 8.02 ± 0.052 (mean ± SD; Table 1).
Table 1:
Water chemistry data measured daily (pCO2, salinity and temperature) and twice weekly (alkalinity and pHtot), during the 50 day exposure experiment
| Parameter | Control | Elevated CO2 |
|---|---|---|
| pCO2 (μatm) | 442 ± 71 | 992 ± 119 |
| Alkalinity [μmol (kg sea water)−1] | 2162 ± 239 | 2149 ± 195 |
| Salinity (practical salinity units) | 25.9 ± 2.3 | 25.9 ± 2.3 |
| Temperature (°C) | 13.1 ± 2.4 | 13.1 ± 2.4 |
| pHtot (calculated) | 8.02 ± 0.05 | 7.69 ± 0.06 |
Abbreviations: pCO2, partial pressure of carbon dioxide; pHtot, total pH. The pCO2, alkalinity, salinity and temperature were measured, while the total pH was calculated with CO2calc (USGS, St Petersburg, Florida, USA). The data are presented as means ± SD.
Behavioural lateralization test
After 40 days of treatment, a double T-chamber was used to evaluate the effect of acidified water on behavioural asymmetry. The chamber measured 50 cm × 50 cm, with a runway 9 cm wide (Fig. 1) and was filled to high 5 cm of water supplied from the holding aquarium from which the fish was taken. Single individuals were introduced into the chamber and left for 3 min to explore the new environment. Subsequently, the fish was gently encouraged with a plastic rod to swim into the runway (unless it was already there) and then along the runway until the end, where the direction in which it turned was scored. It was then encouraged to swim back into the runway and, when reaching the opposing end, the direction of turn was again recorded. This procedure of making the fish swim back and forth in the runway was repeated until 20 turns were recorded. Care was taken to manoeuvre the fish as gently as possible, not touching it with the rod. As described by Jutfelt et al. (2013), the score of each individual's turn preference was used to calculate the relative and the absolute lateralization.
Figure 1:
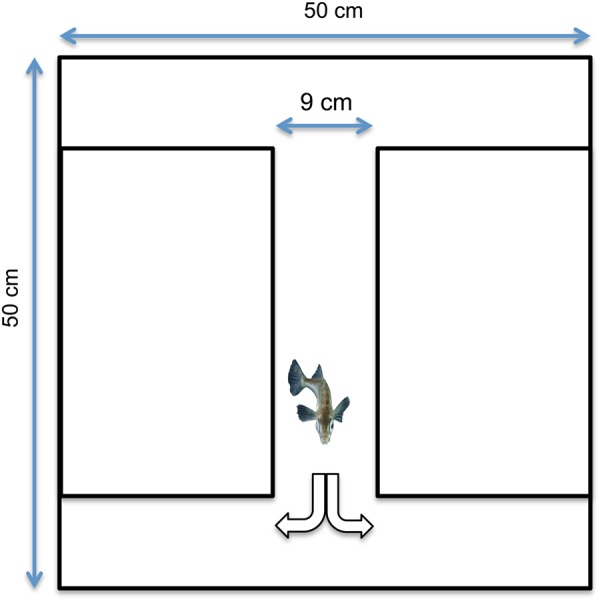
The double T-chamber used for the behavioural lateralization test. Each individual was encouraged to swim in the runway, and the preference on the left or right turn was recorded.
The behavioural lateralization was retested in the same individuals on day 50. This time, the fish were tested twice, once immediately before and once immediately after a 30 min treatment with gabazine. The treatment involved placing each fish in 200 ml jars containing 50 ml seawater with gabazine (4 mg l−1; Sigma-Aldrich Co., St Louis, MO, USA; Nilsson et al., 2012; Chung et al., 2014; Hamilton et al., 2014).
Analysis
Length and weight were measured on days 0, 10, 20 and 50 and analysed using a two-way ANOVA followed by Sidak's multiple comparison post hoc test.
Lateralization indexes were calculated for each fish according to Domenici et al. (2012). In short, the mean for absolute lateralization indices (La) reveals whether there is a significant lateralization at the population level regardless of direction of turn by individuals, and it is derived from the relative lateralization indices (Lr) by removing any negative prefix (individuals with a majority of left turns yield negative Lr values).
The value of Lr was calculated as follows:
Differences in the absolute lateralization between control and high-CO2 fish on day 40 were analysed using Student's two-tailed t-test.
To analyse the effects of high CO2 and gabazine on day 50, two-way ANOVA with Sidak's multiple comparison test as the post hoc test was used. Values are given as means ± SEM if not otherwise stated.
Results
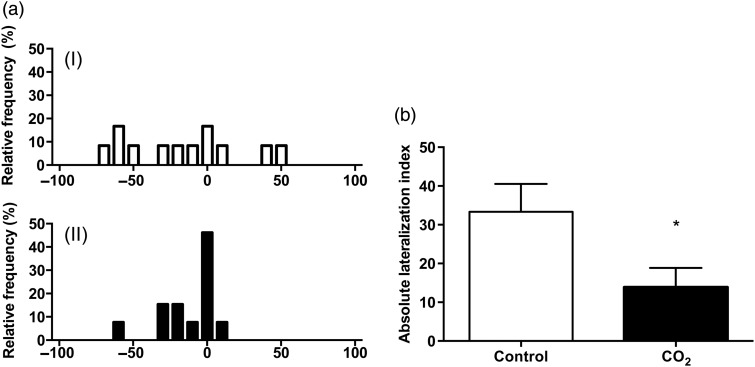
The three-spined stickleback kept in elevated CO2 demonstrated a complete loss of lateralization. Thus, after 40 days in the experimental aquaria, the high-CO2 treatment group had a significantly lower La than the control group (Fig. 2b). The La was 33.3 ± 7.2 in control fish (exposed to 442 μatm CO2) as a result of a significant individual preference for either turning left or right in the lateralization test (but with no population bias because the number of left and right turners were approximately equal). In contrast, fish exposed to 992 μatm CO2 for 40 days showed an La of 13.8 ± 5.0. Also, the Lr was differently distributed among fish exposed to control conditions and high CO2 (F = 4.15, P = 0.0213; Fig. 2a).
Figure 2:
(a) Relative lateralization index (Lr) of fish after 40 days of treatment. (I) Lr in control fish exposed to 442 μatm CO2; n = 12. (II) Lr in the CO2 group exposed to 992 μatm CO2 (n = 13). Comparison among groups was analysed with the F-test. (b) Absolute lateralization index after 40 days of treatment, revealing loss of lateralization after high-CO2 treatment. Carbon dioxide had significant effects on absolute lateralization (Student's two-tailed t-test, P = 0.034). Values are means ± SEM.
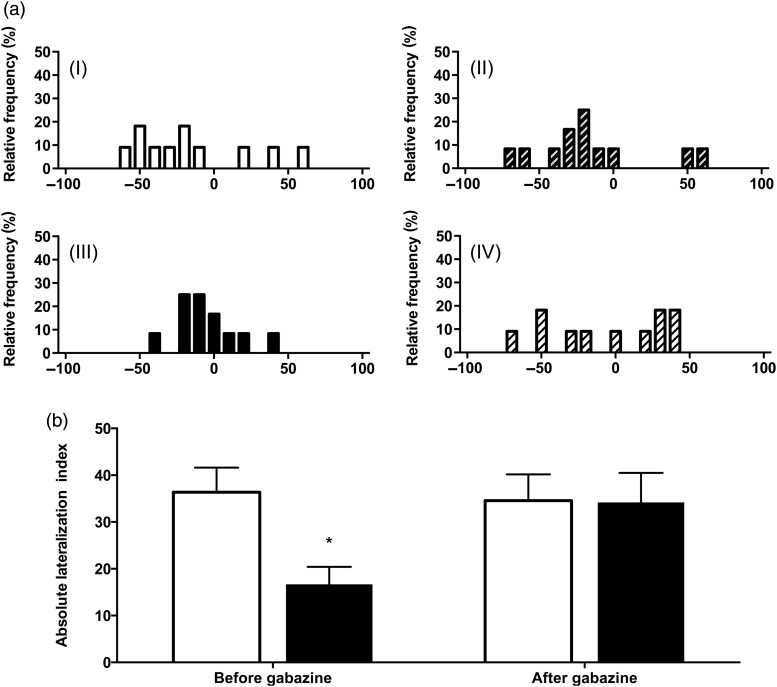
After 50 days in the experimental tanks, the fish were tested for lateralization again (Fig. 3), but this time in two subsequent runs, i.e. before and after treatment with gabazine (4 mg l−1 gabazine in seawater for 30 min). In the first run, control fish (La = 36.3 ± 5.3) and high-CO2 fish (La = 16.7 ± 3.7) showed virtually the same degree of lateralization as they did on day 40, with the suppressive effect of high-CO2 exposure on lateralization being retained (two-way ANOVA, P = 0.0269). In the second run, the lateralization in the high-CO2 group was completely restored by the gabazine treatment and, as a result, there was no difference between the control group treated with gabazine (La = 34.5 ± 5.6) and the high-CO2 group treated with gabazine (La = 34.2 ± 6.3; two-way ANOVA, P = 0.2229).
Figure 3:
(a) Relative lateralization index of fish after 50 days of treatment. The values of Lr are shown for control fish exposed to 442 μatm CO2 before (I) and after (II) being kept in seawater containing gabazine (4 mg l−1) for 30 min (n = 11) and for fish exposed to 992 μatm CO2 before (III) and after (IV) being kept in seawater containing gabazine (4 mg l−1) for 30 min (n = 12). (b) The absolute lateralization index after 50 days of treatment in high CO2 was fully restored by GABAA-receptor antagonist treatment. The treatments had a significant effect (two-way ANOVA, P = 0.0269). *P < 0.05.
Lengths and weights over the experimental period did not differ between control fish and fish exposed to elevated CO2 (two-way ANOVA, P = 0.797 and 0.884, respectively; see Supplementary data). During the 50 day experiment, five control fish and four high-CO2-exposed fish died from unknown causes.
Discussion
The result of the gabazine treatment shows that altered GABAA receptor function is underlying the behavioural abnormalities displayed by stickleback after exposure to high-CO2 levels, exactly like previous findings in coral reef damselfish (Nilsson et al., 2012) and, recently, in rockfish (Hamilton et al., 2014).
Previous studies on coral reef damselfish (reviewed by Munday et al., 2012) have shown that the behavioural effects of high-CO2 treatment set in only after several days of sustained exposure and persist for several days after the high-CO2 exposure has ended, suggesting that alterations in gene expression may be involved. This could include changes in expression of genes for proteins involved in ion and pH regulation as well as the GABAA receptor itself. Exposure to high-CO2 water has been found to reduce the response of the retina to fast stimuli, probably by altering the function of GABAA receptors (Chung et al., 2014), and one may speculate that if other retinal functions are also altered this could contribute the loss of behavioural lateralization observed in this study as well as in previous studies on other species.
Most importantly, the present study suggests that the stickleback will provide us with a very useful model that opens up an avenue of experimental studies, including the examination of underlying molecular changes. For instance, transcriptomic studies will be aided by the annotated genome of this species, and we can also envision transgenerational studies, because this species can be bred readily in aquarium populations.
The three-spined stickleback is regarded as fairly tolerant of various environments, thriving in both freshwater and seawater (Pottinger et al., 2002; Östlund-Nilsson et al., 2006; Barrett et al., 2011), suggesting an extraordinary ion-regulatory plasticity. Nevertheless, its neural functions are apparently sensitive to sustained exposure to predicted future CO2 levels, as shown by the complete loss of behavioural lateralization in the present study, in the study by Näslund et al. (2015) and in other behavioural tests by Jutfelt et al. (2013).
Behavioural lateralization is thought to be a consequence of hemispheric specialization in the brain, allowing animals to carry out parallel processing and simultaneous responses to different stimuli (Bisazza et al., 1998; Dadda et al., 2012). In fish, the left optic lobe is connected to the right eye and vice versa, leading to a corresponding specialization of the eyes. If the right eye is, for example, focused on observing potential threats, pointing the head to the right and turning to the right allows the right eye to observe possible threats coming from behind. At the same time, the left eye can be focused on a different task, such as acquiring resources. Improved schooling performance, escape response, orientation and cognitive tasks are other suggested advantages of brain asymmetry (Bisazza and Dadda, 2005; Sovrano et al., 2005; Vallortigara and Rogers, 2005; Dadda et al., 2010). Loss of lateralization may therefore possibly affect fish fitness and survival.
Initially, it was hypothesized that highly active fish with a need for rapid oxygen uptake, and therefore large respiratory surface areas, would be more at risk for being affected by increases in the water CO2 level (Nilsson et al., 2012). The reason for this is that a large respiratory surface area should also lead to a fast release of CO2 from blood to water, approaching an equilibrium with the ambient water, making the blood carbonate system more sensitive to ambient changes in CO2. Indeed, fish in general have relatively low internal levels of CO2 in comparison to air-breathing animals owing to the high solubility of CO2 in water. Consequently, few would have regarded the stickleback as particularly vulnerable, because it is not a ‘high-performance’ species with a particularly high rate of gas exchange with the environment and because it is able to tolerate a wide range of environmental conditions. A worrying aspect of the present study is therefore that it indicates that altered GABAA-receptor function in response to sustained environmental hypercapnia may affect virtually any fish, at least in marine habitats.
At present, we do not know to what degree natural selection will be able to counteract neural effects of elevated CO2 in the relatively short period during which oceanic CO2 levels have been projected to reach close to 1000 µatm. What the present study shows is that individual acclimation is unlikely to be of help, because the behavioural change was in no way reduced after 50 days in the high-CO2 environment.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by the University of Oslo [to G.E.N. and F.L.], The Formas Research Council [to F.J.], The Swedish Research Council [to F.J. and G.E.N.] and the Royal Swedish Academy of Science [to F.L.].
Author contributions
The study was conceived and designed by G.E.N. and F.J. Experiments and measurements were executed by F.L. All authors contributed to writing the paper.
Supplementary Material
Acknowledgements
The authors thank Erik Lindström for help with fish collection, rearing and water chemistry.
References
- Barrett RDH, Paccard A, Healy TM, Bergek S, Schulte PM, Schluter D, Rogers SM. (2011) Rapid evolution of cold tolerance in stickleback. Proc Biol Sci 278: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A, Dadda M. (2005) Enhanced schooling performance in lateralized fishes. Proc Biol Sci 272: 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A, Facchin L, Pignatti R, Vallortigara G. (1998) Lateralization of detour behaviour in poeciliid fish: the effect of species, gender and sexual motivation. Behav Brain Res 91: 157–164. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. (1987) Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol 385: 243–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner CJ, Baker DW. (2009) Patterns of acid-base regulation during exposure to hypercarbia in fishes. In Glass ML, Wood SC, eds, Cardio-Respiratory Control in Vertebrates. Springer, Berlin, pp 43–63. [Google Scholar]
- Chivers DP, McCormick MI, Nilsson EG, Munday PL, Watson SA, Meekans MG, Mitchell MD, Corkill KC, Ferrari MCO. (2014) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Change Biol 20: 515–522. [DOI] [PubMed] [Google Scholar]
- Chung WS, Marshall NJ, Watson SA, Munday PL, Nilsson GE. (2014) Ocean acidification slows retinal function in a dameselfish through interference with GABAA receptors. J Biol 217: 323–326. [DOI] [PubMed] [Google Scholar]
- Collins M, Knutti R, Arblaster J, Dufresne T, Fichefet P, Friedlingstein X, Gao WJ, Gutowski T, Johns G, Krinner M, et al. (2013) Long-term climate change: projections, commitments and irreversibility. In Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds, Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Dadda M, Koolhaas WH, Domenici P. (2010) Behavioural asymmetry affects escape performance in a teleost fish. Biol Lett 6: 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadda M, Zansonà E, Agrillo C, Bisazza A. (2012) The cost of hemispheric specialization in a fish. Proc Biol Sci 276: 4399–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson DL, Munday PL, Jones GP. (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13: 68–75. [DOI] [PubMed] [Google Scholar]
- Domenici P, Allan B, McCormick MI, Munday PL. (2012) Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett 8: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. (2012) Effects of ocean acidification on learning in coral reef fishes. Plos ONE 7: e31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TJ, Holcombe A, Tresguerres M. (2014) CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABAA receptor functioning. Proc Biol Sci 281: 20132509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu A, Hayashi M, Kikkawa T. (2008) Fishes in high CO2 acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- Jutfelt F, Souza KB, Vuylsteke A, Sturve J. (2013) Behaviour disturbance in a temperate fish exposed to sustained high-CO2 levels. Plos ONE 8: e65825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB. (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106: 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP. (2010) Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA 107: 12930–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, McCormick MI, Nilsson GE. (2012) Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J Exp Biol 215: 3865–3873. [DOI] [PubMed] [Google Scholar]
- Näslund J, Lindström E, Lai F, Jutfelt F. (2015) Behavioural responses to simulated bird attacks in marine three-spined sticklebacks after exposure to high CO2 levels. Mar Freshw Res http://dx.doi.org/10.1071/MF14144. [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson SA, Munday PL. (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Chang 2: 201–204. [Google Scholar]
- Östlund-Nilsson S, Mayer I, Huntingford FA. (2006) Biology of the Three-Spined Stickleback. Taylor & Francis, Boca Raton. [Google Scholar]
- Pottinger TG, Carrick TR, Yeomans WE. (2002) The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. J Fish Biol 61: 207–229. [Google Scholar]
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson D, Gagliano M, Yan HY. (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovrano VA, Dadda M, Bisazza A. (2005) Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav Brain Res 163: 122–127. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. (2005) Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28: 575–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.