Laboratory and field experiments were used to establish a temperature threshold for induction of the cellular and endocrine stress responses in brook trout that is similar to their proposed ecological thermal limit, suggesting that the induction of the stress response by temperature may be a mechanism that limits species distributions.
Keywords: Climate change, cortisol, glucose, heat shock protein, Salvelinus fontinalis, temperature
Abstract
Climate change is predicted to change the distribution and abundance of species, yet underlying physiological mechanisms are complex and methods for detecting populations at risk from rising temperature are poorly developed. There is increasing interest in using physiological mediators of the stress response as indicators of individual and population-level response to environmental stressors. Here, we use laboratory experiments to show that the temperature thresholds in brook trout (Salvelinus fontinalis) for increased gill heat shock protein-70 (20.7°C) and plasma glucose (21.2°C) are similar to their proposed thermal ecological limit of 21.0°C. Field assays demonstrated increased plasma glucose, cortisol and heat shock protein-70 concentrations at field sites where mean daily temperature exceeded 21.0°C. Furthermore, population densities of brook trout were lowest at field sites where temperatures were warm enough to induce a stress response, and a co-occurring species with a higher thermal tolerance showed no evidence of physiological stress at a warm site. The congruence of stress responses and proposed thermal limits supports the use of these thresholds in models of changes in trout distribution under climate change scenarios and suggests that the induction of the stress response by elevated temperature may play a key role in driving the distribution of species.
Introduction
Environmental temperature exerts a primary constraint on the distribution and abundance of species. With predicted global increases in temperature, there is concern over the future of species whose appropriate thermal habitats will shift and shrink. Indeed, a number of studies have documented poleward and elevational shifts in species ranges across a variety of taxa (Parmesan and Yohe, 2003; Root et al., 2003). Models based on the current relationships between distribution and temperature have been used to estimate the change in species' ranges under climate change scenarios. However, uncertainty concerning species' thermal thresholds and the presence of multiple limiting factors may reduce confidence in these predictions. Understanding the congruency between distributional limits and thermal thresholds for the cellular and endocrine stress responses that are directly related to performance and fitness can substantially increase confidence in such models. Several integrative approaches to understanding the physiological mechanisms involved in the role of temperature in altering animal distribution have been proposed (Pörtner, 2010; Sokolova, 2013). These approaches highlight the rapid decrease in growth and swimming performance that occurs above ‘optimum’, which are often accompanied by cellular and physiological stress. Thus, the cellular and endocrine stress responses can serve as effective indicators of exposure to extreme conditions, which can often be transient and difficult to detect.
The eastern brook trout (Salvelinus fontinalis) may be acutely sensitive to climate change, because it is a cold-water species with populations that are spatially constrained to stream networks. Recent models suggest that climate change will lead to a significant loss of brook trout habitat, with the greatest reductions occurring in their southern range (Meisner, 1990; Flebbe et al., 2006). These models are informed by field observations indicating that brook trout are rarely found in streams with 60 day mean temperatures above 21.0°C (Wehrly et al., 2007). This ecological limit is lower than their upper incipient lethal temperature of 25.3°C (Fry et al., 1946; Wehrly et al., 2007), suggesting that sublethal temperatures play a role in limiting the distribution of brook trout.
Endocrine and cellular stress responses are likely mechanisms by which individuals cope with stressfully elevated temperatures and may serve as strong bioindicators for measuring these sublethal effects. Cortisol is the major corticosteroid stress hormone in fish, as it is in other vertebrates (Wendelaar Bonga, 1997; Mommsen et al., 1999). Cortisol plays a critical role in the stress response because, among other functions, it is responsible for adjusting metabolic pathways and mobilizing energy stores in the liver through gluconeogenesis (Vanderboon et al., 1991; Wendelaar Bonga, 1997; Mommsen et al., 1999). In response to a real or perceived stressor, the hypothalamic–pituitary–interrenal axis is activated and releases cortisol (Wendelaar Bonga, 1997; Mommsen et al., 1999). A limited literature suggests an increase in circulating cortisol and glucose in salmonids in response to elevated temperature (Meka and McCormick, 2005; Quigley and Hinch, 2006; Steinhausen et al., 2008). To our knowledge, there has been no previous research examining the relationship of plasma cortisol and stream temperature in wild salmonids.
In addition to the endocrine stress response, there is increasing attention on the use of aspects of the cellular stress response, such as heat shock proteins (HSPs), as potential biomarkers for thermal stress (Iwama et al., 1999; Wikelski and Cooke, 2006). Inducible isoforms of HSPs are upregulated in the presence of denatured proteins, which can result from a variety of environmental stressors, including elevated temperature (Tomanek, 2010; Deane and Woo, 2011). A number of laboratory studies have identified elevated HSP expression in response to temperature increases in a variety of salmonid species (Dubeau et al., 1998; Smith et al., 1999; Mesa et al., 2002; Rendell et al., 2006), including brook trout (Lund et al., 2003). Other factors, including social interaction, may influence HSP concentrations (Currie et al., 2010), and there is a complex interaction between HSPs and the endocrine stress response in fish (Boone et al., 2002). Previous laboratory work on teleosts has established that there are thresholds for HSP concentrations (Dietz and Somero, 1993) and perhaps other indicators of cellular stress, such as AMP-activated kinase activity (Anttila et al., 2013). A significant relationship between stream temperature and HSP70 has been reported in several salmonids in the wild (Lund et al., 2002; Werner et al., 2005; Feldhaus et al., 2010). In this study, we determined thresholds for cellular and endocrine stress in brook trout by exposing them to an acute temperature challenge in the laboratory and then testing whether these same responses were manifested in the field by comparing physiological profiles of fish in streams with widely differing temperature regimens, including several that exceeded laboratory temperature thresholds.
Materials and methods
Laboratory temperature treatment
Juvenile 0+ (11.7–29.0 g) brook trout were obtained from the Sandwich State Hatchery (Sandwich, MA, USA) and transported to the Conte Anadromous Fish Research Center (Turners Falls, MA, USA) in July 2011. Fish were housed in tanks 1.7 m in diameter supplied with 4 l min−1 chilled Connecticut River water (16 ± 2°C) and given supplemental aeration. Fish were fed to satiation with pelleted salmon feed daily (Zeigler Bros, Gardners, PA, USA) with automatic feeders and maintained under natural photoperiod. Fifty-six fish were moved from their rearing tank to one of seven experimental tanks 0.6 m in diameter (n = 8 per tank) and allowed to acclimate for 1 week before the start of the experiment. The fish were fed to satiation once daily, and the tanks were supplied with 16°C Turners Falls, MA dechlorinated city water at a rate of 0.8 l min−1. Each tank received additional heated (∼34°C) city water as needed to achieve the desired target temperature of 18°C. The heated water flowed through solenoid valves (Granzow, Inc., Charlotte, NC, USA) that were controlled by Omega cn7500 controllers (Omega Engineering, Inc., Stamford, CT, USA) with resistance thermometer input installed in each tank. The controllers were optimized to the testing conditions and programmed to pulse the solenoid valves open and shut at varying frequency either to maintain a set point or to achieve a new set point within a predetermined time frame. Each tank was provided with supplemental aeration.
Feed was withheld from the fish for 24 h before the start of the experiment. One tank served as a control and was kept at 18°C throughout the experiment. The remaining six tanks were heated at a rate of 8°C h−1 until the target temperatures of 20, 21, 22, 23, 24 or 26°C were reached (Fig. 1a). The water was then held at these target temperatures for the remainder of the experiment. Dissolved oxygen concentrations were monitored at peak temperature and were found to be above 90% saturation in all tanks. Temperature increases in this experiment were more rapid than would normally occur in nature, but were similar to those used in previous research (Dietz and Somero, 1993) and were necessary for fish to be sampled at a similar time of day.
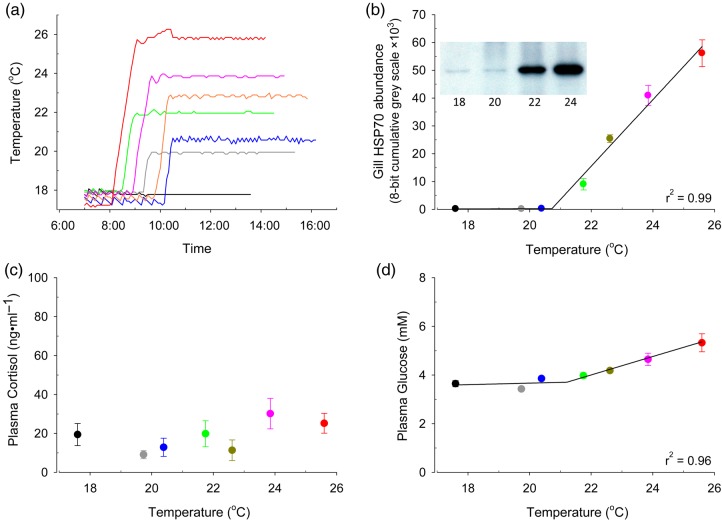
Figure 1:
Temperature profiles of the seven laboratory treatments (a) and effect of temperature on the gill heat shock protein-70 (HSP70; b), plasma cortisol (c) and glucose (d) in brook trout. Inset in (b) is a representative western blot. Water temperatures were elevated at a rate of 8°C h−1 until target temperatures were achieved. Fish were sampled 6 h after initiation of heating. A piecewise regression using temperature as a predictor variable was used to determine temperature thresholds. The relationship between gill HSP70 abundance and temperature showed a threshold for induction of 20.7°C. The relationship between plasma glucose and temperature showed a threshold for induction of 21.2°C. Points represent means ± SEM (n = 6–8).
The fish were killed 6 h after heating was initiated in each group using a lethal dose of anaesthetic (MS-222, 100 mg l−1, pH 7.0; Argent Laboratories, Redmond, WA, USA) so that tissue samples could be taken. Six to eight fish were sampled at each at each temperature regimen. Fish were measured for length (nearest 0.1 cm) and weight (nearest 0.1 g). Blood was collected from the caudal vessels using 1 ml ammonium heparinized syringes within 5 min of tank disturbance. The blood was spun at 3200g for 5 min at 4°C; thereafter, the plasma was aliquoted and stored at −80°C. A biopsy of four to six gill filaments was taken from the first arch and immersed in 100 µl of ice-cold SEI buffer (150 mM sucrose, 10 mM EDTA and 50 mM imidazole, pH 7.3) and stored at −80°C.
Field sampling
This study was conducted in eight small (approximately second order, 3–17 km2 basin size) streams within the Connecticut River basin in western MA, USA (Table 1). In 2010, these sites were sampled on 27–30 July. The 2011 sampling was done on 22–26 July and again on 7–10 November. Six to 12 brook trout were sampled at each site at each of these time points. In July 2011, Atlantic salmon juveniles were also sampled in Roaring Brook (2), where they coexisted with brook trout. At each site, two Hobo pendent temperature loggers (Onset Computer Corporation, Bourne, MA, USA) were placed in the water, one on the upstream and one on the downstream edge of the sampling site, and set to record water temperature at 45 min intervals.
Table 1:
Physical characteristics and population estimates for the eight field sites as measured during July 2010 and July 2011
| Site | Temperature (°C) | Basin size (km2) | Length (m) | Width (m) | Density (fish per km2) |
|---|---|---|---|---|---|
| Lyons Brook | 23.3 | 3.1 | 172.0 | 3.1 | 13.3 |
| Pond Brook | 21.9 | 4.7 | 336.5 | 2.9 | 11.1 |
| Roaring Brook (2) | 20.6 | 8.4 | 175.5 | 5.5 | 8.3 |
| Adams Brook | 20.0 | 17.0 | 90.5 | 5.9 | 18.5 |
| Collar Brook | 19.8 | 3.2 | 95.0 | 3.4 | 30.2 |
| Roaring Brook (1) | 19.3 | 13.1 | 50.0 | 8.3 | 24.0 |
| 4-Mile Brook | 19.3 | 9.5 | 115.0 | 5.0 | 17.4 |
| Buffam Brook | 18.0 | 4.1 | 42.0 | 3.2 | 80.6 |
‘Temperature’ refers to the mean temperature from the 7 days preceding sampling in 2010 and 2011. ‘Length’ refers to the length of the stream section that we sampled. Here, we report width as the mean wetted width of three measurements taken over the length of stream section sampled. We sampled only age 1+ and older fish. The sites are listed here from warmest to coolest.
Sampling was conducted using a one-pass electrofishing technique. The fish ranged in size from 3.5 to 87.4 g, and all fish >8.0 g were sampled non-lethally. There was no significant difference in the size of fish sampled at the sites in either year (P > 0.05, one-way ANOVA). Upon capture, fish were lightly anaesthetized with tricaine methanesulfonate (MS-222, 50 mg l−1, pH 7.0). Fish were measured for length and weight and bled from the caudal vessel using 1 ml ammonium heparinized syringes within 6 min of capture. The blood was spun at 3200g for 5 min; thereafter, the plasma was aliquoted and stored on dry ice. A non-lethal biopsy of four to six gill filaments was taken from the first arch and immersed in 100 µl of ice-cold SEI buffer and frozen on dry ice (McCormick, 1993). Fish were allowed to recover for at least half a hour before being returned to the stream.
Heat shock protein-70 analysis
Gill biopsies were homogenized in 150 µl SEID (SEI buffer and 0.1% deoxycholic acid). After grinding, the samples were spun at 5000g for 5 min at 4°C. A small volume of supernatant was used to determine total protein concentration using the Pierce BCA Protein Assay kit (Thermo Scientific, Rockford, IL, USA). The remaining supernatant was diluted with an equal volume of 2 × Laemmli buffer, heated for 15 min at 60°C and stored at −80°C. Thawed samples were run on a 7.5% SDS-PAGE gel at 2.5 µg per lane with 5 µg Precision Plus protein standards in a reference lane (Bio-Rad Laboratories, Hercules, CA, USA). Following electrophoresis, proteins were transferred to Immobilon PVDF transfer membranes (Millipore, Bedford, MA, USA) at 30 V overnight in 25 mM Tris, 192 mM glycine buffer at pH 8.3. Gels and membranes were periodically checked with Coomassie staining to ensure that proteins were completely transferred. The PVDF membranes were blocked in phosphate-buffered saline with 0.05% Triton X-100 (PBST) and 5% non-fat dry milk for 1 h at room temperature, rinsed in PBST, and probed with an HSP70 antibody (AS05061; Agrisera, Vannas, Sweden) diluted 1:20 000 in PBST and 5% non-fat dry milk for 1 h at room temperature. This antibody is specific to the inducible isoform of salmonid HSP70 and does not recognize the constitutive isoform (Rendell et al., 2006). After rinsing in PBST, blots were exposed to goat anti-rabbit IgG conjugated to horseradish peroxidase diluted 1:10 000 in PBST and 5% non-fat dry milk for 1 h at room temperature. After rinsing in PBST, blots were incubated for 1 min in a 1:1 mixture of enhanced chemiluminescent solution A (ECL A; 396 µM coumaric acid, 2.5 mM luminol and 100 mM Tris–HCl, pH 8.5) and ECL B (0.018% H2O2 and 100 mM Tris–HCl, pH 8.5), then exposed to X-ray film (RPI, Mount Prospect, IL, USA). Digital photographs were taken of films and band staining intensity was measured using ImageJ (NIH, Bethesda, MD, USA); protein abundance is expressed as a cumulative eight-bit grey scale value. A reference sample was run on each gel and was used to correct for interblot differences.
Plasma analysis
Plasma glucose was measured by enzymatic coupling with hexokinase and glucose 6-phosphate dehydrogenase (Carey and McCormick, 1998). Plasma cortisol was measured by enzyme immunoassay as previously described (Carey and McCormick, 1998). Sensitivity as defined by the dose–response curve was from 1 to 400 ng ml−1, and the lower detection limit was 0.3 ng ml−1.
Statistics
All data are presented as means ± SEM. Where necessary, data were logarithmically transformed, and the corresponding P and r2 values were reported. Statistical analyses were performed using SigmaPlot 10.0 and Systat 13.1 (Systat Software, Inc., San Jose, CA, USA). For all analyses, the probability of establishing statistical significance was P < 0.05, and when significant effects were observed the r2 value was reported. For the laboratory portion of this study, we tested the hypothesis of a temperature threshold for induction of cellular and endocrine stress responses by modelling the relationship between mean temperature and response using piecewise regression. This approach was used to demonstrate the presence and level of a threshold temperature and because it resulted in a higher r2 than first-order regression.
For the field portion of this study, we used two approaches. First, we used non-linear regression to test the overall relationship between temperature (2, 3, 10 and 14 day mean) and the three stress responses, analysing site means separately for the 2 years of the study. Second, we used linear mixed models to test whether fish in sites experiencing temperatures above the general thermal distributional limit of 21°C exhibited higher concentrations of stress hormones and higher levels of HSP expression than those in cooler sites throughout the summer. In these models, the random effects accounted for among-site and among-year variation not associated with water temperature, and with water temperature class (separate analyses for 3 and 10 day average temperatures) and individual body mass (due to strong size dependence of many physiological and stress responses) as fixed effects. The Akaike information criterion (AIC) was then used to select the best-supported models, with a difference of 2 AIC units as the criterion for distinction between alternative models. There was strong correlation between mean daily temperature and both cumulative temperature (degree days) and temperature variability among sites that prevented us from independently examining the effect of the latter two factors on physiological responses.
Results
Stress induction in laboratory conditions
There were no mortalities over the course of the experiment. Two of the three stress response indicators demonstrated distinct temperature thresholds for induction in brook trout. The temperature threshold for increased gill HSP70 was estimated at 20.7°C, because there was relatively little gill HSP70 abundance below 20.7°C, after which HSP70 increased rapidly with temperature (Fig. 1b; piecewise linear regression, P < 0.01, r2 = 0.99) and was greatest at the highest temperature of 26°C. There was no significant relationship between temperature and plasma cortisol (Fig. 1c; P = 0.43), whereas plasma glucose increased with temperature (Fig. 1d; piecewise linear regression, P = 0.01, r2 = 0.96), with a threshold for induction of 21.2°C.
Stress induction in field conditions
To demonstrate the efficacy of these biomarkers in wild brook trout and to determine their relationship with overall population density, we sampled fish from eight sites within the Connecticut River watershed in Massachusetts in late July 2010 and 2011 (Fig. 2). Consistent with our expectations, sites differed considerably with respect to summer water temperatures both within and between years. In general, temperatures were higher for a longer period of time in the days preceding the 2011 sampling (Fig. 2c) than they were in 2010 (Fig. 2b). We observed the highest population density at our coldest site, whereas the three lowest population densities were observed at our three warmest sites (Table 1).
Figure 2:
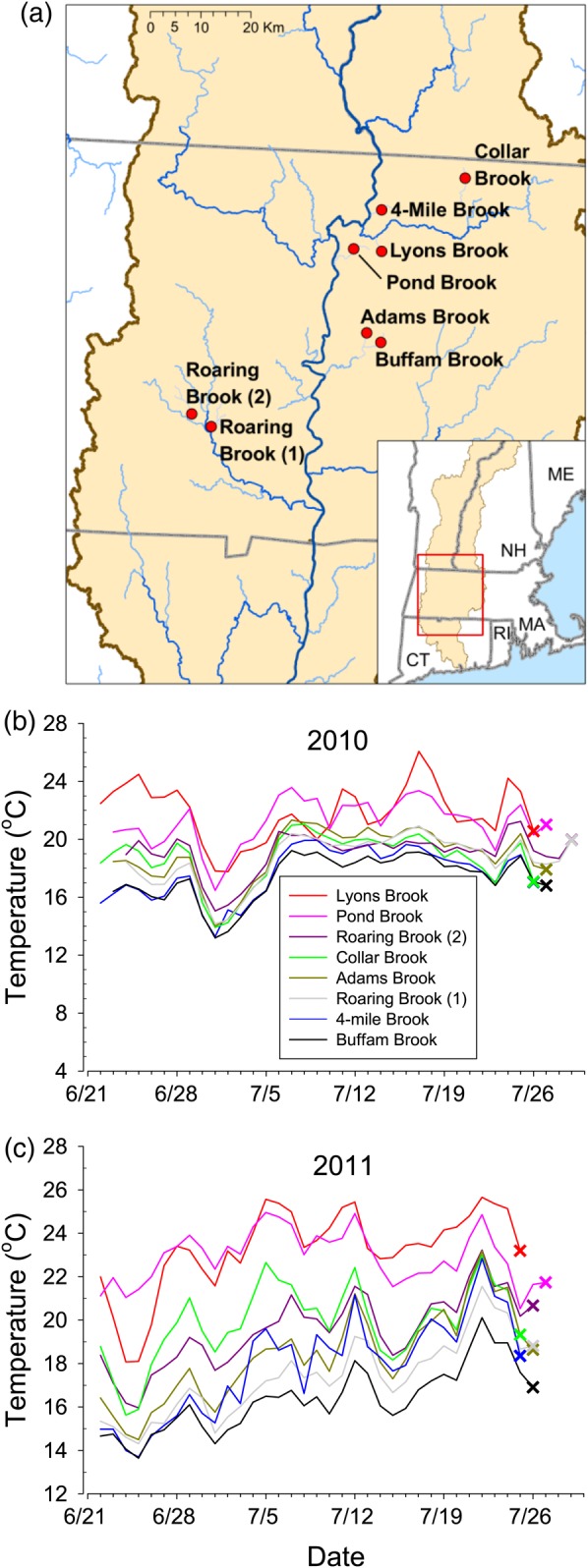
Location (a) and temperature profiles of our eight field sites (b, c). The daily mean water temperature of our field sites was measured in summer 2010 (b) and summer 2011 (c) during the month preceding sampling. The date when each site was sampled is marked by an X.
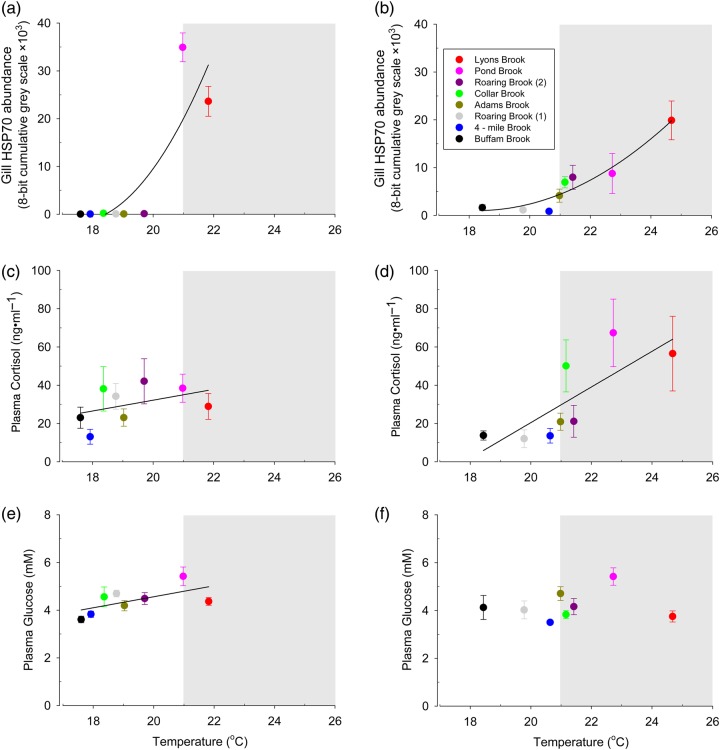
Of the three stress responses, HSP70 showed the strongest and most consistent relationship with temperature. In both 2010 (cooler summer) and 2011 (warmer summer), HSP70 expression increased significantly with increased maximal temperature (Fig. 3a and b; 2010 r2 = 0.82, P < 0.0001; and 2011 r2 = 0.82, P < 0.0001). Consistent with laboratory-derived thresholds for induction and proposed distributional limits, HSP70 expression was close to zero, with low variability amongst individual fish, at sites below 21°C (Fig. 3a and b), but substantially higher and more variable at sites where temperatures were above this threshold, as evidenced by inclusion of the temperature threshold category terms in the best-supported mixed models (Table 2). At the warmest sites (>21.0°C), all fish had elevated HSP70 concentrations. Corresponding to the overall lower maximal temperatures in 2010, only two of the seven sites showed elevated HSP70 (Fig. 3a), while HSP70 was elevated at five of the seven sites in 2011 (Fig. 3b). Size was also determined to be a significant covariate of gill HSP70 concentrations, with a smaller fish tending to have higher concentrations of HSP70 (Table 2), though this effect was small in comparison to temperature (r2 = 0.01 vs. 0.58).
Figure 3:
Effect of temperature on the gill HSP70 (a, b), plasma cortisol (c, d) and plasma glucose (e, f) in wild brook trout from streams samples in 2010 (a, c, e) and 2011 (b, d, f). Points represent means ± SEM (n = 6–12). Quadratic (a, b) and linear regressions (c–f) were used to analyse the data. The mean temperature from the 7 days before sampling was used as a predictor variable.
Table 2:
Results of mixed-model analyses predicting stress response levels as a function of random (site and year) and fixed effects (individual fish mass and temperature class, above or below 21°C)
| Stress response | Model | ΔAIC | Temperature class |
|---|---|---|---|
| HSP70 | Site, year, individual mass, 3 day temperature class | 8.262 | P < 0.001 |
| HSP70 | Site, 10 day temperature class | 8.849 | P < 0.001 |
| Plasma cortisol | Site, 10 day temperature class | 2.005 | P = 0.010 |
| Plasma glucose | Site, year | 4.462 | n.a. |
Effects retained in the best-supported model (based on differences of at least two AIC units) are listed in the second column.Abbreviations: ΔAIC, change in Akaike information criterion; HSP, heat shock protein; n.a., not applicable.
In contrast to the clear effect of temperature on HSP70, the relationships between temperature and the other two stress responses were weaker and less consistent. We observed a general positive relationship between temperature and plasma cortisol in 2010 (Fig. 3c) and 2011 (Fig. 3d), but r2 values were low. Temperature explained more of the variation in cortisol concentrations in 2011 (Fig. 3d; P < 0.01, r2 = 0.19) than it did in 2010 (Fig. 3c; P = 0.03, r2 = 0.06). Variation among individuals was high at all sites. We observed some evidence of an increase in plasma cortisol above 21°C, as evidenced by inclusion of the 10 day temperature threshold in the best-supported mixed model (Table 2), although the 3 day threshold was not included. For plasma glucose, we observed a significant relationship with temperature in 2011 (Fig. 3e; P < 0.01, r2 = 0.17), but again, explanatory power was low, and we did not see a significant relationship in 2011 (Fig. 3f; P = 0.45). Neither 3 day nor 10 day temperature threshold category class was included in the best model for plasma glucose (Table 2).
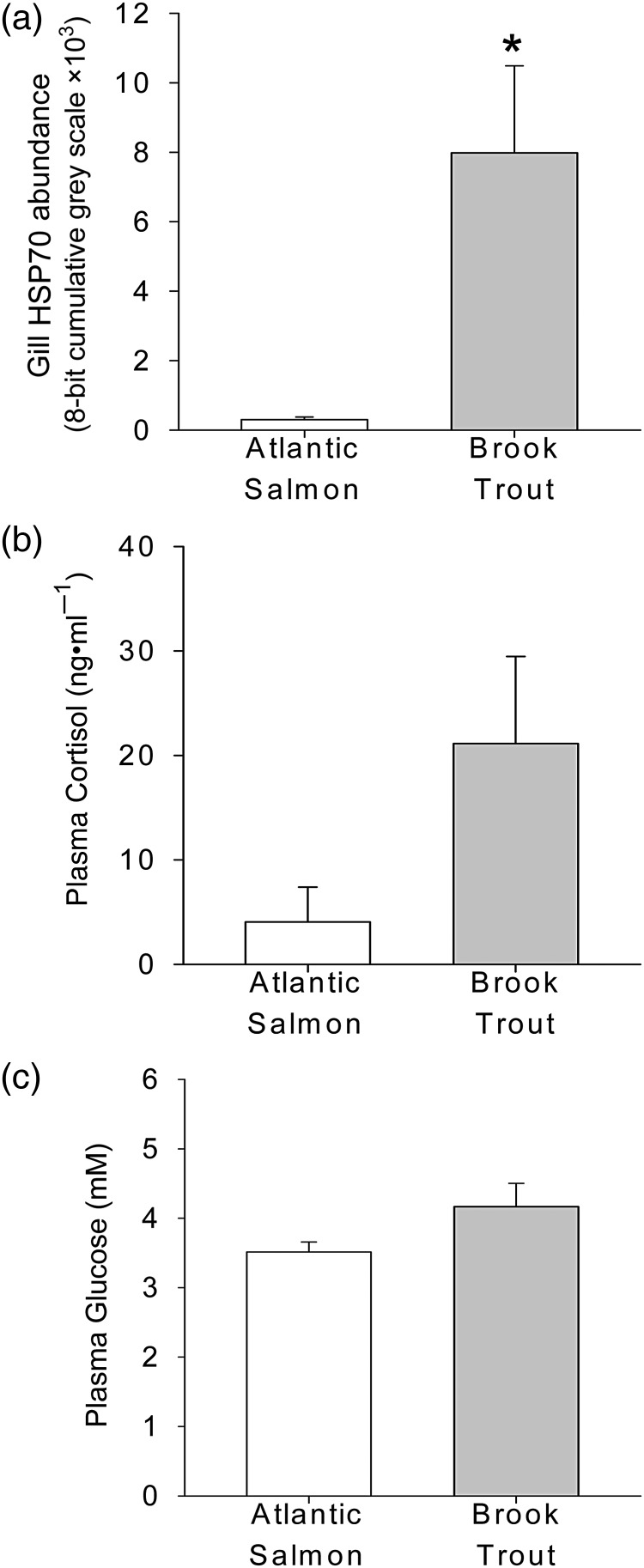
In addition to differences in brook trout stress responses among sites and years, we also observed differences between brook trout and juvenile Atlantic salmon (Salmo salar; Fig. 4a–c). In 2011, in the one site where we sampled both species [Roaring Brook (2)], juvenile Atlantic salmon had significantly lower HSP70 concentrations than brook trout (Fig. 4a), whereas mean values for plasma cortisol and glucose were lower but not significantly different (Fig 4b and c).
Figure 4:
Gill HSP70 (a), plasma cortisol (b) and plasma glucose (c) from wild brook trout (n = 8) and Atlantic salmon (n = 4) from Roaring Brook (2) in 2011. Bars represent means ± SEM, and an asterisk indicates significant differences in means between the two species (P < 0.05 Student's t-test).
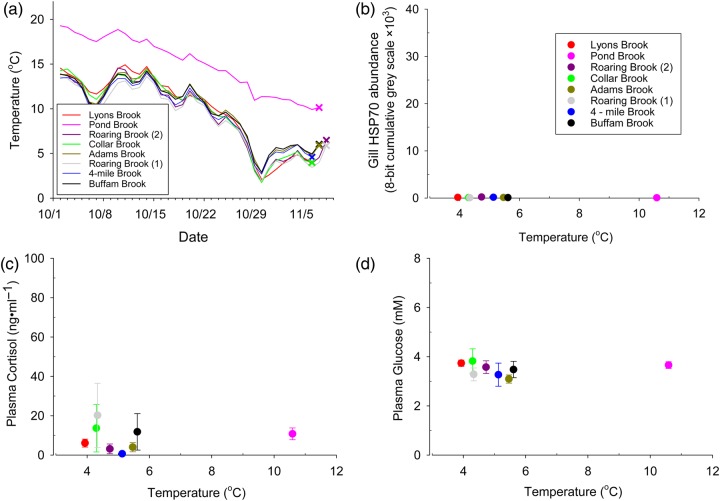
During sampling in November 2011, when temperatures at all sites were <11.0°C (Fig. 5a), all indicators of stress were at or below baseline concentrations from the summer samples, and we did not observe a significant relationship between temperature and gill HSP70 (Fig. 5b; P = 0.06), plasma cortisol (Fig. 5c; P = 0.08) or plasma glucose (Fig. 5d; P = 0.65).
Figure 5:
Effect of temperature on gill HSP70 (a), plasma cortisol (b) and plasma glucose (c) in wild brook trout in autumn 2011. Points represent means ± SEM (n = 6–10). Quadratic (a) and linear regressions (b, c) were used to analyse the data. The mean temperature from the 7 days before sampling was used as a predictor variable.
Discussion
In this study, we used laboratory experiments to demonstrate the induction of endocrine and cellular stress responses by elevated temperature in brook trout. Furthermore, surveys of fish in the field conducted in varying thermal conditions identified a similar response in wild populations at similar temperature thresholds. These temperature thresholds for stress responses may explain the widespread thermal limits for this iconic cold-water species.
In the laboratory, gill HSP70 and plasma glucose increased rapidly at temperatures above 21.2 and 20.7°C, respectively. Although we did not see an effect of short-term exposure to high temperature on plasma cortisol, exposure to increasing temperatures for 8 or 24 days did result in significant increases (J. G. Chadwick Jr and S. D. McCormick, unpublished results), with a threshold of between 20 and 22°C. Field sampling conducted at sites spanning a wide range of temperatures indicated a significant positive relationship of temperature and both gill HSP70 and plasma cortisol as indicated by positive regression analysis (Fig. 3) and linear mixed model (Table 2). In contrast, there was a relatively weak relationship of plasma glucose with temperature in the wild. Gill HSP70 was greatest in abundance at our warmest sites and was above baseline only at sites where the mean temperature exceeded 21.0°C. Plasma cortisol also exhibited generally positive relationships with temperature in the wild, whereas only a weak effect was seen with plasma glucose. This effect of temperature was unlikely to be the result of other, unmeasured differences among sites because samples collected in cooler conditions did not show this pattern of variation. Furthermore, Atlantic salmon, a co-occurring salmonid species with known greater thermal tolerance than brook trout, showed lower indicators of the endocrine and cellular stress responses than brook trout at a warm site where both were sampled. Finally, brook trout were least abundant at our warmest sites and most abundant at our coolest sites. Overall, these results suggest a strong link between high summer stream temperatures and the endocrine and cellular stress responses, and indicate that these responses may be both important mechanisms by which increasing temperatures associated with climate warming affect individuals and populations and an indicator of individual exposure in field conditions.
Temperature thresholds associated with endocrine and cellular stress responses in both our laboratory and our field studies corresponded closely to ecological thermal limits suggested by a number of previous studies. Brook trout are rarely found in streams with 60 day mean temperatures above 21.0°C (Wehrly et al., 2007) and in lentic habitats are limited above 20.0°C (Robinson et al., 2010), despite the fact that their lethal limit is 25.3°C (Fry et al., 1946; Wehrly et al., 2007). Here, we show that the same temperatures that appear to limit brook trout distribution also represent the threshold for the stress response in this species. A number of modelling studies base projections of future brook trout distribution under climate warming scenarios on the predicted loss of cold-water habitat defined by the threshold summer temperatures generally ranging between 20 and 22°C (Meisner, 1990; Flebbe et al., 2006). The concordance between these thermal limits and the stress responses we have observed (which are likely to be highly general across populations and regions) should serve to increase the confidence in model-predicted changes in future distribution, helping managers to justify appropriate adaptation actions.
Factors other than direct mortality are likely to determine the thermal niche of many species, and stress responses provide a potential physiological mechanism by which sublethal effects of increasing temperature influence the current and future species distributions. For cold-dependent salmonid fishes, including brook trout, exposure to stressfully elevated temperatures is associated with altered behaviour (Wurtsbaugh et al., 1975; Curry et al., 1997; Biro, 1998; Baird and Krueger, 2003; Quigley and Hinch, 2006; Breau et al., 2011) and with decreased performance in important aspects of their physiology, such as metabolism (Hartman and Cox, 2008), growth (Pickering, 1990; McCormick et al., 1998; Gregory and Wood, 1999) and fecundity (Robinson et al., 2010). In particular, growth at warmer temperatures is likely to be limited by the stress response, which transfers resources away from somatic growth in order to restore and maintain homeostasis (Vanderboon et al., 1991; Wendelaar Bonga, 1997; Mommsen et al., 1999). In fact, it is well established that fish display reduced growth following a variety of stressors, including elevated temperature (Pickering, 1990). A number of studies have demonstrated decreased appetite in response to elevated cortisol concentrations (Gregory and Wood, 1999; Peterson and Small, 2005; Pankhurst et al., 2008a,b; Leal et al., 2011). Recent work has identified reduced plasma ghrelin concentrations as one mechanism by which cortisol decreases appetite (Pankhurst et al., 2008a,b). The endocrine stress response may also influence endocrine control of growth (Wendelaar Bonga, 1997; Gregory and Wood, 1999; Mommsen et al., 1999). Exogenous cortisol in fish has been shown to decrease growth and plasma concentrations of insulin-like growth factor-I (Kajimura et al., 2003; Peterson and Small, 2005), which is responsible for most of the growth-promoting aspects of growth hormone. In addition to the endocrine stress response, it is likely that the cellular stress response limits growth, because synthesis of HSP consumes cellular resources and occupies cellular machinery that is necessary for growth-promoting biochemical pathways (Feder and Hofmann, 1999; Sokolova, 2013).
Several studies have shown decreased growth in brook trout at temperatures above 16.0°C (Baldwin, 1956; McCormick et al., 1972; Hokanson et al., 1973; Dwyer et al., 1983; McMahon et al., 2007); however, these studies did not include treatment temperatures that were high enough to determine an upper thermal limit for positive growth in this species. Recent work from our laboratory (J. G. Chadwick Jr and S. D. McCormick, unpublished results) strongly supports this idea and indicates that the pejus temperature for growth in brook trout is 20.0°C and the upper thermal limit for positive growth is 23.4°C. Furthermore, there was a strong positive relationship between reduced growth capacity and increased plasma cortisol. There is a well-established relationship between body size and fecundity in salmonids (Thorpe et al., 1984); thus, temperature limitations on growth may also limit reproduction. Furthermore, recent field work demonstrates a negative relationship between elevated temperatures and brook trout reproduction (Robinson et al., 2010).
It is widely accepted that salmonids, including brook trout, exhibit behavioural changes at elevated temperatures. In experimentally heated streams, Chinook salmon had elevated plasma cortisol concentrations and displayed altered behaviours consistent with a stressed state, including erratic swimming, abnormal posture and aggregative behaviour (Quigley and Hinch, 2006). Indeed, a number of studies have shown the propensity for salmonids, including brook trout, to abandon territories during elevated temperatures in order to seek out the refuge of cool-water sources (Wurtsbaugh et al., 1975; Curry et al., 1997; Biro, 1998; Baird and Krueger, 2003; Baird et al., 2006; Breau et al., 2007, 2011). Brook and rainbow trout exhibiting these same behaviours were found to have body temperatures that were cooler than the flow of the main river (Baird and Krueger, 2003). Aggregating at cool water sites led to increased vulnerability to predators and anglers in brook trout (Baird et al., 2006). Some of the variability in HSP70 seen at some sites may be due to variation in the temperature experiences by individual fish. Behavioural changes resulting from elevated temperatures are likely to increase the risk of predation and decrease the ability of brook trout to maximize food resources, contributing to the relationship between temperature and brook trout population density seen in this and other studies.
In addition to informing our basic understanding of how species may respond to a warming climate, the knowledge of such temperature thresholds will also help natural resource managers. This knowledge could be used to identify ‘cool-water’ sites that may be buffered from the harmful effects of climate change. Likewise, it could help to identify populations at risk from rising temperatures. For example, at two sites (Buffam Brook and 4-Mile Brook) temperatures essentially never exceeded threshold values and individuals had consistently lower levels of stress response in both years, while two other sites (Lyons Brook and Pond Brook) exceeded these thresholds and showed consistently elevated stress responses. These latter sites also had relatively low densities of brook trout. Furthermore, our results suggest that the endocrine and cellular stress responses we measured differed with respect to their utility as potential biomarkers for exposure. Expression of HSP70 shows the most promise in this regard. While some individuals from warm sites above the 21.0°C threshold showed little or no expression (possibly as a result of recent immigration from cooler sites or use of cool microhabitats), all of these sites had some individuals with expression well above baseline (in the one most extreme case, all individuals), and essentially none of the fish in cooler sites had HSP70 concentrations above baseline. In contrast, plasma glucose and cortisol concentrations were highly variable among individual fish, even at cooler sites. This variability is likely to be associated with indeterminacy and individual variation in the time scale at which elevated temperatures influence these stress responses.
The time course of physiological responses to thermal stress has an impact on our ability to detect such changes and their utility as an indicator of thermal stress. Gill HSP70 and plasma glucose, but not cortisol, increase following short-term (6 h) exposure of brook trout to high temperature (Fig. 1), whereas after long-term exposure (8–24 days) gill HSP and plasma cortisol are elevated, but plasma glucose is not (J. G. Chadwick Jr and S. D. McCormick, unpublished observations). This differential time course of response may explain why we observed elevated gill HSP70 and plasma cortisol, but not plasma glucose in brook trout in the wild, which had been exposed to elevated temperature for at least several days (Fig. 3).
Previous work has established that there is a potential interaction between the endocrine and cellular stress axes. Cortisol has been shown to induce HSP70 in a number of fish species, though this effect is species and tissue specific and not as robust as the impact of temperature (Deane and Woo, 2011). In rainbow trout (Oncorhynchus mykiss), increased gill HSP70 concentrations were attenuated (but not absent) after exposure to elevated temperature (Basu et al., 2001). Furthermore, the ability of liver to release glucose in response to elevated cortisol is reduced when HSP70 concentrations are high (Boone et al., 2002), which may explain the absence of elevated plasma glucose with temperature in field studies where both plasma cortisol and HSP70 were elevated.
It is of interest to note that there were differences in the magnitude of HSP70 response in the 2 years of study, with higher concentrations at the same average temperature in 2010 than in 2011. There is some indication that prior thermal acclimation can influence the magnitude and/or duration of increases in HSPs (Deane and Woo, 2011; Stitt et al., 2014). However, we did not see any difference in explanatory power when differences between 3 and 10 day running averages were used in our analysis of the relationship between temperature and HSP70. The strong autocorrelation between mean temperature and temperature variation kept us from analysing the latter as a factor in year-to-year or site variation. This difference between years may also be due to other (non-temperature) stressors experienced by these fish in 2010 compared with 2011. Heat shock protein-70 can be induced by a variety of environmental factors (Deane and Woo, 2011), including social stressors and lack of food (Currie et al., 2010). Our results indicate that measures of physiological stress have a threshold response in brook trout and that these responses are related to thermal ecological limits of the species. Heat shock protein-70 appears to be a particularly useful tool to measure thermal stress because it has a very sharp threshold response and large magnitude of response to temperature. Given that the heat shock response appears to be nearly universal in the animal kingdom (Feder and Hofmann, 1999), HSPs may be useful for determining ecological limits in many species. Recent work in brook trout has shown population-specific thermal tolerances that are correlated with induction temperatures of HSP70 (Stitt et al., 2014), further validating its use in determining ecological thresholds. These thermal biomarkers could be used in conjunction with population trend monitoring to justify and evaluate mitigation efforts, including the removal of movement barriers and the preservation and restoration of riparian zones in order to stave off the loss of populations due to a warming climate.
Funding
This research was supported by the National Science Foundation through a Graduate Research Fellowship to Joseph G. Chadwick Jr. Additional support came from the Organismic & Evolutionary Biology Program at the University of Massachusetts Amherst through a student research grant. We thank the USDA Forest Service Northern Research Station and the USDOI Northeast Climate Science Center for support for K. Nislow.
Acknowledgements
We thank Ken Simmons, Craig Lodowsky and the staff of the Sandwich State Hatchery for providing juvenile brook trout. We thank Dave Hallock and Marty Garcia for their help in designing and building our water-heating system. We thank Caleb Slater of MassWildlife for providing temperature and surveying records that allowed us to identify potential field sites. We thank the many people who participated in the field sampling. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Anttila K, Casselman MT, Schulte PM, Farrell AP. (2013) Optimum temperature in juvenile salmonids: connecting subcellular indicators of tissue function and whole-organism thermal optimimum. Physiol Biochem Zool 86: 245–256. [DOI] [PubMed] [Google Scholar]
- Baird OE, Krueger CC. (2003) Behavioral thermoregulation of brook and rainbow trout: comparison of summer habitat use in an Adirondack River, New York. T Am Fish Soc 132: 1194–1206. [Google Scholar]
- Baird OE, Krueger CC, Josephson DC. (2006) Growth, movement, and catch of brook, rainbow, and brown trout after stocking into a large, marginally suitable Adirondack river. N Am J Fish Manage 26: 180–189. [Google Scholar]
- Baldwin NW. (1956) Food consumption and growth of brook trout at different temperatures. T Am Fish Soc 86: 323–328. [Google Scholar]
- Basu N, Kennedy CJ, Hodson PV, Iwama GK. (2001) Altered stress responses in rainbow trout following a dietary administration of cortisol and beta-napthoflavone. Fish Physiol Biochem 25: 131–140. [Google Scholar]
- Biro PA. (1998) Staying cool: behavioral thermoregulation during summer by young-of-year brook trout in a lake. T Am Fish Soc 127: 212–222. [Google Scholar]
- Boone AN, Ducouret B, Vijayan MM. (2002) Glucocorticoid-induced glucose release is abolished in trout hepatocytes with elevated hsp70 content. J Endocrinol 172: R1–R5. [DOI] [PubMed] [Google Scholar]
- Breau C, Cunjak RA, Bremset G. (2007) Age-specific aggregation of wild juvenile Atlantic salmon Salmo salar at cool water sources during high temperature events. J Fish Biol 71: 1179–1191. [Google Scholar]
- Breau C, Cunjak RA, Peake SJ. (2011) Behaviour during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J Anim Ecol 80: 844–853. [DOI] [PubMed] [Google Scholar]
- Carey JB, McCormick SD. (1998) Atlantic salmon smolts are more responsive to an acute handling and confinement stress than parr. Aquaculture 168: 237–253. [Google Scholar]
- Currie S, LeBlanc S, Watters MA, Gilmour KM. (2010) Agonistic encounters and cellular angst: social interactions induce heat shock proteins in juvenile salmonid fish. Proc Biol Sci 277: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry RA, Brady C, Noakes DLG, Danzmann RG. (1997) Use of small streams by young brook trout spawned in a lake. T Am Fish Soc 126: 77–83. [Google Scholar]
- Deane EE, Woo NYS. (2011) Advances and perspectives on the regulation and expression of piscine heat shock proteins. Rev Fish Biol Fisher 21: 153–185. [Google Scholar]
- Dietz TJ, Somero GN. (1993) Species- and tissue-specific synthesis patterns for heat-shock proteins HSP70 and HSP90 in several marine teleost fishes. Physiol Zool 66: 863–880. [Google Scholar]
- Dubeau SF, Pan F, Tremblay GC, Bradley TM. (1998) Thermal shock of salmon in vivo induces the heat shock protein hsp 70 and confers protection against osmotic shock. Aquaculture 168: 311–323. [Google Scholar]
- Dwyer WP, Piper RG, Smith CE. (1983) Brook trout growth efficiency as affected by temperature. Prog Fish Cult 45: 161–163. [Google Scholar]
- Feder ME, Hofmann GE. (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282. [DOI] [PubMed] [Google Scholar]
- Feldhaus JW, Heppell SA, Li H, Mesa MG. (2010) A physiological approach to quantifying thermal habitat quality for Redband Rainbow Trout (Oncorhynchus mykiss gairdneri) in the south Fork John Day River, Oregon. Environ Biol Fish 87: 277–290. [Google Scholar]
- Flebbe PA, Roghair LD, Bruggink JL. (2006) Spatial modeling to project southern Appalachian trout distribution in a warmer climate. T Am Fish Soc 135: 1371–1382. [Google Scholar]
- Fry FEJ, Hart SA, Walker KF. (1946) Lethal temperature relations for a sample of young speckled trout, Salvelinus fontinalis. Univ Toronto Stud Biol Ser 54: 9–35. [Google Scholar]
- Gregory TR, Wood CM. (1999) The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol Biochem Zool 72: 286–295. [DOI] [PubMed] [Google Scholar]
- Hartman KJ, Cox MK. (2008) Refinement and testing of a brook trout bioenergetics model. T Am Fish Soc 137: 357–363. [Google Scholar]
- Hokanson KE, McCormick JH, Jones BR, Tucker JH. (1973) Thermal requirements for maturation, spawning, and embryo survival of brook trout, Salvelinus fontinalis. J Fish Res Board Can 30: 975–984. [Google Scholar]
- Iwama GK, Vijayan MM, Forsyth RB, Ackerman PA. (1999) Heat shock proteins and physiological stress in fish. Am Zool 39: 901–909. [Google Scholar]
- Kajimura S, Hirano T, Visitacion N, Moriyama S, Aida K, Grau EG. (2003) Dual mode of cortisol action on GH/IGF-I/IGF binding proteins in the tilapia, Oreochromis mossambicus. J Endocrinol 178: 91–99. [DOI] [PubMed] [Google Scholar]
- Leal E, Fernández-Durán B, Guillot R, Ríos D, Cerdá-Reverter JM. (2011) Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): a self-feeding approach. J Comp Physiol B 181: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Lund SG, Caissie D, Cunjak RA, Vijayan MM, Tufts BL. (2002) The effects of environmental heat stress on heat-shock mRNA and protein expression in Miramichi Atlantic salmon (Salmo salar) parr. Can J Fish Aquat Sci 59: 1553–1562. [Google Scholar]
- Lund SG, Lund MEA, Tufts BL. (2003) Red blood cell Hsp 70 mRNA and protein as bioindicators of temperature stress in the brook trout (Salvelinus fontinalis). Can J Fish Aquat Sci 60: 460–470. [Google Scholar]
- McCormick JH, Jones BR, Hokanson KE. (1972) Effects of temperature on growth and survival of young brook trout, Salvelinus fontinalis. J Fish Res Board Can 29: 1107–1112. [Google Scholar]
- McCormick SD. (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50: 656–658. [Google Scholar]
- McCormick SD, Shrimpton JM, Carey JB, O'Dea MF, Sloan KE, Moriyama S, Bjornsson BT. (1998) Repeated acute stress reduces growth rate of Atlantic salmon parr and alters plasma levels of growth hormone, insulin-like growth factor I and cortisol. Aquaculture 168: 221–235. [Google Scholar]
- McMahon TE, Zale AV, Barrows FT, Selong JH, Danehy RJ. (2007) Temperature and competition between bull trout and brook trout: a test of the elevation refuge hypothesis. T Am Fish Soc 136: 1313–1326. [Google Scholar]
- Meisner JD. (1990) Potential loss of thermal habitat for brook trout, due to climatic warming in two southern Ontario streams. T Am Fish Soc 119: 282–291. [Google Scholar]
- Meka JM, McCormick SD. (2005) Physiological response of wild rainbow trout to angling: impact of angling duration, fish size, body condition, and temperature. Fish Res 72: 311–322. [Google Scholar]
- Mesa MG, Weiland LK, Wagner P. (2002) Effects of acute thermal stress on the survival, predator avoidance, and physiology of juvenile fall Chinook salmon. Northwest Sci 76: 118–128. [Google Scholar]
- Mommsen TP, Vijayan MM, Moon TW. (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fisher 9: 211–268. [Google Scholar]
- Pankhurst NW, King HR, Ludke SL. (2008a) Relationship between stress, feeding and plasma ghrelin levels in rainbow trout, Oncorhynchus mykiss. Mar Freshw Behav Physiol 41: 53–64. [Google Scholar]
- Pankhurst NW, Ludke SL, King HR, Peter RE. (2008b) The relationship between acute stress, food intake, endocrine status and life history stage in juvenile farmed Atlantic salmon, Salmo salar. Aquaculture 275: 311–318. [Google Scholar]
- Parmesan C, Yohe G. (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- Peterson BC, Small BC. (2005) Effects of exogenous cortisol on the GH/IGF-I/IGFBP network in channel catfish. Domest Anim Endocrinol 28: 391–404. [DOI] [PubMed] [Google Scholar]
- Pickering AD. (1990) Stress and the suppression of somatic growth in teleost fish. Prog Clin Biol Res 342: 473–479. [PubMed] [Google Scholar]
- Pörtner HO. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Quigley JT, Hinch SG. (2006) Effects of rapid experimental temperature increases on acute physiological stress and behaviour of stream dwelling juvenile Chinook salmon. J Therm Biol 31: 429–441. [Google Scholar]
- Rendell JL, Fowler S, Cockshutt A, Currie S. (2006) Development-dependent differences in intracellular localization of stress proteins (hsps) in rainbow trout, Oncorhynchus mykiss, following heat shock. Comp Biochem Physiol Part D Genomics Proteomics 1: 238–252. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Josephson DC, Weidel BC, Kraft CE. (2010) Influence of variable interannual summer water temperatures on brook trout growth, consumption, reproduction, and mortality in an unstratified Adirondack lake. T Am Fish Soc 139: 685–699. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. (2003) Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. [DOI] [PubMed] [Google Scholar]
- Smith TR, Tremblay GC, Bradley TM. (1999) Characterization of the heat shock protein response of Atlantic salmon (Salmo salar). Fish Physiol Biochem 20: 279–292. [Google Scholar]
- Sokolova IM. (2013) Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp Biol 53: 597–608. [DOI] [PubMed] [Google Scholar]
- Steinhausen MF, Sandblom E, Eliason EJ, Verhille C, Farrell AP. (2008) The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J Exp Biol 211: 3915–3926. [DOI] [PubMed] [Google Scholar]
- Stitt BC, Burness G, Burgomaster KA, Currie S, McDermid JL, Wilson CC. (2014) Intraspecific variation in thermal tolerance and acclimation capacity in brook trout (Salvelinus fontinalis): physiological implications for climate change. Physiol Biochem Zool 87: 15–29. [DOI] [PubMed] [Google Scholar]
- Thorpe JE, Miles MS, Keay DS. (1984) Developmental rate, fecundity and egg size in Atlantic salmon, Salmo salar L. Aquaculture 43: 289–305. [Google Scholar]
- Tomanek L. (2010) Variation in the heat shock response and its implication for predicting the effect of global climate change on species' biogeographical distribution ranges and metabolic costs. J Exp Biol 213: 971–979. [DOI] [PubMed] [Google Scholar]
- Vanderboon J, Vandenthillart GEEJ, Addink ADF. (1991) The effects of cortisol administration on intermediary metabolism in teleost fish. Comp Biochem Physiol A Physiol 100: 47–53. [Google Scholar]
- Wehrly KE, Wang LZ, Mitro M. (2007) Field-based estimates of thermal tolerance limits for trout: incorporating exposure time and temperature fluctuation. T Am Fish Soc 136: 365–374. [Google Scholar]
- Wendelaar Bonga SE. (1997) The stress response in fish. Physiol Rev 77: 591–625. [DOI] [PubMed] [Google Scholar]
- Werner I, Smith TB, Feliciano J, Johnson ML. (2005) Heat shock proteins in juvenile steelhead reflect thermal conditions in the Navarro River watershed, California. T Am Fish Soc 134: 399–410. [Google Scholar]
- Wikelski M, Cooke SJ. (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- Wurtsbaugh WA, Brocksen RW, Goldman CR. (1975) Food and distribution of underyearling brook and rainbow trout in Castle Lake, California. T Am Fish Soc 104: 88–95. [Google Scholar]