Small annual temperature fluctuations may limit the plastic responses of tropical fish to thermal stress. We measured the acclimation capacity of an African cichlid to elevated water temperatures by quantifying resting metabolic rate and both thermal and low-oxygen tolerance after short-term exposure to temperatures within and above its natural range.
Keywords: Critical oxygen tension, fish physiology, respirometry, thermal stress, thermal tolerance, tropical fish
Abstract
Tropical inland fishes are predicted to be especially vulnerable to thermal stress because they experience small temperature fluctuations that may select for narrow thermal windows. In this study, we measured resting metabolic rate (RMR), critical oxygen tension (Pcrit) and critical thermal maximum (CTMax) of the widespread African cichlid (Pseudocrenilabrus multicolor victoriae) in response to short-term acclimation to temperatures within and above their natural thermal range. Pseudocrenilabrus multicolor collected in Lake Kayanja, Uganda, a population living near the upper thermal range of the species, were acclimated to 23, 26, 29 and 32°C for 3 days directly after capture, and RMR and Pcrit were then quantified. In a second group of P. multicolor from the same population, CTMax and the thermal onset of agitation were determined for fish acclimated to 26, 29 and 32°C for 7 days. Both RMR and Pcrit were significantly higher in fish acclimated to 32°C, indicating decreased tolerance to hypoxia and increased metabolic requirements at temperatures only slightly (∼1°C) above their natural thermal range. The CTMax increased with acclimation temperature, indicating some degree of thermal compensation induced by short-term exposure to higher temperatures. However, agitation temperature (likely to represent an avoidance response to increased temperature during CTMax trials) showed no increase with acclimation temperature. Overall, the results of this study demonstrate that P. multicolor is able to maintain its RMR and Pcrit across the range of temperatures characteristic of its natural habitat, but incurs a higher cost of resting metabolism and reduced hypoxia tolerance at temperatures slightly above its present range.
Introduction
With the rise in global mean temperatures (Root et al., 2003; IPCC, 2013) species' persistence may depend on their capacity to shift their distributions to more favourable environments, acclimate via phenotypic plasticity or adapt to changes within their current environment. Their vulnerability to rising temperatures may also depend on their mechanism of temperature regulation. Given that an ectotherm has poor insulation and low rates of internal heat production, its body temperature is primarily controlled by environmental heat sources (McNab, 2002). Thus, its overall performance can be greatly influenced by environmental temperature, and its ability to carry out vital functions over a range of temperatures is defined as its performance window (Huey and Stevenson, 1979). This window results from temperature-dependent trade-offs at all levels of functioning (Pörtner, 2010); beyond the upper and lower critical limits of their thermal windows (CTMax and CTMin, respectively), ectotherms will start to exploit their passive range of tolerance but can do so only for a limited time. While increasing temperature can have some positive effects on growth, this only occurs within an optimal range, beyond which growth and other important processes are compromised due to protein denaturation and enzyme inhibition, gradually reducing long-term fitness (Pörtner, 2001, 2010; Pörtner and Knust, 2007).
Given the consequences of environmental temperature on fitness-related traits, an ectotherm's thermal window should strongly overlap with the thermal regimes of its environment (Huey and Kingsolver, 1989; Deutsch et al., 2008). Indeed, many interspecific and intraspecific studies show that the position of the performance window is generally correlated with environmental temperature in ectotherms (Huey and Stevenson, 1979; Huey and Kingsolver, 1989; Tewksbury et al., 2008). However, given shifting thermal regimes owing to climate change, ectotherms, such as fishes, are at risk of being forced to perform at suboptimal temperatures or temperatures beyond their thermal windows. Climate change is expected to favour species with wider thermal windows or thermal generalists over those with narrower windows or thermal specialists (Angilletta et al., 2002; Pörtner and Farrell, 2008; Magozzi and Calosi, 2015). Thus, tropical ectotherms may be particularly sensitive to climate warming relative to temperate species because they experience comparatively small annual temperature fluctuations, often show narrow thermal windows and seem to live relatively close to their maximal thermal tolerance (Janzen, 1967; Stillman, 2003; Deutsch et al., 2008; Tewksbury et al., 2008; Pörtner and Peck, 2010). Small-scale plastic responses (i.e. acclimation) in individuals may help to buffer these thermal stress effects long enough to allow for genetic adaptation within the population or species (Hoffmann and Sgrò, 2011). However, the less seasonal thermal environment of tropical ectotherms is likely to limit their acclimation response relative to taxa from temperate latitudes, a trend supported in salamanders (Feder, 1982), Sceloporus lizards (Tsuji, 1988) and porcelain crabs (Stillman, 2003), but not in European diving beetles (Calosi et al., 2008). In the present study, we measured the acclimation capacity of a widespread tropical fish by quantifying resting metabolic rate and both thermal and low-oxygen tolerance after short-term exposure to temperatures within and above its natural range.
Metabolic rate in fishes and other ectotherms is strongly affected by and tends to increase with temperature (Gillooly et al., 2001; Brown et al., 2004); however, sensitivity to temperature change varies across and within species. In many studies, an increase has been observed in both the standard metabolic rate (SMR, measured as the lowest rate measured over a full diel cycle) and resting or routine metabolic rate (RMR, measured while the fish is completely at rest) in fish with increasing temperature acclimation (SMR: Ott et al., 1980; Schurmann and Steffensen, 1997; Claireaux and Lagardère, 1999; Lapointe et al., 2014; and RMR: Johnston et al., 1991; Clarke and Johnston, 1999; Nilsson et al., 2010; Rummer et al., 2014); however, other studies report reduced metabolic rates with longer acclimation periods (RMR: Donelson et al., 2011, 2012; Sandersfeld et al., 2015; and SMR: Norin et al., 2014), although not always (Nilsson et al., 2010). When a fish reaches a critically high temperature (CTMax) physiological disorganization occurs, leading to loss of equilibrium (Becker and Genoway, 1979; Lutterschmidt and Hutchison, 1997). The CTMax has demonstrated high acclimation capacity with rising temperatures in numerous fish species, including, as examples, shortnose sturgeon Acipenser brevirostrum (Zhang and Kieffer, 2014) and platyfish Xiphophorus maculatus (Prodocimo and Freire, 2001). While a high upper thermal tolerance can aid in short-term persistence at elevated temperature, increases in other parameters, such as SMR or RMR, can limit energy availability for long-term functioning, such as feeding, growth and reproduction, highlighting the importance of integrative studies (Pörtner et al., 2006).
In aquatic systems, the effect of thermal stress on metabolism is closely tied to oxygen availability in water. Many fishes are oxyregulators, keeping their oxygen consumption more or less constant over a wide range of oxygen availability in the water (, oxygen partial pressure); however, when becomes too low to meet oxygen demands, the metabolic rate of the fish will shift from physiological regulation to oxyconformation, transferring to anaerobic metabolism, a level referred to as the critical oxygen tension (Pcrit; Ultsch et al., 1978, 1999; Yeager and Ultsch, 1989). The difference between the Pcrit and ambient represents the excess amount of oxygen available to exploit for aerobic metabolism. Furthermore, Pcrit is predicted to increase with metabolic rate, reflecting the temperature dependence of hypoxia tolerance. Thus, a lower Pcrit may be beneficial in that it may counteract thermally induced hypoxaemia and improve thermal tolerance (Pörtner, 2010). Indeed, hypoxia tolerance and its temperature sensitivity may be a useful predictor of tropical fish persistence with rising temperatures (Sørensen et al., 2014). Specifically, it has been suggested that during severe hypoxia fish survival is no longer determined by its capacity to take up sufficient oxygen, because there is no longer enough oxygen present in the surrounding waters; therefore, the scope for producing energy anaerobically (termed anaerobic scope) could become key to survival (Nilsson and Östlund-Nilsson, 2008). Indeed, temporary hypoxic events in aquatic systems (i.e. overnight hypoxia) are becoming more frequent with global warming as the formation of algal blooms is accelerated and ectothermic respiration rates increase (D'Avanzo and Kremer, 1994; Vaquer-Sunyer and Duarte, 2008; Friedrich et al., 2014). Therefore, understanding relationships between thermal and hypoxia tolerances in fishes may be critical in predicting the response to changing environments (Deutsch et al., 2015).
Here, we combine field and laboratory data to demonstrate the temperature sensitivity and short-term acclimation capacity of resting metabolic rate (RMR), critical oxygen tension, critical thermal maximum and thermal avoidance behaviour in a widespread tropical freshwater fish, the African cichlid Pseudocrenilabrus multicolor victoriae from Lake Kayanja, Uganda, where this fish lives near the upper range of its thermal distribution. Temperature treatments were based on long-term data on water temperature in Lake Kayanja, diurnal temperature profiles measured during the present study and projected climate-warming scenarios for the region. The RMR and Pcrit were measured in P. multicolor acclimated for 3 days in our field laboratory to four temperature treatments both within and slightly above this population's natural thermal range. In a separate laboratory experiment on fish transferred live from Uganda to McGill University, we measured CTMax after a 7 day acclimation to increased temperatures. Together, these data allowed us to investigate the following: (i) to test for evidence of short-term thermal acclimation on metabolic rate and thermal tolerance; (ii) to detect the temperature dependence of hypoxia tolerance; and (iii) to ask whether this equatorial species is living close to the edge of its thermal window.
Materials and methods
Study site, species and specimen collection
Pseudocrenilabrus multicolor victoriae is a small African cichlid, found throughout the Lake Victoria basin of East Africa across a wide range of dissolved oxygen and water temperature regimes (Chapman et al., 2000, 2002, 2008; Reardon and Chapman, 2009). This species is a maternal mouthbrooder, with the eggs and developing young held in the female's mouth for 13–21 days (Reardon and Chapman, 2010b). Across habitats in Uganda, P. multicolor persists under a broad thermal range from 18.1°C in the dense interior of papyrus swamps to 30.8°C in warm ecotonal waters of lake systems (Chapman et al., 2002; Friesen et al., 2012). Thus, this species provides a useful model both for understanding the mechanisms underlying broad thermal distributions and for testing specific predictions related to thermal capacity in tropical ectotherms. This study focused on one population of P. multicolor from the wetland–open water ecotone of Lake Kayanja, Uganda, a population that persists at the upper end of the thermal distribution for this species and is therefore likely to be the most susceptible of the populations in this region to further increase in water temperature.
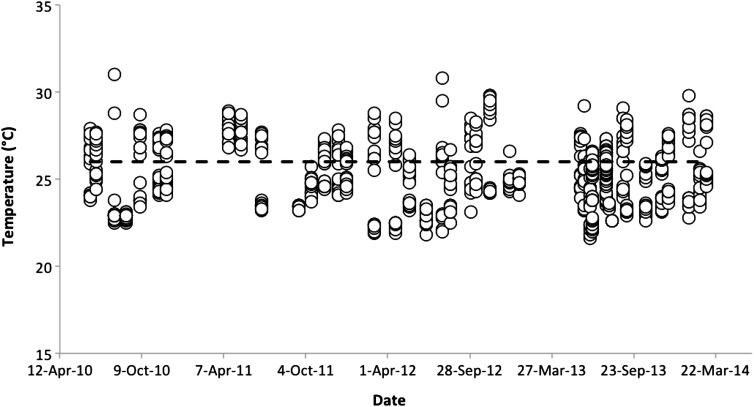
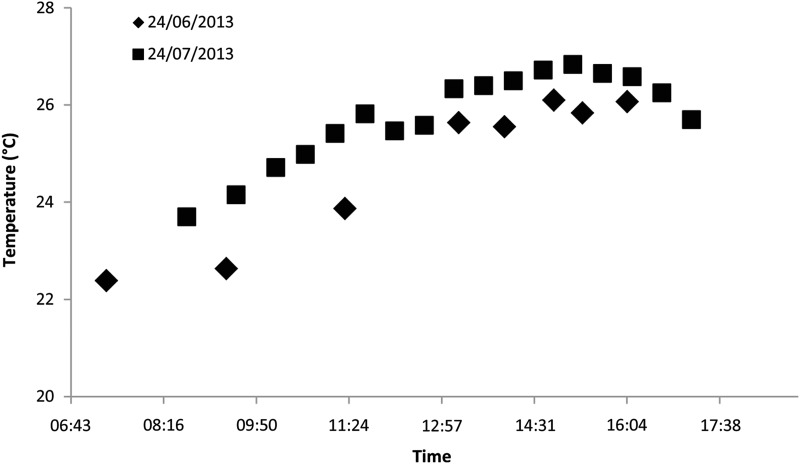
Lake Kayanja (0°16′60.00″S, 31°52′0.00″E) is one of the four small lakes (1.25 km2 in area, with a mean depth of 2.6 m; Sharpe et al., 2012) in the Nabugabo region of Uganda, lying close to Lake Victoria. We defined the current thermal regime of Lake Kayanja to determine the range of temperatures that this population of P. multicolor is likely to experience in its natural habitat. We used two approaches: 4 years of average monthly values; and diurnal profiles to characterize shifts in water temperature over the day. We also recorded the dissolved oxygen concentration (in milligrams per litre) to characterize the oxygen availability in the natural habitat. Two diurnal profiles were conducted a month apart during the fish collection season at Lake Kayanja (24 June 2013 and 24 July 2013). Water temperature and dissolved oxygen were measured at five sites in the lake throughout the day beginning in the morning shortly after sunrise and ending just before sunset. The water temperature data were used to help determine ecologically relevant temperature treatments for acclimations and subsequent physiological experiments with this species. Long-term data show that the water temperature in Lake Kayanja over the past 4 years has ranged between 21.6 and 31.0°C, averaging 25.3°C, with no apparent increase over this time period (Fig. 1). During this same 4 year period, mean dissolved oxygen averaged 7.09 mg l−1. During the two diurnal profiles, 150 measurements were taken at five sites over ∼10 h (∼07.00–15.30 h). Temperature ranged between 22.0 and 27.3°C, and dissolved oxygen remained high (mean 8.09 mg l−1), ranging between 7.10 and 9.03 mg l−1. The mean daily temperature over these 2 days was 25.4°C, rising between sunrise and early afternoon and then plateauing near 26°C until sunset (Fig. 2).
Figure 1:
Monthly water temperature in Lake Kayanja, Uganda from 2010 to 2014. Measurements were made at five sites within the lake, twice daily (once in the morning and once in the afternoon). The dashed line at 26°C represents the midday average used in resting metabolic rate and critical tension experiments, as well as the minimal acclimation temperature used in critical thermal maximum experiments.
Figure 2:
Mean water temperature measurements from five sites in Lake Kayanja, Uganda obtained during two diurnal profiles completed during fish collection in 2013.
Based on these data, the temperature treatments used for this study were 23, 26, 29 and 32°C. These specific temperature treatments were used in order to determine how the metabolism, hypoxia tolerance and thermal tolerance of P. multicolor fluctuates over a range that incorporates the upper end of its existing temperature range as well the lower end of projected increases. Given the relatively wide range of recorded temperatures, it is possible that this population might have been temporarily exposed to temperatures at or very near to 32°C at some point. However, only 0.2% (n = 2) of the total 896 individual temperature measures in Lake Kayanja between 2010 and 2014 fell above 30.0°C. Thus, it is likely that the highest temperature treatment used in the present study (32°C) represents a temperature not naturally experienced by this population for any significant duration.
To test for short-term acclimation effects on RMR and Pcrit, adult male P. multicolor specimens (n = 40) were captured live from Lake Kayanja over 2 months using metal minnow traps. In this study, we did not use brooding females because the metabolic rate is elevated in brooders relative to non-brooders and males (Reardon and Chapman, 2010a). Individuals were transferred to facilities on the shores of Lake Nabugabo, ∼9 km from Lake Kayanja, where we have a small research station. Fish were held for a minimum of 24 h prior to temperature assignment and acclimation. As RMR is known to scale with body size (Clarke and Johnston, 1999), a range of fish lengths and weights were used (2.4–5.3 cm; 0.60–4.31 g; Table 1). During the initial 24 h holding period, fish were held in large, aerated coolers at 26°C (midday average during sampling period) using individual aquarium heaters and randomly assigned to a temperature treatment. Fish were then transferred to smaller coolers, and water temperatures were progressively increased or reduced by 1°C every hour until the target temperature was reached and then held for a minimum of 3 days. Temperatures were monitored four times daily using a hand-held probe to ensure that fluctuations were kept to a minimum (±0.3°C), and fish were fed Tetramin tropical fish flakes once daily to satiation. Although a longer acclimation period may facilitate compensatory mechanisms to offset thermal sensitivities, we used a 3 day acclimation to evaluate short-term physiological compensation abilities. The time frame was appropriate for the logistics of our field setting and informed by a laboratory study on P. multicolor that had been transferred live to McGill University (Canada) to establish effects of acclimation time. In that experiment, groups of four fish were acclimated to temperatures 5°C above ambient for 3, 7 or 14 days, and we detected no significant difference in the RMR or Pcrit (McDonnell, 2015). We opted to conduct the present experiment at our field laboratory to permit fish to be transferred directly from their natural field temperature into the acclimation design.
Table 1:
Mean ±1 SEM mass and standard lengths of Pseudocrenilabrus multicolor victoriae from temperature treatments used in experiments
| Experiment | RMR and Pcrit |
CTMax |
||||
|---|---|---|---|---|---|---|
| Temperature treatment (°C) | n | Mass (g) | SL (cm) | n | Mass (g) | SL (cm) |
| 23 | 10 | 1.89 ± 0.28 | 3.64 ± 0.18 | |||
| 26 | 10 | 1.99 ± 0.36 | 3.61 ± 0.26 | 7 | 2.45 ± 0.44 | 3.92 ± 0.24 |
| 29 | 9 | 1.81 ± 0.30 | 3.72 ± 0.17 | 7 | 2.73 ± 0.52 | 3.99 ± 0.23 |
| 32 | 11 | 2.24 ± 0.33 | 3.94 ± 0.23 | 7 | 2.15 ± 0.26 | 3.78 ± 0.14 |
| Overall | 40 | 1.99 ± 0.16 | 3.73 ± 0.11 | 21 | 2.44 ± 0.24 | 3.89 ± 0.12 |
Abbreviations: CTMax, critical thermal maximum; Pcrit, critical oxygen tension; RMR, resting metabolic rate; and SL, standard length.
Resting metabolic rate and critical oxygen tension
Minimum metabolic rate (MO2Min) is measured when a fish is in a post-absorptive state, during which resources are being used only for maintenance, and provides the best estimation of true basal metabolic rate (Fry, 1971; Brett and Groves, 1979; Clarke, 1993; Schurmann and Steffensen, 1997; Clark et al., 2013). Many studies have used RMR or SMR as the best estimations for an absolute minimal rate (Grantner and Taborsky, 1998; Clarke and Johnston, 1999; Nilsson et al., 2009, 2010; Gardiner et al., 2010; Donelson et al., 2011). We chose to measure RMR because field logistics (availability of power) did not allow for in-chamber acclimations longer than a few hours, ruling out the possibility of recording full diel cycles in the respirometer. Much like SMR, measuring RMR requires a post-absorptive state, but allows for low levels of spontaneous activity (Jobling, 1994).
While it is established that a fish's metabolic rate can be strongly affected by temperature, sensitivity varies across and even within species. An index of sensitivity can be described by the Q10 coefficient, which measures the change in metabolic rate over a given (typically 10°C) temperature range (McNab, 2002). We calculated RMR Q10 values for each temperature increment and across the full temperature range.
Resting metabolic rate measurements were obtained using intermittent flow-through respirometry equipment and software (Swim tunnel and AutoResp 2.0; Loligo Systems, Tjele, Denmark), as previously used to measure resting routine metabolic rate in P. multicolor (Reardon and Chapman, 2010b). The system consisted of a circular, 149 ml glass chamber in which the fish was placed, with a flush pump connected to one end, a motor and speed control instrument connected to the other, and a separate temperature-regulation unit, contributing to a total system volume of 157.0 ml. The system was filled with NovAqua (5 ml l−1) treated water and closed to estimate metabolic rate by following the progression of dissolved oxygen decline. The dissolved oxygen level in the ambient tank was monitored by a galvanic cell oxygen probe (dipping probe; Loligo Systems; 0–100% oxygen) and maintained by an air bubbler to achieve a constant saturation above 90%. The oxygen in the chamber containing the fish was measured using a fibre-optic oxygen probe (Fibox 3; Loligo Systems) and oxygen sensor (oxygen sensor spot; PreSens, Regensburg, Germany). The 100% oxygen setting was recalibrated prior to each trial. The 0% oxygen setting for oxygen probes was recalibrated twice a week with oxygen-free water (as prepared in a solution of 10 g Na2SO3 l−1). Water temperature was monitored continuously by a temperature probe (PT1000; Loligo Systems) and maintained by individual aquarium heaters connected to a temperature-control unit (TMP-REG; Loligo Systems). When the system was open (‘Flush’ setting), the glass chamber was flushed continuously with the aerated water from the ambient tank. Separate experiments were conducted to measure the build-up of waste products during respirometry experiments. The fish were starved for at least 24 h prior to experimentation to ensure a post-absorptive state, and trials were carried out at approximately the same time daily (Clark et al., 2013).
Fish were permitted to rest, covered by an opaque barrier, in the chamber on ‘Flush’ for at least 30 min before beginning the acclimation/loop period and Pcrit trial. During acclimation, the system was set to function on a loop setting: closed to allow oxygen saturation to drop to ∼80%, then flushed until levels return to 95–100%. This acclimation period lasted for a minimum of 2 h, with longer periods if MO2 rates indicated agitation (Nilsson et al., 2009; Gardiner et al., 2010). It was shown in an earlier study using similar techniques that P. multicolor typically recovers from handling stress within 2 h (Reardon and Chapman, 2010a). Consumption rates typically declined with time during this looping period as the fish recovered from handling stress. Once metabolic rate had stabilized to show <10% difference in oxygen consumption between loops (determined by real-time MO2 calculations performed by AutoResp software during acclimation and throughout the trial), the chamber was closed and oxygen was allowed to drop to near-0% levels until the fish reached an obvious critical tension (Pcrit). The RMR values were obtained from the start of this Pcrit trial, measuring MO2 until the oxygen concentration dropped to ∼80% (see ‘Data preparation’). The duration of the Pcrit trial was determined by observing the oxygen consumption vs. time graph in the AutoResp software so as to pinpoint the point of conversion from regulation to conformation (Pcrit was later determined precisely using a modified Yeager and Ultsch program; see ‘Data preparation’), informed by reading MO2 values calculated by the software every 10 min. Afterwards, the chamber was set to ‘Flush’, and the fish was allowed to recover for 30 min before being removed from the chamber, at which point it was weighed (in milligrams) and measured [standard length (SL); in centimetres) before being returned to holding conditions.
Control trials with an empty respirometer were run after the experimental trials (except in the occasional case where power was not available) in order to establish background oxygen consumption rates at each experimental temperature. Average control consumption rates tended to increase with water temperature, but were similar within temperature treatments and were subtracted from values obtained during metabolic trials in order to establish true RMR rates.
Critical thermal maximum
To evaluate short-term thermal acclimation effects on CTMax, male and female P. multicolor from Lake Kayanja (n = 21) were transferred live to our aquatic facility at McGill University and acclimated to three different temperature treatments (26, 29 and 32°C, n = 7 per treatment) for 7 days (at a rate of 1°C/h). Fish were held in captivity for 6 months prior to the experiment (at ∼22–24°C) and were therefore more removed from the field setting. Long-term exposure to slightly cooler and more stable water temperatures than Lake Kayanja may have altered their thermal sensitivities, precluding direct comparisons of the CTMax and RMR/Pcrit experiments. However, the CTMax experiment should be robust as an indicator of acclimation capacity of thermal tolerance. The longer (7 day) acclimation period allowed the experimental protocol to be initiated within a 1 week period for all fish, because only one or two fish could be measured per day. The CTMax was measured on males and non-brooding females following the critical thermal methodology (CTM) described in earlier studies, by gradually increasing the water temperature in the experimental tank at a rate low enough not to shock the fish but high enough to disallow for acclimation (Cox et al., 1974; Lutterschmidt and Hutchison, 1997; Fangue et al., 2006; Chen et al., 2013). After being starved for 24 h, fish were transferred to the experimental tank at their treatment temperature and allowed to acclimate for a minimum of 2 h. The tank was covered with an opaque barrier, and the fish were monitored during acclimation and the CTM trial via webcam in order to minimize disturbance. The experimental tank was supplied with constant aeration to maintain high dissolved oxygen levels (>95% saturation) throughout.
During the trial, individuals were subjected to a constant (0.3°C/min) increase in water temperature that was controlled, monitored and recorded by a temperature-control unit and software (TMP-REG, AutoResp; Loligo Systems). Every individual tested displayed a similar behaviour; during the 2 h acclimation and the beginning of the trial, the fish would rest near cover under the mesh surrounding the circulating pump. As temperatures continued to increase, eventually the fish would emerge and begin swimming around the confines of the tank in a quick, agitated manner. We interpreted this as the fish displaying apparent avoidance behaviour to search for a more favourable thermal habitat, similar to those observed in hypoxia studies (Petersen and Petersen, 1990; Herbert and Steffensen, 2006; Chapman and McKenzie, 2009). The time that this behaviour began was noted, and the exact temperature at that time point was obtained after the trial from the software output. The temperature at the onset of this behaviour, which we refer to as agitation temperature, was also re-confirmed by checking the time-stamped video footage of each trial. The trial was continued until loss of equilibrium, at which point the temperature was recorded and CTMax was designated. At this point, fish were removed from the experimental tank and transferred to a holding tank held at the original treatment temperature and were allowed to recover. After recovery, each individual's mass and SL were measured, and the fish was returned to its original tank.
Data preparation
A modified Yeager and Ultsch program (Reardon and Chapman, 2010a) was used to convert oxygen (percentage saturation) and temperature measures from the AutoResp software output to (in millimetres of mercury), used in calculating Pcrit values, and oxygen concentration (in milligrams per litre) for metabolic rate values. Metabolic rates were recorded during the looping acclimation period; however, the final RMR value was calculated using the start (∼10–15 min) of the Pcrit trial (between 100 and 80% O2 saturation) to standardize methods among trials. As some fish required a prolonged acclimation period, and fish from warmer temperatures underwent shorter ‘Measure’ periods owing to a higher respiration rate, calculating RMR from acclimation loops would have entailed averaging a different number of loops from each individual. However, RMR values calculated from this initial slope were typically very similar (P = 0.732) and highly correlated (r2 = 0.929) with those measured during the last 30 min of the acclimation period.
Statistical analyses and calculations
ANCOVA was used to detect for differences in RMR values among temperature treatments, with body mass as the covariate. There was no difference in the slopes of the bi-logarithmic relationship between RMR and body mass (F3,32 = 1.029, P = 0.393); therefore, the interaction term between treatment and body mass was removed from the model. Both RMR and body mass were logarithmically transformed. The Sidak post hoc test was used to test for differences between any two temperatures, and estimated marginal means (mean values adjusted to the common body mass of all groups) were derived from the ANCOVA model. The Q10 values were calculated by applying the Van't Hoff equation to average mass-adjusted mean RMR values (McNab, 2002) between the 23°C and 32°C treatment groups:
where V2 and V1 represent the metabolic rates at temperatures T2 and T1, respectively. Temperature effects on Pcrit were initially tested with ANCOVA; and ANOVA was applied after determining in the full model that body size was not a significant covariate, nor was the interaction between body mass and temperature (P = 0.185 and P = 0.587, respectively) and therefore these terms were removed from the final model. ANOVA, with sex and temperature as fixed factors and acclimation tank as a random factor, was used to detect acclimation effects on CTMax. SPSS software was used for all statistical analyses (IBM SPSS Statistics, 21.0.0), and all assumptions of the chosen statistical tests were met.
Results
Resting metabolic rate
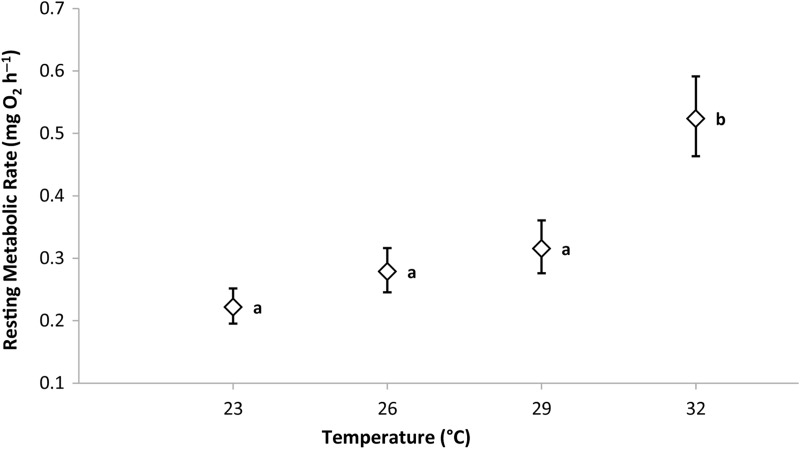
Both temperature and log body mass (covariate) were highly significant and therefore included in the final model for RMR (temperature: F3,35 = 8.608, P < 0.001; body mass: F1,35 = 20.676, P < 0.001). The RMR did not differ significantly among 23, 26 and 29°C but increased dramatically after acclimation to 32°C (Sidak post hoc test, P < 0.001; Fig. 3). Adjusted to the mean common body mass of 1.76 g, the mean RMR of the group tested at 23°C was 0.22 mg O2 h−1 and more than doubled to 0.52 mg O2 h−1 at 32°C.
Figure 3:
Resting metabolic rate (RMR) of Pseudocrenilabrus multicolor victoriae during four temperature treatments. Means are estimated marginal means adjusted to the common mean body mass (1.76 g) with standard errors calculated from ANCOVAs. Symbols that share the same letter are not significantly different (Sidak post hoc tests).
The Q10 value was 2.61 for RMR over the entire 9°C experimental range for this population of P. multicolor. Values for Q10 were also calculated over each 3°C range between treatments, with the caveat that significant differences in RMR were not detected between 23 and 29°C. These Q10 values ranged from 1.53 between 23 and 26°C, to 2.13 between 26 and 29°C, to 5.41 between 29 and 32°C.
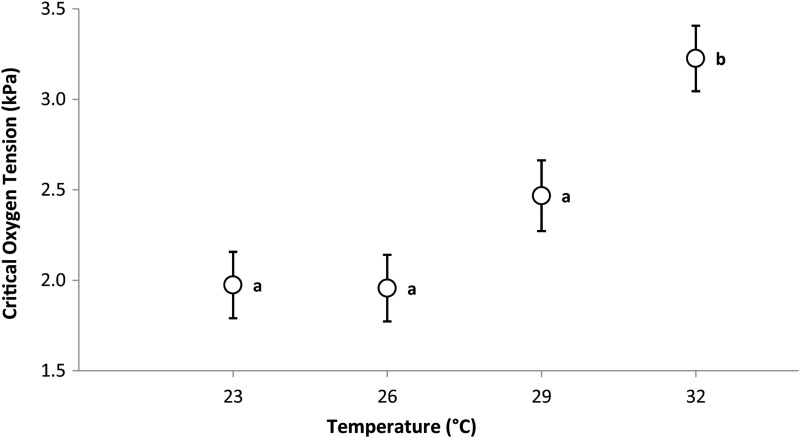
Critical oxygen tension
Temperature was highly significant in the corrected model (F3,36 = 12.279, P < 0.001). The mean Pcrit of the group acclimated to 32°C was significantly higher than those from all three other treatments, which did not differ significantly from one another (Sidak post hoc tests; Fig. 4).
Figure 4:
Mean critical oxygen tension (Pcrit) of P. multicolor during four temperature treatments with standard errors calculated from ANOVAs. Symbols that share the same letter are not significantly different (Sidak post hoc tests).
Critical thermal maximum and agitation temperature
There was no evidence for an increase in agitation temperature with acclimation temperature (F2,17 = 1.06, P = 0.368) and no effect of sex (F1,17 = 2.24, P = 0.153; Fig. 5). However, the mean agitation temperature from all groups (∼38.1°C) was notably lower than CTMax, which may represent the temperature at which behavioural avoidance sets in and therefore a temperature of ecological relevance. In the final model that included temperature, sex and their interaction, only acclimation temperature was significant. The CTMax increased significantly (F2,15 = 158.54, P < 0.001) with each increase in acclimation temperature (Fig. 5).
Figure 5:
Mean ±1 SEM temperature at which critical thermal maximum (black bars) and agitation (white bars) occurred for P. multicolor from three temperature acclimation treatments. Symbols that share the same letter are not significantly different (ANOVA, Sidak post hoc tests).
Discussion
Resting metabolic rate and critical oxygen tension
The mean mass-adjusted RMR of fish acclimated to 32°C was significantly higher than the RMR measured in all lower temperature treatments. Interestingly, RMR showed no significant differences between 3°C temperature increments from 23 to 29°C, temperatures already experienced naturally and frequently in this populations' present thermal regime. While RMR is expected to exhibit a predictable increase in response to increasing temperature and did appear to rise over the tested range (Fig. 3), the Q10 value between the two highest treatments (5.41) in P. multicolor was dramatically higher than general predicted values for teleost fish (∼2.0–3.0, with tropical species falling onto the higher end of that range; McNab, 2002; Seifert and Chapman, 2006) and notably higher than those calculated between the lower temperature treatments. This ability of P. multicolor to maintain a relatively stable RMR over 23–29°C may reflect physiological, biochemical and/or molecular mechanisms of compensation (Bullock, 1955). As observed from the diurnal temperature data (Fig. 2), this population can be exposed to a wide range of temperatures throughout the day (∼5°C on a cloudy day, presumably more on a warmer day); thus, the ability to maintain a stable RMR throughout this range may be beneficial. This suggests that this population of P. multicolor may have optimized maintenance metabolic costs within this range, as compensated for by specific molecular processes, signifying that the fish are well adapted to their present thermal environment (Hazel and Prosser, 1974). The dramatic increase in RMR at 32°C indicates an increase in the cost of maintenance functioning at temperatures only slightly (∼1°C) above the natural thermal range of this population of P. multicolor.
A higher RMR may facilitate rapid growth in conditions that can offset the higher costs of RMR (e.g. high food availability/accessibility, low predation; Burton et al., 2011); however, a high RMR may also induce fitness costs. For example, overwintering juvenile Atlantic salmon (Salmo salar L.) lost energy reserves more quickly when no shelter was available, but this loss was less pronounced in fish that possessed a relatively lower RMR (Finstad et al., 2007). In contrast, even if a low RMR is maintained at high temperatures, this acclimation could also come at the cost of fitness-related traits (Sandersfeld et al., 2015). Some tropical fish reared at temperatures above their environmental temperature range have shown reduced growth and decreased reproductive performance compared with conspecifics reared at lower temperatures, despite showing no significant reduction in metabolic performance (Munday et al., 2008; Donelson et al., 2011, 2014). Other tropical fish held at 29°C [Doederlein's cardinalfish (Ostorhinchus doederleini) and lemon damselfish (Pomacentrus moluccensis)] showed a significant increase in RMR after acute exposure to 32°C, an effect that was not reduced by a longer acclimation time at this high temperature (Nilsson et al., 2010).
A key measure of metabolic performance in fishes and other aquatic ectotherms is aerobic scope (AS), defined as the increase in oxygen consumption of the fish from its standard or resting to maximal metabolic rate (MMR, measured at critical swim speed). Aerobic scope is hypothesized to be tightly linked to the thermal window [e.g. (Pörtner, 2002; Pörtner and Knust, 2007; Farrell et al., 2008), cf. (Gräns et al., 2014; Norin et al., 2014)], described in a theoretical framework known as oxygen- and capacity-limited thermal tolerance (Pörtner and Knust, 2007; Pörtner, 2010). At increased temperatures, oxygen- and capacity-limited thermal tolerance predicts that oxygen delivery systems will not be able to keep pace with the increase in oxygen demands, resulting in a reduced AS of resting metabolism, resulting in a decline in AS (Pörtner and Knust, 2007). This decline in AS is thought to occur because of a greater increase in RMR relative to MMR. For example, in response to thermal acclimation to increased water temperature, several species of coral reef fishes were unable to increase their MMR, while their RMR increased, resulting in an overall decrease in aerobic scope (Nilsson et al., 2009). Thus, the dramatic increase in RMR in P. multicolor in response to acclimation to 32°C may compromise the AS of this population, although we did not estimate MMR in study.
In P. multicolor from Lake Kayanja, hypoxia tolerance, as estimated by Pcrit, decreased at the highest acclimation temperature. However, as would be expected from this species based on other studies demonstrating its high tolerance to hypoxia (Chapman et al., 2002; Reardon and Chapman, 2010b), all fish maintained their RMR down to very low , with groups acclimated to 23 and 26°C reaching 9.6% O2 saturation (2.0 kPa) and groups from 29 and 32°C reaching 11.9% (2.4 kPa) and 16.4% (3.2 kPa), respectively. The Pcrit is predicted to increase with metabolic rate, reflecting the temperature dependence of hypoxia tolerance (Pörtner, 2010; Deutsch et al., 2015); however, experimental exploration of this pattern is still weak, and results are inconsistent. Some studies have shown an increase in Pcrit with temperature (Blažka, 1958; Schurmann and Steffensen, 1997). Others have found no significant increase, indicating that some species may be able to maintain their metabolic rate at low , despite a substantial increase in their RMR at high (Ott et al., 1980; Yamanaka et al., 2013). However, a recent study that measured Pcrit and RMR in two species of coral-reef gobies, Gobiodon histrio and Gobiodon erythrospilus, after a 3–6 day temperature acclimation, found results strikingly comparable to ours, i.e. a significant increase in both parameters between 29- and 33°C-acclimated groups (Sørensen et al., 2014). Likewise, in the Doederlein's cardinalfish and lemon damselfish, Nilsson et al. (2010) found an increase of 79 and 23% in Pcrit, respectively, when fish were acutely exposed to a 3°C temperature increase (29 vs. 32°C), an increase that was not modulated by longer acclimation periods (10 or 22 days). Our results showed a 31% increase in Pcrit between the last two temperature treatments. This elevated Pcrit at the highest temperature treatment in P. multicolor is indicative of a decreased hypoxia tolerance during thermal stress, which is especially important as this species persists under extreme hypoxia in some parts of its natural range (Chapman et al., 2002; Crispo and Chapman, 2008, 2010; Reardon and Chapman, 2010a). Therefore, exploring fish performance below Pcrit may also be a good indicator of persistence under warming temperatures, especially in groups that frequently experience hypoxic conditions. Local adaptation in hypoxia-adapted populations may facilitate a wider thermal window and mitigate the effects of temperature on Pcrit, again emphasizing the importance of interpopulational studies of thermal sensitivities in eurytopic species such as P. multicolor.
Critical thermal maximum and agitation
It has been argued that the upper critical limit of the thermal window is determined by the inability of the cardiorespiratory system to meet increased tissue oxygen demands, which frequently results in an observed decline in aerobic scope at high temperatures (Pörtner, 2001, 2002). However, a recent study subjecting European sea bass to anaemia by reducing haematocrit (thereby, directly lowering oxygen delivery capacity), found that individuals were able to increase their cardiac output to compensate for the rise in metabolic demands at increased temperatures, resulting in only a minimal reduction in CTMax (Wang et al., 2014). In our study, CTMax increased in a linear fashion with temperature treatment, a trend that has been observed in other acclimation studies (Prodocimo and Freire, 2001; Zhang and Kieffer, 2014) and suggests that P. multicolor can adjust its thermal tolerance, at least to some degree, during short-term exposure to high temperatures. However, it is possible that increased thermal tolerance may come at a cost to other functional traits. A review focusing on trade-offs occurring during thermal adaptation points to the importance of developing integrative and large-scale thermal tolerance models that incorporate various levels of biological organization (Pörtner et al., 2006). Indeed, integrative studies may be most ecologically relevant, because CTMax represents a breakdown of whole organismal functioning, but organ systems required for fitness-related activities, such as predator avoidance, are expected to be impacted negatively before CTMax is reached (Anttila et al., 2013; Muñoz et al., 2014).
Agitation temperature, although not generally measured in CTMax experiments, may represent an ecologically significant trait, which is likely to reflect avoidance behaviour. Interestingly, agitation temperature did not increase with acclimation treatment. For example, fish acclimated to 26°C became agitated at 37.7°C and lost equilibrium shortly afterwards, at 38.5°C, whereas fish acclimated to 32°C had a mean agitation temperature of 38.2°C, but did not lose equilibrium until 41.1°C. In both cases, fish began to exhibit avoidance behaviour at the same temperature, which may affect other functions, such as feeding and predator avoidance, emphasizing the importance of both physiological and behavioural indicators of thermal stress. Although, prior to this experiment, agitation temperature had yet to be reported in accordance with CTMax, effects of other environmental stressors on behavioural thresholds highlight the significance of behaviour as an early, yet significant response. For example, a study that prevented several species of marine sculpins from reaching the surface to perform aquatic surface respiration during progressive hypoxia found that all species underwent the same sequence of behaviours, including agitation and attempts to escape, and eventually, quiescence and unresponsiveness (Mandic et al., 2009). Furthermore, the authors found that the oxygen concentration at which agitation was first observed was correlated with Pcrit, linking this stress-induced agitation with a physiological parameter (Mandic et al., 2009). Another study, which measured restlessness in sand gobies in response to decreasing oxygen concentration, found that this behaviour increased significantly when the fish were exposed to levels of oxygen slightly above their critical levels (Petersen and Petersen, 1990). This increase in spontaneous activity/agitation observed in these and other species in response to low oxygen is interpreted as an avoidance behaviour or an attempt to escape to areas of higher oxygen (Petersen and Petersen, 1990; Herbert and Steffensen, 2006; Chapman and McKenzie, 2009). We interpret the agitation response measured in our study as this same type of avoidance behaviour; an effort, possibly at the cost of other behaviours (refuge, feeding, etc.) to reach cooler waters before suffering a physiological loss, which occurs soon after (at CTMax). In our CTMax experiments, P. multicolor remained under cover for the majority of the experimental trial, emerging only at the agitation level, sacrificing their physical refuge, seemingly to search for more favourable thermal habitat.
By integrating field data on the thermal environment of P. multicolor with short-term acclimation experiments, the present study demonstrates the significance of small increases in water temperature above contemporary ambient levels on thermal tolerance, metabolic rate and critical oxygen tension. Certainly, the longer-term response to increasing water temperature via developmental plasticity and/or genetic change may alter thermal sensitivities, and this is a critical area for future study. However, predictions concerning potential effects of climate warming can be formulated based on smaller-scale, shorter-term changes observed within and among species in response to environmental variables that are expected to play a significant role in climate change, such as temperature (Stillman, 2003; Calosi et al., 2010; Verberk and Bilton, 2011; Magozzi and Calosi, 2015). Our results demonstrate that P. multicolor is able to maintain its RMR and Pcrit across a range of temperatures characteristic of its natural habitat, but incurs a higher cost of resting and diminished hypoxia tolerance at temperatures slightly above its present range despite an increase in CTMax. The predicted increase in temperature for the Lake Victoria region is 4°C before the end of the 21st century (Niang et al., 2014), making it very likely that this population will be forced to perform more frequently at temperatures in the higher end of or slightly beyond their present range (29–32°C) over the coming years. These results are the first to suggest that P. multicolor from Lake Kayanja may already be living close to their thermal limit. Future experiments quantifying aerobic scope over a wider range of temperatures will be necessary to characterize the thermal window of performance.
Funding
This work was supported by funds from the Natural Science and Engineering Research Council of Canada (420078-2012 to L.H.M. and Discovery Grant 312201-2010 to L.J.C.), McGill University (00286 to L.H.M.) as well as Canada Research Chair research funds (950-228344 to L.J.C.).
Acknowledgements
We thank Dr D. Twinomugisha for his logistical and field assistance in Uganda, in addition to our field assistant, Kiberu, who helped immensely with fish collection. We thank L. Smith, T. Moulton, D. Hanna, S. Gray and M. Martínez for their assistance in collecting environmental data and fish maintenance at the field station. This research was approved by McGill University Animal Care Committee (AUP 5029) and by the Uganda National Council for Science and Technology. We also wish to thank two anonymous reviewers whose feedback greatly improved an earlier version of this manuscript.
References
- Angilletta MJ, Niewiarowski PH, Navas CA. (2002) The evolution of thermal physiology in ectotherms. J Therm Biol 27: 249–268. [Google Scholar]
- Anttila K, Casselman MT, Schulte PM, Farrell AP. (2013) Optimum temperature in juvenile salmonids: connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol Biochem Zool 86: 245–256. [DOI] [PubMed] [Google Scholar]
- Becker CD, Genoway RG. (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fish 4: 245–256. [Google Scholar]
- Blažka P. (1958) The anaerobic metabolism of fish. Physiol Zool 31: 117–128. [Google Scholar]
- Brett JR, Groves TDD. (1979) Physiological energetics. In Hoar WS, Randall DJ, eds, Fish Physiology, Vol 8 Academic Press, New York, pp 279–352. [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. (2004) Toward a metabolic theory of ecology. Ecology 85: 1771–1789. [Google Scholar]
- Bullock TH. (1955) Compensation for temperature in the metabolism and activity of poikilotherms. Biol Rev 30: 311–342. [Google Scholar]
- Burton T, Killen SS, Armstrong JD, Metcalfe NB. (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc Biol Sci 278: 3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calosi P, Bilton DT, Spicer JI. (2008) Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol Lett 4: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. (2010) What determines a species' geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J Anim Ecol 79: 194–204. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, McKenzie DJ. (2009) Behavioural responses and ecological consequences. In Richards JG, Farrell AP, Brauner CJ. eds, Hypoxia in Fishes. Elsevier, San Diego, pp 26–77. [Google Scholar]
- Chapman LJ, Galis F, Shinn J. (2000) Phenotypic plasticity and the possible role of genetic assimilation: hypoxia-induced trade-offs in the morphological traits of an African cichlid. Ecol Lett 3: 387–393. [Google Scholar]
- Chapman LJ, Chapman CA, Nordlie FG, Rosenberger AE. (2002) Physiological refugia: swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Victoria region. Comp Biochem Physio A Mol Integr Physiol 133: 421–437. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Albert J, Galis F. (2008) Developmental plasticity, genetic differentiation, and hypoxia-induced trade-offs in an African cichlid fish. Open Evol J 2: 75–88. [Google Scholar]
- Chen Z, Anttila K, Wu J, Whitney CK, Hinch SG, Farrell AP. (2013) Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Can J Zool 91: 265–274. [Google Scholar]
- Claireaux G, Lagardère J-P. (1999) Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J Sea Res 42: 157–168. [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F. (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Clarke A. (1993) Seasonal acclimatization and latitudinal compensation in metabolism: do they exist? Funct Ecol 7: 139–149. [Google Scholar]
- Clarke A, Johnston NM. (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68: 893–905. [DOI] [PubMed] [Google Scholar]
- Cox DK, Gibbons JW, Sharitz RR. (1974) Effects of Three Heating Rates on the Critical Thermal Maximum of Bluegill. Oak Ridge National Laboratory, Oak Ridge, TN, USA; Savannah River Ecology Laboratory, Aiken, SC, USA. [Google Scholar]
- Crispo E, Chapman LJ. (2008) Population genetic structure across dissolved oxygen regimes in an African cichlid fish. Mol Ecol 17: 2134–2148. [DOI] [PubMed] [Google Scholar]
- Crispo E, Chapman LJ. (2010) Geographic variation in phenotypic plasticity in response to dissolved oxygen in an African cichlid fish. J Exp Biol 23: 2091–2103. [DOI] [PubMed] [Google Scholar]
- D'Avanzo C, Kremer J. (1994) Diel oxygen dynamics and anoxic events in an eutrophic estuary of Waquoit Bay, Massachusetts. Estuaries 17: 131–139. [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105: 6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C, Ferrel A, Seibel B, Pörtner H-O, Huey RB. (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348: 1132–1135. [DOI] [PubMed] [Google Scholar]
- Donelson JM, Munday PL, McCormick M, Nilsson GE. (2011) Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob Change Biol 17: 1712–1719. [Google Scholar]
- Donelson JM, Munday PL, McCormick MI, Pitcher CR. (2012) Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat Clim Change 2: 30–32. [Google Scholar]
- Donelson JM, McCormick MI, Booth DJ, Munday PL. (2014) Reproductive acclimation to increased water temperature in a tropical reef fish. PLoS ONE 9: e97223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangue NA, Hofmeister M, Schulte PM. (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209: 2859–2872. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Hinch SG, Cooke SJ, Patterson DA, Crossin GT, Lapointe M, Mathes MT. (2008) Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81: 697–709. [DOI] [PubMed] [Google Scholar]
- Feder ME. (1982) Thermal ecology of neotropical lungless salamanders (Amphibia: Plethodontidae): environmental temperatures and behavioral responses. Ecology 63: 1665–1674. [Google Scholar]
- Finstad A, Forseth T, Ugedal O, Naesje T. (2007) Metabolic rate, behaviour and winter performance in juvenile Atlantic salmon. Funct Ecol 21: 905–912. [Google Scholar]
- Friedrich J, Janssen F, Aleynik D, Bange HW, Boltacheva N, Çagatay MN, Dale AW, Etiope G, Erdem Z, Geraga M, et al. (2014) Investigating hypoxia in aquatic environments: diverse approaches to addressing a complex phenomenon. Biogeosciences 11: 1215–1259. [Google Scholar]
- Friesen CN, Aubin-Horth N, Chapman LJ. (2012) The effect of hypoxia on sex hormones in an African cichlid Pseudocrenilabrus multicolor victoriae. Comp Biochem Physiol A Mol Integr Physiol 162: 22–30. [DOI] [PubMed] [Google Scholar]
- Fry FEJ. (1971) The effect of environmental factors on the physiology of fish. In Hoar WS, Randall DJ, eds, Fish Physiology, Vol 6 Academic Press, New York, pp 1–98. [Google Scholar]
- Gardiner NM, Munday PL, Nilsson GE. (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS ONE 5: e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. (2001) Effects of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- Gräns A, Jutfelt F, Sandblom E, Jönsson E, Wiklander K, Seth H, Olsson C, Dupont S, Ortega-Martinez O, Einarsdottir I, et al. (2014) Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J Exp Biol 217: 711–717. [DOI] [PubMed] [Google Scholar]
- Grantner A, Taborsky M. (1998) The metabolic rates associated with resting, and with the performance of agonistic, submissive and digging behaviours in the cichlid fish Neolamprologus pulcher (Pisces: Cichlidae). J Comp Physiol B 168: 427–433. [Google Scholar]
- Hazel JR, Prosser CL. (1974) Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev 54: 620–677. [DOI] [PubMed] [Google Scholar]
- Herbert NA, Steffensen JF. (2006) Hypoxia increases the behavioural activity of schooling herring: a response to physiological stress or respiratory distress? Mar Biol 149: 1217–1225. [Google Scholar]
- Hoffmann AA, Sgrò CM. (2011) Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- Huey RB, Kingsolver JG. (1989) Evolution of thermal sensitivity of ectotherm performance. Trends Ecol Evol 4: 131–135. [DOI] [PubMed] [Google Scholar]
- Huey RB, Stevenson RD. (1979) Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am Zool 19: 357–366. [Google Scholar]
- IPCC (2013) Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA. [Google Scholar]
- Janzen DH. (1967) Why mountain passes are higher in the tropics. Am Nat 101: 233–249. [Google Scholar]
- Jobling M. (1994) Respiration and metabolism. In Jobling M. ed., Fish Bioenergetics. Chapman and Hall, London, pp 121–145. [Google Scholar]
- Johnston I, Clarke A, Ward P. (1991) Temperature and metabolic rate in sedentary fish from the Antarctic, North Sea and Indo-West Pacific Ocean. Mar Biol 109: 191–195. [Google Scholar]
- Lapointe D, Vogelbein WK, Fabrizio MC, Gauthier DT, Brill RW. (2014) Temperature, hypoxia, and mycobacteriosis: effects on adult striped bass Morone saxatilis metabolic performance. Dis Aquat Organ 108: 113–127. [DOI] [PubMed] [Google Scholar]
- Lutterschmidt WI, Hutchison VH. (1997) The critical thermal maximum: history and critique. Can J Zool 75: 1561–1574. [Google Scholar]
- Magozzi S, Calosi P. (2015) Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob Change Biol 21: 181–194. [DOI] [PubMed] [Google Scholar]
- Mandic M, Sloman KA, Richards JG. (2009) Escaping to the surface: a phylogenetically independent analysis of hypoxia-induced respiratory behaviors in sculpins. Physiol Biochem Zool 82: 730–738. [DOI] [PubMed] [Google Scholar]
- McDonnell LH.(2015) Effects of Elevated Temperature on Metabolic Performance and Thermal Tolerance of a Widespread African Cichlid. Master of Science, Department of Biology, McGill University, Montreal, Quebec, Canada. [Google Scholar]
- McNab BK. (2002) The Physiological Ecology of Vertebrates: a View from Energetics. Cornell University Press, Ithaca, New York. [Google Scholar]
- Munday PL, Jones GP, Pratchett MS, Williams AJ. (2008) Climate change and the future for coral reef fishes. Fish Fish 9: 261–285. [Google Scholar]
- Muñoz NJ, Anttila K, Chen Z, Heath JW, Farrell AP, Neff BD. (2014) Indirect genetic effects underlie oxygen-limited thermal tolerance within a coastal population of chinook salmon. Proc Biol Sci 281: 20141082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niang I, Ruppel OC, Abdrabo MA, Essel A, Lennard C, Padgham J, Urquhart P.(2014) Africa. In Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, eds, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, USA, pp 1199–1265. [Google Scholar]
- Nilsson GE, Östlund-Nilsson S. (2008) Does size matter for hypoxia tolerance in fish? Biol Rev 83: 173–189. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Crawley N, Lunde IG, Munday PL. (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol 15: 1405–1412. [Google Scholar]
- Nilsson GE, Östlund-Nilsson S, Munday PL. (2010) Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp Biochem Physiol A Mol Integr Physiol 156: 389–393. [DOI] [PubMed] [Google Scholar]
- Norin T, Malte H, Clark TD. (2014) Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol 217: 244–251. [DOI] [PubMed] [Google Scholar]
- Ott ME, Heisler N, Ultsch GR. (1980) A re-evaluation of the relationship between temperature and the critical oxygen tension in freshwater fishes. Comp Biochem Physiol A Mol Integr Physiol 67: 337–340. [Google Scholar]
- Petersen JK, Petersen GI. (1990) Tolerance, behaviour and oxygen consumption in the sand goby, Pomatoschistus minutus (Pallas), exposed to hypoxia. J Fish Biol 37: 921–933. [Google Scholar]
- Pörtner H-O. (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88: 137–146. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O. (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132: 739–761. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Farrell AP. (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Knust R. (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315: 95–97. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Peck MA. (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Bennett AF, Bozinovic F, Clarke A, Lardies MA, Lucassen M, Pelster B, Schiemer F, Stillman JH. (2006) Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiol Biochem Zool 79: 295–313. [DOI] [PubMed] [Google Scholar]
- Prodocimo V, Freire CA. (2001) Critical thermal maxima and minima of the platyfish Xiphophorus maculatus Günther (Poecillidae, Cyprinodontiformes)–a tropical species of ornamental freshwater fish. Rev Bras Zool 18: 97–106. [Google Scholar]
- Reardon EE, Chapman LJ. (2009) Hypoxia and life-history traits in a eurytopic African cichlid. J Fish Biol 75: 1795–1815. [DOI] [PubMed] [Google Scholar]
- Reardon EE, Chapman LJ. (2010a) Energetics of hypoxia in a mouth-brooding cichlid: evidence for interdemic and developmental effects. Physiol Biochem Zool 83: 414–423. [DOI] [PubMed] [Google Scholar]
- Reardon EE, Chapman LJ. (2010b) Hypoxia and energetics of mouth brooding: is parental care a costly affair? Comp Biochem Physiol A Mol Integr Physiol 156: 400–406. [DOI] [PubMed] [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. (2003) Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. [DOI] [PubMed] [Google Scholar]
- Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL. (2014) Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol 20: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandersfeld T, Davison W, Lamare MD, Knust R, Richter C. (2015) Elevated temperature causes metabolic trade-offs at the whole-organism level in the Antarctic fish Trematomus bernacchii. J Exp Biol 218: 2373–2381. [DOI] [PubMed] [Google Scholar]
- Schurmann H, Steffensen JF. (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- Seifert AW, Chapman LJ. (2006) Respiratory allocation and standard rate of metabolism in the African lungfish, Protopterus aethiopicus. Comp Biochem Physiol A Mol Integr Physiol 143: 142–148. [DOI] [PubMed] [Google Scholar]
- Sharpe DMT, Wandera SB, Chapman LJ. (2012) Life history change in response to fishing and an introduced predator in the East African cyprinid Rastrineobola argentea. Evol Appl 5: 677–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen C, Munday PL, Nilsson GE. (2014) Aerobic vs. anaerobic scope: sibling species of fish indicate that temperature dependence of hypoxia tolerance can predict future survival. Glob Change Biol 20: 724–729. [DOI] [PubMed] [Google Scholar]
- Stillman JH. (2003) Acclimation capacity underlies susceptibility to climate change. Science 301: 65–65. [DOI] [PubMed] [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. (2008) Putting the heat on tropical animals. Science 320: 1296. [DOI] [PubMed] [Google Scholar]
- Tsuji JS. (1988) Thermal acclimation of metabolism in Sceloporus lizards from different latitudes. Physiol Zool 61: 241–253. [Google Scholar]
- Ultsch GR, Boschung H, Ross MJ. (1978) Metabolism, critical oxygen tension, and habitat selection in darters (Etheostoma). Ecology 59: 99–107. [Google Scholar]
- Ultsch GR, Reese SA, Nie M, Crim JD, Smith WH, Leberte CM. (1999) Influences of temperature and oxygen upon habitat selection by bullfrog tadpoles and three species of freshwater fishes in two Alabama strip mine ponds. Hydrobiologia 416: 149–162. [Google Scholar]
- Vaquer-Sunyer R, Duarte CM. (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci USA 105: 15452–15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk W, Bilton DT. (2011) Can oxygen set thermal limits in an insect and drive gigantism? PLoS ONE 6: e22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Lefevre S, Iversen NK, Findorf I, Buchanan R, McKenzie DJ. (2014) Anaemia only causes a small reduction in the upper critical temperature of sea bass: is oxygen delivery the limiting factor for tolerance of acute warming in fishes? J Exp Biol 217: 4275–4278. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Takahara T, Kohmatsu Y, Yuma M. (2013) Body size and temperature dependence of routine metabolic rate and critical oxygen concentration in larvae and juveniles of the round crucian carp Carassius auratus grandoculis Temminck & Schlegel 1846. J Appl Ichthyol 29: 891–895. [Google Scholar]
- Yeager DP, Ultsch GR. (1989) Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol Zool 62: 888–907. [Google Scholar]
- Zhang Y, Kieffer JD. (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can J Zool 92: 215–221. [Google Scholar]