Carotenoids are components of an animal’s diet that are considered beneficial because they typically provide increased immune capacity. However, little research has been done in amphibians. We found that carotenoids can cause lower survival, slower development and growth in amphibians, and no mitigating effects against a common amphibian disease.
Keywords: Amphibian decline, disease ecology, nutritional ecology, parasite, pathogen
Abstract
Carotenoids are considered beneficial nutrients because they provide increased immune capacity. Although carotenoid research has been conducted in many vertebrates, little research has been done in amphibians, a group that is experiencing global population declines from numerous causes, including disease. We raised two amphibian species through metamorphosis on three carotenoid diets to quantify the effects on life-history traits and post-metamorphic susceptibility to a fungal pathogen (Batrachochytrium dendrobatidis; Bd). Increased carotenoids had no effect on survival to metamorphosis in gray treefrogs (Hyla versicolor) but caused lower survival to metamorphosis in wood frogs [Lithobates sylvaticus (Rana sylvatica)]. Increased carotenoids caused both species to experience slower development and growth. When exposed to Bd after metamorphosis, wood frogs experienced high mortality, and the carotenoid diets had no mitigating effects. Gray treefrogs were less susceptible to Bd, which prevented an assessment of whether carotenoids could mitigate the effects of Bd. Moreover, carotenoids had no effect on pathogen load. As one of only a few studies examining the effects of carotenoids on amphibians and the first to examine potential interactions with Bd, our results suggest that carotenoids do not always serve amphibians in the many positive ways that have become the paradigm in other vertebrates.
Introduction
Animal diets provide energy and nutrients that are essential to life. Carotenoids are one such group of nutrients; they consist of more than 600 pigments that are categorized as either carotenes or xanthophylls (both are comprised of hydrocarbon chains, but carotenes contain no oxygen whereas xanthophylls contain oxygen; Pérez-Rodríguez, 2009). Carotenoids function as accessory pigments for photosynthesis and they are synthesized by plants and some species of bacteria and fungi; however, animals cannot synthesize carotenoids and must acquire them from their diet. In addition to producing colours in many animals (commonly in shades of yellow, orange or red), carotenoids are important antioxidants and immune system enhancers (Chew, 1993; Hill, 1995; Vershinin, 1999; Alonso-Alvarez et al., 2004; but see Costantini et al., 2007; Pérez-Rodríguez, 2009). As a result, animals fed diets higher in carotenoids should be less susceptible to many pathogens and diseases; this prediction has been supported in a number of animal species, including humans (Bendich, 1993; Skarstein and Folstad, 1996; Horak et al., 2001; Hill and Farmer, 2005; Babin et al., 2010).
There is broad agreement regarding the benefits of carotenoids, but there is debate over whether carotenoids can cause fitness costs (reviewed by Olson and Owens, 1998). If carotenoids are rare, it may be costly to find and consume a high-carotenoid diet. If carotenoids are abundant, animals may face a cost of consuming a diet that is too high in carotenoids. Excess carotenoids might also be costly if they are directly toxic, if they require a substantial amount of energy to detoxify them or if the breakdown products are toxic (Olson and Owens, 1998; Hartley and Kennedy, 2004). Studies confirming toxicity are rare (Kalariya et al., 2009), but detrimental effects would be more likely to be observed in herbivores than in carnivores because the diets of herbivores typically contain more carotenoids per unit mass than the diets of carnivores (Olson and Owens, 1998).
Compared with other vertebrates, little is known about the effect of carotenoids on amphibians. Researchers have examined how carotenoids contribute to amphibian colouration (Forbes et al., 1973; Frost and Robinson, 1984; Matsui et al., 2003; Bell and Zamudio, 2012) and how such colours may serve to attract mates (Gomez et al., 2009; Richardson et al., 2009); however, only three studies have manipulated carotenoid in the diet. Ogilvy et al. (2012) demonstrated that diets containing different carotenoid concentrations affected red-eyed treefrogs (Agalychnis callidryas); increased carotenoids did not affect larval growth or survival, but they did cause greater post-metamorphic growth, redder skin and increased fecundity. In a second study, Ogilvy and Preziosi (2012) manipulated carotenoid diets in western clawed frogs [Silurana (Xenopus) tropicalis]; increased carotenoids caused more rapid larval development. Finally, in a third study, Dugas et al. (2013) discovered that increased carotenoids in diets resulted in higher reproductive success of captive-bred strawberry poison frogs (Oophaga pumilio).
Despite the important role that carotenoids play in animal immunity, no amphibian studies have examined the effect of carotenoids on amphibian diseases, yet this is an important question given the diversity of pathogens that harm amphibians (Blaustein et al., 2011). One of the most lethal diseases in amphibians is chytridiomycosis, which is caused by the chytrid fungus Batrachochytrium dendrobatidis (hereafter termed ‘Bd’). The fungus grows on tissues containing keratin, which include the mouthparts of tadpoles and the skin of post-metamorphic frogs (Marantelli et al., 2004; Berger et al., 2005). The fungus is found throughout the world and is associated with declines in numerous species (Stuart et al., 2004; Skerratt et al., 2007; Fisher et al., 2009; Olson et al., 2013). Studies suggest that both innate and acquired immunity play a role in determining variation in host responses to Bd; for example, the innate immune system releases antimicrobial peptides and lysozymes onto the skin that can prevent or reduce the amount of Bd infecting amphibian skin cells (Rollins-Smith, 2009). In addition, bacteria growing on amphibian skin can produce antifungal chemicals that may kill Bd (Brucker et al., 2008; Antwis et al., 2014).
When Bd infects the epidermis, the fungus may inhibit lymphocyte proliferation (Rollins-Smith et al., 2011; Fites et al., 2013). While several studies have demonstrated that certain aspects of the acquired immune system defend against Bd infections (Richmond et al., 2009; Ramsey et al., 2010; Savage and Zamudio, 2011), others have not observed an adaptive immune response (Ribas et al., 2009; Cashins et al., 2013). There is general agreement that carotenoids can enhance both the acquired immune system, by increasing lymphocyte development, and the innate immune system, by improving the performace of antigen-presenting cells and serving as an important component for the epithelial cells that produce antimicrobial peptides (see reviews by Chew and Park, 2004; Duriancik et al., 2010; Hall et al., 2011). Given the common role that carotenoids play in stimulating the immune systems of vertebrates, it is important to know whether enhanced carotenoid diets in amphibians might make them more resistant or tolerant to Bd infections. As a result, we tested whether increasing the amount of carotenoids in the diet of amphibians improves tadpole growth and development and decreases the susceptibility of metamorphs to Bd.
Materials and methods
Experimental design
We performed identical experiments with two anuran species, namely wood frogs [Lithobates sylvaticus (Rana sylvatica)] and gray treefrogs (Hyla versicolor). For each species, we used a completely randomized design with a factorial combination of three carotenoid diets (none, low or high) fed during the larval and juvenile stages crossed with a post-metamorphic Bd exposure treatment (exposed or not exposed). The larval experiment was conducted at the Donald S. Wood Field Laboratory at the University of Pittsburgh Pymatuning Laboratory of Ecology; the post-metamorphic exposure to Bd was conducted at Oregon State University (OSU).
Anuran collection and husbandry
In spring 2012, we collected the anurans as recently laid egg masses (seven masses of wood frogs, collected on 14 March) or amplecting adult pairs that subsequently laid eggs (15 masses of gray treefrogs, collected on 9 May). Eggs were kept in 90 l wading pools filled with well water until they hatched. After hatching, animals were fed rabbit chow (Blue Seal Bunny 16) ad libitum until used in experiments (approximately 1–2 weeks). The number of egg clutches, initial tadpole mass and initial developmental stages are presented in the Supplementary material appendix (Table A1).
Experimental set-up
In each experiment, groups of eight tadpoles (drawn from a mixture of all egg masses) were randomly assigned to 14 l plastic tubs and reared on the three carotenoid diets in laboratory conditions [15 h–9 h light–dark photoperiod at a temperature of 22 ± 0.02 (mean ± 1 SD)°C]. As detailed in in the Supplementary material appendix (Table A1), the tadpoles began as recent hatchlings that were small in mass and early in development. Each tub contained 10 l of carbon-filtered, ultraviolet-irradiated water. The three carotenoid treatments were replicated 10 times for wood frogs and 12 times for gray treefrogs. To prevent water fouling, we changed the water in the tubs every 3–4 days when the tadpoles were young and small and every 2 days as they grew into large tadpoles. We raised all tadpoles on a base diet containing fishmeal, wheat flour, rice flour and vitamins (for similar diets fed to amphipods and tadpoles, see Babin et al., 2010; Ogilvy and Preziosi, 2012). This plant- and animal-based diet is appropriate because tadpoles can be omnivorous (Schiesari et al., 2009).
We manipulated the carotenoid content of the diets (1 or 10 mg of carotenoids/g of total dry ingredients) by varying the amount of astaxanthin and lutein (details in the Supplementary material appendix; Tables A2 and A3). To our knowledge, there is no literature on the specific carotenoids found in foods (e.g. periphyton) consumed by wood frog and gray treefrog tadpoles; however, astaxanthin and lutein are commonly found in large quantities in both invertebrate and vertebrate aquatic animals, with the former generally being more prevalent than the latter (Matsuno, 2001; Guerin et al., 2003; Gaillard et al., 2004). On the basis of a previous study on amphibians (Ogilvly and Preziosi, 2012), we chose 10 mg of carotenoid per 1 g of food as our high-carotenoid diet. Such a diet is not unreasonable given that algae, the typical source of carotenoids in aquatic systems, can have carotenoid concentrations >30 mg/g dry biomass (Olaizola and Huntley, 2003).
The carotenoid manipulations were maintained throughout the larval period and after metamorphosis. The tadpoles were fed fresh food ad libitum until they developed forelimbs, at which point they were transferred to 14 l plastic tubs lined with damp sphagnum moss. Individuals were considered to have completed metamorphosis once they had fully absorbed their tail. We defined the time to metamorphosis as the time elapsed from the start of the experiment until full tail resorption. Once the tadpoles metamorphosed, we fed the metamorphs an ad libitum diet of crickets (Acheta domesticus) that had been reared on the same carotenoid diets as the tadpoles. Details regarding cricket maintenance can be found in the Supplementary material appendix.
Once all animals metamorphosed, we shipped them to OSU for Bd exposure. For reasons of shipping, we pooled animals from the same carotenoid treatment and thus lost tub-level identity when analysing for an effect of carotenoid diet on mass. As a result of asynchronous metamorphosis both within and among carotenoid treatments, the metamorphs were held for a maximum of 33 and 43 days for wood frogs and gray treefrogs, respectively, before being shipped to OSU.
After arrival at OSU, all metamorphs were transferred to glass terraria and maintained between 20 and 22°C on a 13 h–11 h light–dark photoperiod. The animals were acclimated to these conditions for 24 h before we initiated experiments. All animals were weighed and then randomly assigned to individual Petri dishes (140 mm × 30 mm), which contained 15 ml of dechlorinated water and had holes in the lid. When quantifying individual mass, we did not weigh each individual on the day it metamorphosed, but instead weighed all animals after they were transported to OSU and immediately before applying the Bd treatment. Once they arrived, the metamorphs continued to be fed crickets that were reared on the different carotenoid diets.
An equal number of animals from each carotenoid treatment were randomly assigned to the Bd and no Bd exposure treatments. Water in the Petri dishes was changed on day 7, and the Petri dishes were not reinoculated. We used the Bd isolate JEL 274; additional details regarding Bd preparation and inoculation can be found in the Supplementary material appendix.
During the Bd exposure, all animals were fed pinhead crickets twice per week under a ration that was related to metamorph mass (one cricket for every 0.1 g of average body weight by species). These crickets were reared using the same range of carotenoid diets as described above.
We monitored animals daily for signs of mortality. In accordance with our Animal Care and Use protocol, we quantified dead animals as those that either died or showed signs of distress (i.e. loss of righting reflex, failure to respond to stimulus, and seizures); any moribund animals were euthanized. Animals that died were immediately removed and individually stored in 95% ethanol to preserve Bd DNA for later analysis. At the end of the experiment, all remaining animals were killed in MS-222 and individually preserved in 95% ethanol.
Assessing B. dendrobatidis infection load
To assess the Bd infection load, we swabbed the ventral abdominal skin and inner thigh skin using fine-tipped sterile rayon swabs (Medical Wire and Equipment, MW&E 113). For each species, we randomly sampled eight Bd-exposed animals from each carotenoid treatment (i.e. 24 animals in total). We also randomly sampled three control animals of each species to ensure that there was no contamination across treatments. Given that many of the wood frogs died but few of the gray treefrogs died, we sampled the Bd loads from the pool of dead and surviving frogs at the end of the experiment. All control animals tested negative for infection.
We used quantitative-PCR (qPCR) to assess the infection load in post-metamorphic amphibians following the methods of Boyle et al. (2004), except that we used 60 μl of Prepman Ultra (Applied Biosystems) instead of 40 μl in all DNA extractions. Extractions were diluted 1:10 and processed in an ABI PRISM 7500 (Applied Biosystems). Each sample was analysed in triplicate, and the mean number of genome equivalents of Bd per animal was calculated. Animals testing negative in at least two of the three replicate wells were considered ‘infection negative’.
Statistical analyses
We conducted separate analyses for the wood frogs and gray treefrogs because the experiments for each species were separated in time (SPSS v. 21 for Mac). To test the effect of carotenoid treatment on survival to metamorphosis, we used a generalized linear model (GENLIN) that included a binomial probability distribution with a logit link function. To test for an effect of carotenoid diet on the time to metamorphosis, we used a GENLIN that included a Poisson probability distribution and a log-link function. In both models, the tub in which the tadpoles were raised was included as a subject variable to account for correlated responses among individuals within tubs. If we found a significant treatment effect in either model, we made pairwise comparisons using sequential Bonferroni tests. To test whether carotenoid diet affected the final mass of the animals immediately before the Bd exposure stage of the experiment, we used a generalized linear model. We used the MIXED procedure in SPSS, even though we do not have a random effect, because it employs a maximum likelihood estimation procedure that produces better parameter estimates for unbalanced designs.
To examine how metamorph survival was affected by Bd exposure, carotenoid diet and their interaction, we used a Cox regression. Mass was included as a covariate in the analysis because mass often correlates with susceptibility to Bd (Searle et al., 2011). Given that none of the second- or third-order interactions with mass was significant (all P ≥ 0.2), we dropped these terms from the analysis. We also ran the model without mass included, and the interpretation of the results was qualitatively the same.
To test for differences in infection load, we conducted an analysis of variance (ANOVA) on the mean number of genome equivalents of Bd per animal. Our initial analyses included mass as a covariate, but mass and the carotenoid-by-mass interactions were not significant, so we excluded these terms from the final analysis. For the gray treefrogs, we removed two data points that were extreme statistical outliers (one from a low-carotenoid treatment and the other from a high-carotenoid treatment). One individual was removed because it had a very high standard deviation among the three swab samples for Bd load (mean = 14.7, SD = 19.38). The other individual was removed after inspecting the box plots; it was three times the interquartile range. Excluding these two data points did not change the interpretation of our results.
Results
Effects of carotenoid diets on life-history traits before B. dendrobatidis exposure
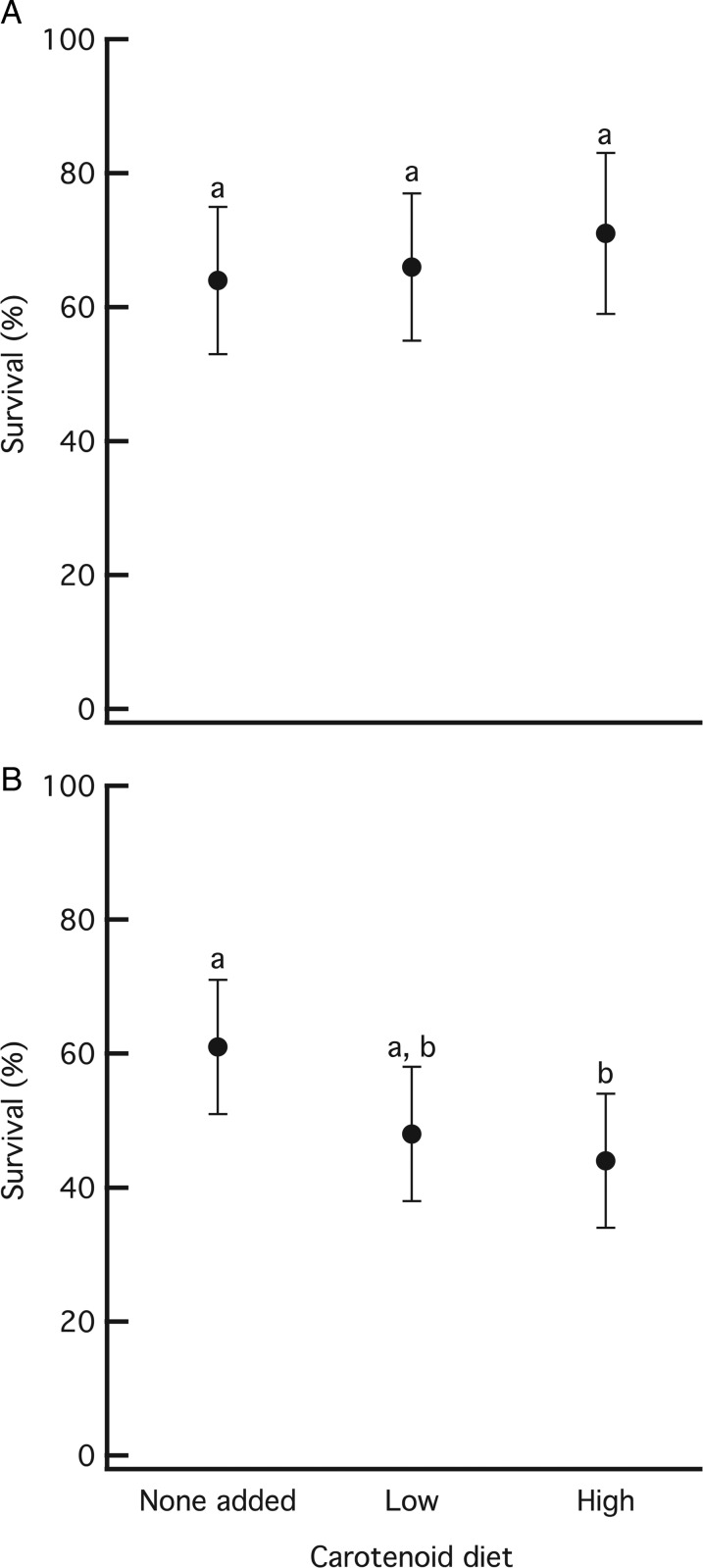
For survival to metamorphosis (Fig. 1), we found no effect of the carotenoid diets on wood frogs (Wald χ2 = 0.872, P = 0.647), but there was an effect on gray treefrogs (Wald χ2 = 6.508, P = 0.039). Gray treefrog survival decreased by nearly 20% when raised on a diet containing high carotenoids compared with a diet containing no added carotenoids (P = 0.038). This mortality was spread throughout the larval period. No other pairwise comparisons were significant (all P ≥ 0.114).
Figure 1:
Survival of wood frogs (A) and gray treefrogs (B) after being raised to metamorphosis on three different carotenoid diets. Data are means ± 95% confidence intervals.
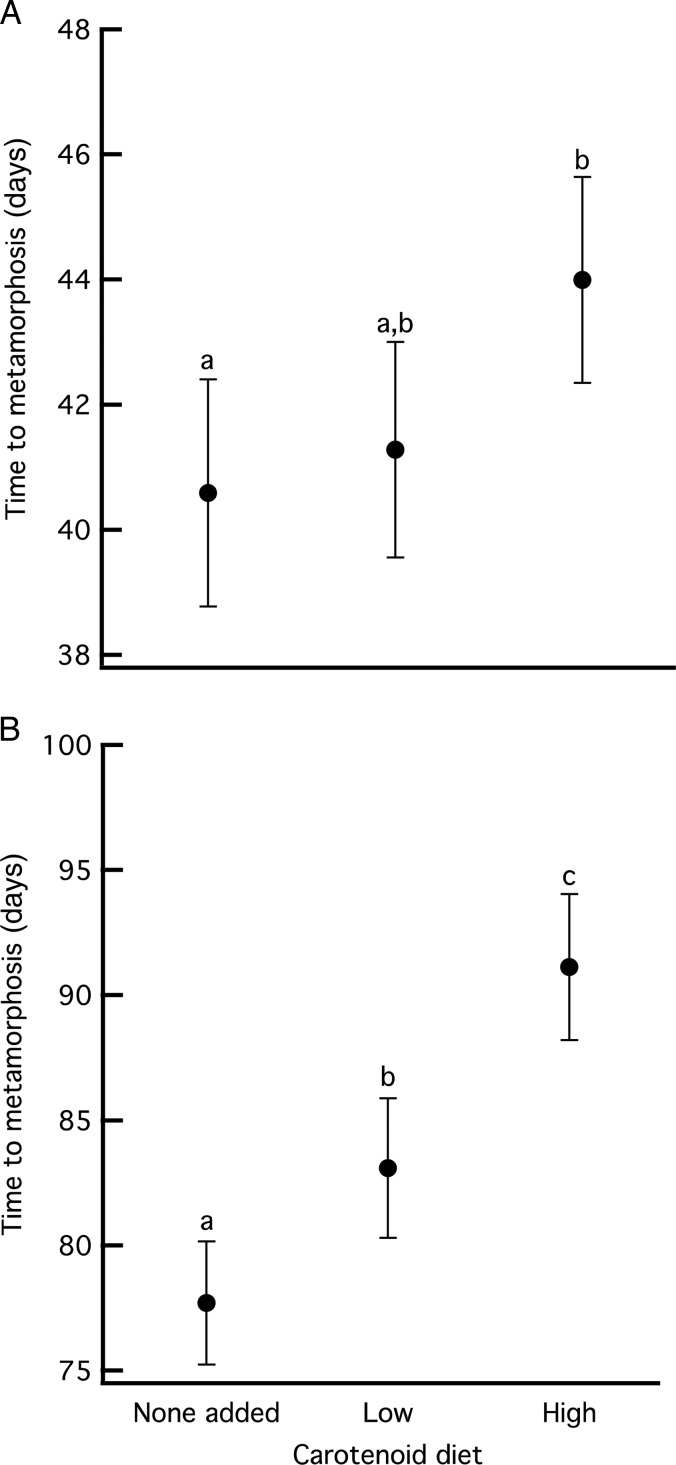
The carotenoid diets affected time to metamorphosis in both species (wood frogs, Wald χ2 = 8.498, P = 0.014; and gray treefrogs, Wald χ2 = 115.92, P < 0.001; Fig. 2). Wood frogs fed a diet containing high carotenoids took an average of 3 days longer to metamorphose than those fed on a diet containing low carotenoids or no added carotenoids (both P < 0.035). The latter two treatments did not differ (P = 0.493). Gray treefrogs fed a diet containing high carotenoids also had a longer time to metamorphosis. In comparison to diets with no added carotenoids, animals took an average of 5 days longer to metamorphose when reared on a low-carotenoid diet and 13 days longer when reared on a high-carotenoid diet (both P < 0.001). The latter two treatments also differed, with the high-carotenoid diet causing gray treefrogs to metamorphose 8 days later than the low-carotenoid diet (P < 0.001).
Figure 2:
Time to metamorphosis of wood frogs (A) and gray treefrogs (B) after being raised to metamorphosis on three different carotenoid diets. Data are means ± 95% confidence intervals.
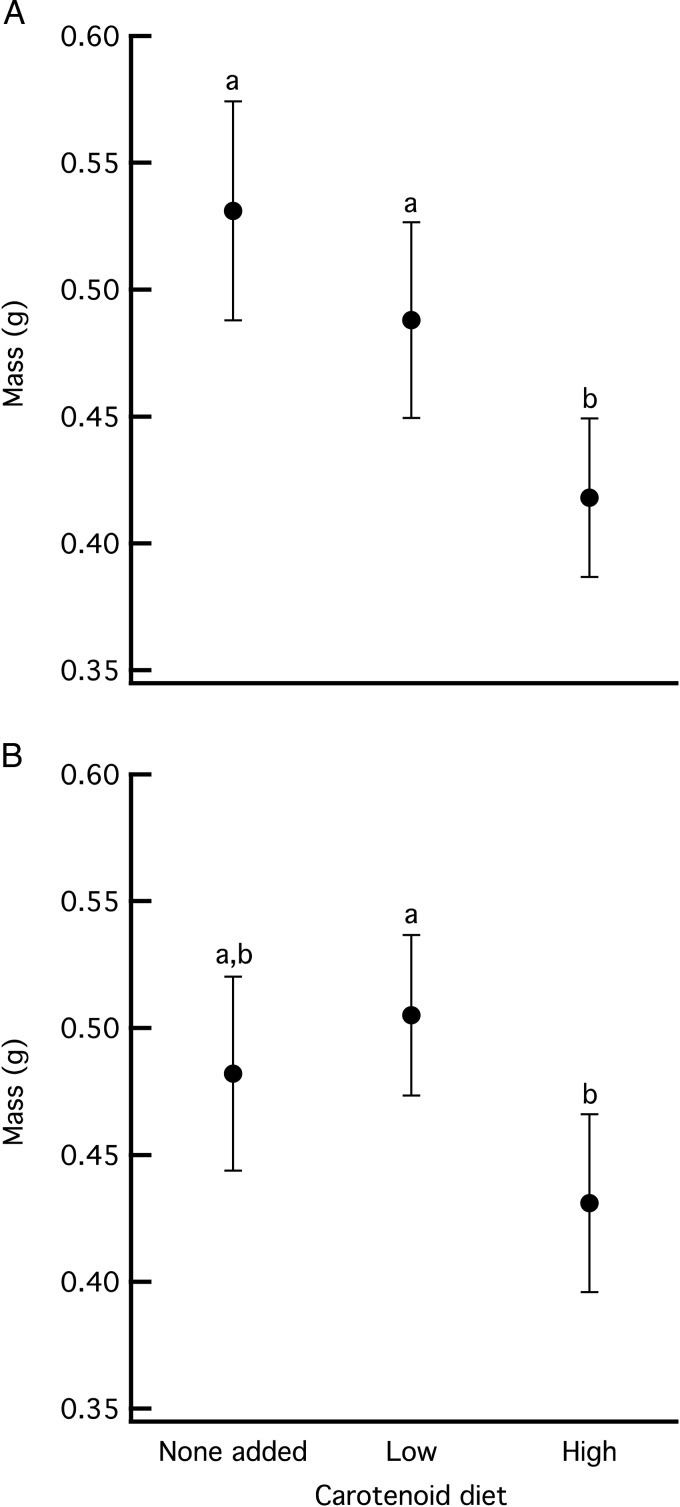
The carotenoid diets also affected the mass of both species (wood frogs, F2,107 = 7.681, P = 0.001; and gray treefrogs, F2,136 = 4.052, P = 0.02; Fig. 3). Wood frog metamorphs reared on a high-carotenoid diet were 17% smaller than those reared on a low-carotenoid diet (P = 0.055) and 27% smaller than those reared on a diet with no added carotenoids (P < 0.001). The latter two treatments did not differ (P = 0.307). Gray treefrog metamorphs reared on a high-carotenoid diet were smaller than those reared on a low-carotenoid diet (P = 0.018) and tended to be smaller than those reared on a diet with no added carotenoids, although the difference was not significant (P = 0.135). The latter two treatments did not differ (P = 1.0).
Figure 3:
Mass of wood frog (A) and gray treefrog metamorphs (B) immediately before being exposed to the Batrachochytrium dendrobatidis (Bd) or no-Bd treatments. Data are means ± 95% confidence intervals.
Effects of carotenoid diet and B. dendrobatidis on metamorph survival
After exposing the metamorphs to the presence or absence of Bd for 2 weeks, we examined metamorph survival (Table 1). For wood frogs, 59% survived in the absence of Bd, whereas 22% survived in the presence of Bd. The Cox regression model showed that the treatments affected survival in the experiment (omnibus test of model coefficients P < 0.001). Animals exposed to Bd were five times more likely to die than unexposed animals (i.e. the hazard ratio was 5.4), and larger individuals were less likely to die than smaller individuals. However, carotenoid diets had no effect on survival (i.e. there was no carotenoid diet or carotenoid diet-by-Bd exposure interaction).
Table 1:
Results from the Cox regression model showing the effects of carotenoid diet, exposure to Batrachochytrium dendrobatidis and their interaction on the survival of wood frog metamorphs
| Wood frogs |
|||
|---|---|---|---|
| Source | χ2 | d.f. | P-value |
| Carotenoid diet | 0.97 | 2 | 0.616 |
| Bd exposure | 11.971 | 1 | 0.001 |
| Interaction | 0.637 | 2 | 0.727 |
| Mass | 6.415 | 1 | 0.011 |
Mass was included as a covariate in the model. The hazard ratio (95% confidence interval) for the effect of Batrachochytrium dendrobatidis (Bd) was 5.4 (2.1–14.0).
For gray treefrog survival, 94% survived in the absence of Bd, whereas 87% survived in the presence of Bd. The Cox regression model showed that the treatments had no effect on survival (omnibus test of model coefficients P = 0.22). Of the 139 metamorphs in the 14 day exposure, only 13 died (nine exposed to Bd and four not exposed to Bd).
Effects of carotenoid diets on B. dendrobatidis infection loads
Our final analysis examined Bd infection loads in the metamorphs. In the ANOVA on wood frogs, we also found no effect on the carotenoid treatments on pathogen load (F2,21 = 0.584, P = 0.567); the mean number of genome equivalents ranged from 116 ± 35 to 186 ± 62. In the ANOVA on gray treefrogs, we found no effect of the carotenoid treatments on pathogen load (F2,19 = 0.774, P = 0.475); the mean number of genome equivalents (±1 SEM) ranged from 86 ± 27 to 158 ± 62. A subsequent power analysis showed that, given our sample size of eight swabbed animals and the variation in genome equivalents among tadpoles, mean differences between carotenoid treatments would have to be around 200–225 genome equivalents for wood frogs and gray treefrogs to achieve statistical significance (at a power of 0.8).
Discussion
Effects of carotenoids on larval amphibians
High-carotenoid diets had no effect on the survival to metamorphosis in wood frogs, but caused a 20% decline in gray treefrog survival. This was particularly surprising given the generally positive effects of carotenoids in a wide range of taxa (Olson and Owens, 1998; Pérez-Rodríguez, 2009; Simons et al., 2012). Amphibian studies are rare, but two other species fed diets with supplemented carotenoids either exhibited no effect on survival or a small increase in survival (Ogilvy and Preziosi, 2012; Ogilvy et al., 2012). Few studies of carotenoid diet manipulations track survival, but a study of three-spined sticklebacks (Gasterosteus aculeatus) observed increased survival in fish that were fed high-carotenoid diets (Pike et al., 2007). These differences in survival responses may reflect the difference in carotenoid concentration, species differences in responses to carotenoids or differences in the ingredients used to manipulate carotenoid concentration.
The decline in the growth and development of the wood frogs and gray treefrogs was counter to our hypothesis that carotenoids would be beneficial. Among studies of amphibians that have manipulated carotenoid diets, Ogilvy et al. (2012) observed no effect on the growth of red-eyed treefrog tadpoles for several weeks post-metamorphosis. In a study using western clawed frogs, Ogilvy and Preziosi (2012) also found no effect of carotenoids on larval growth. In other vertebrate classes, there are few investigations of how carotenoid manipulations affect growth. In an experiment using great tit nestlings (Parus major) that were living in environments with high or low metal pollution, Eeva et al. (2009) found that adding carotenoids caused higher growth in the polluted environment but not in the unpolluted environment. One caveat to our work on growth and development is that animals were not weighed immediately after metamorphosis, but rather once they arrived at OSU before being used in the Bd challenge experiment. Thus, growth differences before and after metamorphosis may both have contributed to the overall mass differences on the day that the frogs were weighted.
We also found that both amphibian species exhibited slower development when fed diets high in carotenoids. Only two other studies have examined carotenoid effects on amphibian development. Ogilvy and Preziosi (2012) found that feeding western clawed frogs a diet high in carotenoids caused more rapid development than when fed a diet without added carotenoids. Dugas et al. (2013) found that female strawberry poison frogs, which produce trophic eggs to feed to their offspring, fed diets supplemented with carotenoids had higher reproductive success than those not fed supplemental carotenoids. This effect was driven by a combination of more successful embryonic development to the tadpole stage and more successful development of tadpoles to the metamorph stage. Negative effects of carotenoids on life-history traits are rare, and the few studies that we discovered are in birds (Costantini et al., 2007; Simons et al., 2014). Clearly, much more research needs to be conducted before we can develop generalities in growth and development responses to carotenoids.
Given that increased carotenoids caused one of our species to exhibit lower survival and both species to exhibit slower growth and development, one obvious question is whether the carotenoids we used are in some way toxic to gray treefrog tadpoles but not to wood frog tadpoles (note that neither species experienced mortality from carotenoids after metamorphosis). The formulation of carotenoids that we used was modelled to be similar to previous amphibian studies (Ogilvy and Preziosi, 2012; Ogilvy et al., 2012). While those studies included β-carotene and our study included astaxanthin (which is a common component of aquatic animal tissues; Matsuno, 2001; Guerin et al., 2003; Gaillard et al., 2004), the effects of different types of carotenoids on the life history and immune function of animals are unknown. While it is known that astaxanthin is better at scavenging free radicals than β-carotene in vitro (Jørgensen and Skibsted, 1993), it is unknown whether astaxanthin is better at scavenging free radicals in vivo. Regardless, the carotenoid diets of Ogilvy and Preziosi (2012), which spanned the same range of concentrations (0–10 mg/g of total diet) as our study, did not have harmful effects on western clawed frogs. In studies using freshwater amphipods (Hyalella spp.), we have used carotenoid diets that were identical to those used for wood frogs and gray treefrogs and have found that the amphipods survived better on diets supplemented with carotenoids (R. D. Cothran, personal observation), so it seems unlikely that the carotenoid diets used in our experiment are generally toxic. They may simply be toxic to certain species of amphibians. This is an important discovery because, as noted by Ogilvy and Preziosi (2012), understanding the effect of carotenoids is a key issue when raising amphibians in captivity to save endangered species from extinction (Moore and Church, 2008).
Effects of carotenoids on metamorphs exposed to B. dendrobatidis
When exposed to Bd after metamorphosis, we observed a dramatic difference in outcome; wood frogs died in large numbers when exposed to Bd, whereas gray treefrogs did not. Such species-level variation in susceptibility has been observed in previous experiments (Gervasi et al., 2013). Our results are consistent with a recent comparative study by Searle et al. (2011), who that found wood frogs exposed to Bd had a much higher hazard ratio (ratio ± SEM = 77.8 ± 0.7) than gray treefrogs (3.0 ± 0.3) because of species-level differences in tolerance. In addition, Searle et al. (2011) found that the risk of mortality declined with body mass of the metamorphs, a result that was also observed in wood frogs but not gray treefrogs in the present study.
A primary focus of our study was to investigate whether carotenoids in the diet throughout the tadpole and early metamorph stages could mitigate mortality caused by Bd. We were unable to assess this hypothesis in gray treefrogs, because they experienced such low mortality after exposure to Bd. However, the lack of a carotenoid effect on pathogen load suggests that carotenoids were not having any mitigating effect in this species. In contrast, the wood frogs experienced high mortality after exposure to Bd, and carotenoids had no effect on their pathogen load or their survival. This was a surprising result given the current paradigm that carotenoids are important in boosting the innate and acquired immune systems (Olson and Owens, 1998; Chew and Park, 2004; Duriancik et al., 2010; Hall et al., 2011). For example, diets higher in carotenoids can increase antioxidant defenses and lower parasitism in birds (Horak et al., 2001; Alonso-Alvarez et al., 2004; Hill and Farmer, 2005), lower rates of oxidative stress in fish (Pike et al., 2007) and provide increased resistance to bacterial infections in amphipods (Babin et al., 2010). We found no evidence for a mitigating effect of carotenoids on Bd in amphibians, which may be because carotenoids do not boost the immune system of amphibians or they boost those components of the immune system that are not relevant to combating Bd infections. Alternatively, it may be that the immune system plays little or no role in combating Bd infections. However, the innate immune system appears to play a key role in producing skin peptides that possess antifungal properties (Rollins-Smith and Conlon, 2005; Woodhams et al., 2006), and the acquired immune system has been shown to play a role in responding to Bd infections in several species (reviewed by Richmond et al., 2009; Ramsey et al., 2010; Savage and Zamudio, 2011), although this is not a universal observation (Ribas et al., 2009; Cashins et al., 2013). Finally, there is the possibility that one might observe alternative outcomes at different concentrations of carotenoids than those used in the present study.
Conclusions
Carotenoids have received a great deal of attention for improving immune systems and serving as indicators of overall animal condition. However, we know relatively little about the effect of carotenoids in amphibians beyond the fact that they can contribute to brighter colouration in some species. We discovered that high-carotenoid diets can decrease survival, development and growth and may not play a role in mitigating resistance or tolerance to the global pathogen, Bd. While our study is the first to examine this question in amphibians, should the result be generalizable to other amphibian species, it suggests that providing carotenoids to amphibians rescued from Bd-positive regions of the world and held in captivity will not necessarily provide increased protection from this deadly pathogen. Clearly, much more work needs to be conducted on other amphibian species to determine the generality of our findings.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This research was funded by a National Science Foundation grant to R.A.R. and A.R.B. (DEB 07-16149, including an REU and RET supplement) and a National Graduate Women in Science Hartley Corporation Fellowship to S.S.G.
Data accessibility
All data and metadata are available at http://dx.doi.org/10.5061/dryad.1qb31
Supplementary Material
Acknowledgements
The research was conducted under the University of Pittsburgh's IACUC animal care protocol #12020108 and Oregon State University's IACUC animal care and use permit #4269.
Footnotes
On page 8, the section ‘Data accessibility’ has been updated.
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G. (2004) An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164: 651–659. [DOI] [PubMed] [Google Scholar]
- Antwis RE, Haworth RL, Engelmoer JP, Ogilvy V, Fidgett AL, Preziosi RF. (2014) Ex situ diet influences the bacterial community associated with the skin of red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 9: e85563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin A, Biard C, Moret Y. (2010) Dietary supplementation with carotenoids improves immunity without increasing its costs in a crustacean. Am Nat 176: 234–241. [DOI] [PubMed] [Google Scholar]
- Bell RC, Zamudio K. (2012) Sexual dichromatism in frogs: natural selection, sexual selection and unexpected diversity. Proc Biol Sci 279: 4687–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. (1993) Biological functions of dietary carotenoids. Annals NY Acad Sci 691: 61–67. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Skerratt LF. (2005) Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Organ 68: 65–70. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Han BA, Relyea RA, Johnson PTJ, Buck JC, Gervasi SS, Kats LB. (2011) The complexity of amphibian population declines: understanding the role of co-factors in driving amphibian losses. Ann NY Acad Sci 123: 108–119. [DOI] [PubMed] [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60: 141–148. [DOI] [PubMed] [Google Scholar]
- Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC. (2008) The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J Chem Ecol 34: 39–43. [DOI] [PubMed] [Google Scholar]
- Cashins SD, Grogan LF, McFadden M, Hunter D, Harlow PS, Berger L, Skerrat LF. (2013) Prior infection does not improve survival against the amphibian disease chytridiomycosis. PLoS ONE 8: e56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew BP. (1993) Role of carotenoids in the immune response. J Dairy Sci 76: 2804–2811. [DOI] [PubMed] [Google Scholar]
- Chew BP, Park JS. (2004) Carotenoid action on the immune response. J Nutr 134: 257S–261S. [DOI] [PubMed] [Google Scholar]
- Costantini D, Fanfani A, Dell'Omo G. (2007) Carotenoid availability does not limit the capability of nestling kestrels (Falco tinnunculus) to cope with oxidative stress. J Exp Biol 210: 1238–1244. [DOI] [PubMed] [Google Scholar]
- Dugas MB, Yeager J, Richards-Zawacki CL. (2013) Carotenoid supplementation enhances reproductive success in captive strawberry poison frogs (Oophaga pumilio). Zoo Biol 32: 655–658. [DOI] [PubMed] [Google Scholar]
- Duriancik DM, Lackey DE, Hoag KA. (2010) Vitamin A as a regulator of antigen presenting cells. J Nutr 140: 1395–1399. [DOI] [PubMed] [Google Scholar]
- Eeva T, Sillanpää S, Salminen J-P. (2009) The effects of diet quality and quantity on plumage colour and growth of great tit nestlings: a food manipulation experiment along a pollution gradient. J Avian Biol 40: 491–499. [Google Scholar]
- Fisher MC, Garner TWJ, Walker SF. (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time and host .Annu Rev Microbiol 63: 291–310. [DOI] [PubMed] [Google Scholar]
- Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Gayek AS, Dermody TS, Aune TM, Oswald-Richetr K, et al. (2013) The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MS, Zaccaria RA, Dent JN. (1973) Developmental cytology of chromatophores in the red-spotted newt. Am J Anat 138: 37–71. [DOI] [PubMed] [Google Scholar]
- Frost SK, Robinson S. (1984) Pigment cell differentiation in the fire-bellied toad, Bombina orientalis I. Structural, chemical, and physical aspects of the adult pigment pattern. J Morph 179: 229–242. [DOI] [PubMed] [Google Scholar]
- Gaillard MJ, Cézilly CF, Perrot-Minnot MJ. (2004) Carotenoids of two freshwater amphipod species (Gammarus pulex and G. roeseli) and their common acanthocephalan parasite Polymorphus minutus. Comp Biochem Physiol B Biochem Mol Biol 139: 129–136. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Gondhalekar C, Olson DH, Blaustein AR. (2013) Host identity matters in the amphibian-Batrachochytrium dendrobatidis system: fine-scale patterns of variation in responses to a multi-host pathogen. PLoS ONE 8: e54490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Richardson C, Lengagne T, Plenet S, Joly P, Léna J-P, Théry M. (2009) The role of nocturnal vision in mate choice: females prefer conspicuous males in the European tree frog (Hyla arborea). Proc Biol Sci 276: 2351–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner KL. (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190. [Google Scholar]
- Guerin M, Huntley ME, Olaizola M. (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21: 210–216. [DOI] [PubMed] [Google Scholar]
- Hall JA, Grainger JR, Spencer SP, Belkaid Y. (2011) The role of retinoic acid in tolerance and immunity. Immunity 35: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RC, Kennedy MW. (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19: 353–354. [DOI] [PubMed] [Google Scholar]
- Hill GE. (1995) Ornamental traits as indicators of environmental health. BioScience 45: 25–31. [Google Scholar]
- Hill GE, Farmer KL. (2005) Carotenoid-based plumage coloration predicts resistance to a novel parasite in the house finch. Naturwissenschaften 92: 30–34. [DOI] [PubMed] [Google Scholar]
- Horak P, Ots I, Vellau H, Spottiswoode C, Møller AP. (2001) Carotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits. Oecologia 126: 166–173. [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Skibsted LH. (1993) Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z Lebensm Unters Forsch 196: 423–429. [DOI] [PubMed] [Google Scholar]
- Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJGM. (2009) Genotoxic effects of carotenoid breakdown products in human retinal pigment epithelial cells. Curr Eye Res 34: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marantelli G, Berger L, Speare R, Keegan L. (2004) Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conserv Biol 10: 173–179. [Google Scholar]
- Matsui K, Takaichi S, Nakamura M. (2003) Morphological and biochemical changes in carotenoid granules in the ventral skin during growth of the Japanese newt Cynops pyrrhogaster. Zool Sci 20: 435–440. [DOI] [PubMed] [Google Scholar]
- Matsuno T. (2001) Aquatic animal carotenoids. Fish Sci 67: 771–783. [Google Scholar]
- Moore RD, Church DR. (2008) Implementing the Amphibian Conservation Action Plan. Intl Zoo Yearbook 42: 15–23. [Google Scholar]
- Ogilvy V, Preziosi RF. (2012) Can carotenoids mediate the potentially harmful effects of ultraviolet light in Silurana (Xenopus) tropicalis larvae? J Anim Physiol Anim Nutr 96: 693–699. [DOI] [PubMed] [Google Scholar]
- Ogilvy V, Preziosi RF, Fidgett AL. (2012) A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red-eyed tree frog. Anim Conserv 16: 480–488. [Google Scholar]
- Olaizola M, Huntley ME. (2003) Recent advances in commercial production of astaxanthin from microalgae. In Fingerman M, Nagabhushanam R, eds, Biomaterials and Bioprocessing. Science Publishers, Boca Raton, FL, pp. 143–164. [Google Scholar]
- Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, The Bd Mapping Group, Fisher MC. (2013) Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE 8: e56802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VA, Owens IPF. (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13: 510–514. [DOI] [PubMed] [Google Scholar]
- Pérez-Rodríguez L. (2009) Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. BioEssays 31: 1116–1126. [DOI] [PubMed] [Google Scholar]
- Pike TW, Blount JD, Bjerkeng B, Lindström J, Metcalfe NB. (2007) Carotenoids, oxidative stress and female mating preference for longer lived males. Proc Biol Sci 274: 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to amphibian declines, in the South African clawed frog, Xenopus laevis .Infect Immun 78: 3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas L, Li M-S, Doddington BJ, Robert J, Seidel JA, Kroll JS, Zimmerman LB, Grassly NC, Garner TWJ, Fisher MC. (2009) Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS ONE 4: e8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C, Popovici J, Bellvert F, Lengagne T. (2009) Conspicuous colouration of the vocal sac of a nocturnal chorusing treefrog: carotenoid-based? Amphibia-Reptilia 30: 576–580. [Google Scholar]
- Richmond JQ, Savage AE, Zamudio KR, Rosenblum EB. (2009) Towards immunogenetic studies of amphibian chytridiomycosis: linking innate and acquired immunity. BioScience 59: 311–320. [Google Scholar]
- Rollins-Smith LA. (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta 1788: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Conlon JM. (2005) Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol 29: 589–598. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Ramsey JP, Pask JD, Reinert LK, Woodhams DC. (2011) Amphibian immune defenses against chytridiomycosis: impacts of changing environments. Integr Comp Biol 51: 552–562. [DOI] [PubMed] [Google Scholar]
- Savage AE, Zamudio KR. (2011) MHC genotypes associate with resistance to a frog-killing fungus. Proc Natl Acad Sci USA 108: 16705–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiesari L, Werner EE, Kling GW. (2009) Carnivory and resource-based niche differentiation in anuran larvae: implications for food web and experimental ecology. Freshwater Biol 54: 572–586. [Google Scholar]
- Searle CL, Gervasi SS, Hua J, Hammond JJ, Relyea RA, Olson DH, Blaustein AR. (2011) Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv Biol 25: 965–974. [DOI] [PubMed] [Google Scholar]
- Simons MJP, Cohen AA, Verhulst S. (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds–a meta-analysis. PLoS ONE 7: e43088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MJP, Briga M, Leenknegt B, Verhulst S. (2014) Context-dependent effects of carotenoid supplementation on reproduction in zebra finches. Behav Ecol 25: 945–950. [Google Scholar]
- Skarstein F, Folstad I. (1996) Sexual dichromatism and the immunocompetence handicap: an observational approach using Arctic charr. Oikos 76: 359–367. [Google Scholar]
- Skerratt L, Berger L, Speare R, Cashins S, McDonald K, Phillott A, Hines HB, Kenyon N. (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4: 125–134. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Vershinin A. (1999) Biological functions of carotenoids—diversity and evolution. BioFactors 10: 99–104. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Rollins-Smith LA, Carey C, Reinert LK, Tyler MJ, Alford R. (2006) Population trends associated with skin peptide defenses against chytridiomycosis in Australian frogs. Oecologia 146: 531–540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and metadata are available at http://dx.doi.org/10.5061/dryad.1qb31