Decay and intra-sample variation of FCMs in tiger faeces were examined. FCM decayed under present climatic conditions (no rainfall and dry conditions) after 48 hours. FCMs were unevenly distributed within the entire faeces. Variation in FCMs can be reduced by homogenizing the entire faecal mass.
Keywords: Conservation physiology, field endocrine sampling, intra-sample variation, natural weathering, physiological stress, tiger (Panthera tigris)
Abstract
Evaluation of physiological stress in the tiger (Panthera tigris) using faecal cortisol metabolite (FCM) enzyme immunoassays (EIAs) provides a powerful conservation physiology tool for the species. However, it is important to validate non-invasive endocrine sampling techniques in field conditions to ensure that the method provides a reliable parameter of physiological stress in the species. This is because endocrine measurements are highly species specific and FCM concentrations can be influenced by environmental factors. Here, we studied the impact of the decay rate of FCMs and intra-sample variation of FCMs using a previously validated EIA. To determine the decay rate of FCMs, we measured FCMs in freshly deposited tiger faeces (n = 8 tigers and 48 scats) that were randomly exposed to the natural environment (dry conditions with no rainfall) for up to 192 h. To determine intra-sample variation in FCMs, we used 10 scats from 10 tigers, divided each sample into four sections and each section into four sub-sections and measured FCMs in each section and sub-section. The results of this decay-rate experiment showed that FCMs in tiger faeces began to decay after 48 h exposure to the environmental conditions available. Thus, FCMs within freshly deposited tiger faeces are influenced by available environmental conditions. Changes in weather conditions (e.g. increased rainfall and humidity) could influence the stability of FCMs. The results of the intra-sample variation study showed that inter-variation among scats accounted for 52% of the variations in FCMs, while intra-sample variation between sections (32%) was greater than the sub-sample variation (16%). Intra-sample variation can be reduced by homogenizing the entire lyophilized faecal sample prior to the EIA. In conclusion, careful evaluation of decay rate and complete homogenization of faeces prior to EIA analysis will increase the reliability of FCMs as a non-invasive index of physiological stress in the tiger.
Introduction
Endocrine assessments of physiological stress are crucial because of the known effects of extreme environmental factors on animal health, immunity, growth, reproduction, behaviour and survival (Sapolsky et al., 2000; Romero, 2004). Quantification of faecal cortisol metabolites (FCMs) in wild animal populations enables conservation biologists to gauge the impacts of extreme environmental factors in the natural environment that are contributing to species declines worldwide (Martin, 2009). Currently, field endocrinology research is increasing in wild and free-ranging populations of threatened animal species using non-invasive endocrinology methods based on faecal sampling (Barja et al., 2012; Breuner et al., 2013). This methodology has not been sufficiently tested for wider field applications, and crucial contextual factors that may influence species-specific FCM measurements remain untested (Millspaugh and Washburn, 2004; Barja et al., 2012). Current research practices have started to use non-invasive endocrine indices, such as FCMs, to monitor physiological stress in threatened wildlife species (e.g. African elephant, Foley et al., 2001; Iberian lynx, Jewgenow et al., 2006; Vargas et al., 2008; greater bilby, Narayan et al., 2012; koala, Narayan et al., 2013b). Despite such a strong emphasis on methodological validation, focus has somewhat been diverted away from the necessity to explore potential sources of error that can arise much earlier in the field sampling process, especially during sample collection and processing (Barja et al., 2012; Descovich et al., 2012; Evans et al., 2013).
Goymann (2012) highlighted the potential contextual factors that require close consideration when designing field endocrine sampling methods in wild animals living in uncontrolled natural environments. Factors such as sex-specific differences in hormone metabolism, diet, changes in environmental temperature, changes in food availability, and bacterial metabolism of hormones in the gut were discussed by Goymann (2012).
Hormone deterioration because of bacterial degradation in faeces is a major source of FCM variation (Beehner and Whitten, 2004). In instances where samples cannot be collected immediately and preserved (such as remote field studies), bacterial enzymes present in the faeces have been reported to degrade or alter hormone metabolite concentrations in samples that are left untreated, which increases the variation in glucocorticoid metabolites (Touma and Palme, 2005; Evans et al., 2013). The increased variation owing to natural weathering effects on faecal samples exposed to the environment can confound results and minimize the reliability of non-invasive endocrine methods for use in field conditions. Researchers are recognizing the influence of contextual environmental factors, such as climatic conditions, on the reliability of hormone metabolite concentrations in animal faeces. Most recently, Mesa-Cruz et al. (2014) studied the effects of natural environmental conditions on FCM concentrations in jaguars (Panthera onca), and their results showed that FCMs were stable for 5 days during the dry season but for <1 day during the wet season. A study by Abáigar et al. (2010) on the stability of FCMs of the Iberian lynx (Lynx pardinus) found that FCMs remained stable for at least 1 week in field conditions. Similar results have been returned from other studies, with no significant effects in FCM concentrations found for up to 1 week when preservation is delayed and samples are exposed to natural weathering conditions (Washburn and Millspaugh, 2002; Beehner and Whitten, 2004; Pappano et al., 2010). Despite this trend, it appears that FCM patterns are species specific. Over prolonged periods (more than 7 days), FCM levels increased in faeces of white-tailed deer (Odocoileus virginianus) and geladas (Theropithecus gelada; Washburn and Millspaugh, 2002; Pappano et al., 2010), but conversely, FCM levels decreased in faeces collected from baboons (Papio spp.; Beehner and Whitten, 2004). These results are possibly a result of the vastly different composition and nature of animal faeces and patterns of metabolism (Descovich et al., 2012). Evans et al. (2013) showed that FCMs were fairly stable to degradation by natural weathering in the endangered greater bilby (Macrotis lagotis); however, they also recommended collection of fresh samples with distinct odour for determining changes in FCMs in samples exposed to the natural environment.
Other studies have investigated the impact of sample treatment and storage conditions on changes in steroid metabolite concentrations resulting from bacterial degradation. Lynch et al. (2003) demonstrated the effects of short-term storage of raw faecal samples (prior to lyophilization) through increased ‘noise’ or temporal change in FCMs. Results showed that FCMs in raw faecal samples of wild baboons (Papio cynocephalus) declined slightly but significantly, highlighting the importance of determining the optimal sample storage conditions for each study based on logistical considerations, such as access to a freezer and a lyophilizer. Terio et al. (2002) demonstrated that storage of faecal samples at room temperature in ethanol was the best alternative to freezing for subsequent analysis of steroid hormone concentrations. Other studies have discussed the influence of various storage methods and long-term storage on the variability of FCMs in faecal samples (Hunt and Wasser, 2003; Kalbitzer and Heistermann, 2013; Davidian et al., 2015). It is also possible that bacterial enzymes present in faeces degrade hormone metabolites and change hormone metabolite concentrations when samples are treated and stored applying these specific conditions but kept for many years (frozen) in the laboratory (as discussed by Davidian et al., 2015).
As well as considering extrinsic sources of variation, researchers have also started to investigate intrinsic factors, such as how the distribution of FCM may vary within entire scats (Millspaugh and Washburn, 2003; Descovich et al., 2012). This consideration is important for the design of sampling methods for FCM monitoring. Hormone metabolites can be unequally distributed among faeces (Millspaugh and Washburn, 2003). For example, Brown et al. (1994) reported that faecal reproductive hormone metabolites (estrogens and progestins) were not evenly distributed within faeces collected from cheetahs (Acinonyx jubatus), snow leopards (Uncia uncia) and clouded leopards (Neofelis nebulosa). Furthermore, FCM concentrations are typically obtained from measuring one small aliquot of the total powdered sample [e.g. 0.2 g of lyophilized and powdered faecal sample used in each enzyme immunoassay (EIA)]. Before taking such an approach, however, we must first establish with confidence that sub-sectioning would not bias the EIA analysis because of unequal distribution of FCMs in the faecal sample.
The technical issues surrounding decay rate of FCMs and variability (intra-sample variation) in FCMs for the tiger (Panthera tigris) have not yet been investigated. The present study was designed and conducted to investigate experimentally the decay of FCMs through time and the variability in FCM measures within faecal samples for the tiger. Specifically, two research questions were investigated in experiments using faeces collected from captive tigers from a zoo in Australia. First, how do cortisol metabolites in tiger faeces change over time when sample preservation is delayed? Second, is there an effect of within-sample variation that would suggest unequal distribution of cortisol metabolites within tiger faeces? Investigation of these contextual sources of variation in FCM measurements are urgently needed to refine field endocrine sampling protocols for monitoring physiological stress in captive and wild tiger populations.
Materials and methods
Experiment 1: natural decay of faecal cortisol metabolites in unpreserved samples
We conducted an experiment to mimic the effect of natural weathering to determine the changes in FCM levels in tiger faeces over time when storage and preservation are delayed. Fresh faeces were collected in April 2013 from eight captive tigers (six males and two females) at the Dreamworld Themepark (Queensland, Australia). Each tiger was observed until it was seen defecating, and faeces were collected immediately (within 3 h of defecation). As captive tigers defecate on average only once a day, and owing to the unpredictability of this event coupled with limited access for sample collection between the hours of 08.00 and 17.00 h with only one observer, it took 7 days to obtain a fresh sample from each individual.
From each fresh scat, a section (approximately one-sixth) was divided and separated, bagged, labelled and immediately frozen at −20°C (0 h sub-sample) as a control. The remaining fresh portion of each sample was placed in an open container (20 cm × 15 cm) and positioned outside, where it was exposed to ambient temperatures and wind, in partial sunlight and shade throughout the day. Each sample was evenly sub-sectioned using a ruler (in millimetres) and pocket knife, with a portion being bagged, labelled and frozen at increasing time intervals (0, 12, 24, 48, 96 and 192 h after defecation) for a total of 8 days, until the last section was preserved at 192 h. In total, eight replicates (individuals), sampled at six time periods (0, 12, 24, 48, 96 and 192 h post-collection), totalling 48 faecal samples were used for the EIA. Mean environmental temperature ranged from 17.8 to 26.4°C, and no rainfall events occurred throughout the experiment, which prevented rehydration of the samples. Samples were dried for 24 h using a lyophilizer freeze dryer (IKEA, Australia). Dried faecal pellets were crushed to a fine powder, and a well-mixed aliquot of 0.2 g was placed in a 15 ml centrifuge tube (Sigma-Aldrich) and stored at −20°C until analysis (no more than 20 days).
Experiment 2: variability of faecal cortisol metabolites among and within tiger scats
A second experiment was designed to measure intra-sample variability in FCM measurements and to examine the distribution of FCMs within each tiger scat. This used faeces collected from ten tigers (six males and four females; the tigers and the fresh faeces were same as the ones sampled for the decay-rate experiment) at the Dreamworld Themepark (Queensland, Australia) in April 2013. One entire scat was collected from each individual tiger immediately after defecation. These samples were labelled (1–10) and immediately frozen at −20°C. In the laboratory, individual scats were divided into four equal sections, placed in separate bags and labelled (e.g. Sample 1A–1D) for testing the variation in FCMs between sections (i.e. within individuals) and faecal samples (i.e. between individuals). Each of these sections was homogenized after drying and pulverization into a powdered substance, and four well-mixed samples from each section were assayed (e.g. Sample 1A.i–1A.iv) to test within-sample variation (see Fig. 1 for diagrammatic illustration of the experimental design).
Figure 1:
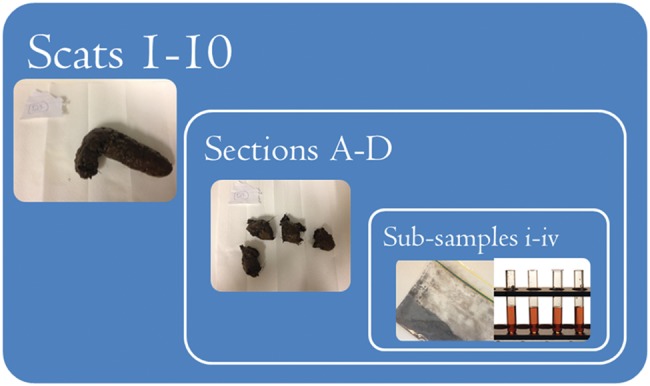
Nested ANOVA design to measure variability within tiger faeces. Ten tiger faecal samples were divided into four sections. Each section (n = 4) per faecal sample was homogenized, and four sub-samples were taken from each of the lyophilized and powered sections to measure faecal cortisol metabolite concentrations.
Enzyme immunoassays
Laboratory methods for the FCM EIA followed protocols described in our recent work (Narayan et al., 2013a). The suitability of this EIA in the tiger species was previously confirmed using biological and laboratory validation in this study (Narayan et al., 2013a).
Statistical analysis
In all analyses, FCM hormone data (expressed as FCM concentration divided by dry weight, in nanograms per gram) is the dependent variable. Data were tested for normality and equal variances to check statistical assumptions. Results were plotted using error bars, box plots, histograms and q-q plots of residuals. As variance in the original values was high and data not normally distributed, data were logarithmically transformed for statistical analysis. P-values were considered significant at α ≤ 0.05, and statistical analysis was conducted in SPSS (PASW version 21).
For Experiment 1, a linear mixed model was used, which treated sample as a random factor (to test for differences among individuals) and time as a repeated measure (testing within-sample effect) to examine the changes in FCM concentrations over time. The autoregressive 1 (AR1) model was selected as the best model to account for unequal distances between measured time intervals due to lowest Akaike information criterion value between ARI, AR2 and AR0 models (P < 0.05). Post hoc testing explored significant differences using least significant difference pairwise comparisons.
Experiment 2 data were analysed using a nested ANOVA to examine the variation in FCM concentrations between samples and the nested effects within samples (section of sample and repeated assays within sub-samples). This nested design partitioned the total variation in FCM among the sample, section and sub-sample.
Results
Experiment 1: natural decay of faecal cortisol metabolites in unpreserved samples
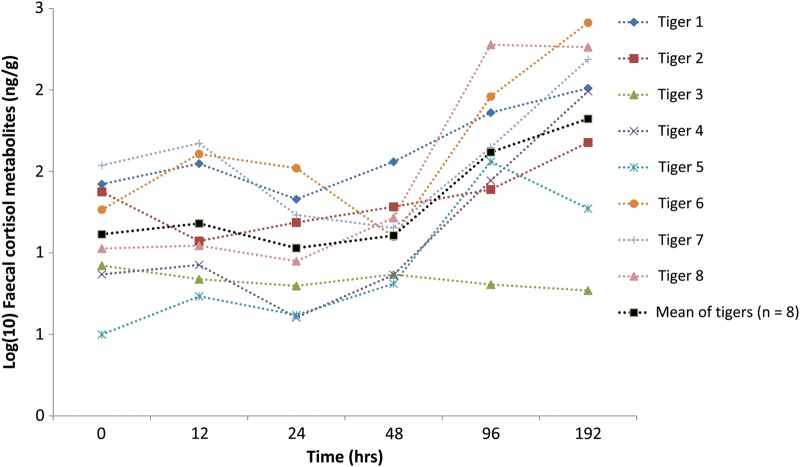
Faecal cortisol metabolite concentrations in tiger (n = 8) faecal samples repeatedly assayed over 8 days were depicted graphically to examine the variation in FCM concentrations detected between sections and between faeces (i.e. individuals) through time (Fig. 2). There was a significant effect of time on FCMs (F5,41 = 8.266; P < 0.001). Post hoc testing confirmed no significant differences in FCM values for samples preserved and assayed between 0 and 48 h, with FCM concentrations remaining relatively stable until this time.
Figure 2:
Change in faecal cortisol metabolite concentrations in samples of tiger faeces (n = 8), measured at time intervals between 0 and 192 h post-defecation. Symbols represent scats collected from each individual tiger.
Experiment 2: variability of faecal cortisol metabolites among and within tiger faeces
There was a large amount of variability in FCMs observed among faecal samples (inter-sample) and within faecal samples (intra-sample section and sub-samples within faecal samples; Fig. 3). Nested-effects ANOVA confirmed a significant effect both among scats (P < 0.001, F3,9 = 60.296) and among sections of scats (F3,9 = 9.059, P < 0.001). The total variance in FCM levels was partitioned in the nested ANOVA. Entire scats accounted for more than half of the variation (51.53%), section was responsible for 32.47% of variation, and 16% of variation was explained by sub-samples within sections.
Figure 3:
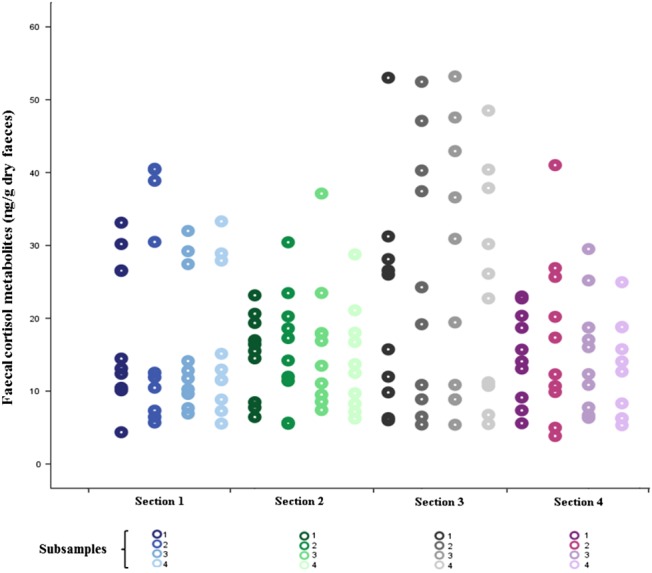
Variation in faecal cortisol metabolite concentrations nested within scats (n = 10). Whole faecal samples were divided into four sections, lyophilized and powered, from which four sub-samples were then assayed.
Discussion
We used a previously validated FCM EIA (Narayan et al., 2013a) to determine the influence of natural weathering and intra-sample variation in FCMs in captive tiger faeces (from an Australian zoo). The results from Experiment 1 demonstrated that concentrations of FCMs in tigers changed significantly as a result of natural weathering (dry conditions without rainfall) after 48 h. Microbial enzymes potentially break down steroidal metabolites in faecal pellets as soon as they are defecated by the animal and exposed to the environment (Khan et al., 2002; Buchanan and Goldsmith, 2004; Descovich et al., 2012), and fluctuations in metabolite concentrations in unpreserved faeces have previously been attributed to microbial degradation (Wasser et al., 1988; Khan et al., 2002). Our results indicate that environmental conditions need to be taken into close consideration, together with the age of faecal samples that are collected from the ground in zoos, semi-natural or wild settings. Results from Experiment 1 indicate that over time of environmental exposure, specificity of the targeted FCM metabolite may be lost. It is possible that cross-reactivity to conjugates of other glucocorticoid metabolites, such as corticosterone, also increases after 48 h (Buchanan and Goldsmith, 2004). Faecal cortisol metabolite concentrations were found to differ significantly after 48 h, and this evidence further emphasizes the importance of collecting and freezing samples as quickly as possible. Other studies have also highlighted the effect of delaying storage of faeces on hormone metabolite analysis, which may significantly alter EIA results, with effects differing between the species and the metabolite of interest being measured (Shutt et al., 2011; Descovich et al., 2012).
Some experiments have reported time to have no significant effect on FCMs measured in samples unpreserved for up to 1 week, in species such as the white-tailed deer, geladas and baboons (Washburn and Millspaugh, 2003; Beehner and Whitten, 2004; Pappano et al., 2010). Evans et al. (2013) detected increases in mean FCMs and variability in faeces from bilbies, but reported that FCM measures were relatively stable for up to 19 days. The rate of change of FCMs during exposure to natural weathering conditions appears to be faster in tiger faeces compared with greater bilby faeces. Shutt et al. (2011) and Möstl et al. (1999) measured variation in FCM values over much shorter periods (12 h) in faeces of the Western lowland gorilla (Gorilla gorilla gorilla) and domestic livestock. They reported increases in FCM concentrations, which showed an almost linear pattern, suggesting that it could be valuable to consider shorter-term changes (within minutes) in metabolite concentrations in faecal samples. Results from the present study indicate that variation in FCMs immediately after defecation would be small compared with the differences detected over longer periods of time (also reported by Narayan et al., 2013a).
As well as considering extrinsic environmental sources of variation, we explored intrinsic variation in FCM concentrations within tiger faeces. Investigating the distribution of FCM within a single scats has remained even less studied in the literature, compared with our knowledge on natural decay of FCMs in degrading samples. Descovich et al. (2012) also suggested that field endocrinology study designs need to quantify the effect of intra-sample variation, which will ensure that sources of error or variation are correctly identified. As expected, we found that the majority of variation in FCMs of tiger faeces was attributed to between-individual faeces (52%). However, the high variability detected between sections of scats (32%) suggests unequal distribution of FCMs in tiger faeces, which must be considered in future sampling design. The variability among sections (32%) was double the variation explained by repeated sub-samples within homogenized sections (16%). Variability associated with intra-sample variation can therefore be reduced by homogenizing entire scats, rather than taking only a section for sampling. This consideration in the sampling design would eliminate the effect of section and remove 32% of the variability associated with FCM measures obtained from only a portion of scats. Following these guidelines will significantly reduce error in the data obtained and increase reliability and accuracy of FCM assessments using the EIA methodology to monitor tigers, giving a value of FCM concentration that is representative of the entire scat.
Reduced variation associated with taking sub-samples from homogenized samples to measure FCMs strengthens the reliability of the EIA methodology that only uses a small portion (e.g. 0.2 g lyophilized and powered faeces) for assaying. We highlight that the detection of low variation in FCM measures from small aliquots of well-mixed homogenized samples (16% in this study) indicates that caution should be taken when interpreting results, and values obtained should not be treated as point values without considering the associated error margin. It is important rather to consider that each sample estimate represents a range that factors in this variation (Washburn and Millspaugh, 2003).
In conclusion, based on the results of our experiments, we recommend the preservation and extraction of glucocorticoid metabolites from tiger faeces to occur within a window of 48 h, provided the weather conditions are similar to the present study. It is important that each field endocrinology study takes the environmental data into consideration for interpretation of results. This is because climatic conditions during exposure of faeces to the natural environment (e.g. variation in rainfall, temperature and humidity) could potentially affect the FCM variation in tigers. For example, based on the results from previous studies (e.g. Washburn and Millspaugh, 2002, who simulated rainfalls), we postulate that precipitations would have accelerated bacterial degradation and hence increased concentrations even more and before 48 h. Therefore, more frequent sampling and sample preservation should be prioritized (i.e. sampling earlier than 48 h). The 48 h window should not be used as a gold standard for all studies because climatic conditions can vary between locations.
Furthermore, if the whole scat is collected for analysis and homogenized, the effect of intra-sample variation is greatly reduced, and derived results will give more accurate FCM measures that are representative of the true concentration contained in the sample. By following these standard sampling methods, we can monitor adrenal activity in tigers and use FCMs as a powerful endocrine tool for the management and conservation of tigers.
Funding
Funding was provided by the School of Environment, Griffith University, Gold Coast Campus, QLD 4222, Australia and Dreamworld.
Acknowledgements
We gratefully acknowledge the cooperation of staff at the tiger captive breeding facility at Dreamworld Themepark (Gold Coast, Australia) for collection of samples. The work represents a chapter of an Honours research project of T.P. supervised jointly by J.-M.H. and E.J.N. We thank two anonymous reviewers for their useful comments on the first submission.
References
- Abáigar T, Domené MA, Palomares F. (2010) Effects of fecal age and seasonality on steroid hormone concentration as a reproductive parameter in field studies. Eur J Wildl Res 56: 781–787. [Google Scholar]
- Barja I, Escribano-Ávila G, Lara-Romero C, Virgos E, Benito J, Rafart E. (2012) Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim Biol 62: 419–432. [Google Scholar]
- Beehner JC, Whitten PL. (2004) Modifications of a field method for fecal steroid analysis in baboons. Physiol Behav 82: 269–277. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Delehanty B, Boonstra R. (2013) Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct Ecol 27: 24–36. [Google Scholar]
- Brown JL, Wasser SK, Wildt DE, Graham LH. (1994) Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in faeces. Biol Reprod 51: 776–786. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Goldsmith AR. (2004) Noninvasive endocrine data for behavioural studies: the importance of validation. Anim Behav 67: 183–185. [Google Scholar]
- Davidian E, Benhaiem S, Courtiol A, Hofer H, Höner OP, Dehnhard M. (2015) Determining hormone metabolite concentrations when enzyme immunoassay accuracy varies over time. Methods Ecol Evol 6: 576–583. [Google Scholar]
- Descovich KA, Lisle AT, Johnston S, Keeley T, Phillips CJ. (2012) Intrasample variation and the effect of storage delay on faecal metabolite concentrations in the southern hairy-nosed wombat (Lasiorhinus latifrons). Aust Mammal 34: 217–222. [Google Scholar]
- Evans N, Narayan E, Hero J-M. (2013) Effects of natural weathering conditions on glucocorticoid metabolite measurements in the greater bilby faeces. Aust J Zool 61: 351–356. [Google Scholar]
- Foley CA, Papageorge S, Wasser SK. (2001) Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv Biol 15: 1134–1142. [Google Scholar]
- Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- Hunt KE, Wasser SK. (2003) Effect of long-term preservation methods on fecal glucocorticoid concentrations of grizzly bear and African elephant. Physiol Biomed Zool 76: 918–928. [DOI] [PubMed] [Google Scholar]
- Jewgenow K, Naidenko SV, Goeritz F, Vargas A, Dehnhard M. (2006) Monitoring testicular activity of male Eurasian (Lynx lynx) and Iberian (Lynx pardinus) lynx by fecal testosterone metabolite measurement. Gen Comp Endocrinol 149: 151–158. [DOI] [PubMed] [Google Scholar]
- Kalbitzer U, Heistermann M. (2013) Long-term storage effects in steroid metabolite extracts from baboon (Papio sp.) faeces – a comparison of three commonly applied storage methods. Methods Ecol Evol 4: 493–500. [Google Scholar]
- Khan M, Altmann J, Isani S, Yu J. (2002) A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen Comp Endocrinol 128: 57–64. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N, Rubenstein N. (2003) Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen Comp Endocrinol 132: 264–271. [DOI] [PubMed] [Google Scholar]
- Martin LB. (2009) Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol 163: 70–76. [DOI] [PubMed] [Google Scholar]
- Mesa-Cruz JB, Brown JL, Kelly MJ. (2014) Effects of natural environmental conditions on faecal glucocorticoid metabolite concentrations in jaguars (Panthera onca) in Belize. Conserv Physiol 2: doi:10.1093/conphys/cou039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE. (2003) Within-sample variation of fecal glucocorticoid measurements. Gen Comp Endocrinol 132: 21–26. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE. (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–199. [DOI] [PubMed] [Google Scholar]
- Möstl E, Messmann S, Bagu E, Robia C, Palme R. (1999) Measurement of glucocorticoid metabolite concentrations in faeces of domestic livestock. J Vet Med A-Physiol Pathol Clin Med 46: 621–631. [DOI] [PubMed] [Google Scholar]
- Narayan E, Evans N, Nicolson V, Mucci A, Hero J-M. (2012) Non-invasive monitoring of physiological stress hormone responses in a captive population of the Greater Bilby (Macrotis lagotis). Endangered Species Res 18: 279–289. [Google Scholar]
- Narayan E, Clark G, Martin-Vegue P, Parnell T, Mucci A, Hero J-M. (2013a) Faecal cortisol metabolite levels in Bengal (Panthera tigris tigris) and Sumatran tigers (Panthera tigris sumatrae). Gen Comp Endocrinol 194: 318–325. [DOI] [PubMed] [Google Scholar]
- Narayan E, Webster K, Nicolson V, Hero J-M. (2013b) Non-invasive evaluation of physiological stress in an iconic Australian marsupial: the koala (Phascolarctos cinereus). Gen Comp Endocrinol 187: 39–47. [DOI] [PubMed] [Google Scholar]
- Pappano DJ, Roberts EK, Beehner JC. (2010) Testing extraction and storage parameters for a fecal hormone method. Am J Primatol 72: 934–941. [DOI] [PubMed] [Google Scholar]
- Romero LM. (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19: 249–255. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Shutt K, Setchell JM, Heistermann M. (2011) Non-invasive monitoring of physiological stress in the western lowland gorilla (Gorilla gorilla gorilla): validation of a fecal glucocorticoid assay and methods for practical application in the field. Gen Comp Endocrinol 179: 167–177. [DOI] [PubMed] [Google Scholar]
- Terio KA, Brown JL, Moreland R, Munson L. (2002) Comparison of different drying and storage methods on quantifiable concentrations of fecal steroids in the cheetah. Zoo Biol 21: 215–222. [Google Scholar]
- Touma C, Palme R. (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046: 54–74. [DOI] [PubMed] [Google Scholar]
- Vargas A, Sanchez I, Martinez F, Rivas A, Godoy JA, Roldán E, Simón MA, Serra R, Perez MJ, Euseñat C, et al. (2008) The Iberian lynx Lynx pardinus conservation breeding program. Dev Zoo World 42: 190–198. [Google Scholar]
- Washburn BE, Millspaugh JJ. (2002) Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer faeces. Gen Comp Endocrinol 127: 217–222. [DOI] [PubMed] [Google Scholar]
- Washburn BE, Millspaugh JJ. (2003) Within sample variation of fecal glucocorticoid measurements. Gen Comp Endocrinol 132: 21–26. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Risler L, Steiner RA. (1988) Excreted steroids in primate faeces over the menstrual cycle and pregnancy. Biol Reprod 39: 862–872. [DOI] [PubMed] [Google Scholar]