Common-garden experiments suggest that the response of Atlantic cod larvae to temperature differs among populations that spawn at different times of year. Populations appear to be adapted to the temperatures experienced during the larval stage at a small spatial scale, despite a lack of physical barriers to gene flow.
Keywords: Atlantic cod, climate change, common-garden experiment, Gadus morhua, genotype-by-environment interaction, thermal adaptation
Abstract
The level of phenotypic plasticity displayed within a population (i.e. the slope of the reaction norm) reflects the short-term response of a population to environmental change, while variation in reaction norm slopes among populations reflects spatial variation in these responses. Thus far, studies of thermal reaction norm variation have focused on geographically driven adaptation among different latitudes, altitudes or habitats. Yet, thermal variability is a function of both space and time. For organisms that reproduce at different times of year, such variation has the potential to promote adaptive variability in thermal responses for critical early life stages. Using common-garden experiments, we examined the spatial scale of genetic variation in thermal plasticity for early life-history traits among five populations of endangered Atlantic cod (Gadus morhua) that spawn at different times of year. Patterns of plasticity for larval growth and survival suggest that population responses to climate change will differ substantially, with increasing water temperatures posing a considerably greater threat to autumn-spawning cod than to those that spawn in winter or spring. Adaptation to seasonal cooling or warming experienced during the larval stage is suggested as a possible cause. Furthermore, populations that experience relatively cold temperatures during early life might be more sensitive to changes in temperature. Substantial divergence in adaptive traits was evident at a smaller spatial scale than has previously been shown for a marine fish with no apparent physical barriers to gene flow (∼200 km). Our findings highlight the need to consider the impact of intraspecific variation in reproductive timing on thermal adaptation when forecasting the effects of climate change on animal populations.
Introduction
In the face of environmental disturbance, the future of a species depends on the extent to which populations respond differently to changes in their environment and the spatial correspondence between the scale of disturbance and the scale of adaptation (Hutchings et al., 2007). Phenotypic plasticity is a primary mechanism by which populations might respond to environmental change in both the short and long term, by serving as a buffer against environmental variability (Canale and Henry, 2010; Nicotra et al., 2010) and facilitating adaptation to new environments (Lande, 2009; Chevin et al., 2010). Genetic variation in reaction norms (the range of phenotypes expressed by a genotype along an environmental gradient; Woltereck, 1909; Schmalhausen, 1949) suggests that plasticity can evolve in response to local environmental regimes (e.g. Liefting et al., 2009; McCairns and Bernatchez, 2010; Baumann and Conover, 2011; De Block et al., 2013). Such population variation in plasticity represents differences in the ways populations are likely to respond to directional environmental change, such as the forecasted increase in temperature due to global climate change. Understanding the mechanisms responsible for shaping population variation in responses and the spatial scales at which adaptive differences in plasticity occur is critical for predicting the persistence of a species in the face of climate change and managing populations effectively to mitigate the potential for population collapse and biodiversity loss.
Intraspecific variation in thermal reaction norms has been documented for a variety of taxa, including insects (e.g. Van Asch et al., 2007; Winterhalter and Mousseau, 2007; Liefting and Ellers, 2008), reptiles (e.g. Sinervo and Adolph, 1994; Bronikowski, 2000), amphibians (e.g. Ficetola and De Bernardi, 2005; Richter-Boix et al., 2010) and fishes (e.g. Conover and Present, 1990; Yamahira et al., 2007; Jensen et al., 2008). Such variation is usually quantified at broad spatial scales and is quite possibly a result of adaptation to seasonality or temperature along a latitudinal cline. Population differences in thermal responses have also been reported at very small spatial scales when thermal regimes differ between nearby habitats [e.g. 5–20 km in the garter snake, Thamnophis elegans (Bronikowski, 2000); <1 km in the soil arthropod, Orchesella cincta (Liefting and Ellers, 2008)] or altitudes [e.g. 50–60 km in the frog, Rana latastei (Ficetola and De Bernardi, 2005)].
Environments can vary both spatially and temporally. For traits related to reproduction or early life stages, intraspecific variation in the timing of reproduction can theoretically promote adaptive variability independent of geographical variation due to temporal variability in selective pressures. Studies of adaptive divergence among groups of individuals that reproduce at different times of year (i.e. isolation-/adaptation- by-time) are seemingly rare, despite variation in reproductive timing existing in a wide variety of taxa (reviewed by Hendry and Day, 2005). Richter-Boix et al. (2013) demonstrated a correlation between larval life-history traits and reproductive timing among moor frog (Rana arvalis) populations that each have an explosive breeding period of a few days within a 22 day span. Lower growth rate and a longer larval period were associated with early breeding and wetlands with warmer water temperatures, despite high gene flow throughout the study area. On a broader temporal scale, Fraser et al. (2011) suggested the potential for adaptive divergence between spring, summer and winter breeding runs within rivers in some salmonids (e.g. Waples et al., 2004), although this has not been explicitly tested. Given that thermal regimes can vary drastically through the year in temperate climates, variation in reproductive timing might promote cryptic genetic variation in thermal responses within species.
We examined genetic variation in early life-history trait plasticity in Atlantic cod (Gadus morhua; hereafter, cod), a demersal marine fish of widespread ecological and socioeconomic importance that is threatened over much of its range by the compound effects of climate change and overfishing (O'Brien et al., 2000; Committee On the Status of Endangered Wildlife In Canada, 2010; Hutchings and Rangeley, 2011). The collapse of Canadian cod stocks in the early 1990s was biologically, socially and economically devastating (Templeman, 2010; Hutchings and Rangeley, 2011). Despite a moratorium on fishing since 1992, most stocks have shown little or no recovery (Hutchings, 2000; Hutchings and Rangeley, 2011). However, variable rates of recovery among stocks underscore the need to understand the underlying genetic differences among stocks for traits that are likely to be influencing recovery.
Cod inhabit coastal waters throughout the North Atlantic characterized by a variety of thermal regimes that promote localized thermal adaptation (Hutchings et al., 2007; Bradbury et al., 2010, 2013). In addition, different groups of cod spawn at different times of year (e.g. Lett, 1980; Brander and Hurley, 1992; Myers et al., 1993). Some management units, such as the Southern designatable unit (DU) on the Southwestern Scotian Shelf in Canada (Committee On the Status of Endangered Wildlife In Canada, 2010), contain multiple spawning components that collectively spawn through most of the year (from September to June; Brander and Hurley, 1992). The temporally stable genetic structure among local spawning groups (reviewed by Ruzzante et al., 1999) and persistent differences in spawning times among populations kept in a common environment over multiple years (Otterå et al., 2006) suggest that spawning time is, in part, heritable in cod, as in other fishes (e.g. Siitonen and Gall, 1989; Danzmann et al., 1994; Sakamoto et al., 1999; Rogers et al., 2006). Therefore, variation in spawning times is expected to manifest in adaptive genetic differences at small spatial scales. Indeed, Marcil et al. (2006) found differences in body shape plasticity between neighbouring spawning components of the Scotian Shelf that experience peak spawning 1 month apart. However, the adaptive significance of these differences is not known.
We constructed thermal reaction norms for two adaptive traits: larval growth (whereby faster growth increases fitness by reducing the duration of the vulnerable pelagic larval phase; Anderson, 1988) and survival. We compared these responses among groups of cod that spawn at different times of year (hereafter, ‘spawning groups’ and ‘populations’ are used interchangeably). Our objectives were to investigate the role of variation in spawning time in promoting adaptive divergence in thermal plasticity in cod and to determine whether genetic variation in plasticity for adaptive traits exists among spawning groups at a smaller spatial scale than that of the current management units. We discuss the short- and long-term implications of our findings in light of predicted changes in climate.
Materials and methods
Study populations
Six common-garden experiments were conducted on the following five populations of cod that were collected between 218 and 1140 km apart (Fig. 1a): (i) Bay of Fundy [Northwest Atlantic Fisheries Organization (NAFO) division 4X; Southern DU]; (ii) Southwestern Scotian Shelf near Sambro, Nova Scotia (NAFO division 4X; Southern DU); (iii) Southern Gulf of St Lawrence (NAFO division 4T; Laurentian South DU); (iv) Bonavista Bay, Newfoundland (NAFO division 3L; Newfoundland and Labrador DU); and (v) Placentia Bay, Newfoundland (NAFO division 3Ps; Laurentian North DU; Committee On the Status of Endangered Wildlife In Canada, 2010). Cod from these areas will be referred to as Fundy, Sambro, Southern Gulf, Bonavista and Placentia, respectively.
Figure 1:
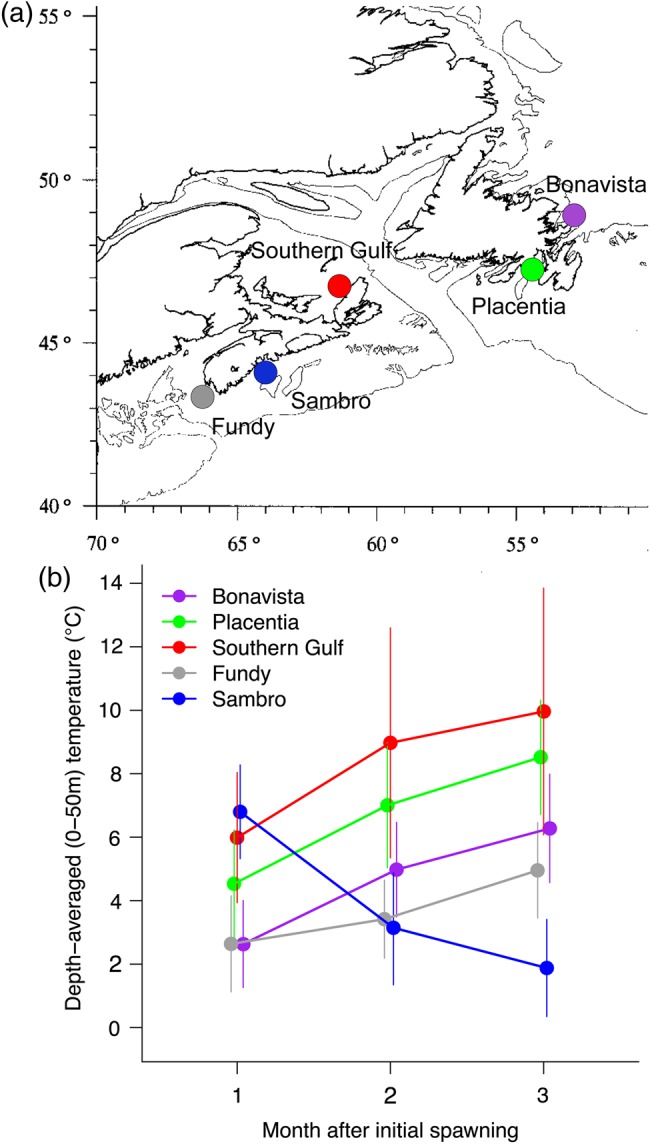
(a) Sampling locations of spawning adults for study populations of Atlantic cod (Gadus morhua). (b) Depth-averaged (0–50 m) water temperatures (in degrees Celsius ± 1 SD) for the first 3 months after the initial peak spawning months [May (Bonavista, Placentia and Southern Gulf), February (Fundy) and November (Sambro)]. Mean temperatures were estimated by using all available data from 1914 to 2009 in the Bedford Institute of Oceanography's Hydrographic Climate Database (http://www.bio.gc.ca/science/data-donnees/base/climate-climat-eng.php).
Fundy and Sambro cod are two of what are thought to be multiple spawning components in the Southwestern Scotian Shelf, based in part on spatial differences in spawning times. Peak spawning of Fundy cod occurs in February (Hutchings et al., 2007), while Sambro cod have a peak spawning period from November to December (Brander and Hurley, 1992; Hutchings et al., 1999). The remaining populations experience peak spawning from May to June (Lett, 1980; Myers et al., 1993). Due to differences in spawning times and locations, populations experience different temperature regimes during early life (Fig. 1b; see Supplementary Fig. 1 for year-round temperature profiles). Fundy and Sambro larvae experience relatively cold temperatures, with more stable temperatures for Fundy compared with Sambro, while Southern Gulf, Bonavista and Placentia larvae experience relatively warm and variable temperatures.
Common-garden experiments
Common-garden experiments on Fundy, Southern Gulf, Bonavista and Placentia cod were conducted by Hutchings et al. (2007) from 2002 to 2003. We performed additional experiments on Sambro and Southern Gulf cod from 2011 to 2012, using similar protocols. Briefly, the methods are as follows.
Between 34 and 73 wild-caught adults from each population were obtained during (Bonavista only) or immediately prior to their respective breeding seasons, i.e. in June 2003 (Bonavista), April 2002 (Placentia), May 2003 and 2011 (Southern Gulf), January 2002 (Fundy) and November 2011 (Sambro). Adult cod spawned undisturbed either in the 684 m3 Pool Tank in the Aquatron Laboratory at Dalhousie University (Fundy, Sambro and Southern Gulf) or at the Oceans Sciences Centre at Memorial University of Newfoundland (Bonavista and Placentia); all cod were held at ∼8°C.
Common-garden experiments took place at Dalhousie University (Sambro and Southern Gulf 2011) or at the Ocean Sciences Centre (Fundy, Southern Gulf 2003, Bonavista and Placentia), to which fertilized eggs from Dalhousie University were transported if necessary. Fertilized eggs were collected 2–4 weeks after they were first observed in mesh collectors positioned at the surface outflows of the tanks. Eggs were incubated at 7°C until hatch, at which time larvae were transferred to 20 l (Dalhousie) or 30 l tanks (Memorial). Larvae were reared at two temperatures (7°C ± 1 and 11°C ± 1°C) with three (Sambro only) or four replicate tanks per treatment and 1200 larvae per replicate. On the day of transfer, all tanks were set to 7°C. The following day, the water in the high-temperature treatments was gradually changed to 11°C over the course of 12 h. Larvae were fed rotifers in excess (4500 prey/litre, corresponding to the high-food treatment of Hutchings et al. 2007), three times per day (at ∼09,00, 13.00 and 17.00 h). Larvae were fed Isochrysis-enriched rotifers from day 1 to 10, Ori-Green (Skretting)-enriched rotifers from day 11 to 31, a 1:1 mixture of rotifers and Artemia from day 32 to 39 and Artemia only from day 40 to 43. Larvae were reared under a light intensity of 2000 lux, and water temperatures were monitored daily. Standard length at hatch was measured for 40–80 (mean = 65) randomly sampled larvae per population using AxioVision image analysis software (Zeiss). Length at 29 days post-hatch was measured for 10 randomly sampled larvae per replicate (except for Fundy and Bonavista, for which five and six larvae were measured per replicate, respectively) and used as a proxy for growth following Hutchings et al. (2007). Survival was quantified as the mean number of larvae alive in each tank on day 43 relative to day 0 and was not corrected for sampling mortality.
Evaluation of the number of families
To evaluate the assumption that a substantial number of families from each population were represented in the experiment, adults (post-spawning) and larvae were genotyped at five to seven microsatellite loci, according to the methods described by Hardie et al. (2006). Genotypes were analysed using PAPA v.2.0 (Duchesne et al., 2002) or COLONY (Jones and Wang, 2010) to assign parentage (see Hutchings et al., 2007; and Supplementary data for further detail). Based on these analyses, the number of families represented in the experiment was at least 21, 29, 31, 15, 71 and 44 for Fundy, Sambro, Southern Gulf (in 2003), Southern Gulf (in 2011), Bonavista and Placentia, respectively. In general, similar minimal numbers of families were identified in samples from the beginning and end of the experiments (excepting Fundy, but note differences in sample sizes between time points) and genetic differentiation between time points within experiments was low (Supplementary data).
Reaction norm analyses
All statistical analyses were performed in R (R Development Core Team, 2012). We performed a one-way analysis of variance (ANOVA) on length at hatch to determine whether it differed among populations and tested for linear relationships between length at hatch and reaction norm slopes. Using a linear mixed-effects model, we constructed reaction norms for larval growth for each population. Population, temperature and their interaction were used as fixed effects and tank as a random effect nested within temperature. To facilitate visual comparison of reaction norm slopes, mean larval length at each temperature was plotted relative to the lowest mean length observed in each population.
Assuming that reaction norm variation is greater among families than within them, there is the potential for pseudoreplication to increase the power of the test artificially for population differences in growth. We invalidated this assumption for Sambro (the only experiment for which family data were available; for details see Supplementary data). We do not know if the same is true for the remaining populations. However, the minimal number of families present at the end of the Bonavista and Placentia experiments (Supplementary data) exceeds the sample size for the growth analysis (n = 40); therefore, the probability of resampling a particular family is low. Thus, the potential impact of pseudoreplication on the test for population differences in growth is likely to be minimal.
To resolve whether variation in reaction norms could be attributed to the fact that experiments were conducted at different times and locations with slightly different protocols, we compared reaction norms for two experiments involving Southern Gulf cod that were carried out in 2003 and 2011. A two-way ANOVA revealed that, although the elevations (i.e. mean trait values) of the reaction norms differed for growth (F = 5.94; P1,82 = 0.017; Supplementary data) and survival (P < 0.001; Supplementary data), the slopes did not (F = 0.957; P1,82 = 0.331 and P = 0.932 for growth and survival, respectively). Thus, comparison of reaction norm slopes among these experiments is unlikely to be confounded by temporal or experimental variation, although the same cannot be said for reaction norm elevations. Given that the mean trait values differed between the Southern Gulf experiments, the data could not be combined. Of the two Southern Gulf experiments, we retained only the 2003 experiment for further analyses due to higher mortality in the 2011 experiment resulting in the early termination of some tanks. This elevated mortality was unrelated to temperature (P = 0.328; Supplementary data).
To evaluate whether variation in density among tanks due to differential survival may have contributed to tank effects observed in the growth model, we examined whether there was a relationship between the magnitude and direction of random effects and tank density. We used survival on day 43 as a proxy for density. A plot of random effect size against survival showed no association (Supplementary Fig. 2); therefore, differential tank density was not considered to be responsible for variation in growth among tanks.
We constructed survival reaction norms using back-transformed model estimates from a generalized linear model with a quasi-binomial distribution and logit link. Population, temperature and their interaction were the fixed effects. Mean survival at each temperature was then plotted relative to the highest mean survival observed in each population. To test for a significant genotype × environment interaction, the identity link was used instead of the logit link so that the reaction norm intercepts did not influence the test.
Results
Reaction norm variation
Larval length at hatch differed among populations (F5 = 117.13; P < 0.001), with Fundy and Sambro larvae generally being larger than the remaining populations (Supplementary data). However, there was no relationship between length at hatch and growth (F = 0.68; P1,3 = 0.47) or survival (F = 0.11; P1,3 = 0.76) responses.
We found substantial population variation in thermal responses for growth (Fig. 2), manifested by a significant population × temperature interaction (F = 4.78; P4,261 = 0.001; Table 1). Plasticity for growth in response to temperature was evident in all populations except for Sambro and was such that larvae grew faster with the high-temperature treatment (Table 2). In contrast, growth of Sambro larvae did not differ between temperature treatments (t = 0.13, P = 0.450). After correcting for multiple comparisons, differences in reaction norm slopes were significant or marginally significant between Sambro and all other populations except Bonavista, for which the difference was significant before the correction (Table 3). Among the populations that exhibited plasticity, the magnitudes of the responses were similar. The uncorrected results suggest that the slope of the response of Placentia larvae was steeper than that of Bonavista (t = 1.79, P = 0.043) and marginally steeper than that of Southern Gulf (t = −1.33, P = 0.097; Table 3). However, these differences were not significant after correcting for multiple comparisons.
Figure 2:
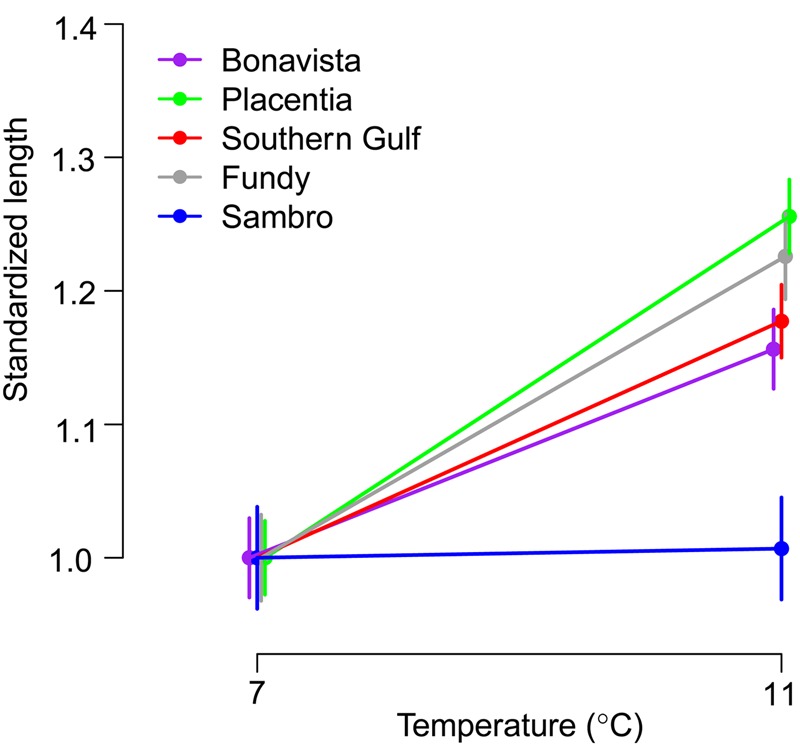
Thermal reaction norms for larval cod growth (±1 SEM), standardized relative to the lowest mean length observed in each population.
Table 1:
Effects of population and temperature on larval cod growth
| Model term | d.f. | Sum of squares | Mean of squares | F | P-value |
|---|---|---|---|---|---|
| Population | 4 | 36.74 | 9.19 | 28.38 | <0.001* |
| Temperature | 1 | 25.92 | 25.92 | 80.07 | <0.001* |
| Population × temperature | 4 | 6.19 | 1.55 | 4.78 | 0.001* |
| Model term | Variance | SD | |||
| Tank | 0.15 | 0.39 | |||
| Residual | 0.32 | 0.57 |
Asterisk denotes significance at α = 0.05.
Table 2:
Effect of temperature on larval growth for five cod populations, where the estimate represents the change in growth from 7 to 11°C
| Population | Estimate | SEM | t | P-value |
|---|---|---|---|---|
| Bonavista | 1.19 | 0.32 | 3.72 | <0.001* |
| Placentia | 1.97 | 0.30 | 6.54 | <0.001* |
| Southern Gulf | 1.38 | 0.33 | 4.25 | <0.001* |
| Fundy | 1.62 | 0.33 | 4.95 | <0.001* |
| Sambro | 0.04 | 0.35 | 0.13 | 0.450 |
Asterisk denotes significance at α = 0.05 after Bonferroni correction.
Table 3:
Pairwise population contrasts of the effect of temperature on larval cod growth
| Bonavista | Placentia | Southern Gulf | Fundy | Sambro | |
|---|---|---|---|---|---|
| Bonavista | — | 0.79 (±0.44) | 0.20 (±0.46) | 0.44 (±0.46) | −1.14 (±0.47) |
| Placentia | 0.043†† | — | −0.59 (±0.44) | −0.35 (±0.45) | −1.93 (±0.46) |
| Southern Gulf | 0.336 | 0.097† | — | 0.24 (±0.46) | −1.34 (±0.48) |
| Fundy | 0.177 | 0.219 | 0.308 | — | −1.58 (±0.48) |
| Sambro | 0.012†† | <0.001** | 0.004* | 0.002** | — |
Estimates (±SEM) are given above the diagonal and P-values below. The point of contrast for the estimates is the row header. Symbols denote significance at the following levels of α: *0.10 and **0.05 (with Bonferroni correction), †0.10 and ††0.05 (without Bonferroni correction). A Bonferroni correction for all contrasts of interest (n = 15) changes the critical P-values to 0.007 (α = 0.10) and 0.003 (α = 0.05).
Significant variation in survival reaction norm slopes was observed between populations (P < 0.001; Fig. 3 and Table 4). Fundy larvae exhibited a high degree of plasticity, with a significant positive relationship between survival and temperature (P < 0.001; Table 5) and 2.5 times greater survival in the high-temperature treatment. The opposite response was observed in Sambro larvae, with survival for the low-temperature treatment being more than three times greater than survival for the high-temperature treatment, although this effect was not significant after correcting for multiple comparisons (P = 0.009). Several populations (Southern Gulf, Bonavista and Placentia) exhibited no plasticity. The slopes of these populations were significantly (Placentia and Southern Gulf; P = 0.031 for both) or marginally significantly (Bonavista; P = 0.108) different from that of Fundy and significantly (Bonavista and Placentia; P = 0.010 and P = 0.044, respectively) or marginally significantly (Southern Gulf; P = 0.092) different from Sambro (Table 6). However, none of these differences was significant after a Bonferroni correction. Only the responses of Fundy and Sambro larvae were significantly different after correcting for multiple comparisons (P < 0.001).
Figure 3:
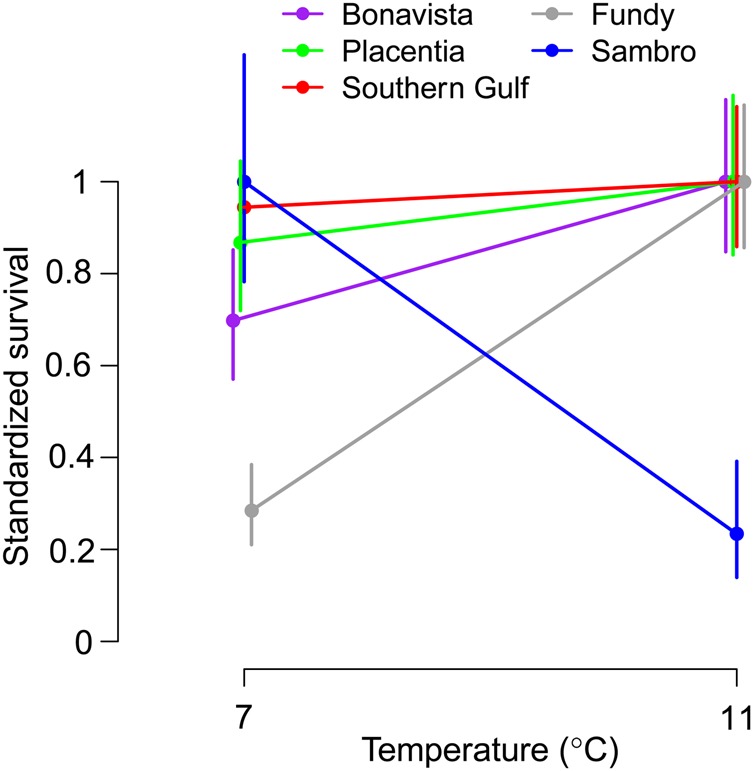
Thermal reaction norms for larval cod survival (±1 SEM), standardized relative to the highest mean survival observed in each population.
Table 4:
Deviance table of the effects of population and temperature on larval cod survival
| Model term | d.f. | Deviance | Residual d.f. | Residual deviance | P-value |
|---|---|---|---|---|---|
| Null | 37 | 608.96 | |||
| Population | 4 | 176.37 | 33 | 432.59 | 0.010* |
| Temperature | 1 | 15.31 | 32 | 417.28 | 0.276 |
| Population × temperature | 4 | 190.38 | 28 | 226.90 | <0.001* |
The P-values were obtained from χ2 tests that were used to determine if the model fit improved significantly by sequentially adding population, temperature and their interaction to the null model. Asterisk denotes significance at α = 0.05.
Table 5:
Effect of temperature on larval survival for five cod populations, where the estimate represents the change in survival from 7 to 11°C
| Population | Estimate | SEM | t | P-value |
|---|---|---|---|---|
| Bonavista | 0.01 | 0.01 | 1.39 | 0.175 |
| Placentia | 0.01 | 0.01 | 0.56 | 0.582 |
| Southern Gulf | 0.00 | 0.01 | 0.27 | 0.792 |
| Fundy | 0.04 | 0.01 | 3.96 | <0.001* |
| Sambro | −0.02 | 0.01 | −2.71 | 0.011† |
Symbols denote significance at α = 0.05 with (*) and without (†) Bonferroni correction.
Table 6:
Pairwise population contrasts of the effect of temperature on larval cod survival
| Bonavista | Placentia | Southern Gulf | Fundy | Sambro | |
|---|---|---|---|---|---|
| Bonavista | — | −0.96 (±1.64) | −1.25 (±1.76) | 2.60 (±1.57) | −4.05 (±1.46) |
| Placentia | 0.563 | — | −0.29 (±1.76) | 3.56 (±1.57) | −3.10 (±1.47) |
| Southern Gulf | 0.483 | 0.870 | — | 3.85 (±1.70) | −2.80 (±1.61) |
| Fundy | 0.108 | 0.031†† | 0.031†† | — | −6.65 (±1.39) |
| Sambro | 0.010†† | 0.044†† | 0.092† | <0.001** | — |
Model estimates (±SEM) are given above the diagonal and P-values below. The point of contrast for the estimates is the row header. Symbols denote significance at the following levels of α: *0.10 and **0.05 (with Bonferroni correction), †0.10 and ††0.05 (without Bonferroni correction). A Bonferroni correction for all contrasts of interest (n = 15) changes the critical P-values to 0.007 (α = 0.10) and 0.003 (α = 0.05).
Discussion
Plasticity in growth and survival
We found variation in thermal reaction norms for larval growth and survival among five putative populations of Atlantic cod in the Northwest Atlantic. Four populations (Fundy, Southern Gulf, Bonavista and Placentia) presented highly plastic growth responses to temperature, growing faster in warmer water, whereas there was no evidence of plasticity for growth in Sambro cod. The magnitude of change might also differ slightly among the plastic growth responses, with Placentia exhibiting the greatest response, but further study will be needed to confirm the significance of this subtle variation. An even greater variety of responses to temperature was observed for survival. Fundy cod experienced much higher survival in warmer water, while the opposite was true for Sambro cod. Survival of Southern Gulf, Bonavista and Placentia larvae was not significantly influenced by temperature.
We interpret these differences in plasticity for larval growth and survival to be largely of genetic rather than maternal origin. This is supported by a lack of relationship between variation in size at hatch (influenced by egg size, the main cause of maternal effects in fishes, Marshall, 2008) and growth or survival, although epigenetic maternal effects cannot be ruled out (e.g. Miller et al., 2012). In a rare example of transgenerational plasticity to temperature in vertebrates, Salinas and Munch (2012) observed optimal growth in larval sheepshead minnows (Cyprinodon variegatus) at temperatures recently experienced by the parents. In contrast, most populations in our study (excepting Sambro) grew more slowly at the colder temperature that was most similar to that at which the parents were held during spawning (8°C) and the temperatures experienced prior to collection (1–4°C; Supplementary Fig. 1). Therefore, transgenerational plasticity does not seem to explain the variation in thermal responses we observed, although it might play an important role in population responses to climate change.
Other potential causes of reaction norm variation in these experiments include variation in larval density among tanks and the fact that experiments were carried out at different times and locations. Stocking densities of 50–300 larvae/litre have been shown to have no effect on survival or growth of cod larvae as long as food is not limiting (Baskerville-Bridges and Kling, 2000), as was the case in our experiment. We also showed that random effects in the growth model were not related to tank density. A lack of association between low survival and high growth further supports our conclusion that variation in density is unlikely to be responsible for the patterns of phenotypic variation observed.
The fact that the common-garden experiments were conducted at different times and locations with slight variation in protocol might have influenced the mean trait values of the reaction norms. However, the reaction norm slopes are unlikely to be significantly affected, as demonstrated by a lack of difference in growth plasticity between the two Southern Gulf experiments. Hutchings et al. (2007) also found no differences in length within treatments between two experiments on Fundy cod that were conducted 1 year apart using the same protocol. Additional replicate experiments are needed to assess the temporal stability of reaction norms and robustness of the common-garden experiments. Perhaps with changing ocean temperatures and sufficient time scales for evolution in plasticity to occur, temporal variation in thermal reaction norm slopes could become apparent (Crozier and Hutchings, 2014).
High mortality is typical of cod larvae in the wild (Sundby et al., 1989; Houde and Zastrow, 1993) and in the laboratory (Gamble and Houde, 1984; Otterlei et al., 1999; Steinarsson and Björnsson, 1999). In our study, survival (uncorrected for sampling mortality) ranged from 0.7 to 5.5% and no population consistently exhibited the highest or lowest survival. If size-selective mortality was responsible for the differences in length observed between groups, we would expect to see a consistent correlation between length and survival among populations, which was not the case. The cause of the exceptionally low survival in the 2011 Southern Gulf experiment is unknown, although survival was not associated with temperature in 2003 or 2011. Interestingly, growth reaction norms did not differ between the two Southern Gulf experiments despite different survival rates. These lines of evidence suggest that variation in survival does not explain the observed differences in growth between temperatures or populations.
Thermal adaptation in plasticity
Three groups are evident based on the types of responses they exhibit to increased temperature: faster growth and higher survival (Fundy); faster growth and similar survival (Southern Gulf, Bonavista and Placentia); and similar growth and lower survival (Sambro). These groups can also be characterized as winter-spawning, spring-spawning and autumn-spawning populations, respectively, each of which experiences a unique pattern of thermal variability in early life (Fig. 1b). While geography and reproductive timing are somewhat interdependent (e.g. the three more northerly populations all spawn in the spring), the two populations with the greatest proximity to one another (Fundy and Sambro) spawn at different times and exhibit the most divergent thermal responses, while the next most proximal populations (Bonavista and Placentia) spawn at the same time and show similar responses. Therefore, variation in reproductive timing rather than geography corresponds better with the variation in thermal responses observed.
The association between thermal reaction norms and the timing of the spawning season raises the question as to the specific mechanism responsible for shaping these diverse responses. Fundy and Sambro cod experience the coldest temperatures overall, yet their reaction norms are the most divergent. Therefore, the average temperature experienced during the larval stage is insufficient to explain the observed reaction norm variation. An association between plasticity and thermal variability is not evident either; Fundy and Sambro both experience relatively low levels of thermal variability compared with the remaining populations due to intense vertical mixing that homogenizes the water column (Garrett et al., 1978; Drinkwater and Gilbert, 2004). The northerly spring-spawning populations could have evolved an intrinsically faster growth rate to compensate for a shorter growing season, as has been found in other fishes (e.g. Conover and Present, 1990; Schultz et al., 1996; Conover et al., 1997), with limited evidence in Northwest Atlantic cod (Hunt von Herbing et al., 1996; Purchase and Brown, 2000). However, growing season does not explain the marked difference in growth response between Fundy and Sambro cod, which both experience the coldest, though unlikely to be growth-limiting, temperatures of the year during the first few months after spawning. Rather, it is the magnitude and direction of the seasonal change in post-spawning temperature that seem to provide the best explanation for the observed reaction norm variability, such that winter- and spring-spawning populations appear to be adapted to increasing temperatures and those populations that experience colder temperatures overall are more sensitive to changes in temperature.
Previous studies have found that cold-water populations experience increasing survival with temperature (Planque and Frédou, 1999; Worm and Myers, 2003; Ottersen et al., 2006). However, Sambro larvae exhibited decreasing survival with temperature, despite experiencing relatively cold temperatures in the wild. What distinguishes the thermal regime typical for Sambro larvae is the decrease in temperature during the larval stage. As a consequence, Sambro cod may not have experienced the selective pressures necessary to shape an adaptive norm of reaction for growth to higher temperatures. This lack of plasticity is probably maladaptive at high temperatures, especially considering the corresponding decrease in survival. The thermal response of Sambro larvae might be the result of a trade-off between having high performance in the natural environment at the expense of low performance in others (i.e. specialist–generalist trade-off), such as observed in Atlantic salmon (Salmo salar; Rungruangsak-Torrissen et al., 1998), or selection for energy savings at low temperatures (Pörtner et al., 2008). Further information about the physiological mechanisms (e.g. gene expression) that underlie the various responses we observed could help distinguish between different trade-offs that might be responsible (Angilletta et al., 2003).
Spatial scale of genetic variation
We found genetic variation in thermal reaction norms between two cod spawning components sampled 218 km apart within the same fisheries management unit (4X). However, the geographical ranges occupied by these spawning groups are not known and may even overlap; therefore, the spatial scale of probable adaptive divergence is likely to be smaller than the distance between collection locations. This is the smallest spatial scale at which genetic variation in adaptive traits has been detected across open waters in a marine fish, that is, waters that are not physically separated by land in some manner, such as along coastal Norway (e.g. Olsen et al., 2008). The finding of this fine-scale biocomplexity in a species that is widely distributed and has high potential for dispersal contradicts traditional notions of genetic homogeneity in marine systems (reviewed by Hilbish, 1996).
The degree to which the autumn- and winter-spawning components of the Scotian Shelf intermix is not known. Given the considerable differences in spawning times, and the limited migration and apparently low levels of gene flow between the winter-spawning components (Ruzzante et al., 1998), differentiation of Sambro cod from winter-spawning components at neutral markers seems likely. However, the significant genetic variation in reaction norms among the remaining populations is not matched by differentiation at neutral markers (Hardie et al., 2006; Hutchings et al., 2007). The lack of correspondence between neutral and adaptive markers provides evidence of genetic structure resulting from selection persisting in the face of apparently high gene flow (Hutchings et al., 2007).
Implications for climate change
Our study suggests that variation in the timing of reproduction has the potential to promote genetic variability in population responses to environmental change in species with high dispersal capabilities at a very small spatial scale. Intraspecific variation in reproductive timing is common in nature and is often correlated with variation in phenotypic traits (reviewed by Hendry and Day, 2005). While variation in reproductive timing can be linked with temperature, allowing populations to track climate change by adjusting breeding times as global temperatures rise (e.g. Charmantier et al., 2008), this is not always the case. In cod, there is no consistent relationship between spawning onset and water temperature in the Northwest Atlantic (Brander and Hurley, 1992; Myers et al., 1993), possibly because the effect of temperature depends on local hydrography and migratory behaviour (Hutchings and Myers, 1994). Thus, it is less likely that cod populations will be able to track climate change by adjusting spawning times.
Even a small, sustained change in ocean temperature could have substantial impacts on population growth rate and recovery considering the high levels of plasticity in life-history traits in some cod populations (Drinkwater, 2005). Our findings suggest that the forecasted 2–4°C rise in ocean temperatures for the study regions (IPCC, 2007) will affect populations differently depending on spawning time (e.g. an increase in productivity for winter- and spring-spawning populations but a decline in that of autumn-spawning populations). Of course, these outcomes will depend on numerous ecosystem variables, such as changes in food availability (Stenseth and Mysterud, 2002; Pörtner and Peck, 2010) and the extent to which cod are able to alter their distribution as temperature changes, given that they are highly mobile (e.g. Neat and Righton, 2007; Freitas et al., 2015). Nonetheless, the thermal responses described in the present study provide a strong empirical basis for predictions of climate change impacts on the abundance and distribution of cod. Furthermore, they highlight the need to consider ecological and behavioural factors that may influence thermal responses in addition to the geographical characteristics typically studied, such as latitude (e.g. Yamahira et al., 2007; Berger et al., 2013; De Block et al., 2013) and altitude (e.g. McKenzie et al., 2013; Vitasse et al., 2013).
The long-term (i.e. evolutionary) consequences of climate change on cod populations will depend on the amount of heritable variation in reaction norms they possess. Variation in adaptive plasticity at the population level increases the likelihood of at least one population having a response that is beneficial in the new environment. The variety of responses observed in the present study alone would suggest that at least one cod population would be well suited to any (small) directional change in temperature, although such a change would most probably result in a loss of intraspecific biodiversity overall through declines (or in the worst case, extinctions) of those populations ill suited to the new environment (Bálint et al., 2011; Pauls et al., 2013). Selection can also act on variation contained within populations to shape a norm of reaction that is adapted to future thermal environments (Ghalambor et al., 2007). Future research should seek to quantify the variation in plasticity that exists within populations (e.g. at the family level) in order to assess the adaptive potential of individual populations (Hutchings 2011).
Many species face additional natural and anthropogenic threats that might interact with climate change, such as habitat fragmentation, pathogens or overexploitation (IPCC, 2014). In harvested species, the negative impacts of climate change can be exacerbated by overexploitation (Hilborn et al., 2003; Hutchings and Reynolds, 2004; Mora et al., 2007). Prevention of further loss of biodiversity and promotion of the recovery of depleted populations will require management strategies that consider both ecological and evolutionary responses of species to their ever-changing environments that are based on appropriate spatial scales (Conover et al., 2006; Hutchings et al., 2007; Olsen et al., 2008). This approach will help to ensure that the adaptive diversity contained in unique populations is preserved and that species have the best genetic tools to cope with their changing environment.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by the Natural Sciences and Engineering Research Council through Strategic and Discovery Grants to J.A.H. and a Canada Graduate Scholarship to R.A.O.; Loblaw Companies Ltd; and the Canadian Wildlife Federation.
Supplementary Material
Acknowledgements
We thank N. Roney, E. Reuchlin-Hugenholtz, P. Debes, C. Kozela, C. Boaler, B. Mosher and the staff at the Aquatron Laboratory at Dalhousie University for their assistance in rearing larval cod. Thanks to V. Yaroshewski and C. Herbinger for assistance with genetic analyses, D. Keith and D. Hamilton for statistical advice, and P. Bentzen, S. Walde, S. Rogers and M. McBride for helpful comments and discussion. Two anonymous reviewers contributed helpful comments and criticism on an earlier version of the manuscript.
References
- Anderson JT. (1988) A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J Northwest Atl Fish Sci 8: 55–66. [Google Scholar]
- Angilletta MJ, Wilson RS, Navas CA, James RS (2003) Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol 18: 234–240. [Google Scholar]
- Bálint M, Domisch S, Engelhardt CHM, Haase P, Lehrian S, Sauer J, Theissinger K, Pauls SU, Nowak C (2011) Cryptic biodiversity loss linked to global climate change. Nat Clim Chang 1: 313–318. [Google Scholar]
- Baskerville-Bridges B, Kling LJ (2000) Larval culture of Atlantic cod (Gadus morhua) at high stocking densities. Aquaculture 181: 61–69. [Google Scholar]
- Baumann H, Conover DO (2011) Adaptation to climate change: contrasting patterns of thermal-reaction-norm evolution in Pacific versus Atlantic silversides. Proc R Soc B Biol Sci 278: 2265–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D, Postma E, Blanckenhorn WU, Walters RJ (2013) Quantitative genetic divergence and standing genetic (co)variance in thermal reaction norms along latitude. Evolution 67: 2385–2399. [DOI] [PubMed] [Google Scholar]
- Bradbury IR, Hubert S, Higgins B, Borza T, Bowman S, Paterson IG, Snelgrove PVR, Morris CJ, Gregory RS, Hardie DC et al. (2010) Parallel adaptive evolution of Atlantic cod on both sides of the Atlantic Ocean in response to temperature. Proc R Soc B Biol Sci 277: 3725–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury IR, Hubert S, Higgins B, Bowman S, Borza T, Paterson IG, Snelgrove PVR, Morris CJ, Gregory RS, Hardie D et al. (2013) Genomic islands of divergence and their consequences for the resolution of spatial structure in an exploited marine fish. Evol Appl 6: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander K, Hurley PCF (1992) Distribution of early-stage Atlantic cod (Gadus morhua), haddock (Melanogrammus aeglefinus), and witch flounder (Glyptocephalus cynoglossus) eggs on the Scotian Shelf: a reappraisal of evidence on the coupling of cod spawning and plankton production. Can J Fish Aquat Sci 49: 238–251. [Google Scholar]
- Bronikowski AM. (2000) Experimental evidence for the adaptive evolution of growth rate in the garter snake Thamnophis elegans. Evolution 54: 1760–1767. [DOI] [PubMed] [Google Scholar]
- Canale CI, Henry P-Y (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43: 135–147. [Google Scholar]
- Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC (2008) Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320: 800–803. [DOI] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8: e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee On the Status of Endangered Wildlife In Canada (2010) COSEWIC Assessment and Status Report on the Atlantic Cod Gadus morhua in Canada. COSEWIC, Ottawa, pp xiii and 105.
- Conover DO, Present TMC (1990) Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83: 316–324. [DOI] [PubMed] [Google Scholar]
- Conover DO, Brown JJ, Ehtisham A (1997) Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Can J Fish Aquat Sci 54: 2401–2409. [Google Scholar]
- Conover DO, Clarke LM, Munch SB, Wagner GN (2006) Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. J Fish Biol 69: 21–47. [Google Scholar]
- Crozier LG, Hutchings JA (2014) Plastic and evolutionary responses to climate change in fish. Evol Appl 7: 68–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzmann RG, Ferguson MM, Heculuck DM (1994) Heterogeneity in the distribution of mitochondrial DNA haplotypes in female rainbow trout spawning in different seasons. Can J Fish Aquat Sci 51: 284–289. [Google Scholar]
- De Block M, Pauwels K, Van Den Broeck M, De Meester L, Stoks R (2013) Local genetic adaptation generates latitude-specific effects of warming on predator–prey interactions. Glob Chang Biol 19: 689–696. [DOI] [PubMed] [Google Scholar]
- Drinkwater K. (2005) The response of Atlantic cod (Gadus morhua) to future climate change. ICES J Mar Sci 62: 1327–1337. [Google Scholar]
- Drinkwater KF, Gilbert D (2004) Hydrographic variability in the waters of the Gulf of St. Lawrence, the Scotian Shelf and the Eastern Gulf of Maine (NAFO subarea 4) during 1991–2000. J Northwest Atl Fish Sci 34: 83–99. [Google Scholar]
- Duchesne P, Godbout M-H, Bernatchez L (2002) PAPA (Package for the Analysis of Parental Allocation): a computer program for simulated and real parental allocation. Mol Ecol Notes 2: 191–193. [Google Scholar]
- Ficetola GF, De Bernardi F (2005) Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Anim Conserv 8: 33–40. [Google Scholar]
- Fraser DJ, Weir LK, Bernatchez L, Hansen MM, Taylor EB (2011) Extent and scale of local adaptation in salmonid fishes: review and meta-analysis. Heredity 106: 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Olsen EM, Moland E, Ciannelli L, Knutsen H (2015) Behavioral responses of Atlantic cod to sea temperature changes. Ecol Evol 5: 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble JC, Houde ED (1984) Growth, mortality and feeding of cod Gadus morhua L. larvae in enclosed water columns and in laboratory tanks. In The Propagation of Cod Gadus Morhua L., Vol 1 pp 123–143. [Google Scholar]
- Garrett CJR, Keeley JR, Greenberg DA (1978) Tidal mixing versus thermal stratification in the Bay of Fundy and Gulf of Maine. Atmos-Ocean 16: 403–423. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407. [Google Scholar]
- Hardie DC, Gillett RM, Hutchings JA (2006) The effects of isolation and colonization history on the genetic structure of marine-relict populations of Atlantic cod (Gadus morhua) in the Canadian Arctic. Can J Fish Aquat Sci 63: 1830–1839. [Google Scholar]
- Hendry AP, Day T (2005) Population structure attributable to reproductive time: isolation by time and adaptation by time. Mol Ecol 14: 901–916. [DOI] [PubMed] [Google Scholar]
- Hilbish TJ. (1996) Population genetics of marine species: the interaction of natural selection and historically differentiated populations. J Exp Mar Bio Ecol 200: 67–83. [Google Scholar]
- Hilborn R, Quinn TP, Schindler DE, Rogers DE (2003) Biocomplexity and fisheries sustainability. Proc Natl Acad Sci USA 100: 6564–6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde ED, Zastrow CE (1993) Ecosystem- and taxon-specific dynamic and energetics properties of larval fish assemblages. Bull Mar Sci 53: 290–335. [Google Scholar]
- Hunt von Herbing I, Boutilier RG, Miyake T, Hall BK (1996) Effects of temperature on morphological landmarks critical to growth and survival in larval Atlantic cod (Gadus morhua). Mar Biol 124: 593–606. [Google Scholar]
- Hutchings JA. (2000) Collapse and recovery of marine fishes. Nature 406: 882–885. [DOI] [PubMed] [Google Scholar]
- Hutchings JA. (2011) Old wine in new bottles: reaction norms in salmonid fishes. Heredity 106: 421–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA (1994) Timing of cod reproduction – interannual variability and the influence of temperature. Mar Ecol Prog Ser 108: 21–31. [Google Scholar]
- Hutchings JA, Rangeley RW (2011) Correlates of recovery for Canadian Atlantic cod (Gadus morhua). Can J Zool 89: 386–400. [Google Scholar]
- Hutchings JA, Reynolds JD (2004) Marine fish population collapses: consequences for recovery and extinction risk. Bioscience 54: 297–309. [Google Scholar]
- Hutchings JA, Bishop TD, McGregor-Shaw CR (1999) Spawning behaviour of Atlantic cod, Gadus morhua: evidence of mate competition and mate choice in a broadcast spawner. Can J Fish Aquat Sci 56: 97–104. [Google Scholar]
- Hutchings JA, Swain DP, Rowe S, Eddington JD, Puvanendran V, Brown JA (2007) Genetic variation in life-history reaction norms in a marine fish. Proc R Soc B Biol Sci 274: 1693–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. RK Pachauri, A Reisinger, eds.IPCC, Geneva. [Google Scholar]
- IPCC (2014) Ocean systems . In Drinkwater K, Polonsky A, eds, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva. [Google Scholar]
- Jensen LF, Hansen MM, Pertoldi C, Holdensgaard G, Mensberg K-LD, Loeschcke V (2008) Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proc R Soc B Biol Sci 275: 2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Wang J (2010) COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour 10: 551–555. [DOI] [PubMed] [Google Scholar]
- Lande R. (2009) Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J Evol Biol 22: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Lett PF. (1980) A comparative study of the recruitment mechanisms of cod and mackerel, their interaction, and its implication for dual stock assessment. Can Tech Rep Fish Aquat Sci 988 Supply and Services Canada, Ottawa. [Google Scholar]
- Liefting M, Ellers J (2008) Habitat-specific differences in thermal plasticity in natural populations of a soil arthropod. Biol J Linn Soc 94: 265–271. [Google Scholar]
- Liefting M, Hoffmann AA, Ellers J (2009) Plasticity versus environmental canalization: population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 63: 1954–1963. [DOI] [PubMed] [Google Scholar]
- McCairns RJS, Bernatchez L (2010) Adaptive divergence between freshwater and marine sticklebacks: insights into the role of phenotypic plasticity from an integrated analysis of candidate gene expression. Evolution 64: 1029–1047. [DOI] [PubMed] [Google Scholar]
- McKenzie DJ, Estivales G, Svendsen JC, Steffensen JF, Agnese J-F (2013) Local adaptation to altitude underlies divergent thermal physiology in tropical killifishes of the genus Aphyosemion. PLoS One 8: e54345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcil J, Swain DP, Hutchings JA (2006) Genetic and environmental components of phenotypic variation in body shape among populations of Atlantic cod (Gadus morhua L.). Biol J Linn Soc 88: 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DJ. (2008) Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89: 418–427. [DOI] [PubMed] [Google Scholar]
- Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Chang 2: 858–861. [Google Scholar]
- Mora C, Metzger R, Rollo A, Myers RA (2007) Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc R Soc B Biol Sci 274: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RA, Mertz G, Bishop CA (1993) Cod spawning in relation to physical and biological cycles of the northern North-west Atlantic. Fish Oceanogr 2: 154–165. [Google Scholar]
- Neat F, Righton D (2007) Warm water occupancy by North Sea cod. Proc R Soc B Biol Sci 274: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F et al. (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15: 684–692. [DOI] [PubMed] [Google Scholar]
- O'Brien CM, Fox CJ, Planque B, Casey J (2000) Climate variability and North Sea cod. Nature 404: 142–143. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Knutsen H, Gjøsaeter J, Jorde PE, Knutsen JA, Stenseth NC (2008) Small-scale biocomplexity in coastal Atlantic cod supporting a Darwinian perspective on fisheries management. Evol Appl 1: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterå H, Agnalt A-L, Jørstad KE (2006) Differences in spawning time of captive Atlantic cod from four regions of Norway, kept under identical conditions. ICES J Mar Sci 63: 216–223. [Google Scholar]
- Otterlei E, Nyhammer G, Folkvord A, Stefansson SO (1999) Temperature- and size-dependent growth of larval and early juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and northeast Arctic cod. Can J Fish Aquat Sci 56: 2099–2111. [Google Scholar]
- Ottersen G, Hjermann DØ, Stenseth NC (2006) Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish Oceanogr 15: 230–243. [Google Scholar]
- Pauls SU, Nowak C, Bálint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22: 925–946. [DOI] [PubMed] [Google Scholar]
- Planque B, Frédou T (1999) Temperature and the recruitment of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 56: 2069–2077. [Google Scholar]
- Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Bock C, Knust R, Lannig G, Lucassen M, Mark FC, Sartoris FJ (2008) Cod and climate in a latitudinal cline: physiological analyses of climate effects in marine fishes. Clim Res 37: 253–270. [Google Scholar]
- Purchase CF, Brown JA (2000) Interpopulation differences in growth rates and food conversion efficiencies of young Grand Banks and Gulf of Maine Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 57: 2223–2229. [Google Scholar]
- R Development Core Team (2012) R: a Language and Environment for Statistical Computing [online]. ISBN 3-900051-07-0. Available from http://www.R-project.org/.
- Richter-Boix A, Teplitsky C, Rogell B, Laurila A (2010) Local selection modifies phenotypic divergence among Rana temporaria populations in the presence of gene flow. Mol Ecol 19: 716–731. [DOI] [PubMed] [Google Scholar]
- Richter-Boix A, Quintela M, Kierczak M, Franch M, Laurila A (2013) Fine-grained adaptive divergence in an amphibian: genetic basis of phenotypic divergence and the role of nonrandom gene flow in restricting effective migration among wetlands. Mol Ecol 22: 1322–1340. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Allen MS, Porak WF (2006) Separating genetic and environmental influences on temporal spawning distributions of largemouth bass (Micropterus salmoides). Can J Fish Aquat Sci 63: 2391–2399. [Google Scholar]
- Rungruangsak-Torrissen K, Pringle GM, Moss R, Houlihan DF (1998) Effects of varying rearing temperatures on expression of different trypsin isozymes, feed conversion efficiency and growth in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 19: 247–255. [Google Scholar]
- Ruzzante DE, Taggart CT, Cook D (1998) A nuclear DNA basis for shelf- and bank-scale population structure in northwest Atlantic cod (Gadus morhua): Labrador to Georges Bank. Mol Ecol 7: 1663–1680. [Google Scholar]
- Ruzzante DE, Taggart CT, Cook D (1999) A review of the evidence for genetic structure of cod (Gadus morhua) populations in the NW Atlantic and population affinities of larval cod off Newfoundland and the Gulf of St. Lawrence. Fish Res 43: 79–97. [Google Scholar]
- Sakamoto T, Danzmann RG, Okamoto N, Ferguson MM, Ihssen PE (1999) Linkage analysis of quantitative trait loci associated with spawning time in rainbow trout (Oncorhynchus mykiss). Aquaculture 173: 33–43. [Google Scholar]
- Salinas S, Munch SB (2012) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15: 159–163. [DOI] [PubMed] [Google Scholar]
- Schmalhausen (1949) Factors of Evolution: the Theory of Stabilizing Selection. Blakiston, Philadelphia. [Google Scholar]
- Schultz E, Reynolds K, Conover D (1996) Countergradient variation among newly hatched Fundulus heteroclitus: geographic differences revealed by common-environment experiments. Funct Ecol 10: 366–374. [Google Scholar]
- Siitonen L, Gall GAE (1989) Response to selection for early spawn date in rainbow trout, Salmo gairdneri. Aquaculture 78: 153–161. [Google Scholar]
- Sinervo B, Adolph SC (1994) Growth plasticity and thermal opportunity in Sceloporus lizards. Ecology 75: 776–790. [Google Scholar]
- Steinarsson A, Björnsson B (1999) The effects of temperature and size on growth and mortality of cod larvae. J Fish Biol 55: 100–109. [Google Scholar]
- Stenseth NC, Mysterud A (2002) Climate, changing phenology, and other life history traits: nonlinearity and match–mismatch to the environment. Proc Natl Acad Sci USA 99: 13379–13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundby S, Bjørke H, Soldai AV, Olsen S (1989) Mortality rates during the early life stages and year-class strength of northeast Arctic cod (Gadus morhua L.). Rapp Procès-verbaux des Réunions Cons Int pour l'Exploration la Mer 191: 351–358. [Google Scholar]
- Templeman ND. (2010) Ecosystem Status and Trends Report for the Newfoundland and Labrador Shelf. Fisheries and Oceans Canada Canadian Science Advisory Secretariat Research Report. [Google Scholar]
- Van Asch M, Van Tienderen PH, Holleman LJM, Visser ME (2007) Predicting adaptation of phenology in response to climate change, an insect herbivore example. Glob Chang Biol 13: 1596–1604. [Google Scholar]
- Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, Scheepens JF, Korner C (2013) Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663–678. [DOI] [PubMed] [Google Scholar]
- Waples RS, Teel DJ, Myers JM, Marshall AR (2004) Life-history divergence in Chinook salmon: historic contingency and parallel evolution. Evolution 58: 386–403. [PubMed] [Google Scholar]
- Winterhalter WE, Mousseau TA (2007) Patterns of phenotypic and genetic variation for the plasticity of diapause incidence. Evolution 61: 1520–1531. [DOI] [PubMed] [Google Scholar]
- Woltereck R. (1909) Weitere experimentelle untersuchungen über Artänderung, speziell über das Wesen quantitativer Artunterschiede bei Daphniden. Verhandlungen der Dtsch Zool Gesellschaft 19: 110–172. [Google Scholar]
- Worm B, Myers RA (2003) Meta-analysis of cod–shrimp interactions reveals top-down control in oceanic food webs. Ecology 84: 162–173. [Google Scholar]
- Yamahira K, Kawajiri M, Takeshi K, Irie T (2007) Inter- and intrapopulation variation in thermal reaction norms for growth rate: evolution of latitudinal compensation in ectotherms with a genetic constraint. Evolution 61: 1577–1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


