Species distribution modeling is the most common method of estimating climate change impacts on biodiversity. In this review, we argue a need for collaboration among physiologists, modelers and conservationists to parameterize models with physiological information in order to increase their accuracy and advance the field of conservation physiology.
Keywords: Climate change, demography, model, physiology, species distribution, temperature
Abstract
Climate change conservation planning relies heavily on correlative species distribution models that estimate future areas of occupancy based on environmental conditions encountered in present-day ranges. The approach benefits from rapid assessment of vulnerability over a large number of organisms, but can have poor predictive power when transposed to novel environments and reveals little in the way of causal mechanisms that define changes in species distribution or abundance. Having conservation planning rely largely on this single approach also increases the risk of policy failure. Mechanistic models that are parameterized with physiological information are expected to be more robust when extrapolating distributions to future environmental conditions and can identify physiological processes that set range boundaries. Implementation of mechanistic species distribution models requires knowledge of how environmental change influences physiological performance, and because this information is currently restricted to a comparatively small number of well-studied organisms, use of mechanistic modelling in the context of climate change conservation is limited. In this review, we propose that the need to develop mechanistic models that incorporate physiological data presents an opportunity for physiologists to contribute more directly to climate change conservation and advance the field of conservation physiology. We begin by describing the prevalence of species distribution modelling in climate change conservation, highlighting the benefits and drawbacks of both mechanistic and correlative approaches. Next, we emphasize the need to expand mechanistic models and discuss potential metrics of physiological performance suitable for integration into mechanistic models. We conclude by summarizing other factors, such as the need to consider demography, limiting broader application of mechanistic models in climate change conservation. Ideally, modellers, physiologists and conservation practitioners would work collaboratively to build models, interpret results and consider conservation management options, and articulating this need here may help to stimulate collaboration.
Predicting impacts of climate change on biodiversity
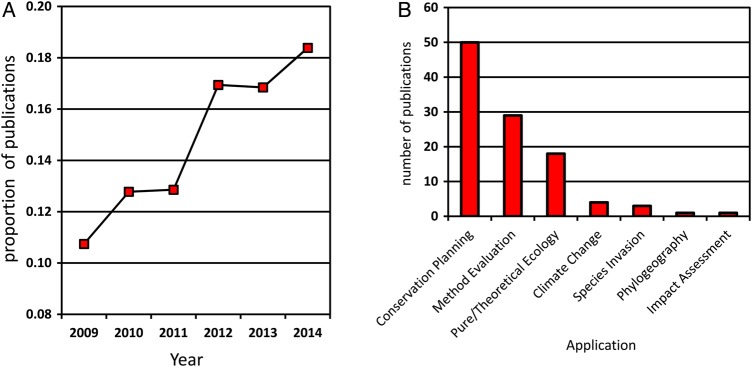
Anthropogenic climate change is recognized as a major threat to global biodiversity, and the ability to predict species' responses to rapid shifts in abiotic conditions has emerged as a conservation priority (Bellard et al., 2012; Cahill et al., 2013). The choice of methods for estimating climate change vulnerability is the result of two overriding factors: (i) the global scale at which climate change is occurring, meaning that very large numbers of species must be evaluated; and (ii) the need to develop conservation interventions quickly given accelerating rates of environmental change. Modelling the distribution of species in future climates is by far the most common means of determining how climate change will influence life on Earth (Kearney et al., 2010), in large part because models can be applied rapidly to diverse taxa over large spatial scales (Pacifici et al., 2015). Use of species distribution modelling within the context of climate change and conservation research also appears to have increased in recent years (Fig. 1A). Importantly, modelled changes in species distribution have become a foundation of climate change conservation planning (Fig. 1B; Dawson et al., 2011; Robinson et al., 2011; Cuddington et al., 2013; Gillson et al., 2013) and are paramount to the design of reserve networks (Araújo et al., 2004; Wilson et al., 2005), planning assisted colonization (Hoegh-Guldberg et al., 2008) and limiting the damage caused by invasive pests (Kearney et al., 2008). In fact, climate change-associated declines in biodiversity predicted by species distribution models have already prompted calls for major conservation interventions, including redesign of protected area systems, development of new areas for restoration and management, and human-assisted migration (Dawson et al., 2011).
Figure 1:
Importance of species distribution models in climate change research and conservation planning. (A) Increasing use of species distribution models within climate change and conservation research. Data are plotted as the number of publications retrieved from the Web of Science database using search terms ‘species distribution model’ AND ‘climate change’ AND ‘conservation’ relative to the number of publications returned using search terms ‘climate change’ AND ‘conservation’. Data apply to a search performed on 29 July 2015. (B) Primary research objective of species distribution models for marine species as determined by Robinson et al. (2011). Data are derived from a search in ISI Web of Science using search topic = ‘species distribution’ OR ‘ecological niche’ OR ‘habitat preference’ OR ‘environmental preference’ OR ‘bioclimate envelope’ OR ‘bioclimate’ OR ‘environmental niche’ OR ‘habitat suitability’ AND ‘model*’ It should be noted that not all research objectives were mutually exclusive. For example, a future species distribution model projection under various climate change scenarios may feed into a conservation planning application, but in these cases the paper was assigned to an application based on the primary objective of the study. Adapted from Robinson et al. (2011).
Simple species distribution models have been applied widely to identify and conserve species affected by climate change; however, awareness of limitations associated with these approaches has prompted appeals to improve methodology (Fordham et al., 2013; Akçakaya et al., 2014; Helmuth et al., 2014; Pacifici et al., 2015). Some have even questioned the utility of models altogether given the uncertainties they entail (Perretti et al., 2013; Schindler and Hilborn, 2015). Incorporating the important role of physiology in defining species distributions is regarded as a means to increase the accuracy of species distribution models and conservation interventions based on these data (Huey et al., 2012; Németh et al., 2013; Helmuth et al., 2014; Sunday et al., 2014; Valladares et al., 2014).
In this review, we propose that physiology is poised to inform conservation decision-making more directly through inclusion of physiological performance metrics in models that forecast the biological consequences of climate change. We begin by iterating the need for modelling in predicting biological responses to climate change and the influence models have on conservation policy. Many physiologists may not appreciate the dependence of climate change conservation planning on predictions derived largely from species distribution models. Next, we discuss the need to parameterize models with physiological data in order to increase their accuracy and the effectiveness of resulting conservation interventions. Descriptions of modelling methodology within this context are intended to highlight gaps in understanding that could be filled by physiological data, rather than to provide a comprehensive evaluation of the many possible variations to modelling species distributions, which has been thoroughly debated elsewhere (see Kearney and Porter, 2009; Morin and Thuiller, 2009; Kearney et al., 2010; Araújo and Peterson, 2012; Dormann et al., 2012; Pacifici et al., 2015). The concept of integrating physiology into species distribution models that predict climate change outcomes is not new; however, progress in this regard appears to be being made by relatively few scientists with expertise in both modelling and environmental physiology (e.g. Buckley et al., 2010; Kearney et al., 2012; Woodin et al., 2013). Given the global scale at which species are affected by climate change, a larger effort is required, and we believe that many opportunities exist for fruitful collaboration between physiologists, modellers and conservationists. As stated by Porfirio et al. (2014) in their evaluation of ways to improve the use of species distribution models in climate change conservation planning and management: ‘Ideally, modellers, species experts and conservation practitioners should work as a team to build the model, interpret results and consider conservation management responses. However, such interdisciplinary exercises are uncommon’ (Porfirio et al., 2014). In emphasizing a role for physiology in species distribution modelling, we examine the underlying question of what physiological metric(s), if any, are strongly correlated with range limits and are therefore most appropriate to integrate into predictive models. We also emphasize the importance of considering the influence of physiology on demography and explore the potential of integrated models that incorporate demographic, physiological and climatic parameters. Lastly, we identify factors currently hindering the use of physiology in predicting future species distributions. From a broader perspective, formalizing the need to incorporate physiological data into models used extensively in conservation will help to advance the burgeoning field of conservation physiology (Table 1). Ensuring that physiological data become and remain useful as a policy tool is the single biggest challenge facing the subdiscipline of conservation physiology (Cooke and O'Connor, 2010; Cooke, 2014). Encouraging physiologists to gather data that are most relevant to current conservation practice, in this instance data that can inform species distribution models, will assist in alleviating this limitation.
Table 1:
Factors constraining the field of conservation physiology addressed in this review
| Constraint for conservation physiology | Priority |
|---|---|
| • Conservation physiology will not always provide information that is needed by managers and policy-makers | High |
| • Determining which of the many possible physiological parameters to measure | Moderate |
| • There has been a general failure to discuss opportunities associated with conservation physiology | Moderate |
Adapted from Cooke and O'Connor (2010).
Forecasting species distributions in future climates
Correlative species distribution modelling is the most commonly applied approach for predicting effects of climate change on biodiversity (Hannah et al., 2007; Pachauri and Reisinger, 2007; Leadley, 2010; Dawson et al., 2011; Fordham et al., 2013; Thuiller et al., 2013; Pacifici et al., 2015; Urban 2015) and has become a cornerstone of climate change conservation policy (Gillson et al., 2013). Correlative modelling is commonly used to project future changes in the geographical ranges of species, estimate extinction rates, examine the efficacy of existing reserve systems and prioritize biodiversity conservation efforts (Porfirio et al., 2014). These models establish statistical relationships between present-day geographical distributions and climate variables, which are then applied to climate change projections to infer climatically suitable habitats for species in the future (Pacifici et al., 2015). Outputs of correlative models are often maps of future climatically suitable regions for a given species, the total area of which can then be compared with current areas of occupancy to estimate vulnerability. Within this framework, species whose area of climatically suitable habitat is expected to decline most in the future are considered to be at the greatest risk for extinction (Thomas et al., 2004; Warren et al., 2013). For example, correlative models of climatic range change applied across 48 786 animal and plant species suggest that 57 ± 6% of plants and 34 ± 7% of animals are likely to lose 50% of their present climatic range by 2080 in the absence of greenhouse gas mitigation. Such severe declines in global biodiversity and ecosystem services argue for prompt and stringent greenhouse gas mitigation to reduce these losses (Warren et al., 2013).
Minimal data requirements, namely current biogeographical range (presence only, presence/absence or abundance records) and coarse climate data (commonly, temperature and precipitation), allow correlative models to be applied widely across taxa (Kearney and Porter, 2009). Such tractability is critical considering that climate change will affect species globally and that conservation decisions often need to be made quickly and without the desired amount of scientific evidence (Cooke and O'Connor, 2010). However, continued use of correlative species distribution models has increased awareness of shortcomings associated with this approach. Correlative models are often criticized for their inability to consider the full range of processes shaping species ranges and their uncertainty in predicting events occurring in the distant future (e.g. Pearson et al., 2006; Tewksbury et al. 2008; Wiens et al., 2009; Thuiller et al., 2013). A key assumption of correlative models is that processes setting range limits will remain fixed in time and space, and many have argued that this assumption will be violated when making predictions about climate change (e.g. Williams and Jackson, 2007; Buckley et al. 2010). Future environments will be likely to involve novel combinations of abiotic (e.g. temperature and precipitation) and biotic (e.g. uneven migration rates among interacting species) variables that fall outside of the range of parameters used to construct the model (Elith et al., 2010; Kearney et al., 2010; Buckley and Kingsolver, 2012a). Past range shifts demonstrate that species with similar climate requirements do not migrate at identical rates or exhibit completely overlapping ranges in their new distribution; trends that are inconsistent with correlative models assuming that species with similar climate requirements will respond in a similar manner to climate change (Buckley, 2010). Correlative models also fail to provide a causal explanation for predicted outcomes. Ecological processes and interactions that lead to successful persistence at a given location are implied in correlative models; however, it remains unclear whether future ranges represent a direct causal relationship with climate, an indirect effect mediated by a biotic interaction, or a direct response to another collinear variable absent from the model (Kearney and Porter, 2009; Dormann et al., 2012). With regard to applying correlative models to conservation, there is concern that having conservation depend largely upon data derived from this single approach increases the risk of policy or management failures (Dawson et al., 2011).
There is a growing consensus on the benefits of using models that include mechanistic variables, so that extrapolated changes in climate can be linked to processes that shape species ranges (Kearney and Porter 2009; Buckley et al., 2010, 2011; Pacifici et al., 2015). The most basic and fundamental constraints on the distribution and abundance of organisms are physiological limitations that set the fundamental niche (Kearney and Porter, 2009). Mechanistic species distribution models (also referred to as process-based models) differ from correlative models in that they consider how the environment constrains physiological performance at a given location. Future distribution is then predicted through a process of elimination, whereby regions that hinder physiological performance to the degree that the capacity for survival, growth or reproduction is compromised are excluded from the final distribution (Kearney and Porter, 2009). For example, cane toad (Rhinella marina) locomotion is confined to temperatures between 13.7 and 37.4°C (Kearney et al., 2008). Consequently, cane toads should be excluded from regions where climate change would cause temperatures routinely to exceed these bounds. As illustrated by this example, mechanistic models contain explicitly defined parameters that have a clear ecological interpretation defined a priori (Dormann et al., 2012) and can therefore provide an improved understanding of the factors underlying responses to environmental change compared with correlative models (Table 2; Kearney and Porter, 2009). An additional advantage is that because mechanistic approaches model species distributions independent of current ranges (and the environmental factors assumed to define current distribution), their predictions do not suffer from the problem of extrapolating to novel climates as correlative models do (Elith et al., 2010; Kearney et al., 2010; Buckley and Kingsolver, 2012a). Mechanistic models have also been argued to be the preferred approach for the majority of management questions given the ability to extrapolate beyond known conditions and isolate traits that determine biogeography (Cuddington et al., 2013). Although it is unlikely that any one modelling approach will offer advantages across all applications (Buckley et al., 2011; Dormann et al., 2012), researchers have routinely called for more widespread use of models that include mechanistic information because of these advantages (Kearney and Porter, 2009; Cuddington et al., 2013; Thuiller et al., 2013). Several authors have also pointed out that the use of different types of models, such as both correlative and mechanistic, provides independent lines of evidence that may confer accuracy to projections where they converge (Hijmans and Graham, 2006; Kearney and Porter, 2009; Morin and Thuiller, 2009).
Table 2:
Comparison of correlative and mechanistic models for predicting climate change outcomes
| Correlative models | Mechanistic models | |
|---|---|---|
| Advantages for predicting climate change outcomes |
|
|
| Disadvantages for predicting climate change outcomes |
|
|
| Data requirements | • Occurrence data (presence only, presence/absence or abundance records) | • Functional traits (e.g. physiological, demographic responses to environmental change measured in laboratory experiments) |
Adapted from Kearney and Porter (2009).
Apparent benefits of mechanistic modelling are tempered by much greater data requirements compared with correlative models. Estimates of physiological performance that form the parameters of mechanistic models must be derived from costly experimental or observational studies of organisms in the field or in the laboratory (Table 2; Buckley et al., 2010; Kearney et al., 2012). Consequently, mechanistic applications are generally restricted to species for which physiology has been studied for a long time (Morin and Thuiller, 2009). Collecting additional physiological data is a requirement to expand use of mechanistic models, and from this need emerges an opportunity for physiologists to collaborate with modellers and conservationists to inform conservation policy more directly and advance the field of conservation physiology. If conservation is to capitalize on the potential benefits of including mechanisms in species distribution models, relevant physiological data will have to be collected across a far greater number of organisms (Gouveia et al., 2014; Violle et al., 2014).
Consequences of model choice
Evaluating the accuracy of either correlative or mechanistic models to predict climate change outcomes is problematic because events being predicted are yet to occur (Araújo et al., 2004). Models are often validated through their ability to recapitulate present-day distributions, but this method offers little assurance that the model will perform similarly well in predicting future distribution given that future climates will probably lack current analogues (Williams and Jackson, 2007). A historic data set for UK butterflies provided a rare opportunity to compare the ability of correlative and mechanistic models to predict range shifts that had occurred as a result of contemporary warming between 1970 and 2004. The comparison provides evidence that mechanistic models may estimate future ranges more accurately. A mechanistic model parameterized with minimal temperature required to complete larval development, an indicator of thermal constraint on development derived from laboratory experiments, more accurately estimated butterfly range shifts compared with correlative models trained with distribution data (Buckley et al., 2011). More generally, leveraging records of past species distribution represents a powerful approach to compare predictions of correlative and mechanistic models empirically. Additional research adopting this methodology will lead to more informed conclusions regarding the accuracy of correlative vs. mechanistic approaches in predicting future distributions.
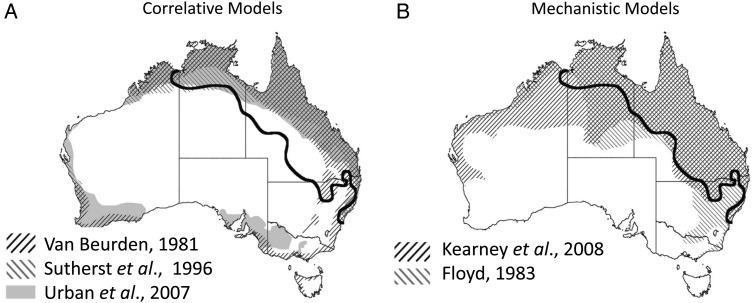
Efforts to model the distribution of a single species both mechanistically and correlatively demonstrate that the two approaches can generate substantially different predictions. Climate change-driven range shifts predicted by correlative and mechanistic models were compared for both the skipper butterfly Atalopedes campestris and the fence lizard Sceloporus undulatus (Buckley et al., 2010). Three mechanistic models were included in the comparison: the first using a minimal energy budget; the second incorporating the effects of temperature on survivorship and fecundity; and the third considering the energetic yield of foraging effort. Comparison of these three models with a single correlative model revealed that all four approaches performed similarly in predicting current distributions. However, mechanistic models predicted larger range shifts for both the skipper butterfly and the fence lizard in response to future climate change. Predictions regarding the future distribution of invasive cane toads in Australia also deviate widely depending on whether correlative or mechanistic models are used (Elith et al., 2010). Mechanistic models of future cane toad distribution, parameterized with thermal constraints on locomotion in the adult stage or limitations on the availability of water for the larval stage, indicate that cane toads will be unable to survive in Southern Australia. Previous experiments using strictly correlative models had predicted this region to be suitable climatically for cane toads in the future (Fig. 2; Kearney et al., 2008; Phillips et al., 2008).
Figure 2:
Comparison of future ranges for cane toads (Rhinella marina) in Australia predicted by correlative (A) and mechanistic (B) models. Maps illustrate results from three studies using correlative models (Van Beurden, 1981; Sutherst et al., 1996; Urban et al., 2007) and two studies using mechanistic models (Kearney et al., 2008; Floyd, 1983). Black line denotes 2007 range edge. Adapted from Phillips et al. (2008).
Discrepancies between predictions generated by correlative and mechanistic models illustrate how model selection could lead to the development of substantially different conservation strategies. A major role for species distributions in conservation planning is to inform the design of reserve networks that help to protect biodiversity. Given that there are limits on the amount of land that can be set aside for nature conservation, reserve design aims to protect species effectively using the minimal possible space (Wilson et al., 2005). A conservationist tasked with defining geographical areas necessary to protect the lizard or butterfly species described above faces the dilemma of having to weigh discordant evidence regarding their likely future ranges. Likewise, efforts to extirpate invasive cane toads are complicated by considerable uncertainty about regions susceptible to future invasions. Rectifying this problem requires that future distributions be modelled as accurately as possible. Improved species distribution models will also assist in developing new reserve design criteria that better account for climate change-related shifts in species distributions (Wilson et al., 2005). There is evidence to suggest that current reserve design criteria do not adequately account for species responses to climate change, and that organisms may shift out of reserve boundaries as ranges track new climates. An analysis of 1200 plant species within a theoretical European reserve network suggests that 5% of species analysed will lose their entire climatic range within the reserve system over the next 50 years (Araújo et al., 2004).
Physiological correlates of species distributions: more than a matter of heat tolerance
Rapid and widespread use of mechanistic models in conservation is dependent on answering a series of complicated questions (Huey et al., 2012). What physiological metric(s) should be measured? Is it necessary to parameterize models with many physiological variables that collectively determine biogeography or are less data-intensive proxies available that can accomplish this task? Can the same proxy traits predict responses to environmental change across phylogenetically diverse species?
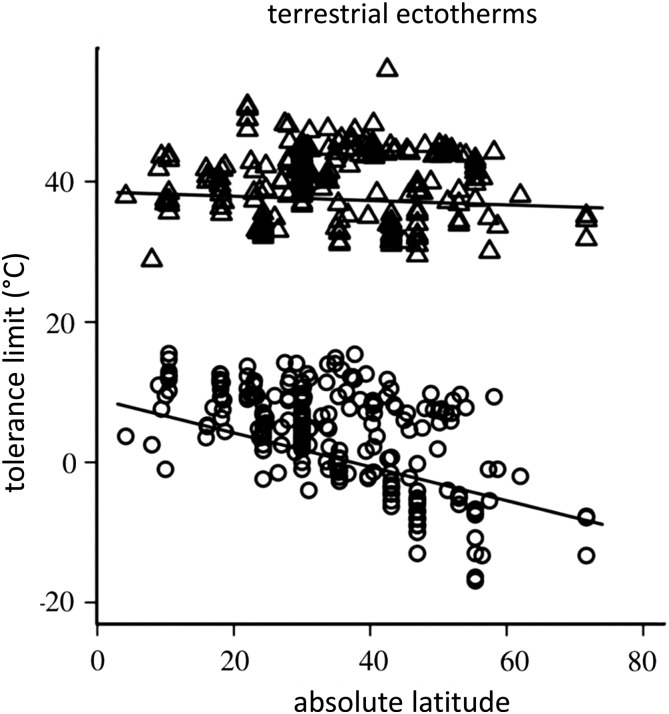
The fact that physiological constraints determine the relationship between abiotic variables and the distributional limits of species is well established, but finding consistent patterns in the traits that influence biogeography across taxa has proved difficult (Bozinovic et al., 2011). Research suggests that no single trait is likely to encapsulate fully the factors that set distribution limits across all species, which will make expanded use of mechanistic models more difficult. This trend is clearly illustrated in efforts to predict climate change outcomes using metrics of heat tolerance. The ability to cope with elevated temperatures is certain to play a role in determining species responses to climate change, and heat tolerance is frequently cited as a defining factor in setting range limits (Roy et al., 2009; Olalla-Tárraga et al., 2011). However, species-specific upper critical temperatures often fail to predict biogeography (Sunday et al., 2012). A meta-analysis of local extinctions associated with climate change determined that physiological tolerance of high temperature was either unrelated or weakly/indirectly related to local extinctions or even population declines (Cahill et al., 2013). Among terrestrial ectotherms, upper thermal tolerances are generally less spatially variable than other physiological responses, such as lower thermal limits (Fig. 3; Sunday et al., 2011; Hoffmann et al., 2013; Gouveia et al., 2014). For example, in Australian Drosophila species, heat tolerance is not correlated with latitude, suggesting that this physiological trait is not the predominant factor in setting range limits for these species. On the contrary, desiccation and cold tolerance are correlated with latitude in both widespread temperate and tropically restricted species, implying that cold tolerance, rather than heat tolerance, may predict range shifts in Drosophilids more accurately as climate changes (Overgaard et al., 2011, 2014). Critical thermal maxima also fail to characterize current species' boundaries in anurans (Gouveia et al., 2014). Links between thermal tolerance and range are further complicated by apparent differences in the factors that set ranges in marine vs. terrestrial environments. The ranges of marine species seem to conform more closely to their limits of thermal tolerance, whereas warmer range boundaries are not at equilibrium with heat tolerance on land (Sunday et al., 2012). Differences in experimental methodology also reduce the effectiveness of using heat tolerance to predict biogeography (Ribeiro et al., 2012). For example, heating rates have repeatedly been shown significantly to affect estimates of upper critical temperatures (Rezende et al., 2011), and although limits may differ among species when assessed at a given heating rate, heat tolerance may be similar when ecologically relevant heating rates are considered for each species (Ribeiro et al., 2012). The duration of the experiment is also a key variable influencing thermal tolerance. Longer experiments can reduce the health of animals in experimental conditions and, in turn, reduce the capacity to withstand heat stress (Ribeiro et al., 2012). Slow heating rates resulting in much longer experimental durations can be associated with greater individual variation in measured heat tolerance (Chown et al., 2009).
Figure 3:
Upper and lower thermal tolerance limits by absolute latitude of collection for terrestrial species. Points indicate upper (triangles) and lower (circles) tolerance limits. Best-fit regression lines from linear mixed-effects model are shown. Adapted from Sunday et al. (2011).
Given the inconsistent correlation between heat tolerance and distribution for many species, mechanistic models that focus exclusively on upper physiological tolerances may fail to characterize responses to future environmental change and provide inaccurate or incomplete information to policy-makers. Diamond et al. (2012) used species-specific thermal tolerances to predict the community responses of ant species to experimental forest-floor warming at the northern and southern boundaries of temperate forests in North America. The authors then compared the predictive ability of thermal tolerance with correlative species distribution models. Thermal tolerances reliably predicted the responses of ant species at southern-most sites where temperatures already approach upper thermal limits, but failed to predict responses at the northern site, where temperatures remain relatively far from ants' upper thermal limit. These data imply that physiological metrics may be most successful in predicting future distributions when current conditions are already close to physiological limits. Importantly, correlative species distribution models were not predictive of ants' responses at either northern or southern sites, again emphasizing the need to improve modelling methodology (Diamond et al., 2012). Likewise, integrating critical thermal maxima into a model predicting the distribution of the marine mussel Mytilus edulis was accurate in predicting current distribution across only a small portion of its total range. Critical thermal maxima were sufficient to predict biogeographical distribution of M. edulis on the east coast of North America, but unable to predict the European distribution of the species accurately (Jones, 2010; Woodin et al., 2013).
Physiological processes most sensitive to environmental change
Upper critical temperatures may not be correlated strongly with biogeography because high temperatures sufficiently limit key physiological processes to exclude species from regions before heat causes mortality (Woodin et al., 2013). Thermal sensitivity often occurs in a hierarchical manner, such that processes most sensitive to environmental change can act as a dominant factor, limiting the overall fitness of an organism. For example, survival is often possible over a wider range of temperatures than locomotion or reproduction (Buckley and Kingsolver, 2012a). Thus, long-term persistence of an organism in a given location is more likely to be defined by thermal constraints on physiological performance than thresholds for heat-induced mortality. Heat transfer and dynamic energy budget models indicate that the distribution of the Mediterranean mussel Mytilus galloprovincialis is not likely to be set by exposure to lethal temperatures, but rather by chronic exposures to sublethal conditions that prevent growth and reproduction. Mussel growth and reproduction are significantly reduced in intertidal habitats that are more frequently exposed to bouts of heat stress caused by aerial exposure at low tide, compared with more environmentally stable subtidal habitats (Sará et al., 2011). In a low-elevation population of Colias butterflies, repeated, sublethal heat treatments applied during the second instar accelerate development but decrease subsequent pupal mass, suggesting that repeated exposure to high temperatures early in development may reduce final size and fecundity in this population (Higgins et al., 2015).
Identifying physiological processes most sensitive to environmental change is a key objective in applying mechanistic species distribution models over a wider range of organisms (Table 3). The concept of a performance curve, which describes the effects of abiotic change on biological rate processes, provides a means of assessing how physiology is affected by the environment. The approach is flexible in that it can be applied widely across taxa and that different abiotic factors or combinations of factors can be used to develop curves. Performance curves tend to take the same general shape regardless of the process measured; performance typically increases, reaches a maximum and then rapidly decreases. Adaptive evolution or phenotypic plasticity can modify performance curves such that different species, populations and life stages differ in how abiotic change influences performance (Schulte et al., 2011). Processes frequently measured using performance curves include rates of locomotion, development or growth, and components of fitness, including survival, fecundity and generation time (Buckley and Kingsolver, 2012a).
Table 3:
Physiological traits and considerations for integration into mechanistic species distribution models
| Trait | Considerations | Examples |
|---|---|---|
| Upper thermal limit |
|
|
| Lower thermal limit |
|
|
| Activity window |
|
|
| Developmental rate |
|
|
| Hypoxia tolerance |
|
|
| Population growth rate |
|
|
| Energetics |
|
|
Performance curves for physiological traits can provide insight into future species distributions and extinction risk, in that species are assumed to be excluded from geographical regions where abiotic conditions severely compromise processes required for growth, development or reproduction. Sinervo et al. (2010) used a thermal performance curve for locomotion to develop a mechanistic model predicting climate change extinction risk for populations of Mexican Sceloporus lizards. The model predicted that future heat stress will limit the duration of activity during the breeding months for many lizard populations, causing local extinctions by reducing their foraging time and preventing accumulation of adequate energy for reproduction (Sinervo et al., 2010; Ceia-Hasse et al., 2014). These results not only provide conservationists with specific geographical regions that will require protection, but also define a temporal window (i.e. the breeding months) when defending lizards against heat stress will be particularly critical. Flight activity of Colias butterflies, which is essential for courtship, mating, nectaring and oviposition, is restricted to body temperatures between 30 and 40°C, with peak performance occurring between 35 and 38°C (Kingsolver et al., 2011). A mechanistic model incorporating thermal constraints on flight predicts that future temperature regimens will limit available flight time for Colias populations occupying areas of low elevation, contributing to population declines and increased extinction risk (Buckley and Kingsolver, 2012b). In this case, the mechanistic model supports conservation interventions that protect lowland butterfly populations. Deutsch et al. (2008) used performance curves to model the effects of temperature increase on population growth in insects. Warming in the tropics, although relatively small in magnitude, is likely to have the most deleterious consequences because tropical insects are relatively sensitive to temperature change and are currently living very close to their optimal temperature. Much empirical evidence indicates that tropical ectotherms are particularly susceptible to future warming (Huey et al., 2009; Dillon et al., 2010; Mitchell et al., 2011; Sunday et al., 2011, 2014; Diamond et al., 2012), and protecting the tropics, where biodiversity also happens to be highest, will be an important component of future conservation efforts.
Using performance curves to develop mechanistic species distribution models offers promise for improving upon correlative models and highlights a pathway for physiologists to contribute to climate change conservation. However, the process of determining which physiological parameter is most sensitive to environmental change is laborious in that several traits may have to be measured before one strongly linked to biogeography is identified. Bioenergetic models that relate climate to metabolism through the concept of ‘scope for growth’, the energy available for growth and reproduction after basic metabolic needs have been met (Widdows and Johnson, 1988), may offer broader applicability. Metabolism is directly linked to climate in both endotherms and ectotherms. In ectotherms, individuals living at higher temperatures use disproportionately more energy per unit body mass than those living in cooler environments (Gillooly et al., 2001), and consequently, more energy is expended in maintenance, imposing bioenergetic constraints on individuals. In endotherms, environmental temperature dictates energy requirements for heating or cooling to maintain constant body temperature, with energy directed toward thermoregulation again reducing the surplus available for growth and reproduction (Kearney and Porter, 2009; Kearney et al., 2010). The direct effect of the environment on metabolism can be used to infer biogeography because an organism will be unable to survive for an extended period in locations where it would be in negative energy balance, that is, possessing insufficient energetic resources to grow and reproduce after accounting for energy consumed through basal metabolism (Kearney and Porter, 2009).
Molnár et al. (2010) attest that energy budget models aimed at predicting reproduction and survival as a function of the environment are needed to improve conservation of polar bears under climate change. Polar bears are vulnerable to climate warming primarily because these animals depend on sea ice as a platform to access prey. Progressively earlier spring ice break-up as a consequence of climate change shortens on-ice feeding and prolongs periods of on-shore fasting. A bioenergetic model used to estimate how long a bear can survive on its energy stores before death by starvation indicates that polar bears incur a major metabolic cost as a function of warming. The model predicts that only 3% of bears are expected to die of starvation with a fasting period of 120 days, typical for the 1980′s. However, early sea ice melt has increased the fasting period by ∼7 days per decade since the 1980s (Stirling and Parkinson, 2006), and when the fasting period is extended to 180 days, the number of polar bears predicted to die of starvation increases to 28%. The authors suggest that this type of mechanistic model will more accurately predict changes in polar bear survival because, unlike correlative approaches, mechanistic models can be formulated independent of environmental conditions. Broad application of this model will assist conservation by identifying particularly vulnerable polar bear populations.
Rising environmental temperatures are expected to increase the metabolic rates of ectotherms, with tangible consequences for species distributions and conservation (Dillon et al., 2010). In marine environments, temperature not only increases metabolic rates of ectotherms, but simultaneously decreases the solubility of oxygen in seawater, potentially restricting their aerobic capacities. Long-term persistence of marine organisms is therefore restricted to regions where oxygen supply exceeds resting metabolic oxygen demand, a relationship that can be exploited in mechanistic models to predict future distributions. Using laboratory-measured hypoxia tolerances for several marine species, Deutsch et al. (2015) provide evidence that marine environments are viable only if they support metabolic rates at least two to five times resting rates. The authors then use this criterion to develop a mechanistic model of future marine species distributions and extinction risks. Continued warming and deoxygenation is expected to drive substantial habitat losses through equatorward range contractions, compression of vertical distributions within the water column and shortening of seasonally inhabited areas. In mid-latitude Northern Hemisphere oceans, where fisheries are often highly productive, climate change is expected to reduce habitat suitability by ∼50%, emphasizing the need to protect these ecosystems.
Metabolic consequences of ocean change are also expected to reduce the size of marine fishes. Cheung et al. (2013) developed a mechanistic model based on the physiological principle that the maximal body weight of marine fishes at a given location is a function of environmental temperature and oxygen supply. Model outputs suggest that ocean warming and deoxygenation will reduce fish body size by 24, 20 and 14% in the Indian, Atlantic and Pacific Oceans, respectively, from year 2001 to 2050 under a high-emission scenario. Results of these models imply major economic and ecological impacts via reduced biomass available for human exploitation, as well as changes in marine food webs that will be compounded by the selective effects of fisheries for larger animals (e.g. Allendorf and Hard, 2009). These data provide a strong incentive for conservation strategies that make commercial fisheries more robust to climate change. Importantly, Cheung et al. (2013) emphasize that the mechanistic model used in this study includes assumptions and simplifications that could be improved by a better understanding of physiology, such as the capacity for phenotypic plasticity to buffer the effects of environmental change (Seebacher et al., 2015).
Physiology, demography and multivariate species distribution models
Integrating multiple approaches and perspectives is advocated as a means to identify habitats and species at risk from a rapidly changing climate most accurately (Cooke and O'Connor, 2010; Dawson et al., 2011). Among climate change-associated local extinctions examined by Cahill et al. (2013), proximate causes were determined to be a mix of thermal limitations on activity time, shifting relationships between temperature and precipitation, physiological traits and species interactions. Multivariate approaches that can account for changes in physiological performance and the interaction of physiology with demography are likely to be required to model species distributions most accurately and infer extinction risks associated with climate change (Fefferman and Romero, 2013; Cooke, 2014). Mechanistic models are poised to link the environment with demography and physiology by incorporating environmental effects on demographic variables such as climate-dependent dispersal, sex ratio and fecundity (Adolph and Porter, 1996; Crozier and Dwyer, 2006; Buckley et al., 2010). The abundance of a species at a particular location is a function of birth, death and migration rates, with persistence occurring at locations in space and time where birth and immigration exceed death and emigration. Physiological traits play an important demographic role by influencing survival and reproduction in a given set of environmental conditions (Chown et al., 2010); nonetheless, demonstrating how physiology influences the balance between births, deaths and migration remains an important knowledge gap in the field of conservation physiology (Cooke, 2014).
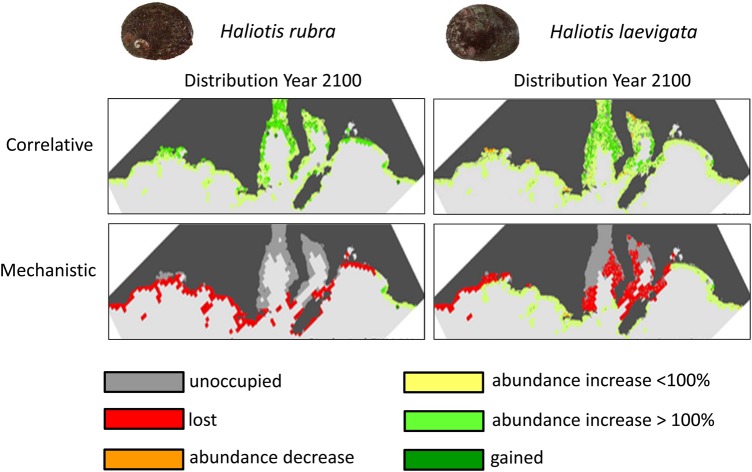
The importance of considering the both demography and physiology when modelling species distributions and extinction risk is highlighted in a study of the impact of climate change on two species of commercially exploited abalone, Haliotis rubra and Haliotis laevigata. Earlier work on these species using correlative modelling suggested that the Australian abalone industry could potentially benefit from climate change. The correlative model implied that warming sea surface temperatures would increase gonad developmental rate and accelerate the development of larvae, leading to greater reproductive output (Grubert and Ritar, 2004). However, when a mechanistic model that accounted for thermal constraints on physiology (i.e. growth and fertility) and demography (i.e. recruitment and mortality) was applied to the same two species, climate change was expected to reduce abalone ranges, rather than increase them as previously forecast (Fig. 4; Fordham et al., 2013). Discrepancy arises in part because the present-day distributions of these species are influenced not only by climate, but also by patterns of human exploitation. Including demographic information helps to account for the effect of anthropogenic harvests on meta-population dynamics, which along with the inclusion of physiological data, is thought to provide a more reliable prediction of future distribution.
Figure 4:
Forecast change in spatial abundance between 2015 and 2100 for the abalone Haliotis rubra and Haliotis laevigata using either correlative models or mechanistic models parameterized with demographic variables. Adapted from Fordham et al. (2013).
Disparate predictions between correlative and mechanistic models have obvious consequences for conservation planning. Correlative models predicting range expansions and population growth provide little incentive for protecting the valuable Australian abalone industry against climate change. In contrast, range contractions and population declines predicted by mechanistic models parameterized with physiological and demographic information support interventions that ensure the long-term stability of abalone fisheries. Analyses of the abalone industry in Australia acknowledge that current understanding is mainly on whole-animal effects of environmental stresses, and very little is known regarding the mechanistic basis of abalone vulnerability to climate change (Morash and Alter, 2015). More broadly, the abalone case study shows that integration of both physiology and demography into a modelling framework is highly relevant to conservation of species in a changing climate. Much like mechanistic models that include physiological parameters, application of more sophisticated models that include demographic indices are presently limited by sparse data (Thuiller et al., 2013). This knowledge gap again presents an opportunity for physiologists to work more closely with modellers, population biologists and aquaculturists to generate data directly contributing to the sustainability of the abalone industry.
Challenges facing mechanistic modelling
Mechanistic modelling is thought to be more robust when extrapolating species distributions into novel climates (Elith et al., 2010), can provide causal explanations for changes range shifts (Kearney and Porter, 2009) and is appropriate for the majority of management questions (Cuddington et al., 2013). However, broadening the use of mechanistic models will be challenging.
A major uncertainty in using physiology to predict climate change outcomes is the rate at which physiological data can be collected, that is, can the necessary information be acquired for a sufficient number of species before climate has already shifted (Schindler and Hilborn, 2015)? The underlying reason that correlative models are favoured in climate change conservation is that predictions can be generated quickly for a large number of organisms, thus allowing time for conservation interventions. To protect biodiversity, rather than individual species, physiologists will need to acquire relevant physiological data rapidly in many more species or develop methods that use physiological data collected in one species to predict the response in another. The concept of phylogenetic niche conservatism, that closely related species are likely to possess similar niche requirements (Wiens et al., 2009), may provide a means to extrapolate physiological or demographic data collected in one species to model responses in closely related species. Should niche conservatism hold true, models of climate change impacts on a few species could be generalized to their relatives (Buckley and Kingsolver, 2012a). However, support for phylogenetic niche conservatism is mixed (Cooper et al., 2011; Olalla-Tárraga et al., 2011), implying that scientists have not yet identified or appropriately quantified the most promising predictive traits. The emerging field of functional biogeography, which considers biota as a continuous distribution of traits and aims to link biogeographical patterns to trait diversity, may assist in the laborious process of screening traits (morphological, physiological, phenological, behavioural or demographic) for those predictive of geographical distribution (Violle et al., 2014). Functional trait approaches have been rapidly developed in plants (Pérez-Harguindeguy et al., 2013) and are expanding in microbe (Krause et al., 2014) and animal systems (Homburg et al., 2014; Pey et al., 2014). Other approaches, such as semi-mechanistic community-level modelling (Mokany and Ferrier, 2011), have emerged recently in an attempt to better predict future distributions across larger number of organisms.
Truly integrated approaches to modelling species distribution will simultaneously consider both physiological traits and demography (Ehrlén and Morris, 2015). However, most demographic models relate climate to abundance at a given locality, whereas both mechanistic and correlative species distribution models predict changes in geographical distribution (Thomas et al., 2004; Moritz et al., 2008). Those interested in modelling responses to climate change must therefore decide which biological response, abundance or distribution, is most relevant to conservation. Abundance and area of occupancy are not necessarily correlated (Fordham et al., 2013), and changes in abundance have been predicted as more ecologically important than shifts in geographical distribution (Ehrlén and Morris, 2015). Increasing or decreasing population sizes may have cascading ecological effects long before a species is extirpated from a particular region. For example, predicting changes in the abundance of commercially harvested species may be more important than identifying climatically suitable regions for these species in the future, given that their economic value is tied to abundance (Bell et al., 2013).
Issues of scale have also been prominent in hindering the application of physiological information to conservation (Cooke et al., 2014). Owing to limits in the resolution of bioclimatic data, many models rely on coarse-scale measurements to define the environmental characteristics of habitats, such as mean annual temperature and precipitation (Hijmans and Graham, 2006; Sears et al., 2011). Nonetheless, organismal performance and fitness are typically influenced by finer-scale variation in the biotic and abiotic environment (Helmuth et al., 2014). Striking differences between air temperature and organism body temperature in many ectotherms exemplifies the sometimes broad capacity to modulate habitat use through behaviour and the over-simplicity of models that assume equivalence between air and body temperature in ectotherms. A recent meta-analysis reports that most ectotherms are incapable of surviving in open habitats through physiological thermal tolerance alone, and thus, must have access to thermal refugia to survive (Sunday et al., 2014). For example, 84% of reptiles have heat tolerance limits that are lower than the highest operative temperatures in the sun and must therefore rely on thermoregulatory behaviours, such as moving into shaded habitats or burrows, to avoid heat death at the warmest times. Collecting environmental data at the microhabitat scale is essential to accurate modelling of responses to climate change (Hannah et al., 2014). The timing and frequency of environmental change can also strongly influence the responses of organisms to climate change, yet these factors are typically left unaccounted when modelling future species distributions. Night-time and seasonally biased warming have been shown to produce different organismal reactions when compared with simple increases in mean temperature (Zhao et al., 2014; Williams et al., 2015). Researchers have also found evidence of organisms responding to the increased frequency of extreme temperature events associated with global change rather than increases in mean temperature (Vasseur et al., 2014). Determining what aspect of the climate is most relevant to predicting impacts on biodiversity is another important question that needs to be answered.
Species distribution models rarely consider ecological interactions such as predation, competition, resource–consumer interactions, host–parasite interactions, mutualism and facilitation, yet species interactions are among the most important forces structuring ecological communities and are commonly climate dependent (Gilman et al., 2010; Wisz et al., 2013). Meta-analyses suggest that climate change influences virtually every type of species interaction (Tylianakis et al., 2008), and consideration of interacting species may be important for mechanistic modelling of distributions under climate change. For example, a growing body of data demonstrates that predation risk (i.e. the effect of the ‘fear’ of being eaten) can elevate the metabolic rates of prey (Rovero et al., 1999; Beckerman et al., 2007; Slos and Stoks, 2008; Miller et al., 2014), thereby altering energy budgets. Bioenergetic models that can account for the change in physiology caused by predation risk may be more accurate than those models that do not consider this variable. An awareness of the importance of biotic interactions has stimulated attempts to incorporate species interactions into distribution modelling frameworks and will probably continue in the future (reviewed by Kissling et al., 2012). However, much like mechanistic modelling in general, there are limitations on the availability of species interaction data to parameterize models with this information across large numbers of species.
A general shortcoming of climate change assessments to date is that few studies subsequently identify the specific conservation action needed to overcome the threats posed by climate change (Watson et al., 2013). As a consequence, modellers are typically unaware of whether or how their data are being used in conservation planning (Guisan et al., 2013). For example, how does one protect valuable fish and fisheries knowing that climate change may reduce suitable habitat by 50% (Deutsch et al., 2015)? Likewise, is it possible to reduce future heat stress in lizard populations vulnerable to continued warming (Sinervo et al., 2010)? Guisan et al. (2013) argue that greater clarity in these issues requires modellers and academics to explain the potential value of their work better to conservation managers, and for conservationists to communicate results of existing model applications better back to scientists. When this collaborative approach is taken, species distribution models can act as valuable pieces of information in developing an appropriate conservation strategy. For example, species distribution models have played key roles in identifying and controlling the spread of invasive species. Species distribution models are systematically used in Australia to classify species as weeds of national significance, to aid decisions about whether to allow the importation of new plant species and to apportion control costs among potentially affected regions (NTA, 2007; Guisan et al., 2013). In Madagascar, species distribution models developed for major biodiversity groups (mammals, birds, reptiles, amphibians, freshwater fishes, invertebrates and plants) were developed by scientists and used by managers to define priority areas for conservation (Kremen et al., 2008). A legal decree from the Madagasscar government prohibited mining and forestry in conservation hotspots identified by the model. Species distribution models have also been applied successfully to management of big horn sheep (Ovis canadensis sierrae) in the Sierra Nevada Mountains of the USA. A model was used to identify suitable sites for reintroductions and translocation by avoiding overlap with existing grazing stock allotments and areas of high predator densities (Johnson et al., 2007). Again, the recurring message may be that there exist many opportunities for collaboration between physiologists, modellers and conservationists to improve the application of models to conservation.
Summary
The burgeoning field of conservation physiology aims to apply physiological concepts, tools and knowledge to understanding and predicting how organisms, populations and ecosystems respond to environmental change (sensu Cooke et al., 2013). The emergence of conservation physiology attests that researchers and stakeholders are aware that physiology is of relevance to conservation (Cooke et al., 2013, 2014; Coristine et al., 2014; Lennox and Cooke, 2014), yet despite this overtone, there is little evidence for physiological data being considered in conservation decision-making (Cooke and O'Connor, 2010; Cooke, 2014). Accurate modelling tools are needed to supply managers and stakeholders with potential species distributions and community structure in response to changing environmental conditions and are major pieces of evidence in conservation planning (Thuiller et al., 2013). However, the accuracy of models currently used in climate change conservation has been widely challenged (Perretti et al., 2013; Schindler and Hilborn 2015), and new approaches to determining climate change sensitivity are needed. Mechanistic models parameterized with physiological information have been suggested as a means of improving model predictions, but are presently limited in their application because the requisite physiological data are available for a comparatively small number of species. This knowledge gap presents an opportunity to physiologists to collaborate with modellers and conservationists to contribute more directly to conservation policy. As stated by Cuddington et al. (2013), the challenges of broadly applying mechanistic models to climate change conservation ‘necessitate a clear line of communication between scientists and managers in developing models for management, and a willingness to alter strategies as models are improved’. Highlighting this need may help to stimulate communication and foster novel and more accurate means of predicting climate change impacts, while advancing the field of conservation physiology.
Funding
The authors would like to thank the Society for Experimental Biology for sponsorship.
Acknowledgements
This review is the result of a symposium presented at the American Physiological Society's 2014 Intersociety meeting: Comparative Approaches to Grand Challenges in Physiology. The authors would like to thank the American Physiological Society for supporting the symposium entitled ‘Responses to Global Change: Acclimatize, Adapt or Die’, symposium organizer Gretchen Hofmann. The authors also thank Ryan Martin and two anonymous reviewers for their constructive comments on this manuscript.
References
- Adolph SC, Porter WP. (1996) Growth, seasonality, and lizard life histories: age and size at maturity. Oikos 77: 267–278. [Google Scholar]
- Akçakaya HR, Butchart SHM, Watson JEM, Pearson RG. (2014) Preventing species extinctions resulting from climate change. Nature Clim Change 4: 1048–1049. [Google Scholar]
- Allendorf FW, Hard JJ. (2009) Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc Natl Acad Sci USA 106: 9987–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo MB, Peterson AT. (2012) Uses and misuses of bioclimatic envelope modeling. Ecology 93: 1527–1539. [DOI] [PubMed] [Google Scholar]
- Araújo MB, Cabeza M, Thuiller W, Hannah L, Williams PH. (2004) Would climate change drive species out of reserves? An assessment of existing reserve-selection methods. Global Change Biol 10: 1618–1626. [Google Scholar]
- Beckerman AP, Wieski K, Baird DJ. (2007) Behavioural vs. physiological mediation of life history under predation risk. Oecologia 152: 335–343. [DOI] [PubMed] [Google Scholar]
- Bell JD, Ganachaud A, Gehrke PC, Griffiths SP, Hobday AJ, Hoegh-Guldberg O, Johnson JE, Le Borgne R, Lehodey P, Lough JM, et al. (2013) Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nature Clim Change 3: 591–599. [Google Scholar]
- Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovic F, Calosi P, Spicer JI. (2011) Physiological correlates of geographic range in animals. Annu Rev Ecol Evol Syst 42: 155–179. [Google Scholar]
- Buckley LB. (2010) The range implications of lizard traits in changing environments. Global Ecol Biogeogr 19: 452–464. [Google Scholar]
- Buckley LB, Kingsolver JG. (2012. a) Functional and phylogenetic approaches to forecasting species' responses to climate change. Annu Rev Ecol Evol Syst 43: 205–226. [Google Scholar]
- Buckley LB, Kingsolver JG. (2012. b) The demographic impacts of shifts in climate means and extremes on alpine butterflies. Funct Ecol 26: 969–977. [Google Scholar]
- Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. (2010) Can mechanism inform species' distribution models? Ecol Lett 13: 1041–1054. [DOI] [PubMed] [Google Scholar]
- Buckley LB, Waaser SA, MacLean HJ, Fox R. (2011) Does including physiology improve species distribution model predictions of responses to recent climate change? Ecology 92: 2214–2221. [DOI] [PubMed] [Google Scholar]
- Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, Hua X, Karanewsky CJ, Yeong Ryu H, Sbeglia GC, Spagnolo F, Waldron JB, Warsi O, et al. (2013) How does climate change cause extinction? Proc Biol Sci 280: 20121890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceia-Hasse A, Sinervo B, Vicente L, Pereira HM. (2014) Integrating ecophysiological models into species distribution projections of European reptile range shifts in response to climate change. Ecography 37: 679–688. [Google Scholar]
- Cheung WWL, Sarmiento JL, Dunne J, Frolicher TL, Lam VWY, Deng Palomares ML, Watson R, Pauly D. (2013) Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nature Clim Change 3: 254–258. [Google Scholar]
- Chown SL, Jumbam KR, Sorensen JG, Terblanche JS. (2009) Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct Ecol 23: 133–140. [Google Scholar]
- Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Jr, Stenseth NC, Pertoldi C. (2010) Adapting to climate change: a perspective from evolutionary physiology. Clim Res 43: 3. [Google Scholar]
- Cooke SJ. (2014) Conservation physiology today and tomorrow. Conserv Physiol 2: doi:10.1093/conphys/cot033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, O'Connor CM. (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Killen SS, Metcalfe JD, McKenzie DJ, Mouillot D, Jørgensen C, Peck MA. (2014) Conservation physiology across scales: insights from the marine realm. Conserv Physiol 2: doi:10.1093/conphys/cou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N, Freckleton RP, Jetz W. (2011) Phylogenetic conservatism of environmental niches in mammals. Proc Biol Sci 1716: 2384–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coristine LE, Robillard CM, Kerr JT, O'Connor CM, Lapointe D, Cooke SJ. (2014) A conceptual framework for the emerging discipline of conservation physiology. Conserv Physiol 2: doi:10.1093/conphys/cou033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier L, Dwyer G. (2006) Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am Nat 167: 853–866. [DOI] [PubMed] [Google Scholar]
- Cuddington K, Fortin MJ, Gerber LR, Hastings A, Liebhold A, O'Connor M, Ray C. (2013) Process-based models are required to manage ecological systems in a changing world. Ecosphere 4: art20. [Google Scholar]
- Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. (2011) Beyond predictions: biodiversity conservation in a changing climate. Science 332: 53–58. [DOI] [PubMed] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105: 6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C, Ferrel A, Seibel B, Pörtner H-O, Huey RB. (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348: 1132–1135. [DOI] [PubMed] [Google Scholar]
- Diamond SE, Nichols LM, McCoy N, Hirsch C, Pelini SL, Sanders NJ, Ellison AM, Gotelli NJ, Dunn RR. (2012) A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93: 2305–2312. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Wang G, Huey RB. (2010) Global metabolic impacts of recent climate warming. Nature 467: 704–706. [DOI] [PubMed] [Google Scholar]
- Dormann CF, Schymanski SJ, Cabral J, Chuine I, Graham C, Hartig F, Kearney M, Morin X, Römermann C, Schröder B, et al. (2012) Correlation and process in species distribution models: bridging a dichotomy. J Biogeogr 39: 2119–2131. [Google Scholar]
- Ehrlén J, Morris WF. (2015) Predicting changes in the distribution and abundance of species under environmental change. Ecol Lett 18: 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J, Kearney M, Phillips S. (2010) The art of modeling range-shifting species. Methods Ecol Evol 1: 330–342. [Google Scholar]
- Fefferman NH, Romero LM. (2013) Can physiological stress alter population persistence? A model with conservation implications. Conserv Physiol 1: doi:10.1093/conphys/cot012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RB. (1983) Ontogenetic change in the temperature tolerance of larval Bufo marinus (anura: Bufonidae). Comp Biochem Physiol A 75: 267–271. [Google Scholar]
- Fordham DA, Akçakaya HR, Araújo MB, Keith DA, Brook BW. (2013) Tools for integrating range change, extinction risk and climate change information into conservation management. Ecography 36: 956–964. [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. (2001) Effects of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- Gillson L, Dawson TP, Jack S, McGeoch MA. (2013) Accommodating climate change contingencies in conservation strategy. Trends Ecol Evol 28: 135–142. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. (2010) A framework for community interactions under climate change. Trends Ecol Evol 25: 325–331. [DOI] [PubMed] [Google Scholar]
- Gouveia SF, Hortal J, Tejedo M, Duarte H, Cassemiro FAS, Navas CA, Diniz-Filho JAF. (2014) Climatic niche at physiological and macroecological scales: the thermal tolerance–geographical range interface and niche dimensionality. Global Ecol Biogeogr 23: 446–456. [Google Scholar]
- Grubert MA, Ritar AJ. (2004) Temperature effects on the dynamics of gonad and oocyte development in captive wild-caught blacklip (Haliotis rubra) and greenlip (H. laevigata) abalone. Invertebr Reprod Dev 45: 185–196. [Google Scholar]
- Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Tulloch AI, Regan TJ, Brotons L, McDonald-Madden E, Mantyka-Pringle C, et al. (2013) Predicting species distributions for conservation decisions. Ecol Lett 16: 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah L, Midgley G, Andelman S, Araújo M, Hughes G, Martinez-Meyer E, Pearson R, Williams P. (2007) Protected area needs in a changing climate. Front Ecol Environ 5: 131–138. [Google Scholar]
- Hannah L, Flint L, Syphard AD, Moritz MA, Buckley LB, McCullough IM. (2014) Fine-grain modeling of species' response to climate change: holdouts, stepping-stones, and microrefugia. Trends Ecol Evol 29: 390–397. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Russell BD, Connell SD, Dong Y, Harley CD, Lima FP, Sará G, Williams GA, Mieszkowska N. (2014) Beyond long-term averages: making biological sense of a rapidly changing world. Clim Chang Responses 1: 6. [Google Scholar]
- Higgins JK, MacLean HJ, Buckley LB, Kingsolver JG. (2015) Growth, developmental and stress responses of larvae of the clouded sulphur butterfly Colias eriphyle to repeated exposure to high, sub-lethal temperatures. Physiol Entomol 40: 189–195. [Google Scholar]
- Hijmans RJ, Graham CH. (2006) The ability of climate envelope models to predict the effect of climate change on species distributions. Global Change Biol 12: 2272–2281. [Google Scholar]
- Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD. (2008) Assisted colonization and rapid climate change. Science 321: 345–346. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Chown SL, Clusella-Trullas S. (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct Ecol 27: 934–949. [Google Scholar]
- Homburg K, Homburg N, Schäfer F, Schuldt A, Assmann T. (2014) Carabids.Org – a dynamic online database of ground beetle species traits (Coleoptera, Carabidae). Insect Conserv Divers 7: 195–205. [Google Scholar]
- Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJÁ, Garland T., Jr (2009) Why tropical forest lizards are vulnerable to climate warming. Proc Biol Sci 276: 1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil Trans R Soc B Biol Sci 367: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H, Bleich VC, Stephenson TR. (2007) Modelling Sierra Nevada Bighorn Sheep habitat: applying resource selection functions to species recovery. Bishop, CA, California Department of Fish and Game. [Google Scholar]
- Jones SJ. (2010). Climate change and biogeography in the marine intertidal. Doctoral dissertation, University of South Carolina, Ann Arbor, MI: ProQuest/UMI No. 3433156, ISBN 9781124384221. [Google Scholar]
- Kearney M, Porter W. (2009) Mechanistic niche modeling: combining physiological and spatial data to predict species' ranges. Ecol Lett 12: 334–350. [DOI] [PubMed] [Google Scholar]
- Kearney M, Phillips BL, Tracy CR, Christian KA, Betts G, Porter WP. (2008) Modeling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31: 423–434. [Google Scholar]
- Kearney MR, Wintle BA, Porter WP. (2010) Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv Lett 3: 203–213. [Google Scholar]
- Kearney MR, Matzelle A, Helmuth B. (2012) Biomechanics meets the ecological niche: the importance of temporal data resolution. J Exp Biol 215: 922–933. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Woods AH, Buckley LB, Potter KA, MacLean HJ, Higgins JK. (2011) Complex life cycles and the responses of insects to climate change. Integr Comp Biol 51: 719–732. [DOI] [PubMed] [Google Scholar]
- Kissling WD, Dormann CF, Groeneveld J, Hickler T, Kühn I, McInerny GJ, Montoya JM, Römermann C, Schiffers K, Schurr FM, et al. (2012) Towards novel approaches to modelling biotic interactions in multispecies assemblages at large spatial extents. J Biogeog 39: 2163–2178. [Google Scholar]
- Krause S, Le Roux X, Niklaus PA, Van Bodegom PM, Lennon JT, Bertilsson S, Grossart H-P, Philippot L, Bodelier PL. (2014) Trait-based approaches for understanding microbial biodiversity and ecosystem functioning. Front Microbiol 5: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, Cameron A, Moilanen A, Phillips SJ, Thomas CD, Beentje H, Dransfield J, Fisher BL, Glaw F, Good TC, et al. (2008) Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320: 222–226. [DOI] [PubMed] [Google Scholar]
- Leadley P. (2010) Biodiversity scenarios: projections of 21st century change in biodiversity, and associated ecosystem services: a technical report for the global biodiversity outlook 3. UNEP/Earthprint, Montreal.
- Lennox R, Cooke SJ. (2014) State of the interface between conservation and physiology: a bibliometric analysis. Conserv Physiol 2: doi:10.1093/conphys/cou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LP, Matassa CM, Trussell GC. (2014) Climate change enhances the negative effects of predation risk on an intermediate consumer. Global Change Biol 20: 3834–3844. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Sgro CM, Hoffmann AA. (2011) Phenotypic plasticity in upper thermal limits is weakly related to Drosophila species distributions. Funct Ecol 25: 661–670. [Google Scholar]
- Mokany K, Ferrier S. (2011) Predicting impacts of climate change on biodiversity: a role for semi-mechanistic community-level modelling. Diversity Distrib 17: 374–380. [Google Scholar]
- Molnár PK, Deroucher AE, Thiemann GW, Lewis MA. (2010) Predicting survival, reproduction and abundance of polar bears under climate change. Biol Conserv 143: 1612–1622. [Google Scholar]
- Morash AJ, Alter K. (2015) Effects of environmental and farm stress on abalone physiology: perspectives for abalone aquaculture in the face of global climate change. Rev Aquacult: doi:10.1111/raq.12097. [Google Scholar]
- Morin X, Thuiller W. (2009) Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90: 1301–1313. [DOI] [PubMed] [Google Scholar]
- Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. (2008) Impact of a century of climate change on small-mammal communities in Yosemite national park, USA. Science 322: 261–264. [DOI] [PubMed] [Google Scholar]
- Németh Z, Bonier F, MacDougall-Shackleton SA. (2013) Coping with uncertainty: integrating physiology, behavior, and evolutionary ecology in a changing world. Integr Comp Biol 53: 960–964. [DOI] [PubMed] [Google Scholar]
- NTA (2007) Northern Territory weed risk management user guide. Natural Resources Division, Department of Natural Resources, Environment, The Arts and Sport, Northern Territory of Australia, Palmerston, NT, Australia. [Google Scholar]
- Olalla-Tárraga MÁ, McInnes L, Bini LM, Diniz-Filho JA, Fritz SA, Hawkins BA, Hortal J, Orme CDL, Rahbek C, Rodríguez MÁ. (2011) Climatic niche conservatism and the evolutionary dynamics in species range boundaries: Global congruence across mammals and amphibians. J Biogeogr 38: 2237–2247. [Google Scholar]
- Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. (2011) Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am Nat 178: S80–S96. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Kearney MR, Hoffmann AA. (2014) Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Global Change Biol 20: 1738–1750. [DOI] [PubMed] [Google Scholar]
- Pachauri RK, Reisinger A. (2007) Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland, p 104. [Google Scholar]
- Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akçakaya HR, et al. (2015) Assessing species vulnerability to climate change. Nature Clim Change 5: 215–224. [Google Scholar]
- Pearson RG, Thuiller W, Araújo MB, Martinez-Meyer E, Brotons L, McClean C, Miles L, Segurado P, Dawson TP, Lees DC. (2006) Model-based uncertainty in species range prediction. J Biogeogr 33: 1704–1711. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte M, Cornwell W, Craine J, Gurvich D. (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61: 167–234. [Google Scholar]
- Perretti CT, Munch SB, Sugihara G. (2013) Model-free forecasting outperforms the correct mechanistic model for simulated and experimental data. Proc Natl Acad Sci USA 110: 5253–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pey B, Nahmani J, Auclerc A, Capowiez Y, Cluzeau D, Cortet J, Decaëns T, Deharveng L, Dubs F, Joimel S, et al. (2014) Current use of and future needs for soil invertebrate functional traits in community ecology. Basic Appl Ecol 15: 194–206. [Google Scholar]
- Phillips BL, Chipperfield JD, Kearney MR. (2008) The toad ahead: challenges of modeling the range and spread of an invasive species. Wildlife Res 35: 222–234. [Google Scholar]
- Porfirio LL, Harris RM, Lefroy EC, Hugh S, Gould SF, Lee G, Bindoff NL, Mackey B. (2014) Improving the use of species distribution models in conservation planning and management under climate change. PloS One 9: e113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende EL, Tejedo M, Santos M. (2011) Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct Ecol 25: 111–121. [Google Scholar]
- Ribeiro PL, Camacho A, Navas CA. (2012) Considerations for assessing maximum critical temperatures in small ectothermic animals: insights from leaf-cutting ants. PLoS One 7: e32083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LM, Elith J, Hobday AJ, Pearson RG, Kendall BE, Possingham HP, Richardson AJ. (2011) Pushing the limits in marine species distribution modeling: lessons from the land present challenges and opportunities. Global Ecol Biogeogr 20: 789–802. [Google Scholar]
- Rovero F, Hughes RN, Chelazzi G. (1999) Cardiac and behavioural responses of mussels to risk of predation by dogwhelks. Anim Behav 58: 707–714. [DOI] [PubMed] [Google Scholar]
- Roy K, Hunt G, Jablonski D, Krug AZ, Valentine JW. (2009) A macroevolutionary perspective on species range limits. Proc Biol Sci 276: 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sará G, Kearney M, Helmuth B. (2011) Combining heat-transfer and energy budget models to predict thermal stress in Mediterranean intertidal mussels. Chem Ecol 27: 135–145. [Google Scholar]
- Schindler DE, Hilborn R. (2015) Prediction, precaution, and policy under global change. Science 347: 953–954. [DOI] [PubMed] [Google Scholar]
- Schulte PM, Healy TM, Fangue NA. (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51: 691–704. [DOI] [PubMed] [Google Scholar]
- Sears MW, Raskin E, Angilletta MJ. (2011) The world is not flat: defining relevant thermal landscapes in the context of climate change. Integr Comp Biol 51: 666–675. [DOI] [PubMed] [Google Scholar]
- Seebacher F, White CR, Franklin CE. (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Clim Change 5: 61–66. [Google Scholar]
- Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, et al. (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328: 894–899. [DOI] [PubMed] [Google Scholar]
- Slos S, Stoks R. (2008) Predation risk induces stress proteins and reduces antioxidant defense. Funct Ecol 22: 637–642. [Google Scholar]
- Stirling I, Parkinson CL. (2006) Possible effects of climate warming on selected populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic 59: 261–275. [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK. (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc Biol Sci 278: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK. (2012) Thermal tolerance and the global redistribution of animals. Nature Clim Change 2: 686–690. [Google Scholar]
- Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci USA 111: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherst RW, Floyd RB, Maywald GF. (1996) The potential geographical distribution of the cane toad, Bufo marinus L. in Australia. Conserv Biol 10: 294–299. [Google Scholar]
- Tewksbury JJ, Huey RB, Deutsch CA. (2008) Putting the heat on tropical animals. Science 320: 1296–1297. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L, et al. (2004) Extinction risk from climate change. Nature 427: 145–148. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Münkemüller T, Lavergne S, Mouillot D, Mouquet N, Schiffers K, Gravel D. (2013) A road map for integrating eco-evolutionary processes into biodiversity models. Ecol Lett 16: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11: 1351–1363. [DOI] [PubMed] [Google Scholar]
- Urban MC. (2015) Accelerating extinction risk from climate change. Science 348: 571–573. [DOI] [PubMed] [Google Scholar]
- Urban MC, Phillips BL, Skelly DK, Shine R. (2007) The cane toad's (Chaunus [Bufo] marinus) increasing ability to invade Australia is revealed by a dynamically updated range model. Proc Biol Sci 274: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Matesanz S, Guilhaumon F, Araújo MB, Balaguer L, Benito-Garzón M, Cornwell W, Gianoli E, van Kleunen M, Naya DE, et al. (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17: 1351–1364. [DOI] [PubMed] [Google Scholar]
- Van Beurden E. (1981) Bioclimatic limits to the spread of Bufo marinus in Australia: a baseline. Proc Ecol Soc Austral 11: 143–149. [Google Scholar]
- Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. (2014) Increased temperature variation poses a greater risk to species than climate warming. Proc Biol Sci 281: 20132612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. (2014) The emergence and promise of functional biogeography. Proc Natl Acad Sci USA 111: 13690–13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R, VanDerWal J, Price J, Welbergen JA, Atkinson I, Ramirez-Villegas J, Osborn TJ, Jarvis A, Shoo LP, Williams SE, et al. (2013) Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nature Clim Change 3: 678–682. [Google Scholar]
- Watson JEM, Iwamura T, Butt N. (2013) Mapping vulnerability and conservation adaptation strategies under climate change. Nature Clim Change 3: 989–994. [Google Scholar]
- Widdows J, Johnson D. (1988) Physiological energetics of Mytilus edulis: scope for growth. Mar Ecol Prog Ser 46: 113–121. [Google Scholar]
- Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. (2009) Niches, models, and climate change: assessing the assumptions and uncertainties. Proc Natl Acad Sci USA 106: 19729–19736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Henry HA, Sinclair BJ. (2015) Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol Rev 90: 214–235. [DOI] [PubMed] [Google Scholar]
- Williams JW, Jackson ST. (2007) Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ 5: 475–482. [Google Scholar]
- Wilson KA, Westphal MI, Possingham HP, Elith J. (2005) Sensitivity of conservation planning to different approaches to using predicted species distribution data. Biol Conserv 122: 99–112. [Google Scholar]
- Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes JA, Guisan A, et al. (2013) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev 88: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin SA, Hilbish TJ, Helmuth B, Jones SJ, Wethey DS. (2013) Climate change, species distribution models, and physiological performance metrics: predicting when biogeographic models are likely to fail. Ecol Evol 3: 3334–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhang W, Hoffmann AA, Ma CS. (2014) Night warming on hot days produces novel impacts on development, survival and reproduction in a small arthropod. J Anim Ecol 83: 769–778. [DOI] [PubMed] [Google Scholar]