Amphibians are in the midst of an extinction crisis, yet there are few tools available to study amphibian immunocompetence under conditions of changing environments, disease and stress. We developed, validated and optimised a practical assay for studying amphibian immunocompetence and tested its efficacy in a real-world scenario of varying environmental conditions.
Keywords: Disease, global environmental change, immunoecology, PHA, phenotypic carry-over, stress
Abstract
The global amphibian biodiversity crisis is driven by disease, habitat destruction and drastically altered ecosystems. It has given rise to an unprecedented need to understand the link between rapidly changing environments, immunocompetence and wildlife health (the nascent field of ecoimmunology). Increasing our knowledge of the ecoimmunology of amphibians necessitates the development of reliable, field-applicable methods of assessing immunocompetence in non-model species. The phytohaemagglutinin (PHA) inflammation assay uses a lectin to elicit localized inflammation that reflects an organism's capacity to mount an immune response. Although extensively used in birds to assess responses to environmental change, stress and disease, its application in amphibians has been extremely limited. We developed, validated and optimized a practical and effective phytohaemagglutinin inflammation assay in phylogenetically distant amphibians and demonstrated its suitability for use in a wide range of ecoimmunological studies. The protocol was effective for all species tested and worked equally well for both sexes and for adult and sub-adult animals. We determined that using set-force-measuring instruments resulted in a ‘compression effect’ that countered the inflammatory response, reinforcing the need for internal controls. We developed a novel method to determine peak response times more accurately and thereby improve assay sensitivity. Histological validation demonstrated considerable interspecies variation in the robustness of amphibian immune defences. Importantly, we applied the assay to a real-world scenario of varying environmental conditions and proved that the assay effectively detected differences in immune fitness between groups of animals exposed to ecologically meaningful levels of density stress. This provided strong evidence that one cost of metamorphic plasticity responses by tadpoles to increasing density is a reduction in post-metamorphic immune fitness and that metamorphosis does not prevent phenotypic carry-over of larval stress to the adult phenotype. This assay provides an effective tool for understanding the role of global environmental change in the amphibian extinction crisis.
Introduction
Recent, resurgent interest in the effects of environmental stress on organisms has been precipitated by the broader biodiversity crisis, which has its roots in anthropogenically driven global environmental change. Global environmental change has been associated with rapid adjustments to environments through threatening processes, such as habitat destruction, invasive species, emerging infectious diseases and climate change (Reich et al., 2012; Pauls et al., 2013; Sutherland et al., 2013). Amphibians, in particular, have been heavily impacted by global environmental change, with disease and habitat loss (among other threatening processes) manifesting as a global amphibian extinction crisis—the worst of any vertebrate class—that has resulted in 50% of the world's amphibian species experiencing decline, with one-third thought to be currently at risk of extinction (Wake, 1991; Houlahan et al., 2000; Stuart et al., 2004; Bishop et al., 2012). Environmental stressors have a multitude of effects on organismal growth, development, fitness and the immune system. Thus, the link between environment, immunocompetence and organismal health has received increasing attention in the fields of ecology and conservation, sparking rapid growth in the newly emerged field of ecoimmunology (Ros et al., 1997; Saino et al., 1997; Zuk and Johnsen, 1998; Acevedo-Whitehouse and Duffus, 2009; Rollins-Smith and Woodhams, 2012; Pigeon et al., 2013).
Knowledge of ecoimmunology is advancing rapidly in the endotherms; however, knowledge in ectothermic groups, including amphibians, is less well advanced, due in part to the limited number of tools available for assessing immunocompetence, particularly in non-model species (Rollins-Smith and Woodhams, 2012). One applicable tool traditionally used in birds and mammals is the delayed-type hypersensitivity (DTH) assay, which uses a lectin (a carbohydrate-binding protein) to elicit localized inflammation that reflects an organism's ability to mount a dynamic innate and adaptive immune response (Goto et al., 1978; Kean and Lamont, 1994; Smits et al., 1999; Kennedy and Nager, 2006; Allen et al., 2009; Vinkler et al., 2010). The most widely used lectin for this purpose is phytohaemagglutinin (PHA; derived from the red kidney bean Phaseolus vulgaris), originally used in ecoimmunological studies on birds (McCorkle et al., 1982; Smits et al., 1999; Grasman, 2002; Martin et al., 2006; Tella et al., 2008). Subcutaneous injection of PHA can result in the influx of neutrophils, eosinophils and macrophages associated with innate immune responses, as well as lymphocytes linked to adaptive cell-mediated immunity (Kennedy and Nager, 2006; Tella et al., 2008; Biard et al., 2009; Vinkler et al., 2010; Brown et al., 2011). By quantifying the level of inflammation that occurs at the site of the PHA injection (for example, by measuring the skin thickness before and after injection) it is possible to quantify the relative immunocompetence of an individual, with a greater swelling response indicating a greater level of immune fitness (Kennedy and Nager, 2006).
Nevertheless, few studies report the use of the PHA assay in amphibians (Gilbertson et al., 2003; Gervasi and Foufopoulos, 2008; Venesky et al., 2012; Fites et al., 2014), and only one study has examined the nature of the inflammatory response histologically (Brown et al., 2011). In the cane toad, Rhinella marina, Brown et al. (2011) demonstrated that PHA injection into the toe webbing, which is particularly prominent and thick in this species, stimulated inflammation associated with the infiltration of leucocytes. However, many species of amphibians have either no toe webbing or webbing that is not large or thick enough to enable intra-dermal injection, making the application of the assay by that method impractical. Indeed, the chosen site for PHA injection has varied in all studies of post-metamorphic amphibians to date, and none is applicable to all species (Gilbertson et al., 2003; Gervasi and Foufopoulos, 2008; Brown et al., 2011; Fites et al., 2014). Furthermore, the inflammatory response to the PHA assay has been found to vary widely between bird species, and there is no reason to expect the situation to be different for amphibians. Indeed, small swelling responses to primary subcutaneous PHA injection in Xenopus laevis prompted Fites et al. (2014) to develop a method in which a secondary intramuscular injection of PHA into the footpad followed intraperitoneal PHA priming in an effort to increase the inflammatory response. Thus, it is important to validate the assay more broadly and to develop a consensus method that optimizes the swelling response and is both practical and applicable to amphibians across diverse taxa and geographies. In addition, whilst the PHA assay has been applied separately to tadpoles, metamorphs and adults in a limited number of amphibian species, there has been no comparative assessment of the PHA-induced inflammation between amphibian sexes or for sub-adults and adults. Such comparisons are important if the assay is to avoid confounding effects when assessing the ecoimmunological status within and across populations. Alternative lectins to PHA that are sometimes used in birds, such as concanavalin A (Con A; derived from the jack bean Canavalia ensiformis; Grasman, 2002), have not been assessed for their effectiveness or potential to elicit stronger responses in amphibians, which might assist researchers and managers in the field to obtain statistically significant data using fewer animals. Importantly, to date there has been no systematic application of the assay to assess its efficacy in reflecting ecologically meaningful levels of environmental stress in amphibians.
We aimed to validate the PHA assay across phylogenetically distant amphibian species and to develop an optimal method of delivery and response measurement that would be both easy to use and widely applicable for amphibians of all sizes. We compared responses between sexes and validated the assay for both adults and juveniles in four species from two families separated by >100 million years of evolutionary history. We used histology to elucidate differences in the nature and magnitude of the swelling response to PHA injection between species and assessed the efficacy of the assay in the presence of ecologically meaningful levels of environmental stress by raising tadpoles at different densities and applying the assay post-metamorphosis. This also provided a rare insight into the effects of stress-related phenotypic carry-over in an endangered model amphibian. We tested a second lectin (Con A) and compared its effectiveness against PHA. Finally, we found that the standard approach used for assessing PHA assays was inadequate for determining peak response times in amphibians and developed a novel method to determine time to peak response more accurately.
Materials and methods
Sources of animals and captive husbandry
Four species of anuran amphibians from two families native to Australia were used for the various validation experiments, including two tree frogs of the family Hylidae (green and golden bell frog, Litoria aurea, and whirring tree frog, Litoria revelata) and two ground frogs of the family Limnodynastidae (striped marsh frog, Limnodynastes peronii, and spotted grass frog, Limnodynastes tasmaniensis). The two lineages are separated by ∼135 million years of evolution (Irisarri et al., 2012; Zhang et al., 2013).
Individuals of L. revelata, Lim. peronii and Lim. tasmaniensis were collected from the wild, with L. revelata and Lim. peronii collected from a pond in the Watagan Mountains (−33.02584°S, 151.37846°E) and Lim. tasmaniensis collected from a pond on Kooragang Island (−32.87235°S, 151.74588°E), New South Wales, Australia. All three species were collected as reproductively mature individuals of both sexes and were transported to the laboratory at the University of Newcastle, where they were housed in groups of 15 in large plastic tubs (approximately 42 cm × 64 cm × 40 cm) lined with leaf litter until they were used in experiments. Access to water and food (brown crickets, Acheta domestica) was provided ad libitum, and environmental conditions (temperature and day length) were partly regulated by air-conditioning and fluorescent lighting in a facility that received partial lighting through external glass windows. Ambient temperatures varied between 16 and 28°C; light–dark cycle was approximately 12 h light–12 h dark.
Litoria aurea were raised from tadpoles that were bred at the University of Newcastle in dedicated breeding facilities. These were housed in groups of 10 tadpoles in plastic trays (approximately 24 cm × 40 cm × 15 cm; each filled with 10 l of water) and fed a mixture of powdered spirulina (Bioglan; Chemist Warehouse, Australia) and trout pellets (Ridley Aqua-feed; Ridley AgriProducts Pty Ltd, Narangba, Queensland, Australia) until metamorphosis, whereby they were transferred to plastic tubs in groups of 15 and fed crickets ad libitum until experimentation. Litoria aurea were used at the juvenile stage to examine the efficacy of the assay on sub-adult animals. All L. aurea juveniles were between 10 and 12 weeks post-metamorphosis at the time of experimentation, which is long enough after metamorphosis to ensure that the animals had developed their adult-type immune system and to ensure that there was no interference from temporarily elevated corticosterone levels that occur during metamorphic climax (Gancedo et al., 1992; Rollins-Smith, 1998, 2001; Denver et al., 2002; Denver, 2009).
Research described in this manuscript was undertaken following approval by University of Newcastle Animal Care and Ethics Committee, which adheres to the NSW Animal Research Act, NSW Animal Research Regulation and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (approval number A-2009-151). Animals were collected under permit from the NSW National Parks and Wildlife Service.
Standard experimental approach for validation of phytohaemagglutinin assays
Animals were housed individually during experiments in small plastic aquaria (20 cm × 12.5 cm × 13 cm) with a gravel substrate and a small amount of water for hydration.
Phytohaemagglutinin assays involved a subcutaneous (lymph sac) injection of the lectin [40 µg delivered in 40 µl of sterile phosphate-buffered saline (PBS)] into the anterior lateral surface of the lower leg just below the knee (Fig. 1A) in one leg, and a control injection (40 µl PBS) into the same position on the contralateral leg (thereby constituting an internal control for each individual). Legs were allocated randomly for injection with the lectin or control solution. The location of the injection site was established in preliminary trials prior to experiments with lectins (data not shown). Injections were made with a 0.3 ml, 31 gauge insulin syringe. Leg thickness was measured at the site of injection using a custom-made 0.4 N set-force dial micrometer (Peacock model G-1A dial thickness gauge with 0.4 N gauging force, accurate to 0.01 mm; Ozaki Manufacturing Ltd, Tokyo, Japan; Fig. 1B) before injections were carried out and at set time periods post-injection. The set-force micrometer was held in position for ∼2 s to allow the dial to stabilize before the measurement was read. Typically, measurements were made at 0 h (prior to injection) and at 6, 12, 24, 48 and 72 h post-injection in triplicate for ‘standard’ validation experiments (dubbed the ‘24 h’ regime), following standard validation protocols (Martin et al., 2006; Brown et al., 2011).
Figure 1:

Photographs of the injection/measuring location (A; frog in photograph is Limnodynastes peronii) and set-force dial callipers used for measuring leg thickness (B; frog in photograph is Litoria revelata).
Specific experimental protocols
Experiment 1: quantification of the ‘compression effect’
From trials prior to experiments with lectins, it was determined that set-force-measuring devices, such as the one used in the present experiments and commonly used for PHA assays in general, have the potential for a compression effect over time whereby the leg being measured becomes marginally thinner with repeated measurements because of an effect of the pressure from the measuring device. This has the potential to oppose the inflammatory response; therefore, the compression effect was quantified in one species, L. aurea, by measuring leg thickness over time without any injections (n = 10), as well as a comparison of no injection in one leg against a control injection of 40 µl of PBS in the contralateral leg to test whether the act of injecting a substance alone exacerbated or negated the observed compression effect (n = 5).
Experiment 2: validation of the phytohaemagglutinin assay
Assessment of inflammatory responses between species, life stages and sexes
The PHA assay was assessed and compared in juvenile L. aurea (n = 25) and in males of the three remaining species (L. revelata, n = 15; Lim. peronii, n = 17; and Lim. tasmaniensis, n = 8). Comparisons were also made between the sexes of two species; Lim. peronii (n = 17 males and n = 8 females) and Lim. tasmaniensis (n = 8 males and n = 6 females). Phytohaemagglutinin was purchased in lyophilized form (Sigma catalogue no. L8754) and made into an injectable solution by diluting into sterile PBS. To establish the true response curves over time due to PHA injection, the average measurements of leg thickness from the internal control (PBS-injected) leg were subtracted from those of the PHA-injected leg to account for the ‘compression effect’ (data from both the raw leg measurements and the corrected curves are presented). All analyses of changes in leg thickness over time due to the PHA injection were carried out using the corrected curves (i.e. PBS leg subtracted from the PHA leg). Dose effects were tested for in all species using three morphometric measurements (snout–vent length, head width and right tibial length) as well as body weight. All body size metrics were recorded immediately before experimentation using plastic dial callipers (accurate to 0.1 mm) and Pesola balances appropriate to weight.
Validation of the assay using histological analysis
To validate the assay (linking inflammation to immune cell infiltration) and assess the nature of the PHA-induced swelling response across species, the lower legs of 10 L. aurea (tree frog model) and six Lim. peronii (ground frog model) were injected with PHA or PBS as described above. Measurements of leg thickness were made at 0 and at 24 h post-injection, and the animals were killed by cardiac excision after MS-222 anaesthesia. The injected lower limbs were dissected prior to overnight fixation in Bouin's fixative, followed by standard histological processing for paraffin embedding, including orientation for injection site with Tissue Marking Dye Blue (Fronine Pty Ltd), sectioning (5 µm) and staining with Haematoxylin and Eosin. Sections from a subset of animals (L. aurea, n = 6; and Lim. peronii, n = 3) were examined for signs of inflammation, including oedema, fibrosis, vasodilatation and leucocyte infiltration, using light microscopy. Blood vessels and leucocytes (including lymphocytes, neutrophils, macrophages, eosinophils and basophils; Cannon et al., 1986) in the lymph sac, and connective tissue (including epimesium, perimysium and endomysium) were counted in five randomly selected fields of view along the border of the dermis, lymph sac and adjacent skeletal muscle on the injected side of the leg at ×400 magnification.
Experiment 3: optimization of the method for determining ‘peak response’
While the standard method of validation for PHA assays used in birds (and described above; see ‘Standard experimental approach for validation of phytohaemagglutinin assays’) is considered suitable for determining whether a reaction to PHA occurs, we found it unsuitable for accurately determining peak response in frogs because of the large time gap between points of measurement after 24 h. As such, we tested a novel validation method in two species (L. aurea, n = 10; and Lim. peronii, n = 12) whereby we measured leg thickness immediately prior to injection (at 0 h) and then once every 4 h until 72 h post-injection, rather than in triplicate at 6, 12, 24, 48 and 72 h post-injection. We then used a curve-smoothing approach (see ‘Data analyses’ below) to produce a more refined curve and more accurately predict where in the 72 h experimental period the peak response occurred. The rationale behind the modified method of data collection and analysis was that we did not want to increase the number of measurements that occurred for each frog over the 72 h period because of the possibility of an increased compression effect (both methods involve 18 measurements of leg thickness in the 72 h period), and by increasing the frequency of data collection we were able to determine the level of variability in the measurements without measuring each time point in triplicate (using generalized additive mixed-effects models, described below; see ‘Data analyses’). We dubbed this modified method the ‘4 h’ regime (as opposed to the ‘24 h’ regime described above; see ‘Standard experimental approach for validation of phytohaemagglutinin assays’).
Experiment 4: comparison of the effectiveness of phytohaemagglutinin and concanavalin A
A second lectin used for DTH assays in birds (Grasman, 2002), Con A (Sigma catalogue no. C7275), was validated on two species of frog (L. aurea, n = 13; and Lim. peronii, n = 6) and compared with the effectiveness of the PHA assay for each species. This was carried out using a randomized, within-individual, paired design whereby the efficacies of the two assays were compared directly to one another by injecting 40 µg PHA into one leg and 40 µg Con A (both delivered in 40 µl of sterile PBS) into the contralateral leg of each individual tested. The two assays were compared in L. aurea using the ‘24 h’ regime and in Lim. peronii using the ‘4 h’ regime.
Experiment 5: experimental validation of the efficacy of the phytohaemagglutinin assay: comparing responses in stressed and non-stressed individuals
The efficacy of the PHA assay was assessed in one species (L. aurea) by rearing tadpoles in conditions of low (1 tadpole l−1) or high tadpole density (4 tadpoles l−1) and applying the assay post-metamorphosis. Density-dependent effects have been shown to occur in tadpoles of L. aurea, with increasing density leading to increased mortality and decreased weight at metamorphosis (Browne et al., 2003). All tadpoles were from a single clutch and were housed in trays approximately 24 cm × 40 cm × 15 cm containing 10 l of water as described above (see ‘Sources of animals and captive husbandry’). Low-density treatments contained 10 tadpoles per tray (1 tadpole l−1), whereas high-density treatments contained 40 tadpoles per tray (4 tadpoles l−1). Tadpoles in both treatments were fed a mixture of one part powdered spirulina to three parts ground trout pellets (30 mg per tadpole per day) and were exposed to the same light and temperature conditions as described above (see ‘Sources of animals and captive husbandry’). Upon metamorphosis (defined as Gosner stage 42, reached when the front limbs emerge; Gosner, 1960), metamorphs were weighed and total length measured, and their interval to metamorphosis was recorded to assess whether there were any plastic effects on the phenotype. Such effects are an indication that the levels of environmental ‘stress’ to which tadpoles were exposed were ecologically meaningful. Metamorphs were then transferred to plastic tubs in groups of 15 (within their respective tadpole treatments) and fed crickets ad libitum until 10–12 weeks post-metamorphosis, at which time they were subjected to the PHA assay as described above (see ‘Standard experimental approach for validation of phytohaemagglutinin assays’).
Data analyses
Linear mixed models were performed in SAS with either one factor (time) for single treatment effects, or two factors (time and treatment) for the various PHA experiments that used the ‘24 h’ regime. The correlations between repeated time measurements were modelled using a residual covariance structure. Several structures were examined to test for non-constant variance and varying correlation over time, and the Akaike information criterion was used to pick the most appropriate structure, which was compound symmetry in all cases.
Using the fitted model, post-hoc Student's one-sample and two-sample t-tests were used to determine the significance of differences with no adjustments or multiple comparisons. To determine when leg thickness first deviated significantly from time 0 h, one-sample tests were used to determine whether the mean of the differences had deviated significantly from time 0 h. The maximal swelling response (i.e. ‘peak response’) was determined when no statistically significant further increase in leg thickness was observed using two-sample tests to determine differences of least-squares means. This approach was also used to determine when the swelling began to subside significantly from one time point to the next (if this occurred at all within the 72 h period).
For the ‘4 h’ regime, generalized additive mixed-effects models were performed in R (mgcv library), using the normal distribution and frog as a random effect. Cubic regression splines were used to fit a smoothed estimate of the relationship between change and time.
To test for differences between time interval to, and size at, metamorphosis between low- and high-density tadpole treatments, generalized linear mixed models were performed in SAS using the glimmix procedure, with fixed effects for tadpole density and a random effect for tadpole tray to account for variability between trays. Differences in inflammatory response to the PHA assay between the tadpole treatments were assessed at 24 and 48 h post-injection, because the peak response to the assay in L. aurea was shown to occur between these times (see Results below). These data were analysed using linear mixed models performed in SAS as described above.
To test for differences in leucocyte numbers between PHA- and PBS-injected legs, we fitted a generalized linear mixed model for the difference between treatments for each cell type and species combination with a Poisson distribution and log link function and with random effect terms for subject differences and replicated field of view differences. The model would not converge with either of the random effects, indicating that the variation due to these sources was not significant. Therefore, a generalized linear model for the difference between treatments (PHA vs. PBS) for each cell type and species combination was used, with a Poisson distribution and log link function. Due to very low cell counts in either PHA- or PBS-injected legs for basophils (total counts for all animals combined: n = 3 vs. n = 0), these cell types were excluded from analyses.
Results
Experiment 1: quantification of the ‘compression effect’
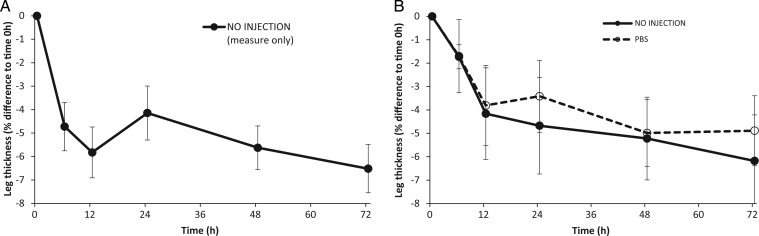
There was a rapid compression effect over time for L. aurea, which resulted in a decrease in leg thickness of up to 6.5% (n = 10, F4,86 = 3.95, P < 0.005; Fig. 2A). The majority of this effect took place within the first 6 h post-injection (following the first round of measurements), although there was some variability to this (Fig. 2A and B). The act of injecting a control substance (PBS) alone did not alter the ‘compression’ effect, with no difference in leg thickness over time observed between legs that were not injected and legs that were injected with a PBS control (n = 5, F1,45 = 0.73, P = 0.39; Fig. 2B).
Figure 2:
Changes in leg thickness of Litoria aurea over time because of the ‘compression effect’. (A) Legs were measured without injection (n = 10). (B) Legs were measured either without injection or following a phosphate-buffered saline (PBS) control injection (n = 5). Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Experiment 2: validation of the phytohaemagglutinin assay
Assessment of inflammatory responses
Phytohaemagglutinin caused visible inflammation (Fig. 3) and a significant increase in leg thickness over time for all species tested (Table 1 and Fig. 4). There were, however, differences between the species in terms of the speed at which they deviated in leg thickness from time 0 h, the time taken to reach peak response and the magnitude of the effect (F3,273 = 19.26, P < 0.001; Figs 4E–H and 5).
Figure 3:

Photograph of Lim. peronii showing visible inflammation in the phytohaemagglutinin (PHA)-injected leg (left-hand side) vs. the PBS-injected leg (right-hand side). Arrow indicates general region of swelling.
Table 1:
Summary of the statistical output for the effect of phytohaemagglutinin injections on the change in leg thickness for all four species over time
| Species | n | d.f. | F-value | P-value |
|---|---|---|---|---|
| Litoria aurea | 25 | 4, 82 | 20.03 | <0.001 |
| Litoria revelata | 15 | 4, 56 | 12.54 | <0.001 |
| Limnodynastes peronii | 17 | 4, 54 | 9.21 | <0.001 |
| Limnodynastes tasmaniensis | 8 | 4, 82 | 6.33 | <0.001 |
Abbreviations: d.f., degrees of freedom; n, number of animals tested.
Figure 4:
Changes in leg thickness for all four species tested over time following injections of PHA and PBS. (A–D) These graphs show the change in leg thickness over time for the PHA- and PBS-injected legs independently. (E–H) These graphs show the adjusted curves whereby the change in leg thickness for the PBS-injected leg was subtracted from the change in leg thickness for the PHA-injected leg at each time point. Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Figure 5:
The adjusted curves (change in leg thickness for PBS-injected leg subtracted from change in leg thickness for PHA-injected leg) for all four species, showing the differences in time to ‘peak response’ and the overall magnitude of the effect. Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Significant inflammation in the legs of the two tree frogs (L. aurea and L. revelata) was not detected until at least 12 h after PHA injection, with significant swelling at 12 h post-injection in L. aurea (t1,45.8 = 2.65, P = 0.011) and at 24 h in L. revelata (t1,16.5 = 2.73, P = 0.015; Fig. 4E and F). There was a further increase in leg thickness between 12 and 24 h post-injection in L. aurea (t1,82.6 = 4.52, P < 0.001; Fig. 4E). Both tree frogs reached peak response by 24 h post-injection, which was a maximum of 7.64% increase in leg thickness for L. aurea and 4.41% for L. revelata (both observed at 48 h post-injection). Only L. aurea experienced a significant decrease in leg thickness after the peak response, decreasing to 5.63% by 72 h post-injection (t1,81.4 = 2.02, P = 0.047).
In the two ground frogs (Lim. peronii and Lim. tasmaniensis), inflammation occurred more rapidly, with the PHA-injected leg thickness increasing in both species by 6 h post-injection (t1,30.7 = 2.42, P = 0.022 and t1,35 = 2.1, P = 0.043, respectively). Both species displayed a further increase in leg thickness from 6 to 12 h post-injection (t1,28 = 2.07, P = 0.048 and t1,28 = 2.55, P = 0.017, respectively), at which point no further significant increases or decreases in leg thickness were observed. The maximal increase in leg thickness was 12.23% for Lim. peronii (observed at 48 h post-injection) and 7.50% for Lim. tasmaniensis (observed at 24 h post-injection). Neither species experienced a significant decrease in leg thickness after the peak response within the 72 h time frame.
There were no sex-related differences in the swelling response to PHA for either of the two species tested (F1,23.1 = 0.22, P = 0.64 for Lim. peronii and F1,12 = 0.11, P = 0.74 for Lim. tasmaniensis; Fig. 6). No dose effects were found for any of the body size metrics measured (snout–vent length, weight, head width or right tibial length) for any of the four species.
Figure 6:
Changes in leg thickness over time of Lim. peronii (A) and Limnodynastes tasmaniensis (B) by sex. All curves are adjusted curves (change in leg thickness for PBS-injected leg subtracted from change in leg thickness for PHA-injected leg). Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Histological analysis
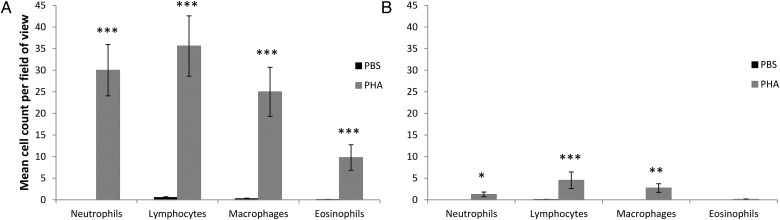
Subcutaneous injection of PHA into the lymph sac of the anterior lateral surface of the lower leg of Lim. peronii resulted in significant inflammation compared with PBS injections after 24 h (mean = 30.2% ± SEM 7.9%; n = 6). In the lymph sac of PHA-injected legs, fibrosis and leucocyte infiltration, especially of lymphocytes, neutrophils, macrophages and a small number of eosinophils, was evident in two of three animals (Fig. 7A and B). Increased extravascular leucocyte infiltration of the connective tissue underlying the stratum compactum of the dermis was evident in all animals (Fig. 7A and B). Leucocyte infiltration of the epimesium surrounding the muscle was evident in all animals (Fig. 7A and B). Significant vasodilatation, oedema, fibrosis and leucocyte infiltration (lymphocytes, neutrophils and macrophages) of the perimesium and endomesium was present in two of three animals, and in these animals leucocyte infiltration and degeneration of some muscle fibres was also evident. Cell counts were significantly higher in PHA-injected legs than in PBS-injected legs for all leucocytes analysed (Table 2 and Fig. 8A).
Figure 7:
Representative photographs from histological sections of legs injected with PBS and PHA, showing significant leucocyte infiltration in the PHA-injected sections. (A) Limnodynastes peronii, PBS. (B) Limnodynastes peronii, PHA. (C) Litoria aurea, PBS. (D) Litoria aurea, PHA. All photographs were taken at ×100 magnification.
Table 2:
Summary of the statistical output for the effect of PHA injections on leucocyte counts between treatment and control legs
| Species | Cell type | n | PBS mean | PHA mean | d.f. | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Lim. peronii | Neutrophil | 3 | 0.0 | 30.0 | 1, 28 | 37.2 | <0.001 |
| Lymphocyte | 3 | 0.53 | 35.6 | 1, 28 | 139.1 | <0.001 | |
| Macrophage | 3 | 0.26 | 25.0 | 1, 28 | 81.6 | <0.001 | |
| Eosinophil | 3 | 0.07 | 9.8 | 1, 28 | 24.7 | <0.001 | |
| L. aurea | Neutrophil | 6 | 0.0 | 1.3 | 1, 58 | 8.5 | <0.01 |
| Lymphocyte | 6 | 0.07 | 4.5 | 1, 58 | 17.92 | <0.001 | |
| Macrophage | 6 | 0.0 | 2.7 | 1, 58 | 14.0 | <0.001 | |
| Eosinophil | 6 | 0.0 | 0.13 | 1, 58 | 0.32 | 0.57 |
Abbreviations: d.f., degrees of freedom; n, number of animals tested; PBS, phosphate-buffered saline; PHA, phytohaemagglutinin.
Figure 8:
Mean counts of leucocytes in legs of Lim. peronii (A) and L. aurea (B) injected with PBS and PHA. Error bars represent ±1 SEM. Statistical significance between counts in PBS- and PHA-injected legs are indicated as *P < 0.01, **P < 0.001 and ***P < 0.0001.
Inflammation-associated increases in leg thickness were lower in L. aurea (mean = 3.9% ± SEM 1.4%; n = 10) than in Lim. peronii. No leucocyte infiltration of the lymph sac was evident in L. aurea (Fig. 7C and D). A limited increase in extravascular leucocyte infiltration of the connective tissue underlying the stratum compactum of the dermis and the epimesium was evident in all animals (Fig. 7C and D). Vasodilatation and oedema were evident to varying degrees in all animals. Modest extravascular leucocyte infiltration within the perimesium and endomesium (predominantly neutrophils, macrophages and lymphocytes) was evident in three of six animals (Fig. 7C and D), with others displaying lower levels of leucocyte infiltration. Collectively, cell counts were significantly higher in PHA-injected legs than in PBS-injected legs for neutrophils, lymphocytes and macrophages, but not for eosinophils (Table 2 and Fig. 8B).
Experiment 3: optimization of the method for determining ‘peak response’
While the standard method of validation above (see ‘Standard experimental approach for validation of phytohaemagglutinin assays’) was suitable for determining whether an inflammatory response to PHA occurred, it was unsuitable for accurately determining peak response because of the large time gap between points of measurement after 24 h. For example, L. aurea was shown to have a peak response of ∼7.5% increase in leg thickness somewhere between 24 and 48 h post-injection, with a slight decrease in leg thickness by 72 h post-injection (Fig. 4E). Thus, it was possible that the true peak response was significantly greater than that observed at either 24 or 48 h post-injection.
The ‘4 h’ method of determining peak response yielded curves of a similar shape to that of the ‘24 h’ method for both species tested, with L. aurea first responding around 12 h and nearing peak response sometime after 24 h (F16,144 = 3.29, P < 0.001; Fig. 9) and Lim. peronii reacting quickly with a sharp increase in leg thickness shortly after 0 h and nearing peak response by ∼12 h post-injection (F16,176 = 8.23, P < 0.001; Fig. 9).
Figure 9:
Change in leg thickness over time for L. aurea and Lim. peronii using the ‘24’ and ‘4 h’ methods. The graphs show the ‘24 h’ method (A) and ‘4 h’ method for L. aurea (B) and the ‘24 h’ method (C) and ‘4 h’ method for Lim. peronii (D). All curves are adjusted curves (change in leg thickness for PBS-injected leg subtracted from change in leg thickness for PHA-injected leg). Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
The smoothed curves modelled using additive mixed-effects models on the ‘4 h’ data more accurately depicted the peak response for both species (Fig. 10). The peak response for L. aurea occurred at ∼34 h post-injection (Fig. 10) and had begun to subside by 57 h post-injection (as determined by the upper 95% confidence interval crossing over with the lower 95% confidence interval of the peak-response curve; Fig. 10). The peak response for Lim. peronii occurred at ∼28 h post-injection and was sustained for most of the remainder of the 72 h period (Fig. 10).
Figure 10:
Smoothed additive model fitted to the differences between the PHA- and PBS-injected legs of L. aurea (A) and Lim. peronii (B) following the ‘4 h’ data collection method. Dashed lines show 2 SEM; vertical dotted line indicates the time at peak response. Changes in leg thickness are expressed as the percentage difference from time 0 h.
Experiment 4: comparison of the effectiveness of phytohaemagglutinin and concanavalin A
Phytohaemagglutinin and Con A performed equally well in stimulating inflammation in L. aurea, with no significant difference in treatment effect observed at any time point (F1,108 = 1.73, P = 0.19; Fig. 11A). However, there was a significant effect of treatment for Lim. peronii (F1,165 = 86.39, P < 0.001; Fig. 11B), with a lower response to Con A than to PHA.
Figure 11:
Change in leg thickness over time for L. aurea (A) and Lim. peronii (B) following injections of PHA and concanavalin A (Con A). Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Experiment 5: experimental validation of the efficacy of the delayed-type hypersensitivity assay: comparison of responses in stressed and non-stressed individuals
Tadpoles raised in conditions of high density metamorphosed significantly earlier than those in conditions of low density, with fitted model means of 42.5 (n = 15) and 56.8 days (n = 25) to metamorphosis, respectively (F1,63 = 7.02, P = 0.01; Fig. 12). There was no difference between treatment for either weight or size at metamorphosis. Animals that were subjected to high tadpole density had a positive reaction to the PHA assay at 10–12 weeks post-metamorphosis, but this was significantly lower in magnitude than for the control animals subjected to low density at both 24 and 48 h post-injection (F1,32 = 4.47, P = 0.04; Fig. 13).
Figure 12:
Days taken for L. aurea tadpoles to reach metamorphosis in conditions of high and low tadpole density. Error bars represent ±1 SEM.
Figure 13:
Change in leg thickness over time for L. aurea exposed to conditions of high and low tadpole density, following injections of PHA and PBS. Changes in leg thickness are expressed as the percentage difference from time 0 h. Error bars represent ±1 SEM.
Discussion
Our study demonstrated that the PHA assay is effective across a range of phylogenetically distant anuran amphibian taxa and, importantly, that it is capable of detecting differences in immunocompetence between groups of animals that have been stressed in changing environmental conditions that are ecologically meaningful. We established a subcutaneous injection site a few millimetres below the knee (into the anterior lateral surface of the lower leg) and a measurement protocol that is easy to use and that worked for all species tested irrespective of body size. We found that the assay worked equally well across the sexes in all species tested and worked for both adult and sub-adult (juvenile) animals. We determined that using set-force-measuring instruments to measure the inflammatory response can result in a compression effect that counters the inflammatory response, reinforcing the need to carry out internal controls. We found PHA to be the lectin of choice when performing the DTH assay because it outperformed or performed equally well to Con A for the two species tested. We also developed an alternative validation technique that more accurately determined the peak response time for each species, improving the functionality and sensitivity of the assay.
The method of performing the assay in this study has overcome the problem of applicability to a wide range of species that exists in the assay as applied by Gilbertson et al. (2003), Gervasi and Foufopoulos (2008) and Brown et al. (2011) by moving the injection site to the lower leg. The lower leg contains less muscle tissue and is relatively thin, and therefore remains more consistent in thickness across species of varying body sizes. Our initial trials in the upper leg (thigh), as performed by Gervasi and Foufopoulos (2008), worked well for very small species but not for larger ones (data not presented). We also found that injecting into the toe (as per Gilbertson et al. 2003) or into the toe webbing (Brown et al. 2011) was not possible for many small species; indeed, many species do not have toe webbing. The low-level (0.4 N) set-force dial callipers we used to measure the leg thickness before and post-injection worked for all species tested and helped to remove the bias associated with investigator-manipulated measuring devices. A study published subsequently to our experimental work by Fites et al. (2014) on the effects of chytrid fungus on local immune responses in Xenopus laevis showed that a significant inflammatory response (∼20% inflammation) to PHA injections could be achieved in that species by injecting intramuscularly in the middle of the foot on the plantar side. The location of this injection site is similar in approach to our injections into the lower leg, and might also have the potential to work for a wider range of species than previous injection sites that have been investigated. Testing the PHA assay on tadpoles was outside the scope of the present study, but a study by Venesky et al. (2012) suggests that it also works for that life stage.
Whilst the assay was effective for all species tested, we found a large amount of variability within and between species. The intra-species variability was similar to that experienced by others (e.g. Gervasi and Foufopoulos, 2008; Brown et al., 2011) and reflects the need for reasonable sample sizes when carrying out these assays, particularly if the goal is to detect potentially small differences between groups or populations. The inter-species variability was also marked and suggests that responses from this assay are not directly comparable between species, although in our trials species that were more closely related appeared to have more similar responses. For example, inflammation occurred more rapidly in the ground frogs (6 h post-injection) compared with the tree frogs (>12 h post-injection) and the magnitude of response was greater in Lim. peronii compared with the other species. Encouragingly, there were no sex differences for any species tested, and the assay worked for both adults and juveniles (10–12 weeks post-metamorphosis). This suggests that the assay is likely to be applicable broadly across populations for a given species irrespective of sex or life stage.
Given the intra-species variability and the differences in the magnitude of inflammation, optimal assay sensitivity will be achieved by determining and comparing peak responses. Thus, the 4 h measurement regime is likely to provide a more accurate estimation of the peak inflammatory response and be more functional compared with the standard assay. The 4 h measurement regime revealed that our ground frog model experienced true peak response at ∼28 h post-injection (as opposed to 12 h estimated by the standard 24 h approach) and our tree frog model experienced true peak response at ∼34 h post-injection (as opposed to 24 h estimated by the standard assay). Maximizing assay sensitivity by determining the peak response time is particularly important in species such as L. aurea that develop only modest PHA inflammatory responses that diminish rapidly. Although establishing the species response curve for the ‘4 h’ method requires more effort and animal handling than the ‘24 h’ method, once established the capacity to take a single peak response measurement in experimental animals would reduce effort. In our experience, the additional handling required to establish the 4 h response curve did not appear to stress the animals. The dynamics of the inflammatory responses in the amphibian species we investigated are broadly comparable with those of R. marina, in which peak response was also observed at ∼24 h (Brown et al., 2011). Gervasi and Foufopoulos (2008) found comparatively small and variable PHA responses in Rana sylvatica, and whilst these investigators nevertheless detected some significant differences in PHA responses to desiccation treatments, their study may have been aided by using a validation assay, such as the 4 h regime, to provide a better estimate of peak responses. We therefore recommend that any PHA assay be validated initially using the 4 h approach to produce the optimal assay for each new amphibian species.
The question as to the necessity for an internal control for PHA assays has been raised many times in the past for birds (Smits et al., 1999; Kennedy and Nager, 2006; Biard et al., 2009; Vinkler et al., 2010) and more recently by Brown et al. (2011) for amphibians. While some have argued that an internal control is not necessary in birds (Smits et al., 1999), we found that it was particularly important when applying our protocol to amphibians. The discovery of a ‘compression effect’ meant that the change in leg thickness from the PHA-injected leg alone did not necessarily reflect the true peak response, and we found that this was highly variable between individuals. Indeed, on occasion the compression effect led to an overall decrease in leg thickness for some individuals despite having a PHA injection. Therefore, we issue a warning against using the PHA assay without a control injection into the contralateral leg, particularly when comparing groups of individuals. We suspect that this phenomenon may also have complicated the study of Gervasi and Foufopoulos (2008), in which some differences in skin thickness were negative. Although the investigators concluded that the negative measurements were not biologically meaningful, they retained the data in the analyses, and as there was no internal control injection used the compression effect could not have been detected.
Phytohaemagglutinin appears to be the lectin of choice for use in amphibian DTH assays. We tested a second mitogenic lectin, Con A, on a ground frog and tree frog model, but found that it only performed equally well (tree frog) or worse (ground frog) than PHA in direct comparison tests. While the possibility cannot be excluded that Con A or other mitogens may be as effective or more effective than PHA in some species of amphibians, the evidence from our study suggests that PHA should be the first choice when validating or using DTH assays in new species.
Inter-species differences in the dynamics of the PHA immune response included variation not only in the maximal response attained, but also in the speed with which inflammation developed and its duration. The inter-species and inter-animal variation was also evident upon histological examination of the tissue. Inflammation in Lim. peronii was characterized by very robust innate immune responses, including vasodilation, oedema and fibrosis as well as significant neutrophil and macrophage infiltration. Significant recruitment of lymphocytes, mediators of the adaptive immune system, also occurred. The location and extent of leucocyte infiltration varied between animals, occurring consistently in the epimesium and connective tissue underlying the dermis that flank the lymph sac where the PHA was injected. However, extensive leucocyte infiltration of the lymph sac, perimesium and endomesium occurred together or separately in different animals. Damage to muscle fibres indicated by leucocyte infiltration also occurred in some animals. The visible histological indicators of inflammation in L. aurea tissue were restricted to modest leucocyte infiltration of the connective tissue underlying the dermis and the epimesium and variable vasodilatation and oedema in the skeletal muscle. Modest leucocyte infiltration of the perimesium and epimesium occurred in only some animals. Although the magnitude, dynamics and location of the immune response was reduced in L. aurea compared with Lim. peronii, leucocyte infiltration in L. aurea nevertheless consisted of both innate cellular mediators (neutrophils and macrophages) and lymphocytes associated with antigen-specific adaptive immune responses. Thus, it is likely that both innate and adaptive immune responses contributed to the PHA-induced inflammation observed in both species in this study.
Brown et al. (2011) demonstrated that PHA-induced inflammation in R. marina was associated initially (6–12 h post-injection) with a rapid infiltration of the innate cellular mediators (eosinophils, neutrophils and macrophages), whereas significant recruitment of lymphocytes took 24 h in PHA-naïve animals. Our study revealed that there are differences in the dynamics and magnitude of the PHA immune response between amphibian species. Initial inflammation (0–12 h) occurred more rapidly and the degree of inflammation was greatest in the ground frogs and lowest in (juvenile) L. aurea than in other species. It would be fruitful to investigate how this inter-species variation relates to differences in investment in immune components and/or signalling pathways and disease resistance/immunocompetence (Kennedy and Nager, 2006; Martin et al., 2006; Vinkler et al., 2010), particularly in conditions of varying environmental stressors. Although the PHA assay has traditionally been assumed to measure T-lymphocyte function, it is increasingly clear that the PHA inflammatory response is complex (Kennedy and Nager, 2006; Martin et al., 2006; Vinkler et al., 2010). Use of this assay in functional analyses of amphibian immune fitness is relatively recent but already suggests that threatening processes, such as xenobiotic exposure and desiccation, alter amphibian immune function (Gilbertson et al., 2003; Gervasi and Foufopoulos, 2008). Our study highlights the importance of carefully validating and optimizing the PHA assay for each species investigated to maximize its sensitivity and significance, as well as to allow meaningful interpretation of the nature of the immune response. Our optimized PHA assay provided a quantitative measure of both the innate and the initial adaptive immune capability of each species. A second PHA challenge would be likely to increase the magnitude of the PHA immune response if the primary goal was to focus on adaptive immune responses (Brown et al., 2011; Fites et al., 2014).
Perhaps the most important outcome of this study was the demonstration that the PHA assay detected differences in L. aurea immune responses between frog populations subjected to ecologically meaningful levels of an environmental stress. This was achieved with relatively small numbers of animals (groups of 25 and 15 individuals) and demonstrates that the PHA assay is effective in ecologically meaningful studies. The frogs used for this study had similar levels of genetic diversity and were raised in identical conditions except for the experimental density variable. Thus, the detected differences in immunocompetence between the experimental groups provide very strong direct evidence that one cost of metamorphic plasticity responses to density stress is a reduction in immune fitness. This also provides compelling evidence to suggest that phenotypic carry-over from larval stress affects the adult phenotype, and thus, metamorphosis is not a new beginning between life stages of species with complex life-cycles. The demonstration that high L. aurea tadpole density was associated with reduced post-metamorphic immunocompetence has significance for the conservation and management of this and other species that have declined to endangered status in the wild. Litoria aurea is currently the subject of a number of captive breed-and-release programmes, where it is assumed that releases of large numbers of tadpoles into waterbodies is advantageous to reintroduction of the species (Stockwell et al., 2008; Mahony et al., 2013; Germano et al., 2015). Our findings provide grounds for caution when determining tadpole stocking densities. By extension, the findings also have important implications for the way that captive-bred tadpoles of other species are raised in similar programmes.
Overall, we found that the PHA protocol developed in this study worked across a broad range of amphibian taxa separated by long evolutionary histories and provides a simple, inexpensive and effective way to quantify immunocompetence in amphibians (and possibly other ectothermic vertebrates), thus facilitating investigations into the effects of environmental change on wildlife health. We applied the assay to demonstrate a reduction in post-metamorphic immune fitness as a consequence of high-density stress in tadpoles and suggest that the PHA assay could be incorporated beneficially into a huge range of ecological and evolutionary studies, which will become more important for managers and researchers as the burden of global environmental change continues to place pressure on the world's amphibian species. However, we issue a warning against the use of the PHA assay in new species without prior validation or without the use of appropriate internal controls.
Funding
This work was supported by the Australian Research Council [ARC Linkage grant LP0989459].
Acknowledgements
Thanks go to Tess Davies for assistance with preliminary trials, to Kim Colyvas for statistical advice and to John Clulow for useful comments on the manuscript.
References
- Acevedo-Whitehouse K, Duffus ALJ. (2009) Effects of environmental change on wildlife health. Philos Trans R Soc Lond B Biol Sci 364: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LC, Turmelle AS, Mendonça MT, Navarra KJ, Kunz TH, McCracken GF. (2009) Roosting ecology and variation in adaptive and innate immune system function in the brazilian free-tailed bat (Tadarida brasiliensis). J Comp Physiol B 179: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biard C, Hardy C, Motreuil S, Moreau J. (2009) Dynamics of PHA-induced immune response and plasma carotenoids in birds: should we have a closer look? J Exp Biol 212: 1336–1343. [DOI] [PubMed] [Google Scholar]
- Bishop PJ, Angulo A, Lewis JP, Moore RD, Rabb GB, Garcia Moreno JG. (2012) The amphibian extinction crisis - what will it take to put the action into the amphibian conservation action plan? S.A.P.I.EN.S 5: 97–111. [Google Scholar]
- Brown GP, Shilton CM, Shine R. (2011) Measuring amphibian immunocompetence: validation of the phytohemagglutinin skin-swelling assay in the cane toad, Rhinella marina. Methods Ecol Evol 2: 341–348. [Google Scholar]
- Browne RK, Pomering M, Hamer AJ. (2003) High density effects on the growth, development and survival of Litoria aurea tadpoles. Aqauculture 215: 109–121. [Google Scholar]
- Cannon MS, Sampson HW, Kapes ED. (1986) The blood leukocytes of Bufo marinus: a light phase-contrast, and histochemical study. Can J Zool 65: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Denver RJ. (2009) Stress hormones mediate environment-genotype interactions during amphibian development. Gen Comp Endocrinol 164: 20–31. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Glennemeier KA, Boorse GC. (2002) Endocrinology of complex life cycles. In Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, eds, Hormones, Brain and Behaviour. Academic Press, San Diego, CA, USA. [Google Scholar]
- Fites JS, Reinert LK, Chappell TM, Rollins-Smith LA. (2014) Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect Immun 82: 4698–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo B, Corpas I, Alonso-Gómez AL, Delgado MJ, Moreale De Escobar G, Alonso-Bedate M. (1992) Corticotropin-releasing factor stimulates metamorphosis and increases thyroid hormone concentration in prometamorphic Rana perezi larvae. Gen Comp Endocrinol 87: 6–13. [DOI] [PubMed] [Google Scholar]
- Germano J, Field K, Griffiths R, Clulow S, Foster J, Harding G, Swaisgood R. (2015) Mitigation-driven translocations: are we moving wildlife in the right direction? Front Ecol Environ 13: 100–105. [Google Scholar]
- Gervasi SS, Foufopoulos J. (2008) Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22: 100–108. [Google Scholar]
- Gilbertson MK, Haffner GD, Drouillard KG, Albert A, Dixon B. (2003) Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environ Toxicol Chem 22: 101–110. [PubMed] [Google Scholar]
- Gosner KL. (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190. [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. (1978) Suppression of phytohemagglutinin skin response in thymectomised chickens. Poultry Sci 57: 246–250. [DOI] [PubMed] [Google Scholar]
- Grasman KA. (2002) Assessing immunological function in toxicological studies of avian wildlife. Integr Comp Biol 42: 34–42. [DOI] [PubMed] [Google Scholar]
- Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL. (2000) Quantitative evidence for global amphibian population declines. Nature 404: 752–755. [DOI] [PubMed] [Google Scholar]
- Irisarri I, San Mauro D, Abascal F, Ohler A, Vences M, Zardoya R. (2012) The origin of modern frogs (Neobatrachia) was accompanied by acceleration in mitochondrial and nuclear substitution rates. BMC Genomics 13: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean RP, Lamont SJ. (1994) Effect of injection site on cutaneous basophil hypersensitivity response to phytohemagglutinin. Poultry Sci 73: 1763–1765. [DOI] [PubMed] [Google Scholar]
- Kennedy MW, Nager RG. (2006) The perils and prospects of using phytohaemagglutinin in evolutionary ecology. Trends Ecol Evol 21: 653–655. [DOI] [PubMed] [Google Scholar]
- McCorkle FM, Simmon DG, Luginbuhl GH. (1982) Delayed hypersensitivity response in Alcaligenes faecalis-infected turkey poults. Avian Dis 26: 782–786. [PubMed] [Google Scholar]
- Mahony MJ, Hamer AJ, Pickett EJ, McKenzie DJ, Stockwell MP, Garnham JI, Keely CC, Deboo ML, O'Meara J, Pollard CJ, et al. (2013) Identifying conservation and research priorities in the face of uncertainty: a review of the threatened bell frog complex in eastern Australia. Herpetol Conserv Biol 8: 519–538. [Google Scholar]
- Martin LB, 2nd, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. (2006) Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol 20: 290–299. [Google Scholar]
- Pauls SU, Nowak C, Balint M, Pfenninger M. (2013) The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22: 925–946. [DOI] [PubMed] [Google Scholar]
- Pigeon G, Bélisle M, Garant D, Cohen AA, Pelletier F. (2013) Ecological immunology in a fluctuating environment: an integrative analysis of tree swallow nestling immune defense. Ecol Evol 3: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N. (2012) Impacts of biodiversity loss escalate through time as redundancy fades. Science 336: 589–592. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. (1998) Metamorphosis and the amphibian immune system. Immunol Rev 166: 221–230. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. (2001) Neuroendocrine-immune system interactions in amphibians: implications for understanding global amphibian declines. Immunol Res 23: 273–280. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Woodhams DC. (2012) Amphibian immunity: staying in tune with the environment. In Demas GE, Nelson RJ, eds, Ecoimmunology. Oxford University Press, New York, NY, USA. [Google Scholar]
- Ros AF, Groothuis TG, Apanius V. (1997) The relation among gonadal steroids, immunocompetence, body mass, and behaviour in young black-headed gulls (Larus ridibundus). Am Nat 150: 201–219. [DOI] [PubMed] [Google Scholar]
- Saino N, Calza S, Møller AP. (1997) Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J Anim Ecol 66: 827–836. [Google Scholar]
- Smits JE, Bortolotti GR, Tella JL. (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13: 567–572. [Google Scholar]
- Stockwell MP, Clulow S, Clulow J, Mahony MJ. (2008) The impact of the amphibian chytrid fungus Batrachochytrium dendrobatidis on a green and golden bell frog Litoria aurea reintroduction program at the Hunter Wetlands Centre Australia in the Hunter Region of NSW. Aust Zool 34: 379–386. [Google Scholar]
- Stuart S, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fishman DL, Waller RW. (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Bardsley S, Clout M, Depledge MH, Dicks LV, Fellman L, Fleishman E, Gibbons DW, Keim B, Lickorish F, et al. (2013) A horizon scan of global conservation issues for 2013. Trends Ecol Evol 28: 16–22. [DOI] [PubMed] [Google Scholar]
- Tella JL, Lemus JA, Carrete M, Blanco G. (2008) The PHA test reflects acquired T-cell mediated immunocompetence in birds. PLoS ONE 3: e3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ. (2012) Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169: 23–31. [DOI] [PubMed] [Google Scholar]
- Vinkler M, Bainová H, Albrecht T. (2010) Functional analysis of the skin-swelling response to phytohaemagglutinin. Funct Ecol 24: 1081–1086. [Google Scholar]
- Wake DB. (1991) Declining amphibian populations. Science 253: 860. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liang D, Mao R-L, Hillis DM, Wake DB, Cannatella DC. (2013) Efficient sequencing of anuran mtDNAs and a mitogenomic exploration of the phylogeny and evolution of frogs. Mol Biol Ecol 30: 1899–1915. [DOI] [PubMed] [Google Scholar]
- Zuk M, Johnsen TS. (1998) Seasonal changes in the relationship between ornamentation and immune response in red jungle fowl. Proc R Soc Lond B Biol Sci 265: 1631–1635. [Google Scholar]