We demonstrate that abundance, reproductive output, and growth rate of Common Gartersnakes living in field and forest habitats are good metrics of habitat suitability, whereas stress hormone levels show no difference in suitability between habitats.
Keywords: Fitness, habitat quality, habitat selection, reproductive output, stress physiology, Thamnophis sirtalis
Abstract
Measuring habitat suitability is important in conservation and in wildlife management. Measuring the abundance or presence–absence of a species in various habitats is not sufficient to measure habitat suitability because these metrics can be poor predictors of population success. Therefore, having some measure of population success is essential in assessing habitat suitability, but estimating population success is difficult. Identifying suitable proxies for population success could thus be beneficial. We examined whether faecal corticosterone metabolite (fCM) concentrations could be used as a proxy for habitat suitability in common gartersnakes (Thamnophis sirtalis). We conducted a validation study and confirmed that fCM concentrations indeed reflect circulating corticosterone concentrations. We estimated abundance, reproductive output and growth rate of gartersnakes in field and in forest habitat and we also measured fCM concentrations of gartersnakes from these same habitats. Common gartersnakes were more abundant and had higher reproductive outputs and higher growth rates in field habitat than in forest habitat, but fCM concentrations did not differ between the same two habitats. Our results suggest either that fCM concentrations are not a useful metric of habitat suitability in common gartersnakes or that the difference in suitability between the two habitats was too small to induce changes in fCM concentrations. Incorporating fitness metrics in estimates of habitat suitability is important, but these metrics of fitness have to be sensitive enough to vary between habitats.
Introduction
Habitat loss is one of the greatest threats to global biodiversity (Sala et al., 2000). For this reason, estimating habitat suitability is an integral part of wildlife management and conservation, allowing for better-informed decisions about what habitats must be preserved to maintain species of conservation concern (Morris, 2003). Traditionally, habitat suitability has been estimated via presence–absence or abundance data in various habitats (Guisan and Zimmermann, 2000). Abundance may not always be a good metric of habitat suitability (Van Horne, 1983); animals are not always most abundant where the population growth rate (the ultimate measure of population success) is the highest. Moreover, habitat occupancy does not always imply habitat quality (Johnson, 2007).
Ideal despotic distributions (Fretwell and Lucas, 1969) are a clear example of a mismatch between abundance and population success. Under an ideal despotic distribution, dominant individuals occupy high-quality habitats and exclude subordinates from such habitats. Therefore, a few dominant individuals experience high fitness in high-quality habitats, whereas many subordinates experience low fitness in lower-quality habitats. For example, dominant European jays live in high-quality older forests at low densities and achieve high fitness, whereas subordinate jays live in poorer-quality habitats at high densities and have lower fitness owing to higher predation (Andrén, 1990). Likewise, a few large, dominant brook trout occupy high-quality pools and exclude many smaller subordinates that then live in lower-quality riffle segments of streams (Knight et al., 2008). Under an ideal despotic distribution, therefore, high density does not imply high population success in a habitat.
Habitat occupancy also can be a misleading metric of habitat quality because metapopulations often have source–sink dynamics (Pulliam, 1988). In a source–sink system, populations in the source habitat increase in size, whereas populations in the sinks decrease in size; dispersal from the source to the sink maintains the population in the sink habitat. For example, Caribbean spiny lobsters occur in source habitats where spawning takes place and where nurseries are located, but local hydrodynamics cause larvae to disperse passively into sink habitats that are not suitable for spawning or as nurseries (Lipcius et al., 1997). Source–sink systems can also be maintained via active dispersal. Checkerspot butterflies actively disperse between habitats throughout the season, and populations in clearing habitats late in the season decrease in size owing to senescence of the host plants (Boughton, 1999). On the contrary, butterfly populations in outcrop habitat late in the season increase in size because the host plant in outcrops does not senesce as early as the host plant in clearings. The outcrop habitat acts as a source for the low-quality clearing habitat. Finally, ‘ecological traps’ have been documented, in which animals prefer habitats where their reproductive success is low (Gates and Gysel, 1978; Battin, 2004; Weldon and Haddad, 2005).
Ideal despotic distributions, source–sink systems and ecological traps exemplify why abundance and occupancy are not necessarily useful measures of habitat suitability. Ecologists have been advocating the use of measures other than abundance to estimate habitat suitability for >30 years (Van Horne, 1983; Homyack, 2010; Cooke et al., 2013), but this advice is still often not heeded (Johnson, 2007). Although ultimate measures of mean population fitness are difficult to obtain, especially in long-lived species, proximate measures of mean population fitness can be more easily obtained. Such proximate measures include individual growth rate (Halpin, 2000; Manderson et al., 2002), fecundity (Morris, 1989; Halliday and Blouin-Demers, 2014; Halliday et al., 2015) or physiological metrics, such as field metabolic rate or concentrations of glucocorticoid stress hormones (Homyack, 2010). For example, corticosterone concentrations of salamanders differ between landscapes with varying human disturbance and in natural vs. anthropogenic habitats (Homan et al., 2003). Corticosterone concentrations also differ among birds living in forests with varying fragmentation (Soursa et al., 2003). Snakes living near roads tend to have a reduced corticosterone stress response compared with snakes living away from roads (Owens et al., 2014). Increased predation (Boonstra et al., 1998) and increased population density in a habitat can also increase stress levels (Montero et al., 1999). In some circumstances, therefore, corticosterone concentrations may be useful indicators of habitat quality.
Corticosterone concentrations are sometimes too variable to be a good indicator of habitat suitability. For example, different levels of human disturbance had no effect on corticosterone concentrations in eastern hellbenders (Hopkins and DuRant, 2011). Corticosterone concentrations may also vary between seasons, and concentrations can differ between habitats in one season but not in another (Marra and Holberton, 1998). Moreover, the relationship between glucocorticoid concentrations and fitness is not consistent across taxa (Bonier et al., 2009). Thus, it remains unclear whether glucocorticoid concentrations can be a reliable indicator of habitat suitability.
In the present study, we evaluated the usefulness of faecal corticosterone metabolite (fCM) concentrations as a proximate measure of habitat suitability for common gartersnakes (Thamnophis sirtalis) living in field vs. forest habitat. We conducted a validation study and confirmed that fCM concentrations indeed reflect circulating corticosterone concentrations. We compared baseline fCM concentrations with abundance, individual growth rate and reproductive output of gartersnakes living in each habitat. Field and forest have very different thermal regimens; field is thermally superior to forest for snakes in our study area (Blouin-Demers and Weatherhead, 2001; Row and Blouin-Demers, 2006; Blouin-Demers and Weatherhead, 2008). For this reason, energy acquisition and assimilation should be higher in field than in forest, which should lead to higher individual growth rates and reproductive success in field than in forest. If fCM concentration is an appropriate proxy for habitat quality in garter snakes, it should reflect the inter-habitat patterns in the other proximate measures of fitness. If fCM concentration is unaffected by thermal or energetic differences, however, it will not vary between habitats. We included body temperature available to snakes to account for its potential direct effect on fCM (Cree et al., 2003).
Materials and methods
Abundance estimates
We monitored the habitat use of common gartersnakes between field and forest habitats at Queen's University Biological Station, Ontario, Canada (44°33′N, 76°21′W). We set up five pairs of 2500 m2 plots in adjacent fields and forests and placed eight plywood coverboards (360 cm2) on each plot (Halliday and Blouin-Demers, 2015). We systematically walked back and forth across each plot and checked under all of the coverboards twice per day over three consecutive days every 2 weeks from 5 May to 16 July 2013. We uniquely marked each individual gartersnake using ventral scale branding (Winne et al., 2006). We counted the number of different individual gartersnakes caught in each field and forest grid throughout the study and compared abundance in field vs. (adjacent) forest habitat using a paired t test in R (package, stats; function, t.test; R Core Team, 2014).
Fitness and faecal corticosterone “metabolite estimates
We examined the growth rate, reproductive output and fCM concentrations of 20 female common gartersnakes in enclosures in Pontiac County, Québec, Canada (45°29′N, 75°55′W). We built 2.67 m × 2.67 m × 1.33 m frames out of lumber and created walls for the enclosure by attaching polyethylene vapour barrier to the frames. We buried the bottom 10 cm of the walls of the enclosures in the ground to prevent snakes from escaping. We built six of these enclosures in field habitat and an additional six in forest habitat. Each enclosure contained a 60 cm × 60 cm plywood coverboard for shelter. In late May 2014, we placed 10 females in forest enclosures and the other 10 females in the field enclosures; snakes were housed either in pairs or on their own. We captured all female snakes for this experiment in fields and wetlands near Ottawa, Ontario (n = 3) and close to our enclosures in Québec (n = 17). In the laboratory, before putting females in the enclosures, we gave each female the opportunity to mate with three or four males collected from the same area. We observed every female mate during these encounters. We fed half of the snakes in each habitat two large earthworms per week and we fed the other half of the snakes four large earthworms per week to determine whether fCM concentrations could be an indicator of habitat quality only with certain caloric intake levels. We systematically placed females in each food and habitat treatment combination based on their body size to ensure that each combination had similar distributions of body sizes, because body size may influence growth, reproductive frequency and reproductive output. We measured the body temperature of each snake with an infrared thermometer immediately before feeding it. We measured the mass and snout–vent length (SVL) of each snake once per week and also attempted to collect a faecal sample from each snake once per week. Faecal samples were stored in individual sealed tubes and were kept on ice until they were returned to the laboratory, where they were frozen at −80°C until they were processed. Snakes started giving birth in late August and finished by early September. We removed all snakes from the enclosures after the snakes gave birth and returned all mothers and their offspring to their point of capture.
We extracted fCMs from faecal pellets following the methods of Berkvens et al. (2013). Briefly, we placed thawed and homogenized (using a metal spatula cleaned with 80% methanol) faeces in 2.0 ml tubes with 80% methanol at a ratio of 0.1 g of faeces per 1.0 ml of methanol. We agitated this mixture on a magnetic stir plate at room temperature for 18 h. Following this period, we centrifuged each tube at 210g for 10 min, decanted the liquid (henceforth referred to as the extract) into a new tube and stored the extract at −80°C until we measured fCM content by commercial radioimmunoassay (RIA). The extraction efficiency for this procedure was estimated by splitting a sample in two, spiking one half of the sample with a known amount of corticosterone, measuring corticosterone/fCM content in each half and comparing recovered corticosterone with the amount added, for six independent samples; using this method, extraction efficiency was 50%. This value is relatively low compared with ∼90% extraction efficiency for the faeces of ground squirrels (Spermophilus beldingi; Mateo and Cavigelli, 2005), but on par with the 49% extraction efficiency reported for faeces of African house snakes (Lamprophis fuliginosus; Berkvens et al., 2013). The present study is the first, to our knowledge, in which fCM concentrations were measured in faecal samples of gartersnakes. For this reason, we conducted a validation experiment (see online supplementary material Appendix S1). Briefly, we compared fCM concentrations with plasma corticosterone concentrations in gartersnakes before and after they were fed corticosterone-injected earthworms. We demonstrated that fCM concentrations reflect plasma corticosterone concentrations.
We measured the fCM content in each sample using a corticosterone double-antibody RIA kit (MP Biomedicals, Santa Fe, CA, USA), which was previously used by Roberts et al. (2009) to measure corticosterone in the plasma of T. elegans. We followed the directions of the RIA kit, except that we mixed 20 μl of extract in 1 ml of steroid diluent rather than the suggested 10 μl of sample in 2 ml of steroid diluent, to take into account the low concentrations of corticosterone metabolites in the samples. All experimental samples were assayed together in a single assay, for which intra-assay variability was 8.7%. In addition, serial dilution of extracted fCM samples yielded a displacement curve parallel to the corticosterone standard curve (online supplementary material Appendix S1).
We calculated the change in mass of snakes as the difference in the mass of a snake between the beginning and the end of the experiment. Likewise, we calculated the change in SVL of snakes from the beginning to the end of the experiment. We compared changes in mass and SVL of snakes in field vs. forest habitat using general linear models in R (package, stats; function, lm; R Core Team, 2014), with habitat, food treatment and their interaction as independent variables. We also included the number of weeks that a snake was in the experiment to control for differences in time to grow (a few snakes escaped the enclosure or were eaten by predators before the end of the experiment) and the starting mass or SVL of the individual to control for size-dependent differences in growth rate. We used bias-corrected Akaike's information criteria (package, qpcR; function, AICc; Spiess, 2014) for model selection and considered the model with the lowest AICc to be the best model. Models within 2 AICc units of the best model were considered to be competing models (Bozdogan, 1987), and we used model averaging between competing models to determine the parameter estimates for the final model (Burnham and Anderson, 2002).
We examined fCM concentrations using mixed-effects models in R (package, nlme; function, lme; Pinheiro et al., 2014), with habitat, food treatment and their interaction as fixed effects and with snake ID nested within week into the experiment as random effects. We again used bias-corrected Akaike's information criteria for model selection. We also examined how fCM concentrations were affected by body temperature using another mixed-effects model with the same random effects. We used the mean body temperature (i.e. mean of the four temperature measurements taken on the four feeding days) of each snake during the week preceding the collection of the faecal sample because the corticosterone metabolites found in faeces should be a function of the corticosterone in the plasma on previous days during which digestion occurred.
Results
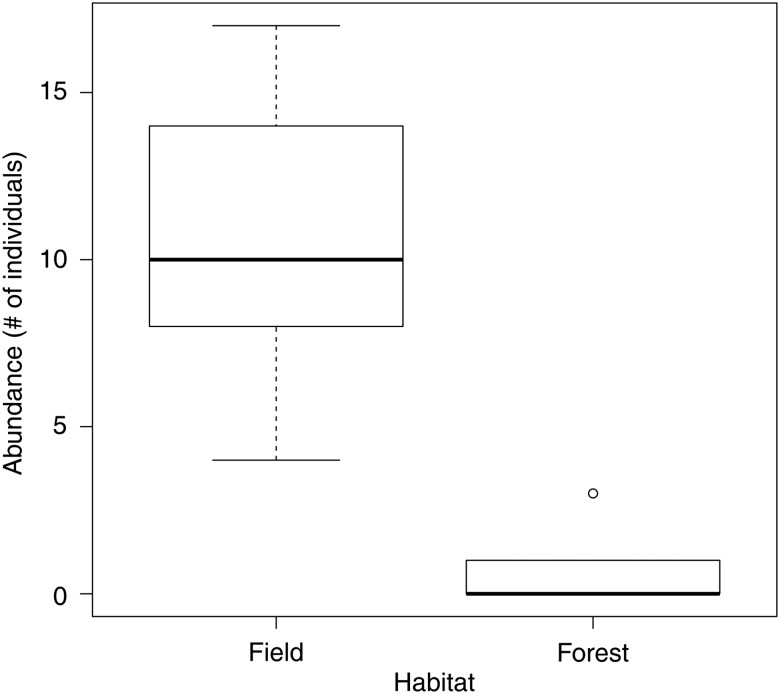
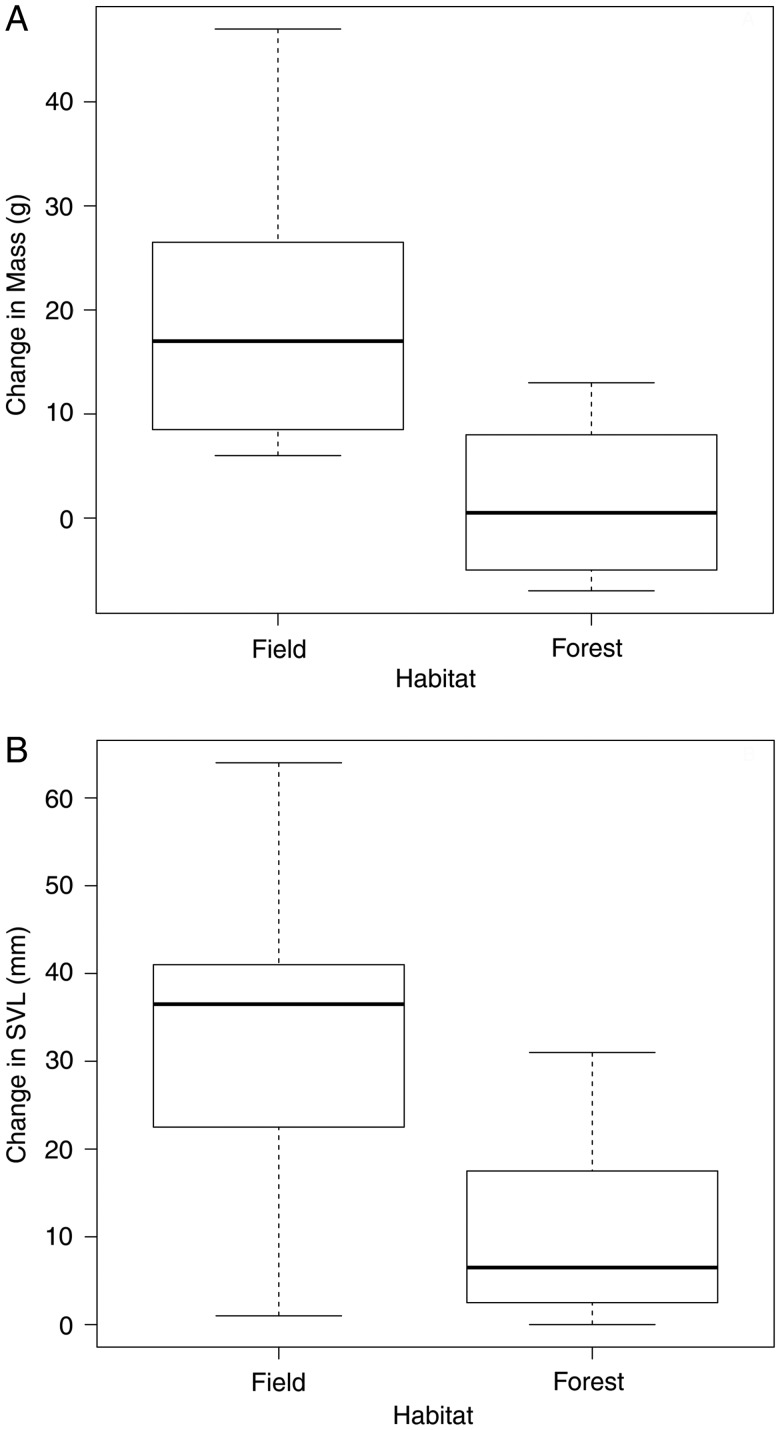
Common gartersnakes demonstrated a strong preference for field over forest habitat (mean abundance in field ± SEM = 10.6 ± 2.3; forest = 0.8 ± 0.6; mean difference = 9.8; t4 = 5.01, P < 0.01; Fig. 1). Four of the 10 female snakes in the field enclosures gave birth (average litter size = 9 ± 1 offspring), whereas no female snakes in the forest gave birth (F14 = 6.07, P = 0.03). Snakes in the field grew more in terms of both mass (field = 20 ± 5 g; forest = 2 ± 3 g; t14 = 3.16, P < 0.01; r2 = 0.42; Table 1 and Fig. 2A) and SVL (field = 33 ± 7 mm; forest = 11 ± 4 mm; t13 = 4.23, P < 0.01; r2 = 0.63; Table 2 and Fig. 2B) than snakes in the forest. Food treatment had no effect on mass gain (t13 = 0.81, P = 0.43) or on SVL increase (t13 = 0.41, P = 0.69).
Figure 1:
Abundance estimates of common gartersnakes (Thamnophis sirtalis) in field and in forest habitat at the Queen's University Biological Station in Ontario, Canada from May to July 2013. The box represents the interquartile range, the line within the box is the median, and the whiskers represent the 95% confidence interval. Points represent outliers outside the 95% confidence interval.
Table 1:
Model selection and final model output for linear models examining the change in mass of female common gartersnakes (Thamnophis sirtalis) living in enclosures in field and forest habitat with different food treatments
| Model | k | AICc | ΔAICc | |
|---|---|---|---|---|
| Mass ∼ Habitat | 3 | 128.02 | 0.00 | |
| Mass ∼ Habitat + Start mass | 4 | 130.25 | 2.23 | |
| Mass ∼ Habitat + Food | 4 | 130.31 | 2.29 | |
| Mass ∼ Habitat + Start mass + Food + Habitat:Food | 6 | 130.50 | 2.48 | |
| Mass ∼ Habitat + Start mass + Food + Habitat:Food + Weeks | 7 | 138.48 | 10.46 | |
| Parameter | Estimate | SE | t | P-value |
|---|---|---|---|---|
| Intercept | 19.63 | 4.02 | 4.88 | <0.01 |
| Habitat (forest) | −18.00 | 5.69 | 3.16 | <0.01 |
Abbreviations: AICc is the bias-corrected Akaike's information criterion value; ΔAICc is the difference in AICc between each model and the model with the lowest AICc; and k is the number of parameters in the model.
Figure 2:
Change in mass (A) and in snout–vent length (SVL; B) of female common gartersnakes (T. sirtalis) living in enclosures in field and in forest habitat in Pontiac County, Québec, Canada from May to August 2014. The box represents the interquartile range, the line within the box is the median, and the whiskers represent the 95% confidence interval.
Table 2:
Model selection and final model output for linear models examining the change in snout–vent length of female common gartersnakes (T. sirtalis) living in enclosures in field and forest habitat with different food treatments
| Model | k | AICc | ΔAICc | |
|---|---|---|---|---|
| SVL ∼ Habitat + Start SVL | 4 | 130.91 | 0.00 | |
| SVL ∼ Habitat + Start SVL + Food + Habitat:Food | 6 | 132.37 | 1.44a | |
| SVL ∼ Habitat + Start SVL + Food | 5 | 132.69 | 1.78a | |
| SVL ∼ Habitat + Start SVL + Food + Habitat:Food + Weeks | 7 | 132.76 | 1.85a | |
| SVL ∼ Habitat | 3 | 137.48 | 6.87 | |
| Parameter | Estimate | SE | t | P-value |
|---|---|---|---|---|
| Intercept | 124.50 | 32.03 | 4.04 | <0.01 |
| Habitat (forest) | −29.75 | 6.43 | 4.23 | <0.01 |
| Start SVL | −0.19 | 0.06 | 3.04 | <0.01 |
| Food | −7.18 | 6.70 | 0.41 | 0.69 |
| Habitat:Food | 15.11 | 12.81 | 1.31 | 0.22 |
| Weeks | 1.23 | 1.20 | 1.03 | 0.33 |
Abbreviations: AICc is the bias-corrected Akaike's information criterion value; ΔAICc is the difference in AICc between each model and the model with the lowest AICc; k is the number of parameters in the model; and SVL is the snout–vent length. aCompeting models that are within 2 AICc units of the best model. Estimates in the final model output are based on model averaging between competing models.
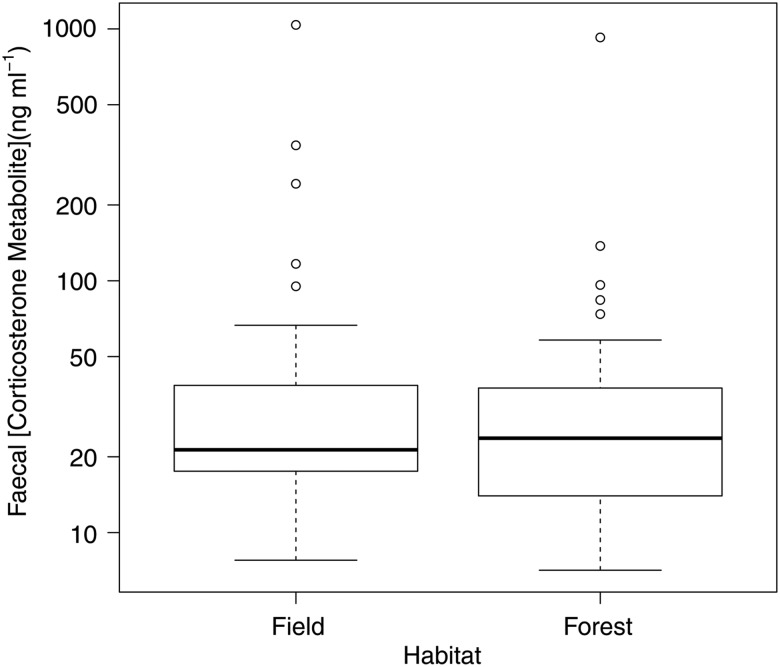
Snakes in field and in forest did not differ in fCM concentrations (field = 65.7 ± 25.0 ng ml−1; forest = 59.1 ± 27.5 ng ml−1; t76 = 0.54, P = 0.60; Table 3 and Fig. 3). Food treatment had no effect on fCM concentrations (t58 = 0.04, P = 0.97), nor were fCM concentrations affected by body temperature (slope = 0.002 ± 0.01 ng ml−1 fCM °C−1; t58 = 0.18, P = 0.86).
Table 3:
Model selection for linear mixed-effects models examining the faecal corticosterone metabolite concentrations of female common gartersnakes (T. sirtalis) living in enclosures in field and forest habitat with different food treatments
| Model | k | AICc | ΔAICc |
|---|---|---|---|
| fCM ∼ 1 | 4 | 53.66 | 0.00 |
| fCM ∼ Habitat | 5 | 58.17 | 4.51 |
| fCM ∼ Temperature | 5 | 63.23 | 9.57 |
| fCM ∼ Habitat + Food | 6 | 63.69 | 10.03 |
| fCM ∼ Habitat + Food + SVL | 7 | 74.32 | 20.66 |
| fCM ∼ Habitat + Food + SVL + All interactions | 11 | 113.22 | 59.56 |
Abbreviations: AICc is the bias-corrected Akaike's information criterion value; ΔAICc is the difference in AICc between each model and the model with the lowest AICc; fCM is the faecal corticosterone metabolite; and k is the number of parameters in the model. The final model contained no predictor variables.
Figure 3:
Faecal corticosterone metabolite concentrations of female common gartersnakes (T. sirtalis) living in enclosures in field and in forest habitat in Pontiac County, Québec, Canada from May to August 2014. The box represents the interquartile range, the line within the box is the median, and the whiskers represent the 95% confidence interval. Points represent outliers outside the 95% confidence interval.
Discussion
To determine habitat suitability, wildlife managers and conservation biologists must use measures of success in different habitats to confirm that a habitat that is occupied also provides high mean population fitness. In this study, we compared fCM concentrations with abundance, individual growth rate and reproductive output of common gartersnakes in field and in forest habitats to test whether fCM concentrations could be a useful measure of habitat suitability. As predicted, abundance, growth rate and reproductive output of gartersnakes were higher in field than in forest habitat. However, fCM concentrations of female gartersnakes did not differ between habitats despite clear indications from our other measures of success that gartersnakes were doing better in field than in forest habitat. For instance, female snakes in the forest did not gain mass as the experiment progressed, whereas females in the field gained mass. It is possible that the fitness differences between habitats in our study were not sufficient to be reflected in fCM concentrations. However, the marked effects of habitat on our other metrics of gartersnake fitness suggest that fCM concentrations are not appropriate indicators of mean population fitness, and thus of habitat suitability for gartersnakes.
The differences in fitness we documented between field and forest habitats are likely to be caused by thermal quality; field provides a more suitable thermal habitat than forest in our study area (W. D. Halliday and G. Blouin-Demers unpublished observations). Yet fCM concentrations in our study did not differ between snakes living in field and in forest habitats, and also did not vary with body temperature. Stress hormones exhibit variable relationships with temperature across different taxa. Fish have demonstrated a consistently positive relationship between cortisol and temperature (e.g. Barton and Schreck, 1987; Davis et al., 2001; O'Connor et al., 2011), whereas reptiles have exhibited positive (e.g. Girling and Cree, 1995; Tyrell and Cree, 1998; Schramm et al., 1999; Cree et al., 2003; Woodley et al., 2003), negative (Dupoué et al., 2013) or no relationship (Dunlap and Wingfield, 1995) between corticosterone and temperature. It is clear that habitat alone does not lead to differences in fCM concentrations, even habitats differing strongly in thermal quality, at least in part because body temperature does not appear to affect fCM concentrations in common gartersnakes.
In animals, important differences between habitats include energetic (food or thermal quality) and structural differences (e.g. presence of cover or nesting sites), which can have different physiological consequences. Structural differences between habitats could lead to differences in predation risk, and animals could then experience increased stress hormones in the riskier habitat (Boonstra et al., 1998). Habitats with different levels of disturbance (human or natural) can also lead to differences in stress hormones (Homan et al., 2003). Habitats differing in energetic potential might cause differences in physiological metrics that are related to energetics, such as field metabolic rate. Given that metabolism is directly affected by temperature in ectotherms (Gillooly et al., 2001; Dubois et al., 2009), the thermal quality of habitats is likely to be an important driver of metabolic differences. Understanding what ecological factors differ between habitats, and then determining how these differences might affect fitness, is the first step in accurately measuring habitat suitability. Physiological metrics are likely to be useful only when they are directly linked to differences in habitat suitability.
In summary, our measures of reproductive output and of growth rate confirm that an increased abundance of gartersnakes in field habitat does indeed reflect higher habitat suitability, whereas our measure of stress hormones in snakes was invariant between habitats. Therefore, researchers should use multiple metrics of fitness when measuring habitat suitability to confirm inferences based on presence or abundance. Researchers planning to use physiological metrics of habitat suitability should carefully select a metric that is likely to reflect fitness differences between habitats, and it would be prudent to assess the usefulness of such physiological metrics in pilot studies.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This project was funded by the University of Ottawa, a Natural Science and Engineering Research Council (NSERC) of Canada Post-Graduate Scholarship to W.D.H., and NSERC Discovery Grants to K.M.G. and G.B.-D.
Supplementary Material
Acknowledgements
We are grateful to L. Dionne-Wilson, P. Fassina, S. Karabatsos and M. Routh for assistance in the field and in the laboratory.
References
- Andrén H. (1990) Despotic distribution, unequal reproductive success, and population regulation in the jay Garrulus glandarius L. Ecology 71: 1796–1803. [Google Scholar]
- Barton BA, Schreck CB (1987) Influence of acclimation temperature on interregnal and carbohydrate stress responses in juvenile Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 62: 299–310. [Google Scholar]
- Battin J. (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18: 1482–1491. [Google Scholar]
- Berkvens CN, Hyatt C, Gilman C, Pearl DL, Barker IK, Mastromonaco GF (2013) Validation of a shed skin corticosterone enzyme immunoassay in the African house snake (Lamprophis fuliginosus) and its evaluation in the eastern massassauga rattlesnake (Sistrurus catenatus catenatus). Gen Comp Endocrinol 194: 1–9. [DOI] [PubMed] [Google Scholar]
- Blouin-Demers G, Weatherhead PJ (2001) Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology 82: 3025–3043. [Google Scholar]
- Blouin-Demers G, Weatherhead PJ (2008) Habitat use is linked to components of fitness through the temperature-dependence of performance in ratsnakes (Elaphe obsoleta). Israel J Ecol Evol 54: 361–372. [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC (2009) Do baseline glucocorticoids predict fitness? Trends Ecol Evol 24: 634–642. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Hik D, Singleton GR, Tinnikov A (1998) The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 68: 371–394. [Google Scholar]
- Boughton DA. (1999) Empirical evidence for complex source-sink dynamics with alternative states in a butterfly metapopulation. Ecology 80: 2727–2739. [Google Scholar]
- Bozdogan H. (1987) Model selection and Akaike's information criterion (AIC): the general theory and its analytical extensions. Psychometrika 52: 345–370. [Google Scholar]
- Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, Berlin. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree A, Tyrrell CL, Preest MR, Thorburn D, Guillette LJ (2003) Protecting embryos from stress: corticosterone effects and the corticosterone response to capture and confinement during pregnancy in a live-bearing lizard (Hoplodactylus maculatus). Gen Comp Endocrinol 134: 316–329. [DOI] [PubMed] [Google Scholar]
- Davis KB, Simco BA, Li MH, Robinson E (2001) The effect of constant and fluctuating temperatures on the confinement-induced plasma cortisol stress response in channel catfish Ictalurus punctatus. J World Aquaculture Soc 32: 422–425. [Google Scholar]
- Dubois Y, Blouin-Demers G, Shipley B, Thomas DW (2009) Thermoregulation and habitat selection in wood turtles (Glyptemys insculpta): chasing the sun slowly. J Anim Ecol 78: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Dunlap KD, Wingfield JC (1995) External and internal influences on indexes of physiological stress 1. Seasonal and population variation in adrenocortical secretion of free-living lizards, Sceloporus occidentalis. J Exp Zool 271: 36–46. [DOI] [PubMed] [Google Scholar]
- Dupoué A, Brischoux F, Lourdais O, Angelier F (2013) Influence of temperature on the corticosterone stress-response: an experiment in the Children's python (Antaresia childreni). Gen Comp Endocrinol 193: 178–184. [DOI] [PubMed] [Google Scholar]
- Fretwell SD, Lucas HL (1969) On territorial behavior and other factors influencing habitat distribution in birds: I. Theoretical development. Acta Biotheor 19: 16–36. [Google Scholar]
- Gates JE, Gysel LW (1978) Avian nest dispersion and fledging success in field-forest ecotones. Ecology 59: 871–883. [Google Scholar]
- Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effect of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- Girling JE, Cree A (1995) Plasma corticosterone levels are not significantly related to reproductive stage in female common geckos (Hoplodactylus maculatus). Gen Comp Endocrinol 100: 273–281. [DOI] [PubMed] [Google Scholar]
- Guisan A, Zimmermann N (2000) Predictive habitat distribution models in ecology. Ecol Model 135: 147–186. [Google Scholar]
- Halliday WD, Blouin-Demers G (2014) Red flour beetles balance thermoregulation and energy acquisition via density-dependent habitat selection. J Zool 294: 198–205. [Google Scholar]
- Halliday WD, Blouin-Demers G (2015) Efficacy of using coverboards for sampling small northern snakes. Herpetol Notes 8: 309–314. [Google Scholar]
- Halliday WD, Thomas AS, Blouin-Demers G (2015) High temperature intensifies negative density dependence of fitness in red flour beetles. Ecol Evol 5: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin PM. (2000) Habitat use by an intertidal salt-marsh fish: trade-offs between predation and growth. Mar Ecol Prog Ser 198: 203–214. [Google Scholar]
- Homan RN, Regosin JV, Rodrigues DM, Reed JM, Windmiller BS, Romero LM (2003) Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim Conserv 6: 11–18. [Google Scholar]
- Homyack JA. (2010) Evaluating habitat quality of vertebrates using conservation physiology tools. Wild Res 37: 332–342. [Google Scholar]
- Hopkins WA, DuRant SE (2011) Innate immunity and stress physiology of eastern hellbenders (Cryptobranchus allegahiensis) from two stream reaches with differing habitat quality. Gen Comp Endocrinol 174: 107–115. [DOI] [PubMed] [Google Scholar]
- Johnson MD. (2007) Measuring habitat quality: a review. Condor 109: 489–504. [Google Scholar]
- Knight TW, Morris DW, Haedrich RL (2008) Inferring competitive behavior from population census and habitat data. Israel J Ecol Evol 54: 345–359. [Google Scholar]
- Lipcius RN, Stockhausen WT, Eggleston DB, Marshall LS Jr, Hickey B (1997) Hydrodynamic decoupling of recruitment, habitat quality and adult abundance in the Caribbean spiny lobster: source–sink dynamics? Mar Fresh Res 48: 807–816. [Google Scholar]
- Manderson JP, Phelan BA, Meise C, Stehlik LL, Bejda AJ, Pessutti J, Arlen L, Draxler A, Stoner AW (2002) Spatial dynamics of habitat suitability for the growth of newly settled winter flounder Pseudo-pleuronectes americanus in an estuarine nursery. Mar Ecol Prog Ser 228: 227–239. [Google Scholar]
- Marra PP, Holberton RL (1998) Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116: 284–292. [DOI] [PubMed] [Google Scholar]
- Mateo JM, Cavigelli SA (2005) A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78: 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero D, Izquierdo MS, Tort L, Robaina L, Vergara JM (1999) High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol Biochem 20: 53–60. [Google Scholar]
- Morris DW. (1989) Density-dependent habitat selection: testing the theory with fitness data. Evol Ecol 3: 80–94. [Google Scholar]
- Morris DW. (2003) How can we apply theories of habitat selection to wildlife conservation and management? Wildlife Res 30: 303–319. [Google Scholar]
- O'Connor CM, Yick CY, Gilmour KM, Van Der Kraak G, Cooke SJ (2011) The glucocorticoid stress response is attenuated but unrelated to reproductive investment during parental care in a teleost fish. Gen Comp Endocrinol 170: 215–221. [DOI] [PubMed] [Google Scholar]
- Owens DAS, Carter ET, Holding ML, Islam K, Moore IT (2014) Roads are associated with a blunted stress response in a North American pit viper. Gen Comp Endocrinol 202: 87–92. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2014) nlme: linear and non-linear mixed effects models. R package version 3.1-118. http://CRAN.R-project.org/package=nlme.
- Pulliam RH. (1988) Sources, sinks, and population regulation. Am Nat 132: 652–661. [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Roberts KA, Vleck C, Bronikowski AM (2009) The effects of maternal corticosterone levels on offspring behavior in fast- and slow-growth garter snakes (Thamnophis elegans). Horm Behav 55: 24–32. [DOI] [PubMed] [Google Scholar]
- Row JR, Blouin-Demers G (2006) Thermal quality influences effectiveness of thermoregulation, habitat use, and behaviour in milk snakes. Oecologia 148: 1–11. [DOI] [PubMed] [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A et al. (2000) Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- Schramm BG, Casares M, Lance VA (1999) Steroid levels and reproductive cycle of the Galapagos tortoise, Geochelone nigra, living under seminatural conditions in Santa Cruz Island (Galapagos). Gen Comp Endocrinol 114: 108–120. [DOI] [PubMed] [Google Scholar]
- Soursa P, Huhta E, Nikula A, Nikinmaa M, Jäntii A, Helle H, Hakkarainen H (2003) Forest management is associated with physiological stress in an old-growth forest passerine. Proc Biol Sci 270: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess A-N. (2014) qpcR: modelling and analysis of real-time PCR data. R package version 1.4-0. http://CRAN.R-project.org/package=qpcR.
- Tyrell CL, Cree A (1998) Relationships between corticosterone concentration and season, time of day and confinement in a wild reptile (tuatara, Sphenodon punctatus). Gen Comp Endocrinol 110: 97–108. [DOI] [PubMed] [Google Scholar]
- Van Horne B. (1983) Density as a misleading indicator of habitat quality. J Wildlife Manage 47: 893–901. [Google Scholar]
- Weldon AJ, Haddad NM (2005) The effects of patch shape on indigo buntings: evidence for an ecological trap. Ecology 86: 1422–1431. [Google Scholar]
- Winne CT, Willson JD, Andrews KM, Reed RN (2006) Efficacy of marking snakes with disposable medical cautery units. Herpetol Rev 37: 52–54. [Google Scholar]
- Woodley SK, Painter DL, Moore MC, Wikelski M, Romero LM (2003) Effect of tidal cycle and food intake on the baseline plasma corticosterone rhythm in intertidally foraging marine iguanas. Gen Comp Endocrinol 132: 216–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.