Abstract
This research was performed based on a comparative study on fungal lipid production by a locally isolated strain Cunninghamella bainieri 2A1 in batch culture and repeated-batch culture using a nitrogen-limited medium. Lipid production in the batch culture was conducted to study the effect of different agitation rates on the simultaneous consumption of ammonium tartrate and glucose sources. Lipid production in the repeated-batch culture was studied by considering the effect of harvesting time and harvesting volume of the culture broth on the lipid accumulation. The batch cultivation was carried out in a 500 ml Erlenmeyer flask containing 200 ml of the fresh nitrogen-limited medium. Microbial culture was incubated at 30 °C under different agitation rates of 120, 180 and 250 rpm for 120 h. The repeated-batch culture was performed at three harvesting times of 12, 24 and 48 h using four harvesting cultures of 60%, 70%, 80% and 90%. Experimental results revealed that nitrogen source (ammonium tartrate) was fully utilized by C. bainieri 2A1 within 24 h in all agitation rates tested. It was also observed that a high amount of glucose in culture medium was consumed by C. bainieri 2A1 at 250 rpm agitation speed during the batch fermentation. Similar results showed that the highest lipid concentration of 2.96 g/L was obtained at an agitation rate of 250 rpm at 120 h cultivation time with the maximum lipid productivity of 7.0 × 10−2 mg/ml/h. On the other hand, experimental results showed that the highest lipid concentration produced in the repeated-batch culture was 3.30 g/L at the first cycle of 48 h harvesting time using 70% harvesting volume, while 0.23 g/L gamma-linolenic acid (GLA) was produced at the last cycle of 48 h harvesting time using 80% harvesting volume.
Keywords: Lipid production, Cunninghamella bainieri 2A1, Agitation, Batch culture, Repeated-batch culture, Gamma-linolenic acid, Harvesting time, Harvesting volume
1. Introduction
In recent years, the production of polyunsaturated fatty acids (PUFAs) such as GLA, arachidonic acids and eicosapentaenoic acids by oleaginous microorganisms has received great interest from researchers. Among these fatty acids, GLA has extensively been used in biomedical products, nutritionals and health supplements (Zikou et al., 2013). Many research studies have been carried out over the last decades to develop lipid production. These attempts have aimed at improving the economic production of microbial lipids rather than plant and animal derived oils. In this view, microbial oils are superior to plant oils and animal fats due to less time required for their circulation in environment, higher possibility for large scale production and higher sustainability under climate changes (Li et al., 2008).
Previous studies have revealed that a high amount of lipid could be accumulated by the fungal species of Cunninghamella depending on the fermentation methods and culture conditions (Fakas et al., 2007, Fakas et al., 2009, Somashekar et al., 2003). Similar studies have shown that a high lipid accumulation is attained by Cunninghamella bainieri 2A1 in the submerged batch culture (Taha et al., 2010). It is well known that C. bainieri 2A1 is capable of producing up to 30% lipid (g/g biomass) which contains 10–15% GLA. In this regard, nutritional intake of GLA and other PUFAs have been used in clinical treatment of human diseases such as blood cholesterol, acute and chronic inflammations, and atopic eczema, hypertension, Crohn’s disease, rheumatoid arthritis and asthma (Shuib et al., 2014, Stoll, 2002, Vadivelan and Venkateswaran, 2014).
The production of lipid by oleaginous fungi is highly dependent on medium composition. It has been observed that lipid production by C. bainieri 2A1 is related to the stress conditions created by the deficiency of nitrogen in the medium. On the other hand, it has been found that lipid synthesis by this strain is affected by carbon and nitrogen concentration in the culture medium (Taha et al., 2010). However, little is known about the effect of agitation rate on simultaneous consumption of nitrogen and glucose of the culture medium in relation to lipid production by C. bainieri 2A1. Agitation rate is an important factor which affects microbial growth, especially in shear sensitive microorganisms. Higher agitation rates result in better oxygen supply, which in turn favors cell growth. Hence, optimization of agitation rates is essential to provide high oxygen supply conditions for the mycelia and to increase their metabolic activities throughout the fermentation process (Abd-Aziz et al., 2008, Sun et al., 2012).
Fungal lipid fermentation could be performed as repeated-batch culture. The repeated-batch culture is a fermentation mode which offers many advantages over the microbial batch culture including the better depletion of medium in the bioreactor at the end of cultivation, the reuse of microbial cells for subsequent fermentation runs, higher cell concentration in the culture and less time required for process operation. Moreover, the repeated-batch culture is expected to increase cell productivity ensuring a high cell growth rate (Huang et al., 2008, Radmann et al., 2007). It has been noted that the repeated-batch culture is affected by operating factors. In this view, it has been observed that the repeated-batch culture is influenced by harvesting times and harvesting volumes of the culture broth (Jin et al., 2011, Masuda et al., 2011).
A number of studies have already been performed to study lipid accumulation by various fungal strains in the batch fermentation (Bellou et al., 2014, Fakas et al., 2009, Gao et al., 2013, Papanikolaou et al., 2004, Zikou et al., 2013). However, much less work has been performed to study fungal lipid synthesis in the repeated-batch cultivation. Current research was performed to investigate lipid production by C. bainieri 2A1 in the batch culture and the repeated-batch culture as a comparative study using a nitrogen-limited medium.
Furthermore, a detailed study on the use of different agitation rates was carried out to investigate the effects of agitation intensities on the depletion of glucose and ammonium tartrate as carbon source and nitrogen source, respectively in the culture medium for the enhancement of lipid production. On the other hand, the effect of two pivotal factors, namely harvesting time and harvesting volume of the culture medium on lipid production by C. bainieri 2A1 in the repeated-batch culture was studied.
2. Materials and methods
2.1. Microorganism and culture medium
C. bainieri 2A1 was obtained from School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia. Stock culture was maintained on potato dextrose agar (PDA) at 4 °C. Inoculum was prepared from the spore suspension containing 106 spores/ml harvested from 7-day-old PDA plates. The nitrogen-limited medium employed by Kendrick and Ratledge (1992) was modified and then utilized in this study with the compositions as follows (in g/L): glucose, 30; ammonium tartrate (C4H12N2O6), 1.0; KH2PO4, 7.0; Na2HPO4, 2.0; MgSO4·7H2O, 1.5; CaCl2·2H2O, 0.1; FeCl3·6H2O, 0.008; ZnSO4·7H2O, 0.0001; CuSO4·5H2O, 0.001; Co(NO3)2·6H2O, 0.0001 and MnSO4·5H2O, 0.0001. The initial pH of the culture medium was adjusted to 6.0 using 1.0 M HCl or 1.0 M NaOH. Seed culture was prepared by transferring 20 ml spore suspension into 180 ml of the growth medium. Seed culture was then incubated at 30 °C and 250 rpm agitation rate for 48 h.
2.2. Batch and repeated-batch cultivation
The batch cultivation was carried out by an addition of 10% (v/v) of seed culture (20 ml) into 180 ml fresh medium in four Erlenmeyer flasks (500 ml) to make a final 200 ml culture medium in each flask. Inoculated butch cultures were incubated at 30 °C on a rotary shaker at 120, 180 and 250 rpm agitation rates for 120 h. The repeated-batch fermentation was run in such a way that the four cycles of the batch culture were continually repeated with same conditions. Three time intervals of fermentation were studied in the repeated-batch culture including 12 h time interval (12 h, 24 h, 36 h and 48 h), 24 h time interval (24 h, 48 h, 72 h and 96 h) and 48 h time interval (48 h, 96 h, 144 h and 192 h). The first cycle of the repeated-batch culture was carried out at 30 °C and 250 rpm agitation rate by transferring 10% (v/v) of seed culture (20 ml) into 180 ml fresh medium in four Erlenmeyer flasks (500 ml) to make a final 200 ml culture medium in each flask. The second cycle to fourth cycle of the repeated-batch culture at each time interval was conducted as described by Dashti et al. (2015). At the end of each cycle determined volumes of culture medium (60%, 70%, 80% and 90% v/v) were harvested which were defined as harvesting volume (ml). The time intervals used for all cycles of the repeated-batch culture were defined as harvesting time (h).
2.3. Analytical methods
The fungal mycelia were harvested by the filtration of 100 ml culture suspension using filter paper (Whatman No. 1). A volume of 5 ml culture medium was obtained after filtration and used for the following glucose and ammonium tartrate analysis. Glucose was determined by using GOD-glucose oxidase kit (Boehringer GOD-PERID test kit). Ammonium tartrate was determined by the indophenols method (Chaney and Marbach, 1962). The filtered mycelia were washed with 200 ml of distilled water, stored at −20 °C for 24 h and then put under freeze-dried conditions (Shell Freeze Dry, LABCONCO LYPH.LOCK6) for 24 h to obtain the dry weight. The dry weight of microbial cells was determined using a balance (AND GR-200). The dry weight of cells was used to determine the biomass, lipid concentration and lipid percentage (lipid content). Dried mycelia were then ground using a pestle and mortar, followed by lipid extraction. Lipid was extracted using a mixture of chloroform and methanol in a ratio of 2:1 (v/v) overnight before filtering. The filtrate was washed with 150 ml NaCl (1% w/v), followed by an addition of 150 ml distilled water (Folch et al., 1957). The chloroform layer was obtained and evaporated using a rotary evaporator (BUCHI Rotavapor R-124). Lipid residue was dissolved in a minimal amount of diethyl ether and transferred to a vial.
3. Results and discussion
3.1. Production of biomass and lipid in the batch culture
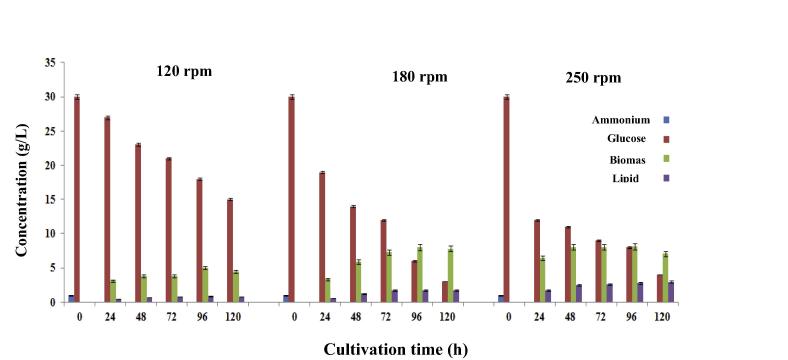
The variations in nitrogen content (ammonium tartrate) of the culture medium at different agitation rates in the batch culture are given in Fig. 1. As can be seen, ammonium tartrate was depleted within 24 h in all agitation rates tested. The results obtained showed that C. bainieri 2A1 could grow in a limited ammonium tartrate concentration (1.0 g/L) with no considerable effect of various agitation rates on nitrogen consumption, indicating the fact that this strain had strong capability of assimilating organic nitrogen compounds.
Figure 1.
The variations in glucose, nitrogen source (Ammonium), biomass and lipid concentration of nitrogen-limited medium in the batch culture of C. bainieri 2A1 at agitation rates of 120 rpm, 180 rpm and 250 rpm for 120 h fermentation.
In order to find out the consumption of glucose by C. bainieri 2A1 in biomass and lipid production process, the variations of glucose consumption in culture medium were investigated with an initial concentration of 30 g/L (Fig. 1). The study of glucose concentration measured in all agitation rates at 24 h cultivation revealed that a high amount of glucose was consumed at agitation rate of 250 rpm and reached a value of 11.9 g/L, compared to glucose consumed at agitation rates of 120 and 180 rpm during 24 h cultivation with the residual glucose concentration of 27 g/L and 19 g/L, respectively. As can be seen, the half of glucose (15.0 g/L) was consumed by C. bainieri 2A1 at the end of 120 h cultivation when agitation rate was set at 120 rpm, compared to glucose consumed at agitation rates of 180 and 250 rpm with higher glucose consumption. The glucose concentration detected at the end of batch culture revealed that with increasing the agitation speed up to 180 rpm glucose consumption concomitantly increased in a shorter time. However, no notable differences in glucose consumed by C. bainieri 2A1 were found at agitation rates of 180 and 250 rpm at the end of cultivation time (Fig. 1). This finding indicated that excessive agitation speed positively could influence glucose consumption, which in turn may affect the biomass and lipid production (Fig. 1). The findings obtained also indicated that glucose concentration of 30 g/L could well support the growth and product formation for C. bainieri 2A1.
Fig. 1 also depicts the levels of biomass concentration and lipid concentration in the batch culture of C. bainieri 2A1 at agitation rates studied. The results obtained revealed that biomass and lipid concentration drastically increased in the first 24 h fermentation. This suggested that the consumption of glucose by C. bainieri 2A1 resulted in the intense synthesis of lipid while nitrogen source was depleting during 24 h cultivation. Subsequently, the level of biomass and lipid accumulation increased to maximum levels. As can be seen, increasing agitation rate from 120 to 250 rpm concurrently had a positive effect on biomass and lipid concentration. It was noted that the highest biomass concentration (8.1 g/L) and lipid concentration (2.96 g/L) were obtained with an agitation rate of 250 rpm at 96 h and 120 h fermentation time, respectively (Fig. 1). These facts suggested the favorable effect of elevated agitation speeds on the process of biomass and lipid production by C. bainieri 2A1. The requirement to nitrogen source for lipid production is diversified depending on the microorganisms. It has been found that nitrogen limitation in culture medium is known as a stimulating factor for microbial lipid biosynthesis in oleaginous microorganisms. Lipid accumulation in oleaginous microorganisms is carried out by microbial cell nitrogen depletion, while glucose continues to be assimilated. During the lipid synthesis phase, the proportion of natural lipid fraction is high; however, it decreases when a decrease in biomass production occurs (Makri et al., 2010, Taha et al., 2010). Accordingly, at the present study nitrogen was used as a limited factor in the culture medium of C. bainieri 2A1. Supporting this view, it has been noted that Cunninghamella sp. has a high ability for lipid production in a nitrogen-limited medium (Gema et al., 2002, Ratledge, 1997, Taha et al., 2010).
During the growth of C. bainieri 2A1, glucose (30 g/L) was intensively utilized when the highest agitation rate (250 rpm) was applied compared to that when other agitation rates tested were applied. This finding was possibly due to a better mixing of culture medium, higher oxygen availability and better nutrient transfer phenomenon for microbial cells at 250 rpm, which resulted in an increase in metabolic activity of C. bainieri 2A1 for lipid synthesis (Abd-Aziz et al., 2008; Fuentes-Grünewald et al., 2012).
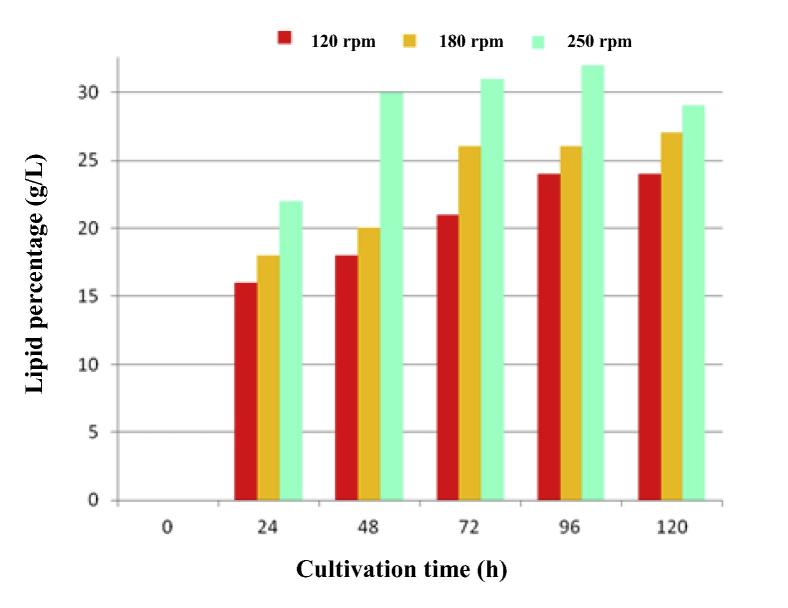
The variations in lipid percentage (lipid concentration/biomass concentration × 100) under the agitation rates studied are depicted in Fig. 2. As is evident, the cultivation of C. bainieri 2A1 at the agitation rate of 250 rpm showed that the highest lipid percentage (32%) was obtained at 96 h cultivation time. This finding indicated that increased agitation rate favored the microbial growth and lipid synthesis by C. bainieri 2A1, however; longer cultivation time higher than 96 h brought about a decrease in lipid percentage at 120 h because of lipid degradation. This was possibly due to turnover phenomenon of lipid produced by C. bainieri 2A1 after the lipogenic phase in which a part of lipid was utilized to produce biomass, accompanying by glucose exhaustion in the growth medium. Thus, lipid formed in biomass started to wane from 96 h to 120 h fermentation (Fakas et al., 2007).
Figure 2.
The lipid percentage measured for the batch cultivation of C. bainieri 2A1 in nitrogen-limited medium at agitation rates of 120 rpm, 180 rpm and 250 rpm for 120 h fermentation.
With consideration of Fig. 2, it is evident that the high lipid percentage was obtained for the agitation rate of 250 rpm at 48 h fermentation time compared to that obtained from agitation rates of 120 and 180 rpm, implying the fact that high lipid content (lipid percentage) could attain in the shorter time at an agitation intensity of 250 rpm. In studies fulfilled by Tao and Zhang (2007) it was revealed that maximum lipid content obtained was 25% by Cunninghamella echinulata at an agitation rate of 150 rpm and 96 h batch cultivation, while Papanikolaou et al. (2004) reported that the highest lipid accumulated by C. echinulata was measured at 170 rpm agitation rate after 310–400 h batch fermentation.
The highest biomass and lipid productivity obtained for all agitation rates were achieved after 24 h cultivation time (Table 1). From the economical point of view, it indicated that in spite of higher lipid and biomass concentration at 96 h, the production of lipid is more cost-effective at 24 h. The productivity of biomass and lipid exhibited a similar trend at 24 h when increasing agitation rates from 120 to 250 rpm were applied. As shown in Table 1, there were no considerable changes in lipid and biomass productivity at 120 and 180 rpm agitation rate with biomass productivity ranging from 12.9 × 10−2 to 13.75 × 10−2 mg/ml/h and lipid productivity ranging from 2.29 × 10−2 to 2.5 × 10−2 mg/ml/h. However, elevated agitation rates up to 250 rpm led to a considerable rise in biomass and lipid productivity with values as high as 31.0 × 10−2 mg/ml/h and 7.0 × 10−2 mg/ml/h, respectively, corroborating the positive effect of increased agitation rates on microbial growth and metabolic activities for biomass and lipid production.
Table 1.
The productivity of biomass, lipid and GLA within different agitation rates at 24 h batch cultivation of C. bainieri 2A1 in nitrogen-limited medium.
| Agitation rate (rpm) | Biomass productivity (mg/ml/h) | Lipid productivity (mg/ml/h) | GLA productivity (mg/ml/h) |
|---|---|---|---|
| 120 | 12.91 × 10−2 | 2.29 × 10−2 | 0.17 × 10−2 |
| 180 | 13.75 × 10−2 | 2.5 × 10−2 | 0.19 × 10−2 |
| 250 | 31.0 × 10−2 | 7.0 × 10−2 | 0.54 × 10−2 |
Table 2 shows the approximate values for the yield of product (lipid) to substrate (Yp/s), yield of product to biomass (Yp/x) and yield of biomass to substrate (Yx/s) which were obtained at 24 h of the batch culture. As can be observed, Yp/s obtained at agitation rates of 120 rpm (5.0 × 10−2) and 180 rpm (5.45 × 10−2) were lower than Yp/s value measured at 250 rpm agitation rate which drastically increased up to the value of 9.4 × 10−2, indicating a limitation in microbial nutrient absorption and gas exchange at low agitation speeds.
Table 2.
The approximate values of Yp/sa, Yp/xb and Yx/sc at different agitation rates at 24 h cultivation of C. bainieri 2A1 in nitrogen-limited medium.
| Agitation rate (rpm) | Yp/s | Yp/x | Yx/s |
|---|---|---|---|
| 120 | 5.0 × 10−2 | 16.0 × 10−2 | 31.0 × 10−2 |
| 180 | 5.45 × 10−2 | 18.0 × 10−2 | 30.0 × 10−2 |
| 250 | 9.4 × 10−2 | 22.0 × 10−2 | 41.0 × 10−2 |
Yp/s: The yield of product to substrate.
Yp/x: The yield of product to biomass.
Yx/s: The yield of biomass to substrate.
As can be seen from the results in Table 2, the highest Yp/x and Yx/s were measured at 250 rpm agitation rate with values as high as 22.0 × 10−2 and 41.0 × 10−2, respectively supporting the fact that the maximum lipid produced in relation to glucose consumed was obtained at agitation speed of 250 rpm. These findings were possibly related to the fact that agitation rate could affect microbial growth in the aerobic process which was responsible for maintaining the required level of dissolved oxygen. Hence, higher oxygen supply to microbial cell brought about higher productivity. However, further study showed that increasing agitation rates higher than 250 rpm brought about a decrease in biomass productivity and Yx/s (data not shown) which corroborated the cost-effectiveness of 250 rpm agitation rate in light of economical production.
3.2. Production of biomass and lipid in the repeated-batch cultivation
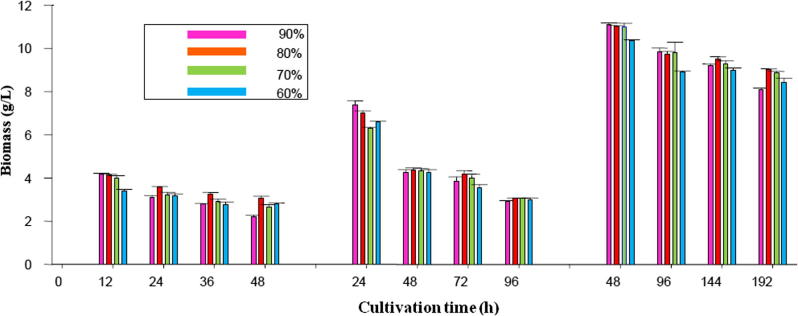
Fig. 3 illustrates the changes in biomass concentration during the repeated-batch culture of C. bainieri 2A1 at 12 h, 24 h and 48 h harvesting times using 60–90% harvesting volumes. This figure reveals that for all samples, the highest level of the biomass concentration was obtained at the first cycle of the repeated-batch culture during three harvesting times tested and fungal cell concentration decreased afterward, indicating the fact that increased batch cycle had an adverse effect on microbial cell growth and biomass concentration.
Figure 3.
The biomass concentration measured in the repeated-batch cultivation of C. bainieri 2A1 at 12 h, 24 h and 48 h harvesting time with 4 cycles of batch repetition using 60%, 70%, 80% and 90% harvesting volume.
From Fig. 3 it can be found that the maximum cell concentrations obtained at 12 h, 24 h and 48 h harvesting times were 4.3 g/L, 7.37 g/L and 11.12 g/L, respectively which were detected at the first cycle of the repeated-batch culture using 90% harvesting volume. However, the last cycle of the repeated-batch using 90% harvesting volume revealed the minimum biomass concentration with the values of 2.2 g/L, 2.9 g/L and 8.1 g/L at harvesting times of 12 h, 24 h and 48 h, respectively. It is obvious that by the comparison of three harvesting times tested, 48 h harvesting time showed the highest amounts of biomass concentration at the first cycle of the repeated-batch using 60%, 70%, 80% and 90% harvesting volume with values as high as 10.35 g/L, 11.0 g/L, 11.02 g/L and 11.12 g/L, respectively.
Furthermore, it was observed that the total biomass concentration of four cycles in 90% harvesting volume (12.14 g/L), 80% harvesting volume (12.81 g/L), 70% harvesting volume (14.02 g/L) and 60% harvesting volume (12.32 g/L) at 12 h harvesting time had high differences with respective values found at 24 h harvesting time with total biomass production of 17.42 g/L, 17.25 g/L, 18.95 g/L and 18.37 g/L as well as 48 h harvesting time with total biomass values of 36.69 g/L, 38.99 g/L, 39.31 g/L and 38.27 g/L for 90%, 80%, 70% and 60% harvesting volume, respectively. These findings implied the considerable effect of harvesting times and harvesting volumes studied on the biomass produced. Furthermore, the results obtained in the repeated-batch culture showed an increase in biomass production (11.12 g/L) compared to the biomass produced in the batch culture (8.1 g/L). Similarly, the study fulfilled by Her et al. (2004) showed that the repeated-batch culture revealed high product formation compared to the batch culture.
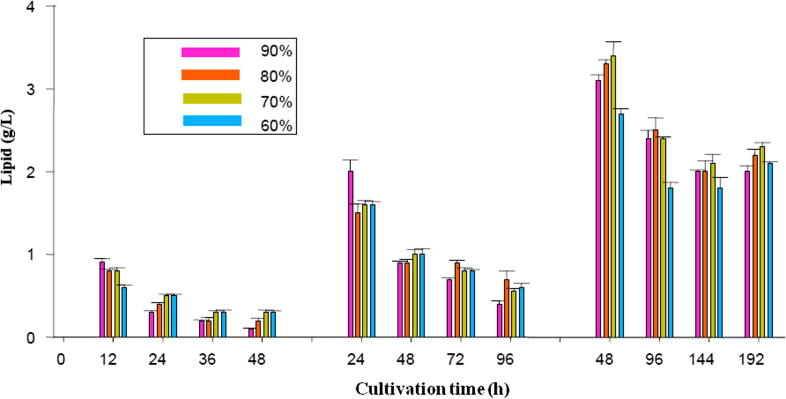
Fig. 4 depicts the lipid concentration of samples during the four cycles of the repeated-batch culture. By the comparison of lipid produced at all harvesting times tested, maximum lipid concentrations were obtained at the first cycle of the repeated-batch culture so that the production of lipid decreased gradually from the first cycle to the last cycle during each harvesting time, implying the fact that the increased repetition of batch cycles had no favorable effect on C. bainieri metabolism for lipid production. Obviously, the highest lipid produced at 12 h and 24 h harvesting times were 0.9 g/L and 2.0 g/L, respectively when 90% harvesting volume was utilized, while the maximum lipid concentration observed at 48 h harvesting time was 3.30 g/L when 70% harvesting volume was used, implying the key role of harvesting time and harvesting volume in lipid production by C. bainieri 2A1 under the repeated-batch culture.
Figure 4.
The lipid concentration measured in the repeated-batch cultivation of C. bainieri 2A1 at 12 h, 24 h and 48 h harvesting time with 4 cycles of batch repetition using 60%, 70%, 80% and 90% harvesting volume.
Fig. 4 also shows that minimum lipid concentration at 12 h and 24 h harvesting time was measured with the values of 0.1 g/L and 0.4 g/L, respectively at the fourth cycle of the repeated-batch culture when 90% harvesting volume was used, while the second and third cycle of the repeated-batch culture at 48 h harvesting time exhibited the lowest lipid concentration with the similar value of 1.8 g/L using 60% harvesting volume. Evidently, a rise in harvesting time from 12 h to 48 h caused a progressive increase in lipid production, indicating the positive effect of increased harvesting time on metabolic activity of C. bainieri 2A1 in lipid production process. The findings of this study showed that the production of lipid was enhanced in the repeated-batch culture (3.30 g/L) compared to that in the batch culture (2.96 g/L).
As mentioned previously, the production of biomass and lipid decreased after the first cycle at three time intervals of 12 h, 24 h and 48 h (Figure 3, Figure 4). These findings were in agreement with the results obtained by Xiao et al. (2011) who showed that a large amount of astaxanthin was produced by Phaffia rhodozyma at the first cycle of the repeated-batch culture and then decreased subsequently in seven cycles. The decrease in biomass concentration and lipid production after the first cycle could be attributed to the formation of pellet after the first cycle, as many fungi mycelia in liquid culture can either disperse or form pellets during microbial growth (Makri et al., 2010, Pazouki and Panda, 2000).
Figure 3, Figure 4 reveal that different harvesting times tested showed the varied effects on biomass and lipid production. Harvesting time could affect cellular growth, product formation, or cellular metabolism, depending on the host system and the range of harvesting time applied. On the other hand, biomass concentration is relatively dependent on cycle time so that microbial productivity could change as a function of cycle time (Bhargava et al., 2005). In this regard, André et al. (2010) noted that harvesting time had important effects on distribution of cellular fatty acids in the various lipids produced. They observed a decrease in biomass concentration (approximately 10–20%) at the end of fermentation runs with three different harvesting times due to the formation of pellets in the repeated-batch culture.
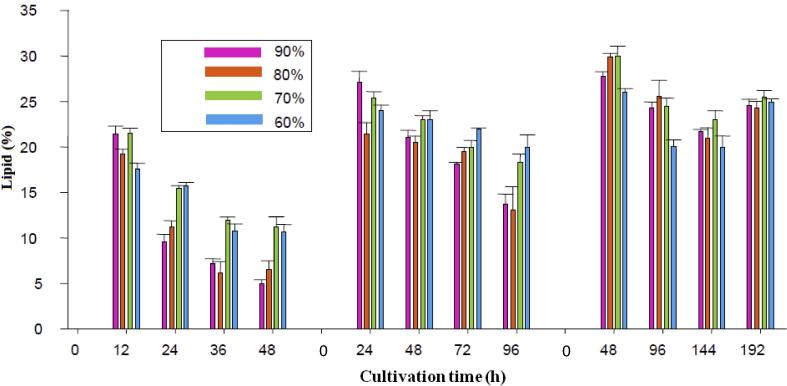
Fig. 5 shows variations in lipid percentage (accumulated lipid in dried biomass) during three harvesting times of the repeated-batch culture. Obviously, the highest lipid percentage was obtained with the value as high as 30% at the first cycle of the repeated-batch culture using 70% harvesting volumes when 48 h harvesting time was applied. On the other hand, the lowest lipid percentage (5%) was measured at the last cycle of the repeated-batch culture using 12 h harvesting time and 90% harvesting volume. As can be seen from Fig. 5, the first cycle of each harvesting time showed the maximum lipid percentage so that an increase in the number of repeating cycles reduced lipid content, showing the deleterious effect of increased repetition of batch cycles on lipid percentage. Similar to lipid production, increasing harvesting time from 12 h to 48 h brought about a rise in lipid percentage (Fig. 5). This figure reveals that the highest lipid percentages of 22.2% at 12 h harvesting time and 27.1% at 24 h harvesting time were produced at the first cycle of the repeated-batch culture using 90% harvesting volume. These findings corroborated the pivotal effects of harvesting time and harvesting volume on lipid percentage. Kim et al. (2006) demonstrated that high harvesting volumes were necessary for maximum production when 12 h harvesting time was applied. Variations observed in harvesting volume and harvesting time tested in the repeated-batch culture could be due to the fact that harvesting volume and harvesting time are related to the characteristics of the selected microorganism and respective microbial cell growth. Hence, varying conditions may provide different effects of harvesting volumes and harvesting times on microorganisms (Radmann et al., 2007).
Figure 5.
The lipid percentage measured in the repeated-batch cultivation of C. bainieri 2A1 at 12 h, 24 h and 48 h harvesting time with 4 cycles of batch repetition using 60%, 70%, 80% and 90% harvesting volume.
3.3. Production of GLA
In terms of the effects of different agitation rates on GLA concentration in the batch culture, it was observed that no considerable differences in GLA concentration were measured at 120 and 180 rpm agitation rates after 24 h batch culture with values of 0.042 g/L and 0.046 g/L, respectively. However, higher agitation speed of 250 rpm enhanced GLA production up to 0.13 g/L after 24 h batch fermentation with a productivity value of 0.54 × 10−2 (Table 1).
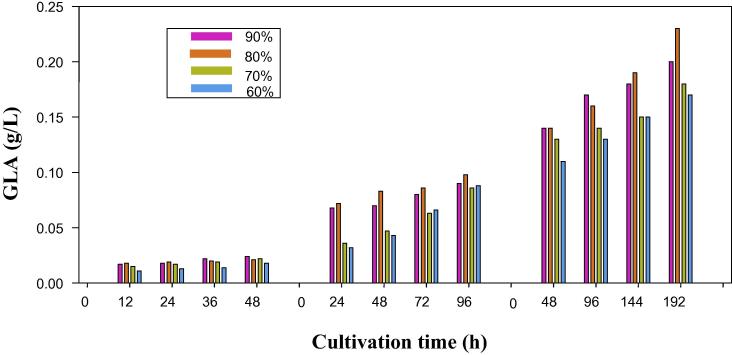
Fig. 6, illustrates the GLA production during three harvesting times in the repeated-batch culture. Experimental results showed a notable difference between GLA produced at three harvesting times tested. As can be seen from Fig. 6, the highest concentration of GLA was measured as high as 0.10 g/L and 0.23 g/L at the fourth cycle of 24 h and 48 h harvesting time, respectively using 80% harvesting volume. However, GLA concentration was maintained with the values less than 0.03 g/L at 12 h harvesting time in all harvesting volumes tested (Fig. 6). Obviously, GLA produced at 24 h and 48 h harvesting time increased gradually from the first cycle to the last cycle in 60%, 70%, 80% and 90% harvesting volumes studied, contrary to that in biomass and lipid production. Moreover, GLA production increased from 12 h to 48 h harvesting time, suggesting the favorable effects of increased harvesting time on GLA production. This finding could be attributed to the fact that reduced biomass and lipid concentration at elevated harvesting time and the repetition of batch cycle resulted in the morphological changes in fungal mycelia and the formation of pellet by C. bainieri 2A1, which resulted in a shift in metabolic activity of the fungal cells to increase GLA production (Dashti et al., 2015).
Figure 6.
The GLA concentration measured in the repeated-batch cultivation of C. bainieri 2A1 at 12 h, 24 h and 48 h harvesting time with 4 cycles of batch repetition using 60%, 70%, 80% and 90% harvesting volume.
Fatty acid composition of lipid produced in the repeated batch culture was determined by measuring fatty acid content of fungal lipid at 48 h harvesting time using 60–90% harvesting volume (Table 3). It is obvious that the main fatty acid produced was oleic acid (Δ9C18:1), followed by palmitic acid (C16:0). Linoleic acid is a PUFA known as an omega-6 fatty acid which has a double bond six carbones away from the omega carbon, while gamma-linolenic acid (GLA) is an omega-3 fatty acid which includes a double bond three carbons away from the omega carbon (Stoll, 2002). It is has been found that the metabolic pathways for the synthesis of omega-6 and omega-3 proceed from same initial step as in stearic acid (C18:0) which results in the synthesis of oleic acid and linoleic acid by the enzyme Δ9 and Δ12-desaturase. Finally, GLA is produced from linoleic acid which is catalyzed by Δ6-desaturase. This enzyme makes a double bond on the sixth carbon counting from carboxyl end (Hagan et al., 2006, Horrobin, 1993).
Table 3.
Fatty acid compositions of lipid produced by Cunninghamella bainieri 2A1 in repeated-batch culture at 48 h harvesting time using different harvesting volumes of 60%, 70%, 80% and 90%.
| Fatty acida |
Δ9,12C18:2 |
Δ9C18:1 |
C18:0 |
C16:0 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harvesting time (h) | Harvesting volume (%) |
|||||||||||||||
| 60 | 70 | 80 | 90 | 60 | 70 | 80 | 90 | 60 | 70 | 80 | 90 | 60 | 70 | 80 | 90 | |
| 48 | 0.30 | 0.41 | 0.43 | 0.35 | 1.20 | 1.50 | 1.49 | 1.37 | 0.45 | 0.41 | 0.42 | 0.63 | 0.65 | 0.59 | 0.60 | 0.83 |
| 96 | 0.28 | 0.32 | 0.31 | 0.30 | 0.82 | 0.99 | 1.14 | 1.09 | 0.22 | 0.38 | 0.37 | 0.44 | 0.38 | 0.50 | 0.46 | 0.49 |
| 144 | 0.24 | 0.20 | 0.24 | 0.23 | 0.86 | 0.98 | 0.98 | 0.92 | 0.24 | 0.35 | 0.30 | 0.34 | 0.42 | 0.50 | 0.45 | 0.48 |
| 192 | 0.20 | 0.12 | 0.24 | 0.21 | 0.87 | 0.98 | 0.91 | 0.92 | 0.26 | 0.31 | 0.30 | 0.27 | 0.41 | 0.46 | 0.41 | 0.42 |
Fatty acid: Δ9,12C18:2, linoleic acid; Δ9C18:1, oleic acid; C18:0, stearic acid and C16:0, palmitic acid.
4. Conclusions
This study showed the enhancement of lipid production in the batch culture of C. bainieri 2A1 grown in the nitrogen-limited medium under elevated agitation rate. The current research work indicated that increasing agitation rate caused higher consumption of glucose which could favor biomass production and lipid accumulation using nitrogen-limited medium. Moreover, this study revealed that the repeated-batch culture is a promising and reliable fermentation system for lipid synthesis compared to the batch culture. The efficiency of the repeated-batch culture was dependent on harvesting time and harvesting volume, which determined biomass synthesis and lipid production. The findings obtained from the repeated-batch culture of C. bainieri 2A1 in the nitrogen-limited medium revealed that the highest concentrations of biomass (11.12 g/L) and lipid (3.30 g/L) were detected at the first cycle of 48 h harvesting time using 90% and 70% harvesting volume, respectively, however, maximum GLA concentration of 0.23 g/L was produced at the last cycle of 48 h harvesting time where 80% harvesting volume was utilized.
Acknowledgement
This work was supported by Kumpulan Bioteknologi Industrri (KBTI) at UKM. The research was also supported by grants 01-03-03-0003-BTK/ER/008 and GUP/BTK-07-14-206 which hereby are acknowledged. The authors wish to thank Associate Professor. Dr. Aidil Abdul Hamid and Professor. Wan Mohtar Wan Yusoff (School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia) for their scientific efforts. The author Marjan Ganjali Dashti expresses her profound gratitude to Dr. Nimah Bahreini Esfahani (Department of Community Nutrition, School of Nutrition and Food Science, Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran) for all her kindly support during Marjan Ganjali Dashti study.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Marjan Ganjali Dashti, Email: ganjali_marjan@yahoo.com.
Peyman Abdeshahian, Email: peyman_137@yahoo.com.
References
- Abd-Aziz S., Fernandez C.C., Salleh M.M., Illias R.M., Hassan M.A. Effect of agitation and aeration rates on chitinase production using Trichoderma virens UKM1 in 2-l stirred tank reactor. Appl. Biochem. Biotechnol. 2008;150:193–204. doi: 10.1007/s12010-008-8140-4. [DOI] [PubMed] [Google Scholar]
- André A., Diamantopoulou P., Philippoussis A., Sarris D., Komaitis M., Papanikolaou S. Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010;31:407–416. [Google Scholar]
- Bellou S., Makri A., Sarris D., Michos K., Rentoumi P., Celik A., Papanikolaou S., Aggelis G. The olive mill wastewater as substrate for single cell oil production by Zygomycetes. J. Biotechnol. 2014;170:50–59. doi: 10.1016/j.jbiotec.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Bhargava S., Wenger K.S., Rane K., Rising V., Marten M.R. Effect of cycle time on fungal morphology, broth rheology, and recombinant enzyme productivity during pulsed addition of limiting carbon source. Biotechnol. Bioeng. 2005;89:524–529. doi: 10.1002/bit.20355. [DOI] [PubMed] [Google Scholar]
- Chaney A.L., Marbach E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962;8:130–132. [PubMed] [Google Scholar]
- Dashti, M.G., Abdeshahian, P., Taha, E.M., Esfahani, N.B., Kalil, M.S., Yusoff, W.M.W., Hamid, A.A., (in press). Mycelial pellet formation in fungal lipid production by Cunninghamella bainieri 2A1 using repeated batch culture, Natl. Acad. Sci. Lett. http://dx.doi.org/10.1007/s40009-014-0341-5.
- Fakas S., Galiotou-Panayotou M., Papanikolaou S., Komaitis M., Aggelis G. Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzym Microb. Technol. 2007;40:1321–1327. [Google Scholar]
- Fakas S., Papanikolaou S., Batsos A., Galiotou-Panayotou M., Mallouchos A., Aggelis G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy. 2009;33:573–580. [Google Scholar]
- Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–506. [PubMed] [Google Scholar]
- Fuentes-Grünewald C., Garcés E., Alacid E., Sampedro N., Rossi S., Camp J. Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J. Ind. Microbiol. Biotechnol. 2012;39:207–216. doi: 10.1007/s10295-011-1016-6. [DOI] [PubMed] [Google Scholar]
- Gao D., Zeng J., Zheng Y., Yu X., Chen S. Microbial lipid production from xylose by Mortierella isabellina. Bioresour. Technol. 2013;133:315–321. doi: 10.1016/j.biortech.2013.01.132. [DOI] [PubMed] [Google Scholar]
- Gema H., Kavadia A., Dimou D., Komaitis M., Aggelis G. Production of γ-linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel. Appl. Microbiol. Biotechnol. 2002;58:303–307. doi: 10.1007/s00253-001-0910-7. [DOI] [PubMed] [Google Scholar]
- Hagan N.D., Higgins T.J.V., Basra A.S. Enhancing the nutritive value of seeds by genetic engineering. In: Basra A.S., editor. Handbook of Seed Science and Technology. Food Products Press; Binghamton: 2006. pp. 171–193. [Google Scholar]
- Her S.L., Duan K.J., Sheu D.C., Lin C.T. A repeated batch process for cultivation of bifidobacterium longum. J. Ind. Microbiol. Biotechnol. 2004;31:427–432. doi: 10.1007/s10295-004-0164-3. [DOI] [PubMed] [Google Scholar]
- Horrobin D.F. Fatty acid metabolism in health and disease: the role of Δ-6-desaturase. Am. J. Clin. Nutr. 1993;57:732S–737S. doi: 10.1093/ajcn/57.5.732S. [DOI] [PubMed] [Google Scholar]
- Huang W.C., Chen S.J., Chen T.L. Production of hyaluronic acid by repeated batch fermentation. Biochem. Eng. J. 2008;40:460–464. [Google Scholar]
- Jin I.H., Jung D., Son C.W., Kim S.K., Gao W., Chung C.H., Lee J.W. Enhanced production of heteropolysaccharide-7 by Beijerinckia indica HS-2001 in repeated batch culture with optimized substitution of culture medium. Biotechnol. Bioprocess Eng. 2011;16:245–255. [Google Scholar]
- Kendrick A., Ratledge C. Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of the novel membrane-bound malic enzyme. Eur. J. Biochem. 1992;209:667–673. doi: 10.1111/j.1432-1033.1992.tb17334.x. [DOI] [PubMed] [Google Scholar]
- Kim C.J., Lee S.J., Chang Y.K., Chun G.T., Yeong Y.H., Kim S.B. Repeated batch culture of immobilized Gibberella fujikuroi B9 for gibberellic acid production: an optimization study. Biotechnol. Bioprocess Eng. 2006;11:544–549. [Google Scholar]
- Li O., Du W., Liu D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008;80:749–756. doi: 10.1007/s00253-008-1625-9. [DOI] [PubMed] [Google Scholar]
- Makri A., Fakas S., Aggelis G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010;101:2351–2358. doi: 10.1016/j.biortech.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Masuda M., Das S.K., Fujihara S., Hatashita M., Sakurai A. Production of cordycepin by a repeated batch culture of a Cordyceps militaris mutant obtained by proton beam irradiation. J. Biosci. Bioeng. 2011;111:55–60. doi: 10.1016/j.jbiosc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Papanikolaou S., Komaitis M., Aggelis G. Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour. Technol. 2004;95:287–291. doi: 10.1016/j.biortech.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Pazouki M., Panda T. Understanding the morphology of fungi. Bioprocess Eng. 2000;22:127–143. [Google Scholar]
- Radmann E.M., Reinehr C.O., Costa J.A.V. Optimization of the repeated batch cultivation of microalga Spirulina platensis in open raceway ponds. Aquaculture. 2007;265:118–126. [Google Scholar]
- Ratledge C. Microbial lipids. Biotechnology. 1997;7:135–197. [Google Scholar]
- Shuib S., Nawi W.N.N.W., Taha E.M., Omar O., Kader A.J.A., Kalil M.S., Hamid A.A. Strategic feeding of ammonium and metal ions for enhanced GLA-rich lipid accumulation in Cunninghamella bainieri 2A1. Sci. World J. 2014 doi: 10.1155/2014/173574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somashekar D., Venkateshwaran G., Sambaiah K., Lokesh B.R. Effect of culture conditions on lipid and gamma-linolenic acid production by mucoraceous fungi. Process Biochem. 2003;38:1719–1724. [Google Scholar]
- Stoll A.L. Simon and Schuster, Fireside, Inc.; New York: 2002. The Omega-3 Connection: The Groundbreaking Antidepression Diet and Brain Program. [Google Scholar]
- Sun J., Zhang L., Rao B., Han T., Chu J., Zhu J., Shen Y., Wei D. Enhanced acetoin production by serratia marcescens H32 using statistical optimization and a two-stage agitation speed control strategy. Biotechnol. Bioprocess Eng. 2012;17:598–605. [Google Scholar]
- Taha E., Omar O., Yusoff W.M.W., Hamid A.A. Lipid biosynthesis in Cunninghamella bainieri 2A1 in N-limited and N-excess media. Ann. Microbiol. 2010;60:615–622. doi: 10.1007/s13213-010-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J.Y., Zhang X.Y. Growth low of gamma-linoleic acid production by Cunninghamella echinulata. J. US-China Med. Sci. 2007;1:55–60. [Google Scholar]
- Vadivelan G., Venkateswaran G. Production and enhancement of omega-3 fatty acid from Mortierella alpina CFR-GV15: its food and therapeutic application. Bio. Med. Res. 2014 doi: 10.1155/2014/657414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A., Ni H., Li L., Cai H. Repeated batch and fed-batch process for astaxanthin production by Phaffia rhodozyma. Chin. J. Biotechnol. 2011;27:598–605. [PubMed] [Google Scholar]
- Zikou E., Chatzifragkou A., Koutinas A.A., Papanikolaou S. Evaluating glucose and xylose as cosubstrates for lipid accumulation and c-linolenic acid biosynthesis of Thamnidium elegans. J. Appl. Microbiol. 2013;114:1020–1032. doi: 10.1111/jam.12116. [DOI] [PubMed] [Google Scholar]