Abstract
Physical inactivity is a leading cause of mortality. Reinforcement interventions appear useful for increasing activity and preventing adverse consequences of sedentary lifestyles. This study evaluated a reinforcement thinning schedule for maintaining high activity levels. Sedentary adults (n=77) were given pedometers and encouraged to walk ≥10,000 steps/day. Initially, all participants earned rewards for each day they walked ≥10,000 steps. Subsequently, 61 participants were randomized to a monitoring only condition or a monitoring plus reinforcement thinning condition, in which frequencies of monitoring and reinforcing walking decreased over 12 weeks. The mean ± SD percentage of participants in the monitoring plusreinforcement thinning condition who met walking goals was 83% ± 24% versus. 55% ± 31% for participants in the monitoring only condition, p < .001. Thus, this monitoring plusreinforcement thinning schedule maintained high rates of walking when it was in effect; however, groups did not differ at a 24-week follow-up. Monitoring plus reinforcement thinning schedules, nevertheless, hold potential to extend benefits of reinforcement interventions at low costs.
Keywords: contingency management, reinforcement schedule, walking, sedentary adults
Physical inactivity is now the fourth leading risk factor for mortality worldwide (World Health Organization, 2010). The American College of Sports and Medicine recommends 30 minutes or more of moderate intensity cardiorespiratory exercise at least 5 days per week (Garber et al., 2011), and this level of activity can help prevent cardiovascular diseases, Type 2 diabetes, and obesity (Boone-Heinonen, Evenson, Taber, & Gordon-Larsen, 2009; Haskell et al., 2007; Hu, Li, Colditz, Willett, & Manson, 2003; Hu et al., 1999). One form of exercise that is convenient and widely accessible is walking. Walking a minimum of 10,000 steps per day is usually equivalent to meeting the prescribed moderate intensity exercise levels (Le-Masurier, Sidman, & Corbin, 2003) and is recommended in public-health activity guidelines (Tudor-Locke & Bassett, 2004; Tudor-Locke et al., 2011; Tudor-Locke, Hatano, Pangrazi, & Kang, 2008). Despite the benefits of walking, it is estimated that less than 5% of the US adult population engages in the recommended level of physical activity (Trojano et al., 2008).
Many interventions that promote physical activity use pedometers (see Tudor-Locke et al., 2011 for a review). Pedometers are light, unobtrusive, and relatively inexpensive monitors that continuously measure the number of steps taken throughout the day(s). Recently, some behavior analytic research has used pedometers to increase physical activity in adults and children (see Van Camp & Hayes, 2012, for a review). For example, VanWormer (2004) and Normand (2008) implemented treatment packages composed of pedometers, self-monitoring, goal setting, and contingent praise to increase the number of daily steps taken. Although the sample sizes were small, the reported interventions increased the number of steps taken per day.
A recent study using procedures parallel to the contingency management reinforcement-based procedures developed for reducing drug use (Peirce et al., 2006; Petry, Barry, Alessi, Rounsaville, & Carroll, 2012; Petry, Martin, Cooney, & Kranzler, 2000; Petry, Weinstock, & Alessi, 2011; Petry et al., 2005) found that interventions in which tangible reinforcers are provided contingent on ambulatory activity can increase such activity. Petry, Andrade, Barry, and Byrne (2013) randomized 45 sedentary older adults to either an intervention comprising pedometers and guidelines to walk > 10,000 steps per day or to the same intervention plus chances to win monetary prizes contingent upon meeting walking goals. Participants randomized to the reinforcement contingency condition walked substantially more, meeting target goals on 82.5% of days compared to 55.2% of days for those in the control group. Furthermore, participants exposed to the reinforcement contingency showed greater reductions in blood pressure and weight, as well as improvements in other fitness indices, relative to participants in the non-reinforcement group.
Finkelstein, Brown, Brown, and Buchner (2008) also evaluated the efficacy of pedometers and a monetary reinforcement procedure to increase walking in 51 adults. Participants randomized to a treatment condition involving monetary reinforcement contingent upon reaching walking goals were more active relative to participants in a control group, whose behavior was not reinforced. However, this study lasted for only 4 weeks, so the question remains as to whether the intervention would sustain walking levels over longer periods of time. Furthermore, walking was reinforced only once, after study completion. Given that delays to reinforcement reduce reinforcer effectiveness (e.g., Lussier, Heil, Mongeon, Badger, & Higgins, 2006), reinforcing behavior more immediately might increase the proportion of individuals who respond to a reinforcement intervention. In the Finkelstein et al. (2008) study, only 38% of participants assigned to the reinforcement intervention met the public health recommendations for moderate physical activity based on steps.
The monitoring schedule plays a prominent role in reinforcement-based interventions. In substance abuse contingency management treatments based on these principles, monitoring usually occurs at a relatively high frequency (e.g., twice or thrice weekly monitoring schedules for 12 weeks; Lussier et al., 2006; Petry, 2000; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). Thus, opportunities for the behavior to be reinforced also occur frequently. Such high-density reinforcement schedules exert strong control over behavior, and they generate behavior change and maintain behavior while the contingency is in place. These high-density schedules, however, are usually expensive and labor intensive. Further, compared to less intensive schedules, high-density schedules appear very distinct from the naturally occurring contingencies of reinforcement that may control the target behavior after the intervention is withdrawn. Such discrepancies between experimenter controlled and natural contingencies might reduce the likelihood of treatment generalization (Stokes & Baer, 1977).
To increase the likelihood of generalization (i.e., maintenance of treatment effects), schedule thinning, in which the density of reinforcement is gradually decreased over time, can be incorporated after the target behavior is effectively modified (LeBlanc, Hagopian, Maglieri, & Poling, 2002). One way to thin a reinforcement schedule is to implement more intermittent schedules of reinforcement. A conceptually important feature of intermittent schedules is the unpredictable availability of the reinforcer (Stokes & Baer, 1977). Use of an intermittent schedule once a new behavior pattern has been established might promote sustainable effects at relatively low costs, as the behavior is reinforced less frequently.
The present study evaluated the effects of reinforcement schedule thinning in the context of a reinforcement intervention on the maintenance of increased ambulatory activity in sedentary adults. The schedule thinning was comprised of a variable interval (VI) schedule for which the interval gradually increased over time. In the context of this article, the term “variable interval” is used to designate a monitoring system (and the corresponding opportunity for reinforcement) that occurs. at intervals that were variable and increased over time. Specifically, after achieving high rates of walking using a fixed-interval (FI) monitoring plusreinforcement schedule, participants were randomly assigned to a monitoring-only condition (with no tangible reinforcement contingent on exercising) or to a monitoring plus reinforcement thinning condition. In this latter condition, the frequency with which walking was monitored and reinforced decreased gradually over a 12-week period, down to an average of once per month. The specific aim was to assess whether this monitoring plus reinforcement thinning condition would sustain high rates of walking relative to the monitoring-only condition throughout the period in which it was in effect. If successful in maintaining behavioral gains, such a schedule would reduce the cost and time burdens associated with frequent attendance required by fixed monitoring schedules. The long-term effects of this monitoring plus reinforcement thinning system were also evaluated to assess whether benefits on walking were maintained 9 weeks after the end of the intervention period.
Method
Participants
Participants were recruited through advertisements stating that individuals were sought for a study of methods to promote walking. Participants were eligible if they were 18 years or older and walked fewer than 6,000 steps per day, on average, as assessed by a pedometer, although this criterion was not disclosed to potential participants. This criterion is similar to the index of sedentary activity used in another study (Petry et al., 2013) but slightly higher than that applied in some other studies (<5,000, e.g., Tudor-Locke et al., 2008; Tudor-Locke & Bassett, 2004). The 6,000-step criterion was used in this study to ensure the inclusion criteria were not too stringent and to increase the potential for generalization of results to a larger range of persons. Participants were ineligible if they had a major uncontrolled psychiatric illness (e.g., psychosis, suicidality), had a physical condition that could interfere with walking 10,000 steps per day (e.g., back or leg problem, recent heart attack), or were in recovery from pathological gambling due to the potential similarity between gambling and the treatment intervention (cf., Petry et al., 2006; Petry & Alessi, 2010). Initial screening occurred over the phone, and potentially eligible individuals were scheduled for two in-person assessment interviews, scheduled eight days apart. During the initial in-person assessment, potential participants provided written informed consent, as approved by the University Institutional Review Board.
Procedure
Baseline (week 0)
Those who were interested in the study and appeared eligible were instructed at an initial baseline assessment to engage in their usual activities for the next 7 days while wearing a pedometer (Omron model HJ-112; Kyoto, Japan) at all times, except when bathing and sleeping. This pedometer was chosen because it contains a memory feature that records and stores total number of steps walked daily for up to 7 consecutive days, and because it has been independently validated (Hasson, Haller, Pober, Staudenmayer, & Freedson, 2009). This pedometer automatically resets step counts daily at midnight, weighs 32 grams, and measures 7.3 cm long by 5.4 cm wide by 1.6 cm high.
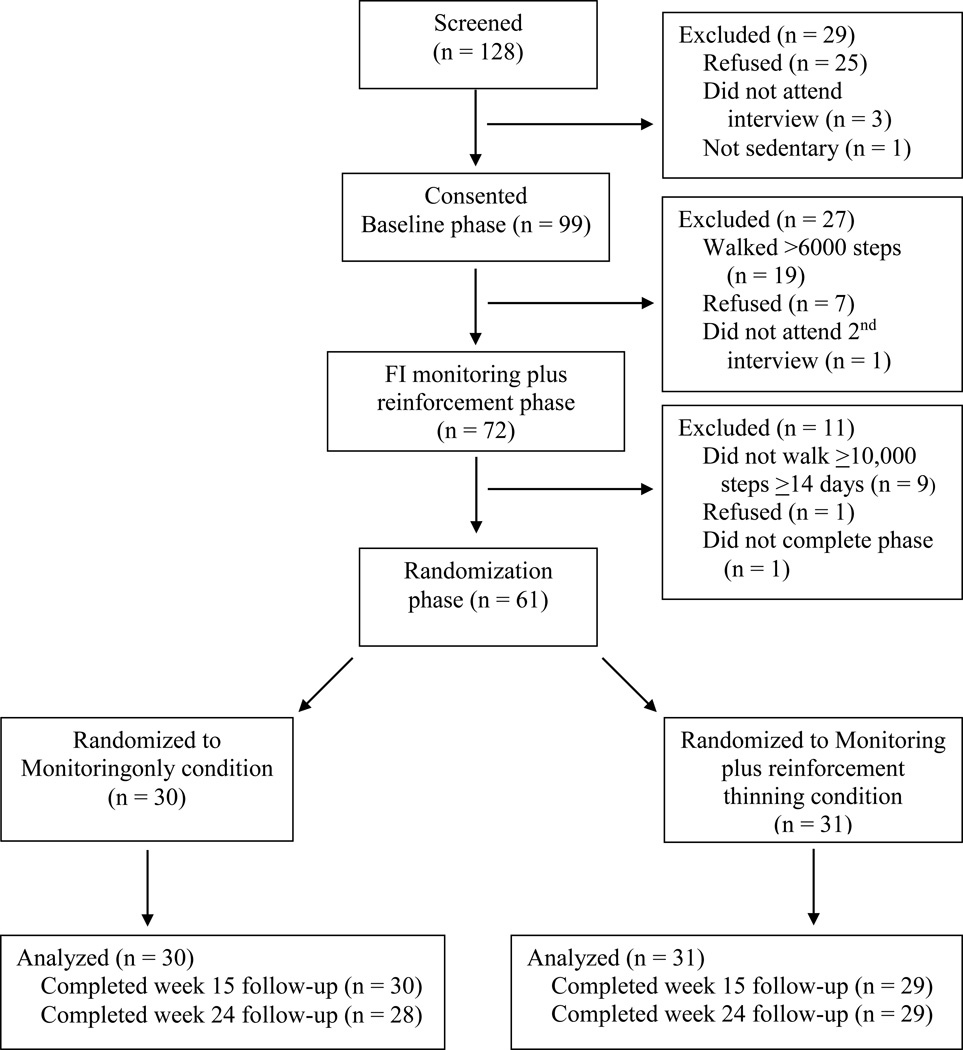
After wearing the pedometer for 7 days, participants attended a second baseline assessment, at which steps taken in the past week were evaluated. Those who walked >6,000 steps per day on average were thanked for their time and provided additional resources regarding methods to improve their physical activity levels. Those who walked <6,000 steps on average completed the remainder of the structured baseline assessments and continued to the 3-week FI monitoring plusreinforcement phase of the study described below. Figure 1 shows the flow of participants through the study phases. Participants were compensated with a $10 gift card for completing structured evaluations at baseline, week 3, week 15, and week 24, with >93% of follow-ups completed (Figure 1). In these evaluations, information regarding demographics, medical history, psychiatric distress, and physical activity levels was collected.
Figure 1.
Flowchart of participants in the study.
Fixed interval (FI) monitoring plusreinforcement (weeks 1–3)
Following the baseline assessment, all remaining eligible participants (n = 72) were exposed to a FI monitoring-reinforcement condition for 3 weeks. They were instructed to continue wearing the pedometer daily and encouraged to walk ≥10,000 steps per day. Participants were scheduled to meet with a research assistant three times per week (e.g., Mondays, Wednesdays and Fridays) for three consecutive weeks. In each 15-min meeting, pedometer data were examined and reinforcement was delivered contingent on walking ≥10,000 steps per day. (For the purposes of this study, we refer to this condition as FI monitoring plusreinforcement phase because there are two intertwined schedules embedded in this condition: the monitoring schedule and reinforcement schedule, both of which were fixed in this phase. However, some might consider the reinforcement contingency to involve a differential reinforcement of high rate behavior [DRH] schedule). The reinforcers were opportunities to draw from a bowl and win prizes ranging from $1 to $100 in value. The bowl contained 500 slips of papers, of which 50% were “winning” slips. Of these, 209 slips (41.8%) were small prizes, 40 (8.0%) were large prizes, and 1 (0.2%) was a jumbo prize. The other 250 (50.0%) non-winning slips were composed of an encouraging message, “Good job!” Small prizes were worth about $1, such as food items, toiletries, and $1 gift certificates. Large prizes were worth up to $20, and they consisted of retail items such as clothing, watches, and gift cards to stores and restaurants. The jumbo prize was worth up to $100, and consisted of items such as iPods, e-readers, and gift cards. Throughout the study, new prizes were frequently made available according to participants’ preference.
All participants earned one draw for each day they walked ≥10,000 steps. To promote sustained behavior change, participants also earned bonus draws if they walked ≥10,000 steps on the two to four consecutive days since their last visit. Bonus draws started at two, and increased by two draws at each visit up to a maximum of eight draws. Bonus draws were reset if patients failed to reach 10,000 steps on any day since the last visit or if patients missed a scheduled appointment. Similar types of escalating schedules with a reset contingency have been used effectively in contingency management treatment targeting drug abstinence (e.g., Higgins, Wong, Badger, Haug Ogden, & Dantona, 2000; Petry et al., 2005; Silverman, Robles, Mudric, Bigelow, & Stitzer, 2004).
At the end of the 3-week interval monitoring plus reinforcement phase, participants who walked ≥10,000 steps per day on at least 14 of the 21 days were eligible to move to the randomization phase. This subsample was chosen because these participants had demonstrated initial behavior change and would therefore be in a position to demonstrate durable behavior change. Those who failed to meet the 10,000 steps criterion on more than 7 of the 21 days were thanked for participation and informed about other methods to increase walking (e.g., varying the routine, making it social, etc.), but they did not continue in the study (see Figure 1).
Randomization (weeks 4–15)
Participants were randomized to a monitoring-only condition or to a monitoring plus reinforcement thinning schedule for the next 12 weeks. To ensure balance between the two conditions, a computerized urn randomization program (Stout, Wirtz, Carbonari, & Del Boca, 1994) balanced group assignment based on whether participants attended all sessions during the FI monitoring plus reinforcement phase and whether they walked ≥10,000 steps on 18 or more of the 21 days during that phase.
Participants assigned to both conditions were instructed to continue wearing the pedometer and were encouraged to walk ≥10,000 steps per day for the next 12 weeks. During this phase, participants selected 2 potential meeting days each week separated by at least 72 hours (e.g., Mondays-Fridays, Mondays-Thursdays, or Tuesdays-Fridays) during which they would be available to meet if the day was selected as a meeting day. Days were randomly selected as meeting days, but participants were unaware of which days were randomly selected as a meeting day until the morning of that day. In the mornings of randomly selected meeting days, research staff contacted participants by phone and informed them that they were due to meet that day.
Monitoring-only condition
Participants assigned to this condition earned a $5 gift card for attending meetings on randomly selected meeting days. To earn the gift card, participants also needed to bring their pedometers, with step data recorded for at least the past 4 days. Receipt of this $5 gift card, however, was not contingent upon how many steps were walked. Participants were congratulated for each day in which they walked ≥10,000 steps, but tangible reinforcers were no longer provided.
Monitoring plus reinforcement thinning condition
Participants assigned to this condition earned the same $5 gift card for attending randomly selected meeting dates and bringing the pedometer with steps recorded in the past four days. In addition, these participants continued earning “bonus” draws contingent on walking ≥10,000 steps on the prior four days. Bonus draws increased for consecutive periods of time in which ≥10,000 steps were walked in the past 4 days. The difference in this phase relative to the FI monitoring plus reinforcement phase was that pedometer readings were not always available between contiguous meetings (e.g., large gaps could occur between randomly selected meeting days; see below). Thus, bonuses were earned so long as steps walked were ≥10,000 in at least the 4 days prior to the randomly selected meeting date. Participants continued earning the same bonuses that they had earned during the FI monitoring plusreinforcement phase (range, 2–8 draws). Bonuses were reset if participants failed to attend a randomly selected meeting day or if steps decreased below 10,000 on any of the 4 days prior to a randomly selected meeting date. Once reset, bonuses could again escalate once walking resumed to ≥10,000 steps per day on the 4 days prior to a randomly selected visit.
In both conditions, a Microsoft Excel macro determined specific meeting days for each participant. The probabilities of scheduling a meeting on any potential day started at 50% and decreased by 50% across every 4-week period, with the restriction that the total number of scheduled visits was ≥7 during the 12-week period of the randomization phase (and averaged 7.1 ± 0.6 for those assigned to the monitoring-only condition and 7.0 ± 0.3 for those assigned to the monitoring plus reinforcement thinning condition). Specifically, the probability of scheduling a meeting in any of the two potential meeting days during weeks 4–7, 8–11, and 12–15 was 50%, 25%, and 12.5%, respectively; on average, the number of scheduled visits during these periods were four, two, and one. Participants were not informed about the tapering schedule or average number of meetings; they were only told that meeting days were randomly determined and could occur between 1 and 24 times over the 12-week randomization phase.
Follow-up (weeks 16–24)
At the end of the intervention period, participants in both conditions were given their pedometers to keep. They were encouraged to continue wearing them to monitor their steps daily and to walk ≥10,000 steps per day. They were scheduled for a 24-week follow-up evaluation, and a week before the evaluation, they were telephoned and reminded of their upcoming appointment and to wear the pedometer daily for the week preceding the evaluation.
Results
Initially, differences in baseline characteristics were evaluated between participants who were later assigned to monitoring only and the monitoring plusreinforcement thinning conditions. Independent t-tests were used for normally distributed continuous variables, Mann Whitney U tests for non-normally distributed variables, and chi-square tests for categorical variables. Two primary outcomes were defined a priori: (a) percentage of days on which ≥10,000 steps were taken, and (b) average number of steps per day, each assessed via pedometer readings. Initially, independent group t-tests evaluated differences in changes in these walking indices between baseline and 3 weeks later, the period during which all participants contacted reinforcement for walking ≥10,000 steps per day (i.e., the FI monitoring plus reinforcement). Change scores were utilized (baseline minus post-baseline values) because they were normally distributed.
The primary analyses focus on between-group differences in change from baseline scores during the 12-week randomization phase. Again, independent group t-tests were used to evaluate differences between the two treatment conditions. We also present descriptive data on the average number of steps registered weekly throughout the study period. Missing data throughout the randomization phase were not included, because they were relatively infrequent and did not differ between treatment groups; only 14.8% and 9.2% of randomly selected sessions were not attended in the monitoring-only and monitoring plus reinforcement thinning condition, respectively, t (59) = 0.95, p = .35.
Finally, independent t-tests evaluated group differences in follow-up change from baseline values for the primary walking data. These analyses were conducted twice, both considering missing data as missing, and using a 0 (no change from baseline) for participants who failed to complete the follow-up evaluation (n = 2 and 2 for each group). Because including missing data as a 0 did not impact results, only analyses from follow-up completers are presented. Thus, missing data were not included in any of the analyses herein reported.
Participant Characteristics
The sample comprised mostly females (90%), who described themselves as non-Hispanic (95%) and White (82%). Mean (± standard deviation) age and annual income were 48 (±9.5) years and $59,913 (±$21,972), respectively. At baseline, participants walked on average 4,444 (±1,108) steps per day. There were no significant differences between the participants assigned to the monitoring only and monitoring plusreinforcement thinning groups on any demographics or baseline characteristics.
Response to Experimental Contingencies
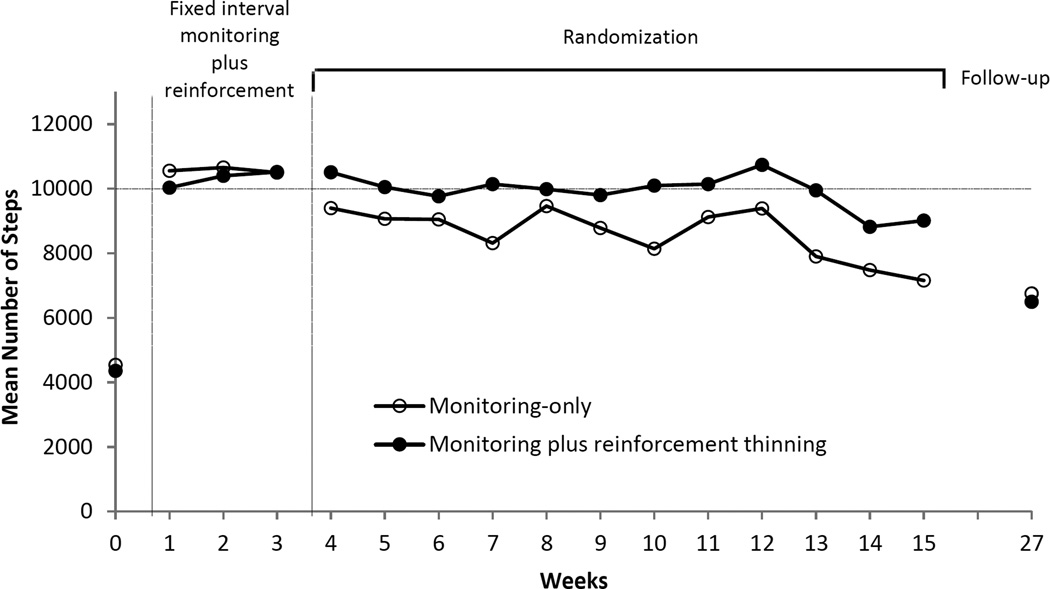
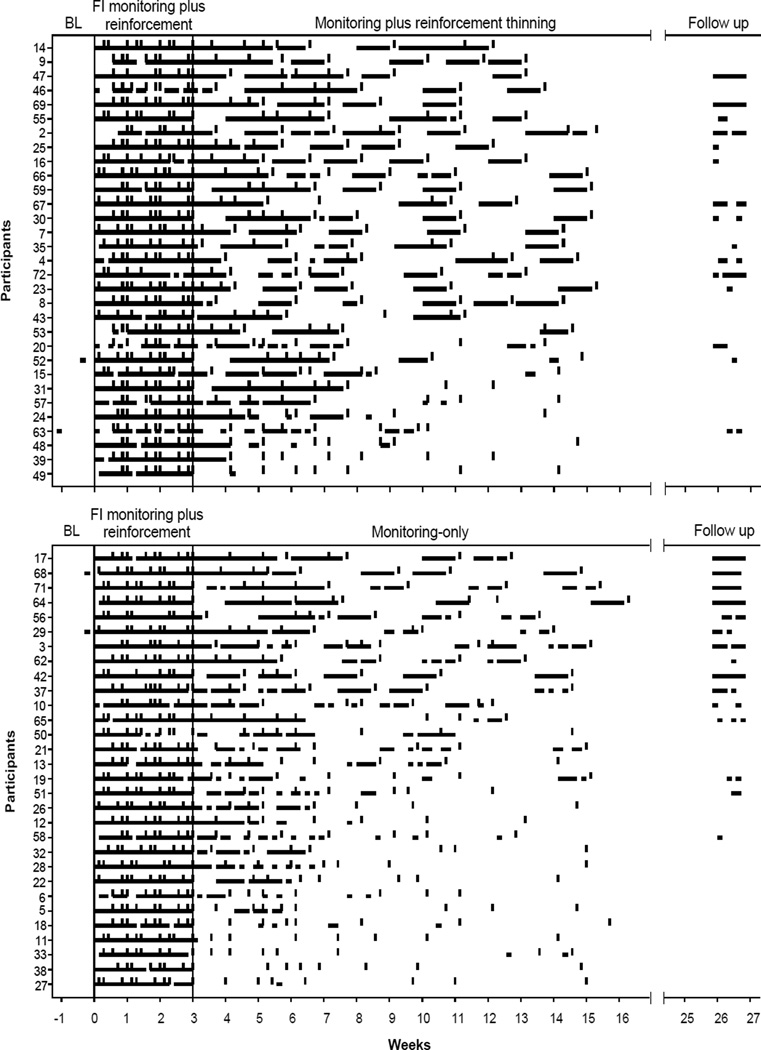
Figure 2 depicts the mean number of steps registered weekly across the 24-week study period for each group. Figure 3 depicts data for individual participants in the monitoring plus reinforcement thinning group and the monitoring-only group separately. The horizontal lines indicate days on which > 10,000 steps were logged on the pedometer, and vertical dashes indicate days on which monitoring visits were scheduled. Due to the memory capacity of pedometers, there were missing data when monitoring visits were scheduled more than seven days apart. Missing data occurred more often toward the end of the randomization phase than in earlier parts of the phase because of the nature of the thinning schedule, but missing data did not differ between groups, as noted earlier. As seen in Figure 3, participants from both groups exhibited similar patterns of walking ≥10,000 steps per day during the BL and FI monitoring plus reinforcement phases (i.e., prior to randomization). Only 4 participants had any days of walking ≥10,000 steps per day during BL, and in each case, steps exceed 10,000 on only one day during BL. All participants walked substantially more during the 3-week FI monitoring plusreinforcement phase, and they met walking goals on almost all of the days of this phase. More specifically, the percent of days meeting target goal was 96% and 92% for participants in the monitoring-only and monitoring plus reinforcement thinning groups, respectively (see Table 1).
Figure 2.
Average number of steps registered weekly across the study. Filled symbols refer to participants randomized to the monitoring plus reinforcement thinning condition during the Randomization phase, and unfilled symbols refer to participants randomized to the monitoring-only condition during the Randomization phase; all participants received reinforcement during the FI monitoring plus reinforcement phase. Values represent group means collected each week, but not all participants provided data at each week during the Randomization phase. During the Randomization phase, participants met with research staff on average 4 visits during the first four week period (weeks 4–7), on average twice during the second four week period (weeks 8–11), and on average once during the last four week period (weeks 12–15). See text for further details. BL = Baseline
Figure 3.
Consecutive days each participant in the monitoring-only and monitoring plus reinforcement thinning groups met walking goals. Horizontal lines depict days on which > 10,000 steps were logged into the pedometer of each participant across conditions. Vertical dashes represent days monitoring visits were scheduled. The thinner horizontal lines depict missing data that were considered as meeting the walking criteria when the monitoring visits were scheduled more than 7 days apart and if the participant met walking goals on both ends of the data string. In each panel, participants are arranged with those showing the greatest number of days meeting walking goals on the top to participants showing the least number of days meeting walking goals on the bottom. The numerals on the y-axis represent participant numbers. See text for further details.
Table 1.
Primary walking outcomes obtained at each assessment.
| Variable | FI monitoring reinforcement (Wks 1–3) |
Statistics (df) | Randomization (Wks 4–15) |
Statistics (df) | Follow-up (Week 24) |
Statistics (df) |
|---|---|---|---|---|---|---|
| Percent days walked ≥10,000 steps | t (59)= 1.73, p=.09 | t (59) = 3.88, p<.001 | t (55) =0.67, p=.51 | |||
| Monitoring-only | 96.1 (6.0) | 55.3 (31.0) | 31.1 (37.5) | |||
| M + R thinning | 91.6 (11.4) | 82.6 (23.5) | 24.9 (33.0) | |||
| Mean steps/day | t (59) = 0.16, p=.88 | t (59) = 2.98, p=.004 | t (55) = .29, p= .77 | |||
| Monitoring-only | 10,571 (721) | 8,428 (2,033) | 6,754 (3,159) | |||
| M + R thinning | 10,349 (682) | 9,561 (1,570) | 6,498 (2,408) |
FI= Fixed interval. M + R thinning = Monitoring plus reinforcement thinning. Values represent means (standard deviations). Statistics refer to differences between treatment groups with respect to change from baseline values.
During the randomization phase, the overall performance from the participants in the two groups differed. As indicated by the horizontal lines appearing in top graph relative to bottom graph, participants exposed to the monitoring plus reinforcement thinning contingency met walking goals more often than their counterparts exposed to monitoring alone. In addition, participants in the monitoring plus reinforcement thinning group achieved longer periods of walking > 10,000 steps per day than participants in the monitoring-only group. For example, 19 of the 31 (61%) participants assigned to the monitoring plus reinforcement thinning condition met walking goals for at least 3 consecutive weeks during the randomization phase versus only 8 of 30 (26%) participants assigned to the monitoring-alone condition.
Participants from both groups exhibited similar performance during the follow-up phase. About half of the participants from each group (15 from the monitoring-only group and 16 from the monitoring plus reinforcement thinning group) met the ≥10,000 step goal on at least one of the 7 days. Although days in which participants walked ≥10,000 steps during the follow-up were lower than during the randomization phase, participants in both groups registered more days with > 10,000 steps at follow-up than in baseline.
Visual inspection of the average group data (Figure 2) also shows that participants in the monitoring plus reinforcement thinning group sustained higher levels of walking indices throughout the randomization phase compared to those in the monitoring-only group. However, activity levels in both groups seemed to decrease during the last 2–3 weeks of the randomization phase, i.e., the period during which participants were exposed to the leanest monitoring or monitoring plus reinforcement schedule (12.5% of chance of having a visit scheduled). Table 1 depicts walking outcomes and statistical analyses comparing groups at each phase of the study. There were no differences between groups during the pre-randomization phase, when all participants contacted reinforcement for walking. Significant differences between the two groups in changes from baseline emerged on both primary walking indices during the randomization phase, ps < .004. The increases in the percent of days participants walked ≥10,000 steps as well as the average number of steps walked per day were significantly higher in the monitoring plus reinforcement thinning group compared to the monitoring-only group. However, these significant between-group differences during the randomization phase were not maintained at the 24-week follow-up evaluation.1
Reinforcement Earned and Adverse Events
During the 3-week FI monitoring plus reinforcement phase, participants who were later assigned to the monitoring-only condition earned an average of 68 ± 13 draws, resulting in $155 ± $48 in prizes, compared with an average of 64 ± 20 draws and $140 ± $59 in prizes for those who were later assigned to the monitoring plus reinforcement thinning condition, t (59) = 1.05 and 1.07, ps >.29. During the 12-week randomization phase, participants in the monitoring plus reinforcement thinning condition earned an average of 36 ± 20 draws and $77 ± $68 in prizes. No study-related adverse events occurred.
Discussion
This study found that continued reinforcement on a monitoring plus reinforcement thinning schedule maintained ambulatory activity relative to an abrupt cessation of reinforcement during the 12 weeks in which the thinning reinforcement schedule remained in effect. Nevertheless, long-term walking outcomes were similar between conditions. Sustaining high levels of walking beyond 12–15 weeks may require even longer durations of reinforcement-based interventions.
Results from this study also demonstrate that walking, once established, can be maintained with lower levels of reinforcement. On average, participants earned about $7 per day during the initial reinforcement period (about $150 over 21 days). This amount was selected because it is consistent with levels of reinforcement reported to alter substance use, weight loss, and medication adherence (Petry et al. 2005; Petry, Barry, Pescatello, & White, 2011; Petry, Rash, Byrne, Ashraf, & White, 2012), whereas lower monetary amounts appear ineffective in engendering initial behavior change (Petry et al., 2004; Petry, Barry, et al., 2012). Throughout the randomization phase of this study, less than $1 per day in reinforcement ($77 over 84 days) was sufficient to sustain high rates of walking.
The monetary amount used during the randomization phase of this study was also lower than that used in other randomized studies using monetary incentives to reinforce walking. In Finkelstein et al.’s (2008) study, for example, participants could earn up to $150 during the 4 weeks of the study. In Petry et al.’s (2013) study, participants earned an average of $375 for increased walking during the 12-week intervention phase, and this study used a monitoring-reinforcement procedure that resembled the one used in the initial reinforcement phase of the current study. One important difference, however, was that participants were not exposed to schedule thinning in the Petry et al. (2013) study; instead, they were monitored and walking was reinforced on a set weekly schedule during the entire intervention phase. The present study demonstrates that monitoring and reinforcement can occur relatively infrequently yet sustain behavior change. During the randomization phase, the average number of monitoring-reinforcement visits was only seven (four, two, and one in each 4-week period), and participants earned a total of $77 dollars in prizes during this phase. The density of reinforcement during each consecutive four-week period was on average $44, $22, and $11, respectively.
Data from the FI monitoring plus reinforcement phase of this study show that the reinforcement contingencies substantially increased the number of steps taken per day. Compared to baseline, participants, on average, increased their steps per day by about 6,000 steps, up from 4,000 per day at baseline to 10,000 per day during this phase. These results suggest that a program comprising pedometers, daily step goals, monitoring, and tangible reinforcers can promote increased levels of walking. Eighty-five percent (61 of 72) of individuals exposed to the FI monitoring plus reinforcement procedures walked ≥10,000 steps on at least two-thirds of the days and thus qualified for the randomization phase of the study.
Using a randomized design, Finkelstein et al. (2008) also reported increased activity levels among participants who received reinforcement for reaching walking goals compared to participants who did not. In that study, however, the percentage of participants who met the public health guidelines for moderate physical activity was substantially lower than in the current study (38% vs. 85%). This difference could relate to many factors including different populations and settings, or to design characteristics of the reinforcement interventions. For example, Finkelstein et al. provided slightly lower magnitude reinforcement than the initial FI monitoring plus reinforcement phase in the present study. Further, in the Finkelstein et al. study, all reinforcement was provided at the end of the study period, whereas reinforcement occurred up to three times per week in the FI monitoring plus reinforcement phase of the current study.
The studies by VanWormer (2004) and Normand (2008) demonstrated that a self-management treatment package could increase the number of steps taken by participants, even without programmed reinforcement contingencies. Direct comparisons between these studies and the current one, however, are difficult due to methodological differences. For example, in the current study the target behavior (i.e., walking goals) was the same for all participants (≥10,000 steps), whereas in the other studies the walking goals varied widely across participants in an individualized manner. Further, the prior studies were of shorter durations than this evaluation.
In the current study, participants increased walking by approximately 6,000 steps per day under the reinforcement contingencies. Whether this large increase in physical activity produces health benefits that outweigh its costs is an empirical question, but the present study demonstrates methods that can minimize personnel and reinforcement costs while maintaining behavior change. Although future studies are needed to identify the most efficacious and least time intensive approaches to delivering reinforcement, this study is among the first to address the minimum frequency of monitoring and reinforcement necessary for maintaining clinically important behavior change. Studies that implement similar procedures, such as contingency management treatments for substance use disorders, might implement schedule-thinning procedures to maintain treatment gains as well.
The ecological validity of the current study might also be questioned. Although the sample resembles those in other observational studies and randomized clinical trials (see Bravata et al. 2007), at least regarding gender and age, we cannot determine whether the effects observed here will generalize to other populations or settings. Some other limitations should be considered when interpreting the results from this study. First, the sample was composed primarily of white well-educated women with middle incomes or higher, so the findings might not generalize to men or other less educated groups or to individuals of other racial or ethnic groups. Furthermore, participants responded voluntarily to advertisements and were willing to be available on two to three potential meeting days each week. Results might differ with individuals who do not self-select to participate in programs that enhance walking. Nevertheless, the average number of steps taken daily at baseline by this sample was lower than U.S. national average and below the average number of steps taken by women of the same age range in general (Bassett, Wyatt, Thompson, Peters, & Hill, 2010).
Other limitations should be considered in addition to those related to the sample characteristics. The efficacy of reinforcement to initiate behavior change was not directly assessed in this study, and subsequent studies should evaluate the minimal reinforcement levels needed to engender walking at high rates. Furthermore, objective physical measurements (e.g., weight, blood pressure) were not taken, and thus this study did not determine if the observed increases in walking impacted physical health or fitness indices. It is also possible that participants may have given pedometers to others to wear, although none reported doing so at the follow-up evaluation. Including individuals who self selected to increase walking and conducting assessments in-person (as opposed to remote computerized uploading of pedometer readings) may guard against “cheating” in this context, but the possibility of deceit must always be considered when designing and implementing reinforcement interventions (Petry, 2012).
Although this research relied on government funding, the current procedures may eventually facilitate adoption of this type of intervention. Because the cost was low, some individuals interested in increasing and sustaining high levels of exercise may be willing to fund their own treatment. For example, participants could make monetary deposits that would be reimbursed contingent upon meeting the target goals. Alternatively, employers, health care or other organizations (e.g., retirement communities or schools) may cover costs of reinforcers if improved performance, health, or other outcomes were noted in conjunction with increased activity levels.
In summary, this study demonstrates that a monitoring plus reinforcement thinning schedule using the prize reinforcement system has the potential to maintain high rates of ambulatory activity in sedentary adults. Effects were achieved with relatively low levels of reinforcement, delivered at infrequent intervals. These aspects of the intervention are likely to enhance the dissemination and acceptability of contingency-management interventions more generally, and these interventions might ultimately prove to be cost-beneficial for improving health, especially in high-risk patient populations.
Acknowledgments
We thank Amy Novotny for assistance in conducting this study. This research and preparation of this report were funded in part by NIH grants P30-DA023918, R01-DA027615, R01-DA022739, R01-DA13444, P50-DA09241, P60-AA03510, R01-HD075630, R01-DK097705, and T32-AA07290.
Footnotes
In addition to visual inspection (Figures 2–3) and t-tests of between treatment groups effects (Table 1), multilevel modeling analyzed the step data as a discontinuous growth model. These analyses, conducted on SAS proc MIXED, used maximum likelihood estimation methods, in which missing data are taken into account in the process of estimating the covariance matrices (Singer & Willett, 2003). The time variable days (starting at baseline and running through the 24-week follow-up) was divided into study phases. Both the intercept and the day variables were included as random effects.
The number of steps was influenced by the reinforcement offered during study phases. The effect from Baseline to the FI monitoring plus reinforcement phase was very large, reflecting the increase in steps taken when walking was reinforced, F (4536) = 253.11, p < .001. The slope of steps over time during this phase remained flat, F (4536) = 0.00, p >.90, indicating no change in steps during the 3-week FI monitoring plus reinforcement phase. As expected, no treatment condition effects emerged at this point, prior to randomization, in terms of differential level of steps, F (4536) = 1.97, p > .15, or slopes between groups, F (4536)=2.37, p > .15.
The number of steps during the Randomization phase was also elevated with respect to baseline, F (4536) = 406.05, p < .001, and again the slope during this period was flat, F (4536) = 0.35, p >.50. At the transition to the Randomization phase, a significant treatment condition effect emerged, F (4536) = 4.22, p <.05, such that participants assigned to the monitoring plus reinforcement thinning condition evidenced a higher mean number of steps than those assigned to the monitoring-only condition. A significant treatment condition X slope effect also emerged during this period, F (4536) = 7.61, p < .01, accounted for by the decline over time in steps recorded by participants in the monitoring-only condition.
Steps recorded at the 24-week follow-up were not significantly different from that recorded during the Randomization phase, F (4536) = 0.05, p > .80, and the slope during this 7-day period remained flat, F (4536) = 0.02, p > .80. There were no differences in steps, F (4536) = 0.01, p > .90 or treatment condition X slope effects during the follow-up period F (4536) = 0.01, p > .90. Data not reported; available from authors.
References
- Bassett DR, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Medicine and Science in Sports and Exercise. 2010;42:1819–1825. doi: 10.1249/MSS.0b013e3181dc2e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone-Heinonen J, Evenson KR, Taber DR, Gordon-Larsen P. Walking for prevention of cardiovascular disease in men and women: a systematic review of observational studies. Obesity Reviews. 2009;10:204–217. doi: 10.1111/j.1467-789X.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Sirard JR. Using pedometers to increase physical activity and improve health: A systematic review. The Journal of the American Medical Association. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Brown DS, Brown DR, Buchner DM. A randomized study of financial incentives to increase physical activity among sedentary older adults. Preventive Medicine. 2008;47:182–187. doi: 10.1016/j.ypmed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I, Swain DP. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Medicine and Science in Sports and Exercise. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hasson RE, Haller J, Pober DM, Staudenmayer J, Freedson PS. Validity of the Omron HJ-112 pedometer during treadmill walking. Medicine and Science in Sports and Exercise. 2009;41:805–809. doi: 10.1249/MSS.0b013e31818d9fc2. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Haug Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. The Journal of the American Medical Association. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. The Journal of the American Medical Association. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- LeBlanc LA, Hagopian LP, Maglieri KA, Poling A. Decreasing the intensity of reinforcement-based interventions for reducing behavior: Conceptual issues and a proposed model for clinical practice. The Behavior Analyst Today. 2002;3:289–300. [Google Scholar]
- Le-Masurier GC, Sidman CL, Corbin CB. Accumulating 10,000 steps: Does this meet current physical activity guidelines? Research Quarterly for Exercise and Sport. 2003;74:389–394. doi: 10.1080/02701367.2003.10609109. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Normand MP. Increasing physical activity through self-monitoring, goal setting, and feedback. Behavioral Interventions. 2008;23:227–236. [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58:9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM. Contingency Management for Substance Abuse Treatment: A Guide to Implementing this Evidence-based Practice. New York, NY: Routledge/Taylor & Francis; 2012. [Google Scholar]
- Petry NM, Alessi SM. Prize-based contingency management is efficacious in cocaine-abusing patients with and without recent gambling participation. Journal of Substance Abuse Treatment. 2010;39:282–288. doi: 10.1016/j.jsat.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Andrade LF, Barry D, Byrne S. A randomized study of reinforcing walking in older adults. Psychology and Aging. 2013;28:1164–1173. doi: 10.1037/a0032563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. Journal of Consulting and Clinical Psychology. 2012;80:276–285. doi: 10.1037/a0026883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pescatello L, White WB. A low-cost reinforcement procedure improves short-term weight loss outcomes. The American Journal of Medicine. 2011;124:1082–1085. doi: 10.1016/j.amjmed.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JR, Stitzer ML, Hamilton JA. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83:269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: Contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Li R. Effect of Prize-Based Incentives on Outcomes in Stimulant Abusers in Outpatient Psychosocial Treatment Programs: A National Drug Abuse Treatment Clinical Trials Network Study. Archives of General Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Petry NM, Rash CJ, Byrne S, Ashraf S, White WB. Financial reinforcers for improving medication adherence: Findings from a meta-analysis. The American Journal of Medicine. 2012;125:888–896. doi: 10.1016/j.amjmed.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. Journal of Consulting and Clinical Psychology. 2011;79:686–696. doi: 10.1037/a0024813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. Journal of Consulting and Clinical Psychology. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. London: Oxford University Press; 2003. [Google Scholar]
- Stokes TF, Baer DM. An implicit technology of generalization. Journal of Applied Behavior Analysis. 1977;10:349–367. doi: 10.1901/jaba.1977.10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol and Drugs. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Bassett DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Medicine. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, Blair SN. How many steps/day are enough? For adults. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:79. doi: 10.1186/1479-5868-8-79. Retrieved from: http://www.ijbnpa.org/content/8/1/79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting “How many steps are enough?”. Medicine and Science in Sports and Exercise. 2008;40:S537–S543. doi: 10.1249/MSS.0b013e31817c7133. [DOI] [PubMed] [Google Scholar]
- Van Camp CM, Hayes LB. Assessing and increasing physical activity. Journal of Applied Behavior Analysis. 2012;45:871–875. doi: 10.1901/jaba.2012.45-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWormer JJ. Pedometers and brief e-counseling: Increasing physical activity for overweight adults. Journal of Applied Behavior Analysis. 2004;37:421–425. doi: 10.1901/jaba.2004.37-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: WHO; 2010. http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ [PubMed] [Google Scholar]