Abstract
Background
Massive transfusion (MT) is classically defined as >10units of packed RBCs in 24 hours. This fails to capture the most severely injured patients. Extending the prior work of Savage and Rahbar, a rolling hourly rate-based definition of MT may more accurately define critically injured patients requiring early, aggressive resuscitation.
Methods
The Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) trial collected data from ten level-1 trauma centers. Patients were placed into rate-based transfusion groups by maximal number of PRBC's transfused in any hour within the first 6 hours. A nonparametric analysis using classification trees partitioned data according to mortality at 24-hours using a predictor variable of maximum number PRBC units transfused in an hour. Dichotomous variables significant in previous scores and models as predictors of MT were used to identify critically ill patients: a positive FAST exam, GCS <8, HR >120, SBP <90, penetrating mechanism of injury, INR >1.5, Hg <11 and BD >5. These critical indicators were then compared among the nodes of the classification tree. Patients omitted included those who did not receive PRBC's (n=24) and those who did not have all 8 critical indicators reported (n=449).
Results
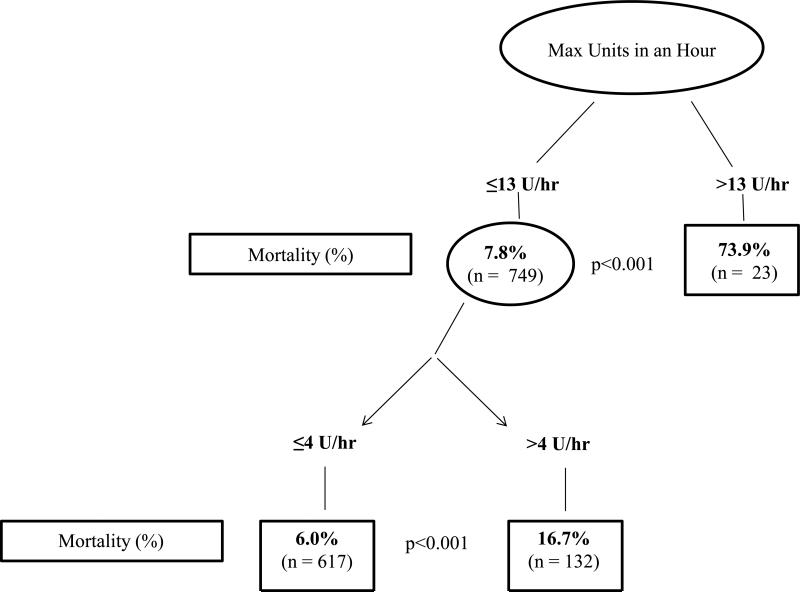
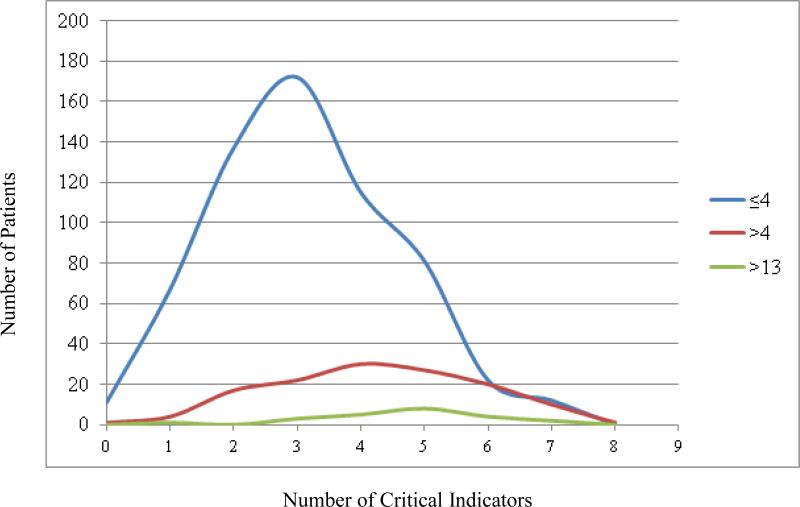
In a population of 1245 patients, the classification tree included 772 patients. Analysis by recursive partitioning showed increased mortality among patients receiving greater than 13U/hr (73.9%, p<0.01). In those patients receiving ≤13U/hr, mortality was greater in patients who received more than 4U/hr (16.7% vs 6.0%; p<0.01) (Figure 1). Nodal analysis showed the median number of critical indicators for each node were: 3 (2,4) (≤4U/hr), 4(3,5) (>4U/hr and ≤13U/hr) and 5(4,5.5) (>13U/hr).
Conclusions
A rate-based transfusion definition identifies a difference in mortality in patients who receive >4U/hr of PRBC's. Redefining MT to >4U/hr allows early identification of patients with a significant mortality risk who may be missed by the current definition.
Level of Evidence
Level III
Study Type
Original Article, observational study
Keywords: Trauma, Massive Transfusion, Rate
Background
Approximately 10% of all injured trauma patients are transfused one or more units of blood. Of those patients receiving blood, up to 30% of patients require a massive transfusion (MT).1,2 The traditional definition of massive transfusion (>10 units per 24 hours) fails to capture the true populace; excluding exsanguinating patients who expire prior to 10 units of PRBC's and including those who are not exsanguinating but require transfusions throughout the 24 hour period.2 The ability to assign objective data for MT triggers has proved difficult but such scoring and models do exist including the Trauma Associated Severe Hemorrhage (TASH) or Assessment of Blood Consumption (ABC) models which use dichotomous variables including HR, SBP, positive FAST exam, etc. to determine if a patient meets a certain criteria for MT.3-5
As the current MT definition fails to capture the most severely injured patients it introduces a significant survival bias. To minimize this bias patients receiving a massive transfusion would have to be studied and in doing so, account for both rate and time. Accounting for rate and time allows a new MT definition to become more relevant in the clinical setting.6 Up to 60% of patients arriving in the emergency department are often coagulopathic.4 The past decade has focused on improvements in mortality using a balanced ratio of PRBC, FFP and platelets.7-9 Life-saving fluid resuscitation does not occur without consequences and complications. It remains important to identify patients in need of a MT but also those who are not, in order to save patients from complications which can include DVT, infection, transfusion reactions and organ injury.4
Each of the existing models was developed retrospectively, identifying patients through chart review. PROMMTT allows for prospective data collection to compare the validity of these models to predict MT. The purpose of this study is to demonstrate a paradigm shift from the traditional definition of massive transfusion to one which incorporates an hourly interrogation of the patients’ status and the interventions administered. We hypothesized that by calculating a dynamic rate based MT definition, critical patients that require implementation of a massive transfusion protocol could be identified early. Once this new definition is identified it can be translated into the clinical setting, allowing for earlier identification of patients at risk for significant hemorrhage and earlier initiation of an appropriate massive transfusion protocol.
Patients and Methods
The PROMMTT study was an observational clinical study conducted at 10 Level 1 trauma centers which aimed to identify practices leading to improved survival. Prospective data on 1,245 adult trauma patients (>16 years old) who required a MT were collected. This study was approved by the local institutional review board at each study site, as well as the Data Coordinating Center (DCC) and the US Army Human Research Protections Office.
Patients enrolled in the PROMMTT study were highest level trauma activations who survived for greater than 30 minutes and received at least 1U PRBCs within 6 hours of admission. Patients who died within 30 minutes were excluded because it was believed that they had irreversible causes of death and it was not possible to give plasma in most trauma centers within 30 minutes of arrival in most trauma centers. Exclusion criteria included transfers from another facility, CPR >5mins, >20% TBSA burn, inhalation injury, pregnancy, incarceration or death within 30 minutes of admission. Infusion data were collected in real-time until active resuscitation ended or patients were deemed ineligible for the study. Post resuscitation data such as in hospital mortality, complications, and other variables were recorded daily. Patient demographics (gender and age) along with Injury Severity Score (ISS), injury mechanism, emergency department (ED) vital signs, admission laboratory values (basic metabolic panel, hemoglobin, hematocrit, Platelets, prothrombin Time (PT), International Normalized Ratio (INR), partial thromboplastin time (PTT), fibrinogen) and outcomes (morbidity and survival) were collected by direct observation or by record abstraction. Treatment sites were assigned a number (1 through 10) to allow evaluation by site.
All data were analyzed with the Statistical Package for the Social Sciences (SPSS), Version 19 (IBM, Armonk, New York) or the R Project (R Foundation for Statistical Computing, 2013). Patients were placed into rate-based transfusion groups by maximal number of PRBC's transfused in any hour within the first 6 hours. Those patients whose transfusions were stopped or who died in less than 1 hour were assigned a maximum number of units corresponding to what was actually received to that point; no extrapolation was made. Reported data were cumulative number of RBCs received at half hour increments, so overlapping hour long windows could be constructed from these (units received between 0 and 60 minutes, 30 and 90 minutes, 60 and 120 minutes, etc.). Separately, we were able to use this data to look at maximum number of PRBC units received in a half hour, but results from this analysis were not appreciably different and we chose to use per hour thresholds because they are more widely used. The half hour level cumulative PRBC data only extended to 6 hours, so our threshold represents only what was achieved in the first 6 hours. Twenty-four hour cumulative PRBC transfusions were also reported, but were not included in the rate-based determination as there was no way of knowing when (between 6 and 24 hours) these additional units were delivered and under what circumstances. Patients in these groups did not overlap. Patients who did not receive PRBCs were omitted (n=24).
A nonparametric analysis using classification trees (recursive partitioning) was used to partition data according to mortality at 24 hours using a predictor variable of maximum number of units of PRBCs transfused in an hour. Using a classification tree eliminates the need to define a cut-point a priori but rather the branch points are based on groups that are most different in terms of 24 hour mortality. This statistical modality is a technique that does not require further validation as it self-selects in a non-biased manner the partitioning at a point of statistical significance. Recursive partitioning was chosen because it omits and limits the bias placed on other statistical techniques. By using this statistical technique in addition to excluding the patients who expired within 30 minutes of hospital admission, we minimize survival bias as these patients would likely not have survived any type of fluid resuscitation. Dichotomous variables found to be significant in previous scores and models as predictors of MT were used to identify critically ill patients: a positive FAST exam, GCS <8, HR >120, SBP <90, penetrating mechanism of injury, INR >1.5, Hg <11 and BD >5.5,8,10-14 The current definition of MT was solely used to recognize indicators of critically ill patients so that the groups created by the breakpoints could be compared with existing measures of critical injury. Critical injury was assessed by assigning each patient 1 point per critical indicator for a maximum of 8 points. Applying critical indicators previously found to be sensitive and specific predictors for patients requiring a massive transfusion allows the validation of how many of these variables the patients who fall within the scope of the new definition meet. This is important as it allows for clinical applicability as the dichotomous variables are translatable at the bedside. These critical indicators were then compared among the nodes of the classification tree. Patients who did not have all 8 critical indicators were omitted (n=449). A significant difference was defined as p ≤ 0.05. Mann-Whitney U tests were used to assess all non-parametric continuous data. Chi-squared tests were used to analyze the nominal data.
Results
In a population of 1245 patients, the classification tree included 772 patients. Analysis by recursive partitioning showed increased mortality in patients receiving greater than 13U/hr (73.9%, p<0.01). In those patients receiving ≤13U/hr, mortality was greater in patients who received more than 4U/hr (16.7% vs 6.0%; p<0.01) (Figure 1). Nodal analysis showed that the median number of critical indicators for each node was: 3 (2,4) (≤4U/hr), 4(3,5) (>4U/hr and ≤13U/hr) and 5(4,5.5) (>13U/hr) (Figure 2). Forty-four percent of patients with >3 critical nodes expired.
Figure 1.
Recursive partitioning demonstrating 24 hour mortality using maximum number of PRBC's transfused in an hour.
Figure 2.
Distribution of critical indicators for patients within each node of the recursive partitioning model
This classification based on transfusion rate was then compared to the classic massive transfusion definition (Table 1). Everyone in the >13u/hr PRBC group met the classic MT definition as expected. Among the lowest and middle groups, 9% and 71% met the classic MT definition. This demonstrates that in the group that received of >4u/hr and ≤13u/hr there was a mortality rate of 17% and 29% of patients are potentially missed by the classic MT definition.
Table 1.
Number of cases and percent's, first within transfusion rate group and second within classic massive transfusion group; first set of percentages add to 100% vertically, second add to 100% horizontally.
| Classic Massive Transfusion Definition | ||
|---|---|---|
| Transfusion Rate Group (max units/hr) | NO | YES |
| ≤ 4 | 563 (91%) | 54 (9%) |
| > 4 and ≤ 13 | 38 (29%) | 94 (71%) |
| > 13 | 0 (0%) | 23 (100%) |
To determine if the patients in the new MT definition group truly had a higher risk of mortality, mortality at 24 hours was compared for each group (Table 2). Within both classic massive transfusion status groups, mortality increased with increasing transfusion rate. Within each of the two lower transfusion rate groups, the 24 hour mortality rate was lower for those that met the classic massive transfusion criteria than those that did not. We believe the reason for this is a survivor bias. To reach the threshold for the classic massive transfusion criteria, a patient must survive long enough to receive sufficient total units. If the patient survives longer, then it is more likely that the patient survives to 24 hours. This final trend shows the utility of this proposed transfusion rate classification: a patient can be identified as high risk sooner and does not have to survive for a prolonged time period.
Table 2.
Mortality rate at 24 hours for each combination of transfusion rate group and classic massive transfusion definition.
| Classic Massive Transfusion Definition | ||
|---|---|---|
| Transfusion Rate Group (max units/hr) | No | Yes |
| ≤ 4 | 6.40% | 1.90% |
| > 4 and ≤ 13 | 21.10% | 14.90% |
| > 13 | n/a | 73.90% |
Discussion
Exsanguinating hemorrhage continues to be one of the leading causes of death for trauma patients and the majority of these deaths occur in the first 6 hours after injury.15-17 MT as currently defined is needed in a small subset of the most critically ill patients.18 The practice of transfusing blood during trauma resuscitations is based on a perceived clinical need rather than definition.19 The current MT definition serves as a tool to study the most critically ill. As both military and civilian trauma studies have shown, “damage control resuscitation” or equivalent replacement 1:1:1 or 1:1:2 resuscitation has proven beneficial as high ratios of FFP and platelets is associated with improved survival and decreased overall PRBC need.9,20,21 PROMMTT is a unique study in that it is prospective and allows for variance of administration of blood and blood products, thus allowing a perfect medium for reevaluating MT. As noted by Savage, the search for redefining MT has failed due to an inability to account for changes in transfusion patterns over time and because the current definition of MT lends exclusion and bias to those who do not reach 10U/24hrs.22 To date, all alternatively proposed definitions have been difficult to adopt clinically due to time consuming calculations or models that prove impractical for bedside applicability, and the current definition of MT remains at 10U PRBCs in 24 hours. Therefore, we chose to compare our model with the classic and still up-to-date MT definition in hope to create a more applicable definition used to trigger a balanced resuscitation.
PROMMTT allowed for data to be collected in half hour increments and therefore a rolling-time over 6 hours was gathered on individual patients, thus omitting all arbitrary timelines.22
PROMMT includes both penetrating and blunt trauma omitting patients who expired within 30 minutes of arrival. In this paper we do not mention cause of death specifically but account for the sickest persons requiring MT to identify a new definition based on mortality. We address timing of initial PRBC transfusion which was universally started in the Emergency Department. Incorporating the use of preexisting predictors of MT allowed for a more accurate model than those developed previously due to the prospective nature of the variables. Therefore, the timing of when to initiate the 1:1:1 or 1:1:2 resuscitation can be made real-time compared to previous methods that often use complex retrospective calculations or technology that is not necessarily feasible to determine the patients’ actual need for transfusion.6,13,22,23 Recursive partitioning allowed for identification of significant mortality rates, >3 critical indicators and >4U/hr PRBC's, pinpointing areas when a MT and consequently damage control resuscitation should be started. These data permit early activation of a clinical massive transfusion protocol making the definition more practical and accurate both from clinical and research perspectives.
There are several limitations to our study. This was an analysis of data accumulated over 10 geographically distinct trauma centers from a study in which the primary focus was to investigate in-hospital trauma transfusion protocols. As there were 10 different sites it was the opinion of the attending physician when a patient was to receive blood and although speculation can be made as to when and why a patient was transfused the criteria were not uniform over all 10 centers and all attendings. Secondly, PROMMTT was an observational study that did not impose standardized procedures, and thus data were often missing for patients who did not undergo certain tests. This resulted in exclusion of a large portion of the population. Lastly, by applying critical indicators which allowed for validity and ease of clinical translation of the study this resulted in omission of a large number of patients.
Conclusion
In conclusion, >4U/hr PRBC transfusion is an improved dynamic rate-based MT definition. A subset of patients are identified who are at elevated risk for death that are not identified by the current MT definition. Redefining MT to >4U/hr allows early identification of patients with a significant mortality risk who may be missed by the current definition and permits earlier activation of an appropriate massive transfusion protocol.
Acknowledgements
T Hansberry II
This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148.
Footnotes
This study was presented at the 45th annual meeting of the Western Trauma Association, March 1–6, 2015, in Telluride, Colorado.
Portions of this paper were presented as a poster at the Military Health System Research Symposium on August 19, 2014 in Fort Lauderdale, Fl
There are no conflicts of interest
Contributor Information
Alexis Moren, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239.
David Hampton, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239 moren@ohsu.edu Phone: 503-494-5300 Fax: 503-494-6519.
Brian Diggs, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239 moren@ohsu.edu Phone: 503-494-5300 Fax: 503-494-6519.
Laszlo Kiraly, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239 moren@ohsu.edu Phone: 503-494-5300 Fax: 503-494-6519.
Erin Fox, The University of Texas Health Science Center at Houston 6410 Fannin St Houston, Tx 77030 erin.e.fox@uth.tmc.edu Phone: 713-500-7965 Fax: 713-500-0683.
John Holcomb, The University of Texas Health Science Center at Houston 6410 Fannin St Houston, Tx 77030 john.holcomb@uth.tmc.edu Phone: 713-500-7965 Fax: 713-500-0683.
Mohammad Rahbar, The University of Texas Health Science Center at Houston 6410 Fannin St Houston, Tx 77030 mohammad.h.rahbar@uth.tmc.edu Phone: 713-500-7965 Fax: 713-500-0683.
Karen Brasel, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239 moren@ohsu.edu Phone: 503-494-5300 Fax: 503-494-6519.
Mitchell Cohen, 513 Parnassus Ave University of California San Francisco San Francisco, Ca 94143 mcohen@sfghsurg.ucsf.edu Phone: 415-306-8673 Fax: 415-206-5484.
Eileen Bulger, University of Washington 325 Ninth Avenue Seattle, Wa 98104 ebulger@uw.edu Phone: 206-520-5000.
Martin Schreiber, Oregon Health & Science University 3181 SW Sam Jackson Park Rd Portland, OR 97239 moren@ohsu.edu Phone: 503-494-5300 Fax: 503-494-6519.
References
- 1.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44(6):809–813. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 2.Malone DL, FAU HJ, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. The Journal of trauma. 2009;60:S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. 60:S91-S96. [DOI] [PubMed] [Google Scholar]
- 3.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: Simple as ABC (assessment of blood consumption)? J Trauma. 2009;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 4.Maegele M, Brockamp T, Nienaber U, Probst C, Schoechl H, Görlinger K, Spinella P. Predictive models and algorithms for the need of transfusion including massive transfusion in severely injured patients. Transfus Med Hemother. 2012;39(2):85–97. doi: 10.1159/000337243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yucel N, Lefering RF, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, Neugebauer EA, Wappler F, Bouillon B, Rixen D. Polytrauma Study Group of the German Trauma Society. Trauma associated severe hemorrhage (TASH)-score: Probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60:1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 6.Rahbar E, Fau FE, del Junco DJ, Harvin JA, Holcomb JB, Wade CE, Schreiber MA, Rahbar MH, Bulger EM, Phelan HA, et al. PROMMTT Study Group Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;74(7):S16–23. doi: 10.1097/TA.0b013e31828fa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Nunez TC, Dutton WD, May AK, Holcomb JB, Young PP, Cotton BA. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion. 2010;50(9):1914–1920. doi: 10.1111/j.1537-2995.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: Directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, Holcomb JB. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, Zaydfudim V, Johnston M, Arbogast P, Young PP. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(Suppl 1):S33–9. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 13.Vandromme MJ, Griffin RL, McGwin G, Weinberg JA. Prospective identification of patients at risk for massive transfusion: An imprecise endeavor. Am Surg. 2011;77:155–161. [PubMed] [Google Scholar]
- 14.Rainer TH, Ho AM, Yeung JH, Cheung NK, Wong RS, Tang N, Ng SK, Wong GK, Lai PB, Graham CA. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011;82(6):724–729. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Callcut RA, Johannigman JA, Kadon KS, Hanseman DJ, Robinson BR. All massive transfusion criteria are not created equal: Defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011;70:794–801. doi: 10.1097/TA.0b013e3182127e40. [DOI] [PubMed] [Google Scholar]
- 16.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, et al. The coagulopathy of trauma: A review of mechanisms. J Trauma. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 17.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: Its pathophysiology and treatment in the injured patient. World J Surg. 2007;31(5):1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 18.Dente CJ, Shaz BH, Nicholas JM, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. Journal of Trauma-Injury Infection & Critical Care. 2009;66(6):1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 19.Mitra B, Cameron PA, Gruen RL, Mori A, Fitzgerald M, Street A. The definition of massive transfusion in trauma: A critical variable in examining evidence for resuscitation. Eur J Emerg Med. 2011;18(3):137–142. doi: 10.1097/MEJ.0b013e328342310e. [DOI] [PubMed] [Google Scholar]
- 20.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. The American Journal of Surgery. 2009;197(5):565–570. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 22.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):396–402. doi: 10.1097/TA.0b013e31827a3639. [DOI] [PubMed] [Google Scholar]
- 23.Stanworth SJ, Morris TP, Gaarder C, Goslings JC, Maegele M, Cohen MJ, König TC, Davenport RA, Pittet JF, Johansson PI, et al. Reappraising the concept of massive transfusion in trauma. Crit Care. 2010;14(6):R239(1466–609). doi: 10.1186/cc9394. [DOI] [PMC free article] [PubMed] [Google Scholar]