Abstract
Foxp3+ regulatory T (Treg) cells are essential for preventing autoimmunity and uncontrolled inflammation, and also modulate immune responses during infection and development of cancer. Accomplishing these tasks requires the widespread distribution of Treg cells in both lymphoid and non-lymphoid tissues, and the selective recruitment of Treg cells to different tissue sites has emerged as a key checkpoint that controlling tissue inflammation in autoimmunity, infection, cancer development and in the context of allograft acceptance or rejection. Additionally, Treg cells are functionally diverse, and it has become clear that some of this diversity segregates with Treg cell localization to particular tissue sites. In this article, I will review progress in understanding the mechanisms of Treg cell trafficking, and discuss factors controlling their homeostatic maintenance and function in distinct tissue sites.
The discovery of ‘dominant tolerance’ mediated by different populations of Treg cells approximately 20 years ago initiated a flurry of research into the cellular and molecular basis for the function of these cells. A key discovery occurred when several groups found that the transcription factor Foxp3 is essential for the proper development and function of Treg cells (1). Indeed, loss of Treg cell function due to mutations in Foxp3 results in fatal systemic autoimmunity in both mice and humans, and defects in the development, function or maintenance of Treg cells have been implicated in the pathogenesis of a host of autoimmune and inflammatory diseases. Conversely, Treg cells can inhibit pathogen clearance and promote chronic infection, and Treg cells represent a significant barrier to effective tumor immunotherapy. Therefore, understanding the control of Treg cell homeostasis and function has significant therapeutic implications.
Based on the discovery of Foxp3 as a ‘master’ transcription factor, a number of experimental tools were developed that have allowed for the precise identification and molecular characterization of Foxp3-expressing cells, resulting in unparalleled insights into the biology of Treg cells. A central theme that has emerged from these studies is that like conventional CD4+ helper T cells, Treg cells are phenotypically and functionally diverse, and that their localization and maintenance in different tissue sites is essential for their ability to interact with and modulate their cellular targets. This brief review will cover recent advances in understanding the control of Treg cell localization, homeostasis and function in lymphoid and non-lymphoid tissue sites, with particular emphasis on how manipulation of these pathways could be therapeutically beneficial in the contexts of autoimmune disease, cancer and transplantation.
Phenotypic and functional diversity of Treg cells
Two pathways exist for Treg cell development. Differentiation of thymic-derived Treg cells (tTreg cells) depends on high-affinity interactions with self-peptide/MHCII complexes during T cell development in the thymus (2, 3), whereas peripheral-derived Treg cells (pTreg cells) develop in the periphery from naïve T cell precursors that upregulate Foxp3 when activated by foreign antigens in toleragenic conditions. Specifically, activation of naïve T cells in the presence of TGF-β and the absence of inflammatory cytokines such as IFN-γ, IL-4 or IL-6 results in pTreg cell development (4), and as such pTreg cells are particularly important for tolerance at mucosal surfaces against commensal micro-organisms and harmless environmental antigens. However, definitive markers differentiating tTreg and pTreg cells have not been identified, and thus in most cases the relative contributions of tTreg and pTreg cells to the Treg cell pool in different tissues and inflammatory settings have not been determined.
Initial analysis of homing receptor expression by Treg cells indicated that rather than having a uniform phenotype, Treg cells could be sub-divided into distinct populations that expressed adhesion and chemoattractant receptors that would target them to a range of tissues and inflammatory sites (5). These included cells that would be targeted to secondary lymphoid organs, to specific non-lymphoid tissues such as the skin and intestines, and to sites of Th1, Th2 or Th17-mediated inflammatory responses. Accordingly, Treg cells are broadly distributed in lymphoid and non-lymphoid tissue sites, even in the absence of any overt inflammation (6), and many studies have demonstrated that Treg cells function in both lymphoid and non-lymphoid tissues to either prevent initiation of aberrant immune responses or to dampen ongoing inflammatory responses, respectively.
Treg cells are known to occupy their own homeostatic ‘niche’, evidenced by the ability of small numbers of Treg cells to expand dramatically when transferred into Treg cell-deficient hosts (7). However, the presence of significant populations of Treg cells in multiple lymphoid and non-lymphoid organs raises the question of whether Treg cells in different tissues are maintained by distinct homeostatic mechanisms. Indeed, despite the incredibly complex patterns of homing receptor expression by Treg cells, based on differential expression of the activation marker CD44 and the lymph node homing receptor CD62L, Treg cells can broadly divided into CD44loCD62L+ ‘central’ Treg cells (cTreg cells) and CD44hiCD62Llo/- ‘effector’ Treg cells (eTreg cells) that display distinct homeostatic behaviors (8). Whereas cTreg cells are quiescent, express high-levels of anti-apoptotic molecules such as Bcl-2 and Mcl-1, and recirculate through the secondary lymphoid tissues, eTreg cells are highly proliferative, prone to apoptosis due to decreased expression of Bcl-2 and Mcl-1, and are the dominant Treg cell population in non-lymphoid tissues where they relatively tissue-resident. These data and others have led to a model in which there is a 'division of labor' cTreg cells and eTreg cells that are specialized for functioning either within the secondary lymphoid tissues to inhibit T cell priming, or in specific non-lymphoid tissues and inflammatory sites to dampen effector cell responses, respectively (9, 10). However, function of both cTreg cells and eTreg cells likely depends on their precise positioning that facilitates the cellular interactions that promote Treg cell function and homeostasis.
Treg cell function and maintenance in secondary lymphoid organs
Before exiting the thymus, tTreg cells upregulate expression of the homing receptors CD62L and CCR7, which together target them to secondary lymphoid tissues (11). Several imaging studies have examined Treg cell behavior in secondary lymphoid tissues, and found that they were highly mobile cells that were present throughout the central T cell zones (12–15). However, in the presence of self-antigen, for instance when islet-antigen specific Treg cells from BDC2.5 TCR transgenic mice are examined in the pancreatic lymph nodes, Treg cells arrested and formed stable contacts with antigen-bearing DCs (13). The interactions between Treg cells and DCs subsequently prevented the stable interactions between naive T cells and DCs that are required for T cell activation and effector differentiation (12, 13). Thus, a primary mode of Treg cell function in secondary lymphoid tissues may be through inhibition of DC activation and function, thereby blocking the inappropriate priming of autoreactive T cells. Consistent with this, depletion of Treg cells caused a rapid increase in the activation state of DCs in secondary lymphoid tissues (16).
The actual mechanisms by which Treg cells control DC activation in secondary lymphoid organs are still not entirely clear. However, Treg cell expression of CTLA-4 appears to be one important component. Treg cells are characterized by constitutive expression of CTLA-4, which as an inhibitory cell surface receptor has a higher affinity for the co-stimulatory ligands CD80 and CD86 than its co-stimulatory counterpart CD28 (17). Thus, CTLA-4 expression by Treg cells can inhibit DC function by masking CD80 and CD86, or even by stripping CD80 and CD86 out of the membrane of DCs via a process known as trans-endocytosis (18). Additionally, ‘reverse signaling’ via CTLA-4 interactions with CD80 and CD86 induced DC expression of the immunosuppressive enzyme indolamine 2,3-dioxygenase (IDO) (19). Consistent with this model, loss of CLTA-4 expression in Treg cells resulted in lymphoproliferative disease marked by aberrant T cell activation, and severe lymphadenopathy and splenomegaly (20). Interestingly, CTLA-4 also mediates direct interactions between Treg cells and effector T cells in lymph nodes, and although the functional importance of these interactions is not clear, they may also contribute to inhibition of T cell priming (15).
In contrast to naïve T cells, Treg cells in secondary lymphoid organs express low levels of the IL-7 receptor component CD127 (21), and therefore do not rely on IL-7 for their homeostatic maintenance. Instead Treg cells are characterized by constitutive expression of the high-affinity IL-2 receptor component CD25. Indeed, IL-2 serves many similar functions for Treg cells that IL-7 does for naïve T cells. For example, as naïve T cells respond to paracrine IL-7 produced by fibroblastic reticular cells (FRCs), Treg cells cannot produce their own IL-2, and instead rely on paracrine IL-2 produced by activated T cells (22, 23). Additionally, as IL-7 helps maintain naïve T cells without driving robust homeostatic proliferation, IL-2 signaling in Treg cells in secondary lymphoid tissues is limited to quiescent cTreg cells, and not associated with the high-level of homeostatic proliferation observed in eTreg cells (8). Thus, cTreg cells are particularly dependent on IL-2 for their homeostatic maintenance, whereas eTreg cell maintenance and proliferation are largely IL-2-independent. The ability of cTreg cells to selectively access IL-2 in vivo was due to their CCR7-dependent migration into organized T cells zones in secondary lymphoid tissues (8). Interestingly, FRCs are the primary sources of the CCR7 ligands CCL19 and CCL21 in the secondary lymphoid tissues (24). Therefore, it is tempting to speculate that whereas FRCs control naïve T cell homeostasis via direct production of IL-7, they indirectly regulate cTreg cell homeostasis by bringing together CCR7-expressing cTreg cells, DCs and effector T cells to facilitate effector T cell activation and paracrine IL-2 cross-talk between effector and regulatory T cells. It should be noted that in addition to its role in cTreg cell maintenance, IL-2 is also influences Treg cell function. Thus, increasing Treg cell numbers in IL-2 deficient mice by knocking out the pro-apoptotic molecular Bim failed to rescue the inflammatory phenotype in these animals, and this was associated with impaired Treg cell expression of CTLA-4 (25).
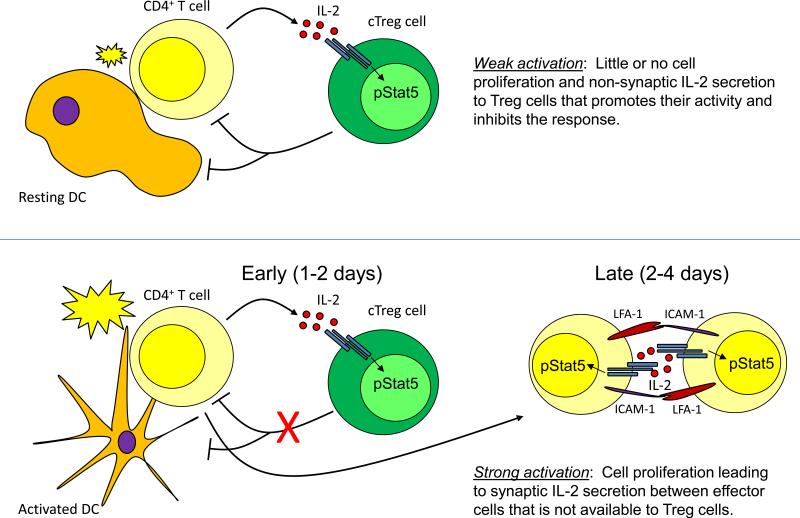
In addition to its impacts on Treg cells, IL-2 also potentiates effector T cell proliferation and differentiation. This raises the questions of how IL-2 signaling is directed toward different cell types during the development and resolution of immune responses, and how this contributes to the pro- or anti-inflammatory functions of IL-2? In the steady-state, high-level expression of CD25 is limited to Treg cells, and thus these cells have a competitive advantage for responding to IL-2 (8, 26). However, activated T cells rapidly upregulate CD25, allowing them to compete with Treg cells for locally produced IL-2. Additionally, to facilitate IL-2 signaling effector T cells can form clusters characterized by multi-focal, LFA-1-dependent T cell-T cell ‘synapses’ that allow for direct delivery of IL-2 between the engaged cells (27). In addition to increasing the local concentration of IL-2, it is possible that this synaptic cross-talk between effector T cells may function to ‘hide’ IL-2 from nearby cTreg cells, thereby helping to potentiate effector cell responses. Thus, synaptic vs. non-synaptic signaling could help explain the dual pro- and anti-inflammatory nature of IL-2. In this scenario, IL-2 production following initial T cell activation would act primarily on Treg cells, enhancing their function and acting as a break on self-limiting responses. However, if responding T cells reach a critical density, T cell-T cell synapses can form, leading to direct exchange of IL-2 between effector T cells, thereby promoting their proliferation and function (Fig 1). Consistent with this model, cTreg cells can access IL-2 in vivo even without stimulation by their cognate antigen (and the resulting upregulation of LFA-1 expression and affinity), indicating that at least in the steady-state Treg cells are unlikely to be receiving IL-2 through synaptic signaling (8, 28). Additionally, IL-2 produced early during viral infection acts predominantly on Treg cells, and not on responding effector T cells (26). However, various aspects of the timing, extent and localization of IL-2 signaling in regulatory vs. effector T cells during the development of normal and dysregulated immune responses have not been carefully examined, and therefore the extent to which synaptic vs. non-synaptic production/signaling influence the pro- and anti-inflammatory functions of IL-2 are not clear.
Figure 1.
Model for inflammatory and non-inflammatory functions of IL-2. Weak activation of CD4+ effector T cells in non-inflammatory environments results in low-level ‘non-synaptic’ IL-2 secretion that promotes Treg cell function and blunts the response (Top). During strong activation in inflammatory conditions, this early inhibition is overcome, resulting in cell proliferation and formation of T-T synapses that facilitate paracrine IL-2 signaling between effector T cells and exclude Treg cells, promoting effector and memory T cell formation.
Treg cell migration, function and homeostasis in non-lymphoid tissues
In addition to the ability of cTreg cells to inhibit T cell priming in the secondary lymphoid tissues, eTreg cells are widely distributed in non-lymphoid tissues where they can modulate the activity of a variety of effector cell targets to dampen inflammatory responses and prevent collateral tissue damage and autoimmunity. Indeed, eTreg cells express patterns of adhesion and chemoattractant receptors expected to differentially target different eTreg cell subsets to specific non-lymphoid tissues or inflammatory sites, and highly express immunosuppressive molecules such as IL-10 that are important for maintaining tolerance in non-lymphoid tissues (10). Non-lymphoid tissues also contain populations of ‘memory’ Treg (mTreg) cells that can develop in response to transient expression or encounter with antigens, such as antigens contacted at barrier surfaces such as the skin, or paternal antigens recognized during allogeneic pregnancy, and are maintained in the absence of continued antigen recognition (29–31). In many cases, the importance of individual receptors for Treg cell migration to distinct tissue sites has been demonstrated in vivo, with corresponding impacts on Treg cell function and immune homeostasis in these sites (32). That Treg cells have a fundamental role in controlling autoimmune and inflammatory responses in a wide-range of non-lymphoid tissues has been well established and reviewed elsewhere (33), and therefore will only be discussed here in the context of specific tissues and inflammatory conditions that demonstrate some of the fundamental principles of Treg cell migration, function and homeostasis.
As barrier organs, the skin and the intestines present unique challenges to the immune system. Although both are portals of entry for pathogens, these must be identified amongst the large number of commensal micro-organisms present in these sites. Additionally, both the skin and intestines are exposed to harmless environmental and food-derived antigens that are contacted or ingested, and breakdown in the appropriate regulation of immune responses in these tissues leads to development of diseases such as psoriasis, atopic dermatitis, contact hypersensitivity, inflammatory bowel disease and food allergy. Given this immunological balancing act, it is not surprising that although Treg cells are widely distributed in non-lymphoid tissues, both the skin and intestine harbor large populations of specialized Treg cells. Moreover, cutaneous and intestinal Treg cells utilize many of the same molecules to access these sites as their effector cell counterparts. For instance, constitutive Treg cell migration to the skin requires the chemokine receptor CCR4 and surface expression of carbohydrate ligands for P- and E-selectin that are expressed by vascular endothelial cells in the skin, and consequently Treg cells lacking CCR4 or the endothelial selectin ligands fail to properly regulate cutaneous immune responses (6, 34, 35). Treg cell migration to the intestine is not as well characterized, though as with effector T cells it is dependent on expression of β7 family integrins (α4β7 or αeβ7) (36). Recently, however, the orphan G-protein coupled receptor GPR15 was identified as an essential factor selectively mediating regulatory T cell migration to the intestine (37), and consequently loss of GPR15 results in dysregulated intestinal immune responses.
Expression of cutaneous vs. intestinal homing receptor expression by Treg cells appears to be controlled by specific factors present in the immune environment of these organs and their associated lymphoid tissues. For instance, the intestine harbors a specialized population of CD103 (αE integrin)-positive dendritic cells that selective express the RALDH enzymes capable of converting dietary vitamin A into retinoic acid (RA) (38, 39). Signaling through cellular retinoic acid receptors (RAR), RA drives T cell expression of the intestinal homing receptors α4β7 integrin and CCR9 (40). Additionally, short-chain fatty acids (SCFA) such as butyrate produced by commensal bacteria can both promote pTreg cell differentiation and enhance the proliferation and accumulation of existing intestinal Treg cells via signaling through GPR43 (41–43). In contrast to RA and SCFA, inflammatory cytokines such as IL-6 that are produced following recognition of bacterial DNA via TLR9 inhibits pTreg cell differentiation and function in the intestine (44, 45). This highlights the concept that the abundance and activity of Treg cells within the intestine are controlled by a complex regulatory circuit that can monitor the microbiota in the intestine and respond by altering Treg cell differentiation, homeostasis, migration and function.
Although the development and homeostasis of cutaneous Treg cells is not as well characterized as that of intestinal Treg cells, many of the same concepts are thought to apply. The skin is home to a diverse microbial flora, and interaction with these organisms can directly influence the size and functionality of the cutaneous Treg cell pool as evidenced by the dramatically elevated numbers of Treg cells in the skin of germ-free mice that can dampen responses to cutaneous infection with Leishmania major (46). Moreover, cutaneous Treg cells accumulate near invaginations in the epithelium associated with hair-follicles that are specific points of interaction between the skin micro-flora and the cutaneous immune system (47). The signals that direct Treg cell expression of cutaneous homing receptors responsible for this positioning have not been precisely defined, however interaction with cutaneous DCs can promote expression of the fucosyltransferase IV and VII enzymes that generate ligands of P- and E-selectin (48).
In addition to the Treg cell populations constitutively found in the tissues such as the skin and intestines, eTreg cells are rapidly recruited to inflamed tissues where they can help resolve the inflammatory response. The ability of Treg cells to access inflamed tissues is due to the wide-array of receptors for inflammatory chemokines they express (5, 33). These include receptors directing cells to sites of Th1-, Th2- or Th17-mediated inflammatory responses such as CXCR3, CCR8 and CCR6, as well more general inflammatory receptors such as CCR2 and CCR5. In several cases, deficiency in individual receptors has had severe impacts on Treg cell function during inflammation (49). For instance, loss of CCR6 prevents Treg cell migration to inflamed joints in a model of rheumatoid arthritis (50, 51), whereas CCR5 broadly helps direct Treg cell localization in the contexts of infection, allograft rejection and inflammatory bowel disease (52–54). In addition to these chemokine receptors, Treg cells express surface integrins that influence their localization and function during inflammation. For instance, Treg cells express high levels of αLβ2 integrin (LFA-1) and α4β1 integrin (VLA-4), and together these can help direct Treg cell migration to inflamed tissues (55).
Interestingly, Treg cells in certain non-lymphoid tissues have proposed tissue support functions beyond their well-established roles in immune regulation. These include metabolic regulation by Treg cells in visceral adipose tissue (56), and regulation of tissue repair by Treg cells in skeletal muscle (57). Although, the signals responsible for the development, tissue-specific migration and maintenance of these resident Treg cell populations are still poorly characterized, Treg cells in adipose tissue depend on the transcription factors IRF4 and BATF, and the cytokine IL-33 (58).
Generally speaking, the homeostatic mechanisms that maintain Treg cells in non-lymphoid tissues are distinct from those supporting cTreg cells. Thus, rather than being IL-2-dependent eTreg cell abundance appears to be controlled largely by signals through the TCR and associated co-stimulatory receptors such as ICOS (8, 28, 59). Accordingly, deletion of the TCR in mature Treg cells or antibody-mediated blockade of ICOSL resulted in rapid decline of the eTreg cell population, whereas IL-2 blockade has little effect on the abundance or proliferation of these cells. The molecular mechanisms by which continued TCR and co-stimulatory receptor signaling support eTreg cell homeostasis are poorly understood, but may involve activation of pro-survival signaling pathways such as the PI3-kinase pathway that counteract the proapoptotic functions of Foxp3 (60), and maintenance of the eTreg cell transcriptional signature, which is at least partly dependent on the transcription factor Blimp-1 (28, 61). Additionally, new data indicate that IL-33 is an important homeostatic factor for eTreg cells in multiple tissue sites (58, 62, 63). In contrast to eTreg cells, mTreg cells (by definition) are maintained in non-lymphoid tissues in the absence of cognate antigen stimulation. However, at least in the skin, mTreg cell maintenance was also IL-2-independent, and instead relied on IL-7/IL-7R signaling (64). Additionally, new data indicate that IL-33 is an important homeostatic factor for eTreg cells in multiple tissue sites (58, 62, 63). Importantly, most eTreg and mTreg cells retain CD25 expression (albeit generally at lower levels than cTreg cells), and respond well to IL-2 in vitro and in vivo (8, 64). This strongly indicates that the lack of IL-2 signaling in these cells likely occurs as a result of their localization in tissues and environments that have a low T cell density and lack the cellular organization that facilitates paracrine IL-2 signaling between effector and regulatory T cells. This has important clinical implications, as it indicates that eTreg and mTreg cells would still be responsive to IL-2 based therapies for boosting Treg cell abundance.
Treg cell homeostasis and migration in disease
Because the outcome of a given immune response depends in part on the relative numbers and function of effector and regulatory cells, understanding Treg cell migration and homeostasis is important in the contexts of organ-specific autoimmune diseases, transplantation and cancer development. In each of these settings, therapeutically manipulating Treg cell function could be beneficial for inducing or restoring tolerance, or for promoting effective anti-tumor responses and successfully implementing new anti-cancer immunotherapies.
Paradoxically, due to the effective recruitment of Treg cells to sites of inflammation discussed above, the number and frequency of Treg cells is often dramatically elevated in inflammatory infiltrates associated with autoimmune disease in both mouse and human (65–67). However, the inability of these Treg cells to control disease indicates that they are insufficient to fully restore immune tolerance and ameliorate the inflammatory response. This inability to control disease indicates that these Treg cells are either quantitatively or qualitatively insufficient to fully restore immune tolerance and ameliorate the inflammatory response. Efforts to quantitatively restore Treg cells have focused on either expansion of existing Treg cells via IL-2, or re-infusion of ex vivo expanded Treg cells. A major hurdle to successful implementation of IL-2-based therapies lies in the fact discussed above that in addition to its effects on Treg cells, IL-2 is a potent trophic factor for pro-inflammatory effector T cells that upregulate CD25 upon activation. As a result, the effectiveness of IL-2 in treating disease in pre-clinical models of autoimmunity had varied greatly depending on the dose and timing of administration (68). Whereas low-doses of IL-2 given prior to or early in disease have proven effective, higher-doses of IL-2 given later have actually exacerbated or accelerated disease development (67, 69, 70). To circumvent this issue, IL-2 can be combined with immunosuppressive agents such as rapamycin that selectively inhibit effector T cell proliferation and function. However, although administration of IL-2 + rapamycin did boost Treg cells, this was generally not associated with clinical benefit but with a worsening on disease in a recent clinical trial in type-1 diabetes (71).
In addition to efforts to boost Treg cells via IL-2, several protocols have been developed for the isolation and ex vivo expansion of large numbers of both polyclonal and antigen-specific Treg cells for subsequent clinical use (72). However, the relatively limited size of the homeostatic Treg cell niches in vivo could limit the ability of these infused cells to achieve long-term engraftment and provide substantial clinical benefit. If this is the case, combining Treg cell infusion with IL-2 therapy to expand the Treg cell niche may prove to be more efficacious than either therapy alone. Additionally, since eTreg cell maintenance depends on continued TCR and costimulatory signals, maintenance of antigen-specific Treg cells would likely be far more efficient than that of polyclonal Treg cells. However, because Treg cell-derived cytokines such as TGF-β and IL-35 can promote regulatory function in other T cells (73, 74), Treg cell infusion may provide long-term clinical benefit even if the infused cells are present for only a short time via induction of ‘infectious tolerance’.
In the application of Treg cell-based cellular therapies, a second important factor to consider is the ability of the infused cells to migrate to the appropriate tissues after transfer. The complexity of Treg cell migration during suppression of unwanted immune responses is highlighted by a study demonstrating in an islet allograft model that suppression by transferred Treg cells required their choreographed and sequential migration from the blood into the inflamed graft and then to the draining lymph node, which depended on Treg cell expression of a CCR2, CCR4, CCR5, CCR7 and P-/E-selectin ligands (52). In cases where the signals that direct expression of specific homing receptors are known, these could be added to expansion cultures to tune the tissue tropism of the resulting cells. Thus, RA could be added to promote Treg cell migration to the intestines via α4β7 integrin and CCR9 (75), whereas IFN-γ or IL-27 could be used to direct Treg cell expression of CXCR3 and facilitate their localization to sites of Th1-mediated inflammation where its ligands CXCL9 and CXCL10 are abundantly produced (76, 77).
Qualitatively, Treg cell function can be compromised in inflamed tissues by cytokines such as IL-1, IL-6 or type-1 IFNs that either directly inhibit Treg cells or promote effector T cell resistance to suppression by Treg cells (45, 78–80). Additionally, the inflammatory environment can promote Treg cell instability and the differentiation of Foxp3− ‘ex-Treg’ cells, which due to their autoreactivity and production of effector cytokines may actually contribute to disease pathology and exacerbation (81). This represents a significant barrier to effective implementation of Treg cell-based immunotherapies, and suggests that combination therapies aimed at simultaneously inhibiting inflammatory cytokines and boosting Treg cell function will have the greatest chance of therapeutic efficacy in blocking unwanted immune responses (82).
In addition to positively promoting Treg migration and accumulation in inflamed tissues to dampen autoimmunity and prevent graft rejection, inhibition of Treg cells function in tumors is key to initiating robust anti-tumor responses and could help improve the efficacy of cellular tumor immunotherapy. That Treg cells actively inhibit effective anti-tumor immune responses is supported by the fact that Treg cell accumulation within some tumors is associated with poor clinical prognosis, and that depletion of Treg cells can promote tumor rejection in animal models (83). Additionally, new immunomodulatory therapies for cancer treatment such as pembrolizumab (anti-PD1) and ipilimumab (anti-CTLA4) target molecules prominently expressed by Treg cells, and may function in part by inhibition of Treg cells. However, therapies generally targeting Treg cells are likely to encounter side-effects related to loss of tolerance and development of autoimmune or inflammatory diseases, as has been observed with development of severe IBD in melanoma patients treated with anti-CTLA4 (84). Thus, specifically inhibiting Treg cell migration into the tumor as an adjunct to cellular immunotherapy may be advantageous. Indeed, the homing receptors used by Treg cells and effector CD8+ T cells to access some tumors may be distinct, thereby providing the opportunity to selectively disrupt Treg cell migration and boost anti-tumor immunity. Specifically, Treg cell infiltration into several tumor types appears to be dependent on CCR4 (85–87), whose ligands CCL17 and/or CCL22 can be produced by tumor cells themselves or by tumor-associated macrophages. Importantly, CCR4 is generally not highly expressed or utilized by effector CD8+ T cells, suggesting that targeting CCR4 may selectively inhibit Treg cell function in certain tumors, thereby boosting anti-tumor immune responses (87, 88). Similarly, inhibiting CCR10-mediated recruitment of Treg cells to tumors could be used to augment anti-tumor immunity (89).
Concluding remarks
Due to the potent impact Treg cells have on development of immunity vs. tolerance, the manipulation of Treg cell activity has tremendous therapeutic potential. Although much progress has been made, realizing this potential still requires a better understanding of the basic mechanisms of Treg cell biology. As Treg cell activity in a given tissue site is a function of their migration to that tissue, the abundance of homeostatic factors that govern their proliferation and survival, and the presence of cytokines and other factors that promote or inhibit their function , better understanding each of these processes will not only allow for the manipulation of endogenous Treg cells, but also improve the prospects of Treg cell-based cellular therapies.
Footnotes
The author's lab has been supported in part by grants AR055695, DK072295, HL098067 and AI067750 from the NIH.
References
- 1.Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 2014;14:343–349. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh C-S, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl SM. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huehn J, Siegmund K, Lehmann JCU, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Bräuer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J. Immunol. Baltim. Md 1950. 2007;178:301–311. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 12.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T Cells Reversibly Suppress Cytotoxic T Cell Function Independent of Effector Differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Matheu MP, Othy S, Greenberg ML, Dong TX, Schuijs M, Deswarte K, Hammad H, Lambrecht BN, Parker I, Cahalan MD. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nat. Commun. 2015;6 doi: 10.1038/ncomms7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 17.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LSK, Sansom DM. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell Extrinsic Function of CTLA-4. Science. 2011 doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre M-L, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 20.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amado IF, Berges J, Luther RJ, Mailhé M-P, Garcia S, Bandeira A, Weaver C, Liston A, Freitas AA. IL-2 coordinates IL-2–producing and regulatory T cell interplay. J. Exp. Med. 2013;210:2707–2720. doi: 10.1084/jem.20122759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. Baltim. Md 1950. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long M, Adler AJ. Cutting Edge: Paracrine, but Not Autocrine, IL-2 Signaling Is Sustained during Early Antiviral CD4 T Cell Response. J. Immunol. 2006;177:4257–4261. doi: 10.4049/jimmunol.177.7.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ, Krummel MF. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow Z, Banerjee A, Hickey MJ. Controlling the fire — tissue-specific mechanisms of effector regulatory T-cell homing. Immunol. Cell Biol. 2015 doi: 10.1038/icb.2014.117. [DOI] [PubMed] [Google Scholar]
- 33.Gratz IK, Campbell DJ. Organ-specific and memory Treg cells: specificity, development, function, and maintenance. Immunol. Mem. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. J. Exp. Med. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schön MP, Scheffold A, Lowe JB, Hamann A, Syrbe U, Huehn J. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J. Immunol. Baltim. Md 1950. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 37.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, Sarpel U, Rifkin DB, Xu R, Littman DR. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science. 2013;340:1456–1459. doi: 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg P-L, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 44.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 46.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH-Y, Anthony BA, Sverdrup FM, Krow-Lucal E, Mackenzie TC, Johnson DS, Meyer EH, Löhr A, Hsu A, Koo J, Liao W, Gupta R, Debbaneh MG, Butler D, Huynh M, Levin EC, Leon A, Hoffman WY, McGrath MH, Alvarado MD, Ludwig CH, Truong H-A, Maurano MM, Gratz IK, Abbas AK, Rosenblum MD. Memory regulatory T cells reside in human skin. J. Clin. Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 Regulates the Migration of Inflammatory and Regulatory T Cells. J. Immunol. Baltim. Md 1950. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang SG, Piniecki RJ, Hogenesch H, Lim HW, Wiebke E, Braun SE, Matsumoto S, Kim CH. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966–981. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Glatigny S, Duhen R, Arbelaez C, Kumari S, Bettelli E. Integrin alpha L controls the homing of regulatory T cells during CNS autoimmunity in the absence of integrin alpha 4. Sci. Rep. 2015;5 doi: 10.1038/srep07834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 59.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, Kroczek RA, Hutloff A. ICOS controls the pool size of effector-memory and regulatory T cells. J.Immunol. 2008;180:774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 60.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, Singer A. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 62.Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, Turnquist HR. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J. Immunol. Baltim. Md 1950. 2014;193:4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Löhning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gratz IK, Truong H-A, Yang SH-Y, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: Memory Regulatory T Cells Require IL-7 and Not IL-2 for Their Maintenance in Peripheral Tissues. J. Immunol. Baltim. Md 1950. 2013 doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J. Immunol. Baltim. Md 1950. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 66.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central Role of Defective Interleukin-2 Production in the Triggering of Islet Autoimmune Destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayer AL, Pugliese A, Malek TR. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol. Res. 2013;57:197–209. doi: 10.1007/s12026-013-8452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wesley JD, Sather BD, Perdue NR, Ziegler SF, Campbell DJ. Cellular Requirements for Diabetes Induction in DO11.10xRIPmOVA Mice. J. Immunol. 2010;185:4760–4768. doi: 10.4049/jimmunol.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long SA, Buckner JH, Greenbaum CJ. IL-2 therapy in type 1 diabetes: “Trials” and tribulations. Clin. Immunol. Orlando Fla. 2013;149:324–331. doi: 10.1016/j.clim.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 2013;3 doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni H-T, Artis D, Turk MJ, Vignali DAA. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J. Exp. Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 76.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno EDT, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. The Cytokines Interleukin 27 and Interferon-γ Promote Distinct Treg Cell Populations Required to Limit Infection-Induced Pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 2014;211:961–974. doi: 10.1084/jem.20131556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srivastava S, Koch LK, Campbell DJ. IFNαR signaling in effector but not regulatory T cells is required for immune dysregulation during type I IFN-dependent inflammatory disease. J. Immunol. Baltim. Md 1950. 2014;193:2733–2742. doi: 10.4049/jimmunol.1401039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M, Buckner JH. In Active Relapsing-Remitting Multiple Sclerosis, Effector T Cell Resistance to Adaptive Tregs Involves IL-6–Mediated Signaling. Sci. Transl. Med. 2013;5:170ra15–170ra15. doi: 10.1126/scitranslmed.3004970. [DOI] [PubMed] [Google Scholar]
- 81.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-Driven Activation Induces Instability of Regulatory T Cells during an Inflammatory Autoimmune Response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long SA, Buckner JH. CD4+FOXP3+ T Regulatory Cells in Human Autoimmunity: More Than a Numbers Game. J. Immunol. 2011;187:2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014;27C:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 86.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, Durand I, Léon-Goddard S, Blay JY, Caux C, Ménétrier-Caux C. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71:6143–6152. doi: 10.1158/0008-5472.CAN-11-0573. [DOI] [PubMed] [Google Scholar]
- 87.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, Karbach J, Jäger E, Sakaguchi S. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, Batteux F, Sandoval F, Adotevi O, Chiu C, Garcia S, Tanchot C, Lone Y-C, Ferreira LC, Nelson BH, Hanahan D, Fridman WH, Johannes L, Tartour E. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood. 2011;118:4853–4862. doi: 10.1182/blood-2011-01-329656. [DOI] [PubMed] [Google Scholar]
- 89.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]