Abstract
Cannabis use disorder (CUD) occurs in up to 42% of patients with schizophrenia and substantially worsens disease progression. The basis of CUD in schizophrenia is unclear and available treatments are rarely successful at limiting cannabis use. We have proposed that a dysregulated brain reward circuit (BRC) may underpin cannabis use in these patients. In the present pilot study, we used whole-brain seed-to-voxel resting state functional connectivity (rs-fc) to examine the BRC of patients with schizophrenia and CUD, and to explore the effects of smoked cannabis and orally administered delta-9-tetrahydrocannabinol (THC) on the BRC. 12 patients with schizophrenia and CUD and 12 control subjects each completed two fMRI resting scans, with patients administered either a 3.6% THC cannabis cigarette (n=6) or a 15mg THC capsule (n=6) prior to their second scan. Results revealed significantly reduced connectivity at baseline in patients relative to controls, with most pronounced hypoconnectivity found between the nucleus accumbens and prefrontal cortical BRC regions (i.e., anterior prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex). Both cannabis and THC administration increased connectivity between these regions, in direct correlation with increases in plasma THC levels. This study is the first to investigate interregional connectivity of the BRC and the effects of cannabis and THC on this circuit in patients with schizophrenia and CUD. The findings from this pilot study support the use of rs-fc as a means of measuring the integrity of the BRC and the effects of pharmacologic agents acting on this circuit in patients with schizophrenia and CUD.
Keywords: schizophrenia, cannabis use disorder, brain reward circuitry, nucleus accumbens, fMRI, resting state functional connectivity
1. Introduction
Cannabis use disorder (CUD) occurs in up to 42% of patients with schizophrenia (SCZ), a rate nearly ten times greater than in the general population (Green et al., 2008; Henquet et al., 2005). Patients with SCZ and co-occurring CUD have a significantly worse prognosis than those with SCZ alone (Buckley et al., 2009; Swendsen et al., 2011); their cannabis use leads to symptom exacerbation (Buckley et al., 2009), an increased likelihood of psychotic relapse, and an overall poorer global functioning (Henquet et al., 2010; Hides et al., 2006; Swendsen et al., 2011; Zammit et al., 2008).
Despite the elevated prevalence and deleterious consequences of CUD in SCZ, there are no safe and effective therapies available for limiting their cannabis use. Neither psychosocial treatment programs nor most of the antipsychotic medications appear to decrease their cannabis use (Hjorthoj et al., 2009). While clozapine has shown some ability to decrease cannabis use in SCZ, (Drake et al., 2000; Green et al., 2003; Zimmet et al., 2000), it is used infrequently because of its side effect profile.
We and others have suggested that a dysfunctional brain reward circuitry (BRC) underpins cannabis (and other substance) use in SCZ, and that use of cannabis by these patients may serve to transiently improve functioning of this brain circuit (Chambers et al., 2001; Green et al., 1999; Krystal et al., 2006). Dysfunction of the BRC has been implicated in a number of behavioral (Heerey et al., 2007; Moberg et al., 2003), and task-based neuroimaging studies (Juckel et al., 2006; Nielsen et al., 2012) investigating patients with SCZ.
Resting state functional connectivity (rs-fc) is a neuroimaging method that detects functionally related patterns of neuronal activity, and is increasingly used to delineate the intrinsic architecture of functional brain networks (Fox and Raichle, 2007; Raichle, 2011; Whitfield-Gabrieli and Nieto-Castanon, 2012). To date, few studies have reported on rs-fc of the BRC in healthy or clinical samples. Di Martino and colleagues (Di Martino et al., 2008) examined cortical-striatal circuits, including a ‘motivational circuit’ comprised of the NAc, anterior cingulate cortex (ACC) and lateral orbitofrontal cortex in healthy adults. Their findings were consistent with both anatomical white matter projections in non-human primates as well as a meta-analysis of task based fMRI studies of these BRC subregions (Di Martino et al., 2008). Moreover, Cauda and colleagues (2011) examined rs-fc of the NAc in healthy adults, and found significant connectivity with dorsal striatum, insula, amygdala, hippocampus, ACC and orbitomedial prefrontal cortex.
In this pilot study, we investigated BRC connectivity in patients with SCZ and co-occurring CUD as compared to healthy controls. We predicted that decreased connectivity within BRC regions would be found in patients relative to controls. We also assessed the effects of smoked cannabis and oral THC (delta-9-tetracannabinol, the primary psychoactive ingredient in cannabis) on BRC connectivity in these patients. In line with our theory that BRC dysfunction underpins cannabis use in patients with SCZ (Chambers et al., 2001; Green et al., 2008; Green et al., 1999), we anticipated that both smoked cannabis and oral THC would improve functional connectivity within the BRC.
2. Methods
2.1 Subjects
Twelve patients with SCZ and CUD and twelve healthy control subjects participated in the study. All patients met diagnostic criteria for SCZ and a current diagnosis of CUD as determined by the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 2002). In order to participate in the study, patients were required to meet criteria for cannabis abuse and/or dependence with recent use of cannabis (within the past month based upon the Timeline Follow-Back interview). While a history of alcohol or illicit substance use disorder (other than cannabis) was not an exclusion criterion for patient participants, they were required to be substance free for at least 7 days prior to their participation in the study. Since up to 90% of patients with schizophrenia smoke cigarettes (Kalman et al., 2005), current smokers were included in the patient group. Patients were required to be on a stable dose of antipsychotic medication for a minimum of one month prior to enrollment, and were maintained on the same dose for the duration of study participation. Patients taking clozapine were excluded, given its apparent ability to decrease alcohol and cannabis use in patients with SCZ (Green et al., 2008). The control group was matched to the patient sample on age, gender and handedness. A SCID was administered to control subjects in order to ensure that they did not have a history of an Axis I disorder, including any substance use disorder. Pharmacological treatment for addiction (disulfiram, naltrexone, acamprosate, topiramate or varenicline), mental retardation, history of head injury or factors that contraindicate the use of fMRI served as exclusion criteria for all subjects. The protocol was approved by the Committee for the Protection of Human Subjects (IRB) at Dartmouth College, and informed consent was obtained from all subjects prior to initiation of the study.
2.2 Procedures
Subjects completed an initial screening session in which SCID and Timeline Follow-Back (TLFB) assessments for substance use (Sobell et al, 1996) were performed. If they met inclusion criteria, they were scheduled for two scan sessions (T1 and T2), and were instructed to remain abstinent from cannabis, alcohol and other substance use (except tobacco or caffeine) for the seven day period prior to each session. To facilitate abstinence, patients came to the clinic three times in the week prior to each scan session where abstinence was reinforced with contingency management techniques.
At the baseline scan session (T1), all patients were assessed with the TFLB for self-report of substance, nicotine and caffeine use (Sobell et al., 1996), and underwent urine/breath tests for substances. If continued abstinence of substances was confirmed, T1 scanning was performed. All female subjects underwent a pregnancy test prior to scanning. Patients who were tobacco smokers were asked to smoke a cigarette 90 minutes prior to scanning. This timeframe was based upon pharmacokinetics of smoked tobacco – in order to avoid the effects of either nicotine intoxication or nicotine withdrawal at the time of scanning (Benowitz et al., 2009). Prior to the scan, each subject had an intravenous line inserted (to mimic the procedure for patients with SCZ and co-occurring CUD at T2), and venous blood was obtained from all patients to measure serum THC and THCC. During the 8-minute resting scan, subjects were told to think of nothing in particular, to keep their eyes open, and to stay awake.
The T2 scanning session, performed at least 7 days after T1, with continued abstinence, was identical to T1, except for an intervention component: patients were randomized into either a THC or cannabis group. Those in the THC group were asked to swallow a 15 mg THC capsule 3 hours prior to scanning, and to smoke a placebo cannabis cigarette immediately prior to scanning; those in the cannabis group were given a placebo THC capsule (3 hours prior to scanning) and smoked an active 3.6% THC cannabis cigarette immediately prior to scanning. The timing of oral THC administration and cannabis smoking was designed such that subjects in both groups would have similar THC levels during the T2 scan (Grant et al., 2012; Huestis, 2005). Smoking of both placebo and active cannabis cigarettes was conducted using a custom made smoking apparatus, and followed the Foltin method (Foltin et al., 1987). Cannabis (and placebo) cigarettes were obtained from the National Institute on Drug Abuse; the THC capsule (dronabinol) and matching placebo capsules were provided by the Dartmouth Hitchcock Medical Center Pharmacy. Control subjects underwent an identical procedure at both T1 and T2, except they did not receive any pharmacologic intervention and did not have blood taken during the T2 session.
2.3 Resting State fMRI Acquisition and Analysis
Resting state fMRI data were acquired using a 3T Phillips Achieva fMRI scanner with an 8 channel head coil. The resting state data were acquired transverse to the AC-PC plane, with a T2*-weighted single shot echo planar imaging (EPI) pulse sequence designed to measure whole brain BOLD contrast with optimal temporal and spatial resolution [repetition time (TR) = 2000ms; echo time (TE) = 30ms; Flip angle (FA) = 90 degrees; field of view (FOV) = 240 mm; Slice thickness=2.5 mm; Slice skip 0.5; Slice location = Pat. Spec; Fat saturation=SPIR; Reps=240; NEX=1; yielding 36 contiguous transverse functional images in an 80×80 matrix with an isotropic resolution of 3.0 mm3]. T1-weighted anatomic reference images were acquired in the same planes and thickness immediately following the resting scans.
Data were analyzed using a seed driven approach with in-house, custom software (Whitfield-Gabrieli and Nieto-Castanon, 2012), using methods that minimize the influence of motion artifact and allow for valid identification of correlated networks (Behzadi et al., 2007; Chai et al., 2012; Whitfield-Gabrieli and Nieto-Castanon, 2012). Data were slice time corrected, realigned, coregistered, normalized and spatially smoothed with a 6-mm kernel. To address the spurious correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART (http://www.nitrc.org/projects/artifact_detect). An image was defined as an outlier image if the head displacement in x, y, or z direction was greater than .5mm from the previous frame, or if the global mean intensity was greater than 3 standard deviations from the mean image intensity for the entire resting scan. Outliers were included as regressors in the first level general linear model along with motion parameters. Physiological and other spurious noise sources were estimated using the aCompcor method (Behzadi et al., 2007). The residual BOLD time-series was band-pass filtered over a low-frequency window of interest (0.009Hz<f<0.08Hz). Correlation maps were produced by extracting the residual BOLD time course from bilateral NAc seeds which were anatomically defined using 6 mm spheres around peak coordinates (Figure 1) (Di Martino et al., 2008; Juckel et al., 2006). Pearson’s correlation coefficients were then calculated between the NAc time course and the time course of all other voxels in the brain. Correlation coefficients were converted to normally distributed scores using Fisher’s transformation to allow for second-level General Linear Model analyses. Second-level within-group (one sample t-tests) and between group (ANOVA) analyses were performed on the average Z-maps from NAc source ROIs. Using the Conn toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), we performed a paired t-test to examine the change of resting state connectivity for time 2 (T2) versus time 1 (T1) for the patients, and then performed a correlation analysis between BRC connectivity (at T2) and the change in THC plasma levels, as well as the T2 THC plasma level itself.
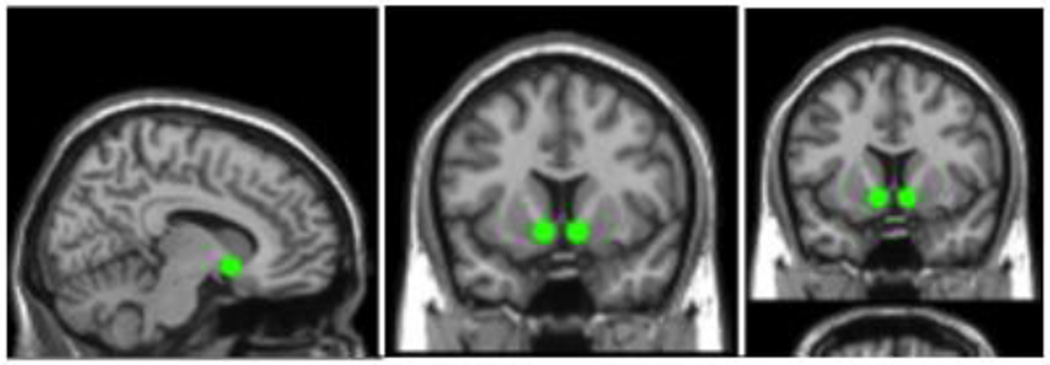
Figure 1.
Bilateral anatomically defined 6mm source ROIs used in the rs-fc analysis: sagittal; coronal; and axial slices are shown. MNI coordinates of the left NAc: −11, 9, −8; and for the right NAc: 8, 9, −8. Brain images are neurologically oriented.
2.4 T2 Plasma THC, Physiological, and Symptom Measures
Venous blood draws were obtained from patients for the assessment of THC prior to administration of the THC capsule (‘baseline’), as well as just prior to and after the resting scan. Heart rate and blood pressure were measured at regular intervals during T2 to ensure safety. At three time points, the Marijuana Craving Questionnaire (MCQ) (Heishman et al., 2001), Cannabis Withdrawal Scale (CWS) (Budney et al., 1999), and Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) were administered. Change in these measures was analyzed using repeated measures analysis of variance (ANOVA) with group as the between group factor and time as the within-subjects factor followed by post-hoc analyses using Tukey’s LSD test for pairwise comparisons.
3. Results
3.1 Demographic Data
Table 1 summarizes study participants’ demographic data. There was no significant group difference with respect to age (t (21) = 0.87, p =0.39), mean parental education (t (19) = 0.16, p = 0.87), gender (x2 (1) = 0.1, p = 0.75), handedness (x2 (1) = 0.1, p = 0.75) or ethnicity. All patients were taking a stable dose of one of the following antipsychotic medications: aripiprazole (n=2), haloperidol (n=1), olanzapine (n=1), paliperidone (n=2), paliperidone palmitate (n=2), or risperidone (n=2). The mean chlorpromazine equivalent was 300 ± 261 mg/day, with no significant difference in dose between the cannabis and THC groups (p = 0.1). All patients remained on the same medications, with no changes in antipsychotic dose between the T1 and the T2 scan.
Table 1.
Participant Demographic Information
| Characteristic | Healthy Controls (N=12) |
Cannabis (N= 6) |
THC (N=6) |
|---|---|---|---|
| *Age (years) | 33.5 ± 7.8 | 36.2 ± 9.60 | 32.17 ± 8.32 |
| Education (years) | 16.1 ± 1.7 | 9.6 ± 1.50 | 11.83 ± 1.34 |
| *Mean parental education | 14.2 ± 2.9 | 13 ± 1.41 | 12.83 ± 2.19 |
| *Gender | 3 F, 9 M | 1 F, 5 M | 2F, 2M |
| *Handedness | 1 L, 11 R | 1 L, 5 R | 0L, 6R |
| *Ethnicity (n): Caucasian | 12 | 6 | 6 |
No statistically significant between group difference.
Abbreviations: F, female; M, male; L, left; R, right. Data are reported as mean ± standard deviation where applicable.
3.2 Substance Use in Patient Groups
All patients met criteria for current diagnosis of cannabis use disorder, which included both cannabis dependence (n=10) or cannabis abuse (n=2). Patients smoked an average of 1.8 ± 1.2 cannabis joints per week prior to enrollment in the study. All except one patient were daily tobacco users meeting criteria for dependence. They smoked an average of 18.15 ± 10.7 cigarettes per day, with no difference noted in number of cigarettes per day between the patient subgroups (THC = 15 ± 10.8; cannabis = 19.3± 7.5; p = 0.46). Two-thirds (9/12) of the patients had a lifetime history of other substance use disorder per the SCID, and were counterbalanced into cannabis and THC interaction subgroups. Seven subjects met criteria for alcohol dependence, in remission, and one patient met criteria for current alcohol abuse; in addition, one patient had a past history of cocaine dependence and another methamphetamine dependence, although both were in full remission. All patients refrained from any substance use (except nicotine and caffeine) for at least 7 days prior to the initial scanning session.
3.3 Plasma THC, Physiological, and Symptom Measures
THC measures at T1 in the cannabis (18.2 ± 16.1) and THC (17.25 ± 14.4) were slightly higher (not statistically significant) when compared to baseline THC measures at T2 in the cannabis (17.2 ± 19.7) and THC (14.7 ± 15.0) groups prior to the intervention, suggesting that patients had remained abstinent in the interval between scans at T1 and T2. Moreover, THCC levels were 11.3 ± 11.62 in the cannabis group and 9.2 ± 8.16 in the THC group at baseline T1 and 9.2 ± 8.16 in the cannabis group and 10.2 ± 9.0 in the THC group at baseline T2. (Note: The T2 scan of one of the patients within the cannabis group was excluded from the data analysis because of an elevated plasma THC level prior to intervention, indicating recent use of cannabis.)
Following intervention, at T2, the THC plasma levels increased to 44.8 ± 12.2 ng/ml and 43.4 ± 23.1 ng/ml (for the cannabis smoking and THC capsule groups, respectively) immediately prior to resting scan acquisition. There was no significant effect of intervention on blood pressure at T2, although there was a trend for an increase in heart rate ((F (5, 90) =2.13, p = 0.07).
There were no significant differences in craving, withdrawal symptoms or symptom severity at T2 (prior to the intervention) as compared to T1. Moreover, there were also no significant changes in symptoms following either the cannabis or THC intervention.
3.4 Quality Assurance
Using the art tool, we found, no statistically significant difference (p = 0.37) between the total number of outliers in motion and global signal intensity in patients with SCZ and CUD (5.39 ± 7.8) as compared to healthy control subjects (4.6 ± 3.5). Within-group comparison across sessions also found no significant difference in number of outliers (p=0.42).
3.5 T1 Functional Connectivity
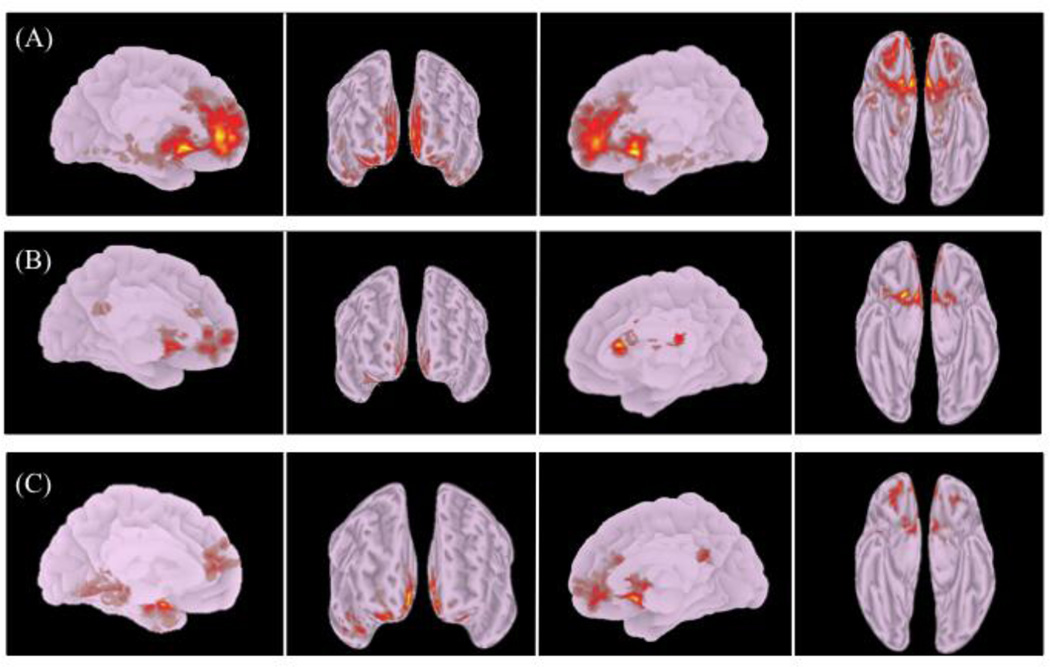
Within-group whole brain seed-to-voxel analysis using an averaged bilateral NAc source ROI and a false discovery rate (FDR) correction for multiple comparisons of p<0.05 revealed significant connectivity between the NAc and reward related cortical-regions in both patients and healthy controls. These regions included the ventral anterior cingulate cortex (vACC), orbitofrontal cortex (OFC), anterior prefrontal cortex (aPFC), parahippocampal cortex, entorhinal cortex and insular cortex. Significant connectivity was also detected between the NAc and regions involved in selection of behavioral output including the premotor cortex and inferior frontal gyrus. Figure 2 shows connectivity results for healthy comparison subjects (2a), as well as for patients with SCZ and co-occurring CUD (2b).
Figure 2.
Brain regions showing significant (p<0.05, FDR corrected) rs-fc to bilateral NAc source ROIs in (a) healthy control subjects and (b) patients with SCZ and CUD. (c) Between group contrast of controls > patients. Left medial, frontal, right medial, ventral views are depicted (left to right). Brain images are neurologically oriented.
Between group comparison revealed significantly greater connectivity (p<0.05, FDR corrected) between the NAc and the vACC, OFC, aPFC, dorsolateral prefrontal cortex, insular cortex, premotor cortex and the parahippocampal cortex in control subjects as compared to patients (Table 2). A cluster comprised of the secondary visual cortex (BA18) showed greater connectivity (cluster p <0.05, FDR-corrected) with the NAc in the patient group as compared to control subjects (not shown).
Table 2.
Between-Group Bilateral NAc Seed-to-Voxel Rs-fc Results (T1).
| X | Y | Z | BA | L/R | K | p-FDR | |
|---|---|---|---|---|---|---|---|
| Cluster 1 | −14 | 14 | 26 | - | - | 4110 | <0.01 |
| Ventral Anterior Cingulate Cortex | −2 | 40 | 16 | 32 | L | 447 | |
| Ventral Anterior Cingulate Cortex | 14 | 34 | 24 | 32 | R | 343 | |
| Orbitofrontal Cortex | −10 | 56 | 24 | 11 | L | 336 | |
| Anterior Cingulate Cortex | −4 | 0 | 50 | 24 | L | 332 | |
| Anterior Cingulate Cortex | 3 | −22 | 38 | 24 | R | 238 | |
| Anterior Prefrontal Cortex | 12 | 48 | 10 | 10 | R | 223 | |
| Anterior Prefrontal Cortex | −8 | 68 | 10 | 10 | L | 110 | |
| Orbitofrontal Cortex | 18 | 48 | −22 | 11 | R | 98 | |
| Dosolateral Prefrontal Cortex | −28 | 30 | 36 | 9 | L | 227 | |
| Subgenual Cortex | −2 | 16 | −22 | 25 | L | 67 | |
| Cluster 2 | 44 | 0 | −40 | - | - | 1071 | <0.01 |
| Parahippocampal Cortex | 20 | 6 | −24 | 34 | R | 150 | |
| Entorrhinal Cortex | 34 | −12 | −20 | 36 | R | 92 | |
| Cluster 3 | −22 | −30 | 18 | - | - | 909 | 0.014 |
| Parahippocampal Cortex | −30 | −29 | −22 | 36 | L | 112 | |
| Insular Cortex | 34 | −24 | 16 | 13 | R | 92 | |
| Insular Cortex | −34 | 10 | −10 | 13 | L | 76 | |
| Cluster 4 | −44 | −2 | 50 | - | - | 430 | 0.037 |
| Premotor Cortex | −42 | −2 | 48 | 6 | L | 288 | |
| Primary Motor Cortex | −16 | −36 | 68 | 4 | L | 52 |
Significant clusters surrounding peak MNI (x, y, z) coordinates are listed, as are the cortical components detected within each cluster. All reported values reached cluster extent threshold FDR-corrected significance of p<0.05. K values correspond to spatial extent (i.e., number voxels). L/R indicates laterality of findings.
3.6 Effect of Cannabis and THC (T2)
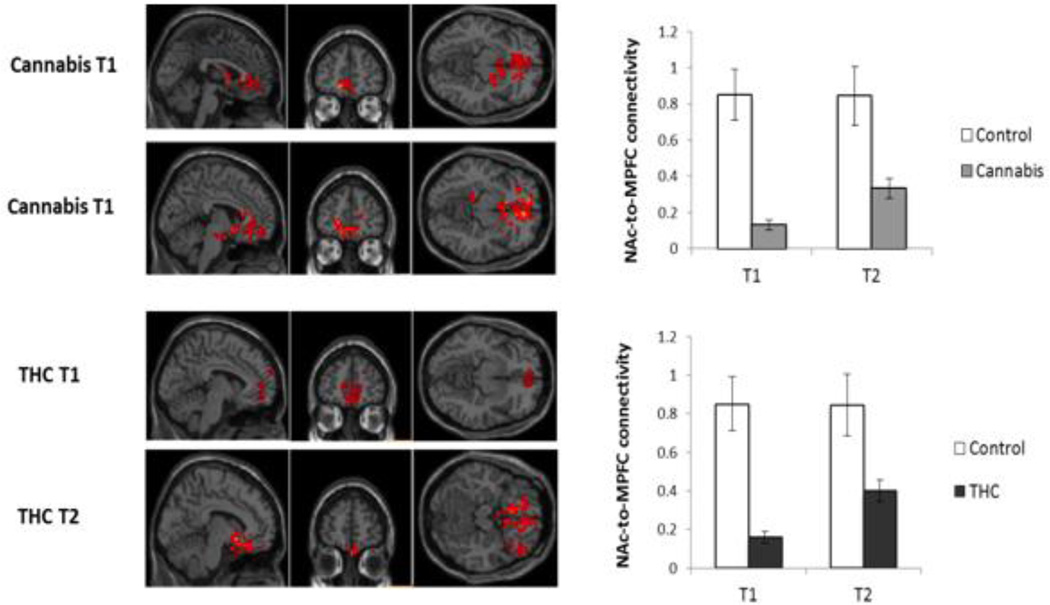
Subsequent to both cannabis and THC administration, significant (p<0.05, FDR-corrected) improvement in BRC functional connectivity was found. Smoked cannabis increased connectivity between the bilateral NAc seed and the aPFC, OFC, and vACC, cortical regions that were hypoconnected with the NAc relative to control subjects at T1. Following THC administration, increases in connectivity were detected between the NAc and cortical components of the BRC. As shown in Figure 3, hypoconnectivity between the NAc and medial PFC regions of the BRC (i.e., vACC/OFC cluster) was less prominent after cannabis and THC administration. Moreover, BRC functional connectivity subsequent to cannabis and THC intervention (in a combined sample, N=11) directly correlated with elevation in plasma THC (Table 3, Figure 4), as well as with the level of THC at T2. Although there was some variability among the patients with respect to their response to the THC and smoked cannabis, as reflected in their ratings of high, liking and craving, there were no significant correlations between these ratings and measures of connectivity.
Figure 3.
Cannabis and THC induced increases in functional connectivity between the NAc and medial prefrontal regions. At baseline, functional connectivity between the NAc and a cluster comprised of the vACC and OFC was significantly reduced in patients with SCZ and CUD as compared to the control group. Subsequent to both cannabis and THC administration, functional connectivity between these regions of the BRC significantly increased, though connectivity remained decreased relative to controls. Error bars indicate standard deviation in Z score measures of NAc-to-MPFC connectivity at T1 and T2 in both patient groups. Functional connectivity values are depicted as z scores. Brain images are neurologically oriented.
Table 3.
NAc Rs-fc Correlation with Increases in Plasma [THC] at T2
| X | Y | Z | BA | L/R | K | p-FDR | |
|---|---|---|---|---|---|---|---|
| Cluster 1 | 6 | 16 | −10 | − | − | 556 | <0.01 |
| Ventral Anterior Cingulate Cortex | −6 | 38 | 28 | 32 | L | 151 | |
| Orbitofrontal Cortex | −2 | 44 | −24 | 11 | L | 132 | |
| Anterior Cingulate Cortex | −6 | 22 | 30 | 24 | L | 88 | |
| Dorsolateral Prefrontal Cortex | 50 | 41 | 28 | 46 | R | 149 | |
| Cluster 2 | −4 | 68 | 10 | − | − | 477 | |
| Anterior Prefrontal Cortex | −28 | 60 | 24 | 10 | L | 255 | |
| Dorsolateral Prefrontal Cortex | −46 | 38 | 28 | 9 | L | 36 | |
| Anterior Prefrontal Cortex | 40 | 54 | 22 | 10 | R | 26 | |
| Cluster 3 | 18 | −20 | 34 | − | − | 395 | <0.01 |
| Ventral Anterior Cingulate Cortex | 14 | 30 | 28 | 32 | R | 154 | |
| Insular Cortex | 46 | 10 | 8 | 13 | R | 93 | |
| Insular Cortex | −34 | 10 | 10 | 13 | L | 77 | |
| Dorsal Posterior Cingulate Cortex | 10 | −498 | 24 | 31 | R | 53 | |
| Cluster 3 | −42 | −76 | 26 | -- | -- | 354 | 0.02 |
| Associative Visual Cortex | −46 | −74 | 32 | 19 | L | 94 | |
| Angular Gyrus | −44 | −78 | 28 | 39 | L | 86 | |
| Angular Gyrus | 46 | −60 | 24 | 39 | R | 81 | |
| Associative Visual Cortex | 48 | −66 | 22 | 19 | R | 32 | |
| Cluster 5 | −14 | −24 | −20 | − | − | 322 | 0.03 |
| Parahippocampal Cortex | −14 | −6 | 22 | 36 | L | 90 | |
| Parahippocampal Cortex | 20 | 8 | −24 | 36 | R | 83 | |
| Cingulate Cortex | −12 | 30 | 29 | 30 | L | 64 | |
| Perirhinal Cortex | −19 | −12 | −29 | 35 | L | 26 | |
| Perirhinal Cortex | 24 | −11 | −27 | 35 | R | 25 |
Significant clusters surrounding peak MNI (x, y, z) coordinates are listed, as are the cortical components detected within each cluster. All reported values reached cluster extent threshold FDR-corrected significance of p<0.05. K values correspond to spatial extent (i.e., number voxels). L/R indicates laterality of findings. Data shown are for combined patient sample at T2 (N=11) following either cannabis or THC administration.
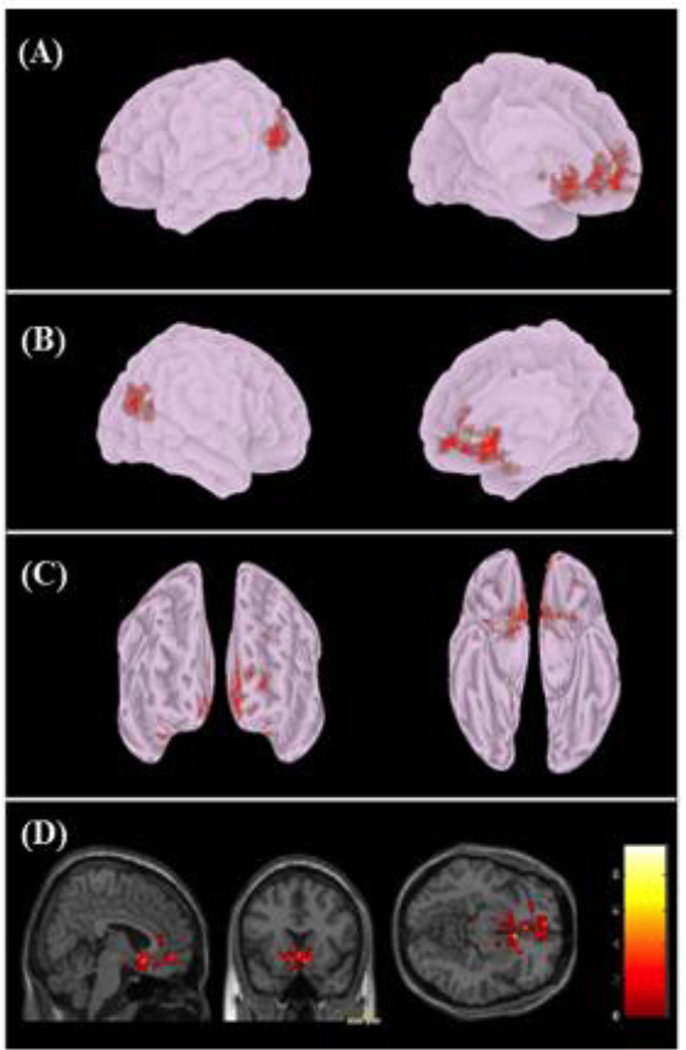
Figure 4.
Correlation between plasma THC and NAc rs-fc. BRC connectivity following cannabis and THC administration at T2 show a direct correlation with increases in plasma THC. (a) Left lateral and medial, (b) Right lateral and medial, (c) rostral and ventral views are shown as well as (d) bilateral NAc cluster (Peak MNI coordinates 6,16, −10) showing greatest positive correlation with plasma THC levels. Brain images are neurologically oriented.
4. Discussion
To our knowledge, this pilot study is the first to use resting-state functional connectivity analysis to assess the integrity of connectivity within the brain reward circuit (BRC) in patients with schizophrenia and cooccurring CUD, and to explore the effects of smoked cannabis and oral THC on this circuit. By assessing regions functionally correlated with the nucleus accumbens (NAc), our analysis revealed impairment in connectivity with prefrontal cortical regions involved in reward processing in patients relative to controls. Interestingly, the patient group also had decreased connectivity between the NAc and the dorsolateral PFC, the source of projections involved in exerting “top-down” modulation (executive control) over the NAc and other subcortical BRC regions (40).
Both smoked cannabis and oral THC significantly improved connectivity between the NAc and prefrontal BRC components (vACC, OFC and PFC) that were hypo-connected in patients relative to the controls. The observed increases in connectivity induced by cannabis and THC were detected between BRC regions with high CB1 receptor density (Gardner, 2005; Oleson and Cheer, 2012). Importantly, no significant change in heart rate, blood pressure, or symptom severity followed the intervention. Moreover, comparing measurements obtained at T1 and T2 (prior to intervention), there was no evidence of marijuana withdrawal at T2.
While rs-fc findings have been shown to align with task-based fMRI studies of the BRC (Di Martino et al., 2008), the paradigm employed in the present study does not directly measure response to reward, and the brain regions implicated in the BRC are also involved in processes other than reward. Thus, while definitive conclusions cannot be made, especially given our small sample size, our findings provide some support for the theory that a dysfunctional BRC underpins cannabis use in schizophrenia (Chambers et al., 2001; Green et al., 1999; Krystal et al., 2006; Roth et al., 2005).
Moreover, our pilot findings that cannabis and THC improve connectivity of the BRC suggest that these patients may resort to cannabis or other substance use as a means of ameliorating dysfunction within the BRC (even though chronic cannabis use ultimately worsens long-term prognosis) (Green et al., 2008; Green et al., 1999; Henquet et al., 2010; Swendsen et al., 2011). If confirmed by further research, low doses of THC (that do not increase psychotic symptoms in patients with SCZ), or other agents that ameliorate BRC dysfunction, might be considered as candidate agents to lessen cannabis use in SCZ.
While the majority of studies investigating BRC dysfunction in SCZ have relied predominantly on behavioral and task-based neuroimaging approaches, the present study provides evidence that rs-fc can be used to detect circuit-level BRC impairment in schizophrenia beyond the localized dysfunction within specific regions, assessed in the majority of task-based neuroimaging studies (Esslinger et al., 2012; Fornito et al., 2009; Juckel et al., 2006). Our findings also suggest a potential applicability of rs-fc for use as a measure of circuit-level alteration in connectivity induced by pharmacologic agents. Rs-fc may thus prove to be a useful tool in assessing drug effects on the BRC, as it is not susceptible to task-based confounds such as differential performance levels or ceiling and floor effects (Raichle, 2011; Whitfield-Gabrieli and Ford, 2011). This is of particular relevance for the investigation of psychiatric patient populations where cognitive and behavioral deficits may negatively impact task performance.
Interpretation of these findings should be considered in light of the study’s limitations. This was a pilot study, involving a small number of subjects, especially within the cannabis and THC subgroups. Significant findings, however, were observed using correction for multiple comparisons and were in line with our theoretical formulation. A second limitation was the lack of a comparison group of patients with SCZ, but without CUD, as a well as one comprised of individuals with CUD without schizophrenia. Future studies should include such comparison groups. Third, since patients were taking antipsychotic medication, we cannot rule out the possibility that our findings may have been impacted by treatment, although we note that impaired BRC activation has been observed in medication-naive patients with SCZ (Nielsen et al., 2012) (Juckel et al., 2012). And fourth, while the majority of patients were cigarette smokers (consistent with 70–90% prevalence of cigarette smoking in SCZ (Kalman et al., 2005)), none of the control subjects smoked cigarettes. Further studies should include a sample of nicotine-dependent healthy controls to assess whether BRC dysfunction may relate, in part, to cigarette smoking (Sweitzer et al., 2012). In addition, while no acute exacerbations of psychotic symptoms were observed using 15mg THC, doses of 10mg have been reported to worsen psychotic symptoms (Koethe et al., 2006) (Bhattacharyya et al., 2009). Thus, a dose-response study may be helpful in determining whether a lower dose of THC would also improve BRC connectivity in patients with SCZ. Lastly, alterations in blood flow induced by THC and cannabis could have confounded the interpretation of drug effects on neural activity. Future studies should include arterial spin labeling as a means of investigating the potential impact of any vascular changes induced by THC and cannabis.
5. Conclusion
To our knowledge, the present pilot study is the first to assess resting state functional connectivity of the BRC in patients with SCZ and co-occurring CUD. Decreased connectivity between regions of the BRC was found in the patients relative to controls. Particularly prominent were reductions in connectivity between the NAc and prefrontal cortical regions of the BRC (vACC, OFC and aPFC) implicated in higher-order reward processing. The administration of both cannabis and THC improved BRC connectivity in the patients, primarily between those regions that were hypoconnected at baseline relative to controls, and BRC connectivity after administration directly correlated with increases in plasma THC and in the level of plasma THC itself. These pilot study findings provide some support the proposition that BRC dysfunction may underpin cannabis use in patients with SCZ, and if confirmed by subsequent investigation, suggest that THC (or other agents that improve BRC connectivity) should be tested in patients with SCZ and co-occurring CUD in an attempt to limit their cannabis use. Future studies investigating BRC connectivity are needed to confirm and expand upon the present findings as well as to determine whether this method could serve as an indicator of clinical outcome and/or response to prospective treatments aimed at limiting cannabis use in SCZ.
Acknowledgments
This study was supported by DA026799-01 (AIG) from the National Institute on Drug Abuse. We would like to thank Douglas Noordsy, M.D., Kari Barton, Michael Pearl, Christopher O’Keefe, M.A., and Joseph Rancourt for their assistance in implementation of the study protocol, and to Jibran Khokhar, Ph.D. for help with the preparation of this manuscript. We also thank clinicians at West Central Behavioral Health and the Mental Health Center of Greater Manchester for conducting contingency management visits with patients to ensure substance use abstinence during the seven days prior to scanning.
In the past three years, Dr. Green has received grants from Novartis and Janssen to support research studies, and he has served on a Data Safety Monitoring Board for Eli Lilly and as a consultant to Otsuka Pharmaceutical and Alkermes (both without financial compensation). He is a co-inventor of two patent applications regarding treatment of substance abuse. Dr. Brunette has received support from the Bristol Meyer Squibb Foundation. Dr. Roth serves as a consultant to Shire Pharmaceuticals Inc.
Role of Funding Source
The funding source is NIDA. NIDA had no role in the execution of the study, the analysis of the data or the writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Brunette assisted with subject recruitment and study implementation. Dr. Roth was involved with study implementation and report writing. Dr. Whitfield-Gabrieli was involved in data analysis and report writing. Dr. Fischer, wrote the first draft of the manuscript, was involved in study implementation, data analysis and report writing. Dr. Green designed the study, and was involved in all aspects of implementation, data analysis and report writing. All authors contributed to and have approved the final manuscript.
Financial Disclosures
Dr. Whitfield-Gabrieli and Dr. Fischer have no disclosures.
References
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob PI. Nicotine chemistry, metabolism, kinetics, and biomarkers. In: JE H, editor. Hanbdbook of Experimental Pharmacology. Berlin Heidelberg: Springer-Verlag; 2009. pp. 29–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, Allen P, Seal ML, Fletcher PC, Crippa JA, Giampietro V, Mechelli A, Atakan Z, McGuire P. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Archives of general psychiatry. 2009;66(4):442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35(2):383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Cauda F, Cavanna AE, D'Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. Journal of cognitive neuroscience. 2011;23(10):2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441–449. doi: 10.1093/oxfordjournals.schbul.a033464. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Englisch S, Inta D, Rausch F, Schirmbeck F, Mier D, Kirsch P, Meyer-Lindenberg A, Zink M. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophrenia research. 2012;140(1–3):114–121. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P), Patient Edition. Washington, D.C.: American Psychiatric Publishing, Inc.; 2002. [Google Scholar]
- Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20(1):87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Wood SJ, Bechdolf A, Carter S, Adamson C, Velakoulis D, Saling MM, McGorry PD, Pantelis C. Anterior cingulate cortex abnormalities associated with a first psychotic episode in bipolar disorder. The British journal of psychiatry : the journal of mental science. 2009;194(5):426–433. doi: 10.1192/bjp.bp.107.049205. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacology, biochemistry, and behavior. 2005;81(2):263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Grant I, Atkinson JH, Gouaux B, Wilsey B. Medical marijuana: clearing away the smoke. The open neurology journal. 2012;6:18–25. doi: 10.2174/1874205X01206010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophrenia research. 2003;60(1):81–85. doi: 10.1016/s0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, O'Keefe C. Substance abuse and schizophrenia: Pharmacotherapeutic intervention. Journal of Substance Abuse Treatment. 2008;34:61–71. doi: 10.1016/j.jsat.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harvard review of psychiatry. 1999;6(6):287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognitive neuropsychiatry. 2007;12(3):213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96(7):1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Henquet C, van Os J, Kuepper R, Delespaul P, Smits M, Campo JA, Myin-Germeys I. Psychosis reactivity to cannabis use in daily life: an experience sampling study. The British journal of psychiatry : the journal of mental science. 2010;196(6):447–453. doi: 10.1192/bjp.bp.109.072249. [DOI] [PubMed] [Google Scholar]
- Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospective study. The British journal of psychiatry : the journal of mental science. 2006;189:137–143. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- Hjorthoj C, Fohlmann A, Nordentoft M. Treatment of cannabis use disorders in people with schizophrenia spectrum disorders - a systematic review. Addictive behaviors. 2009;34(6–7):520–525. doi: 10.1016/j.addbeh.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handbook of experimental pharmacology. 2005;(168):657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y, Knutson B, Wrase J, Brune M, Heinz A, Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66(1):50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koethe D, Gerth CW, Neatby MA, Haensel A, Thies M, Schneider U, Emrich HM, Klosterkotter J, Schultze-Lutter F, Leweke FM. Disturbances of visual information processing in early states of psychosis and experimental delta-9-tetrahydrocannabinol altered states of consciousness. Schizophrenia research. 2006;88(1–3):142–150. doi: 10.1016/j.schres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Gallinat J, Driesen N, Abi-Dargham A, Petrakis I, Heinz A, Pearlson G. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotoxicity research. 2006;10(3–4):235–252. doi: 10.1007/BF03033360. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, Gur RE, Turetsky BI. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160(10):1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, Glenthoj B. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biological psychiatry. 2012;71(10):898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harbor perspectives in medicine. 2012;2(8) doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain. Brain connectivity. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Brunette MF, Green AI. Treatment of substance use disorders in schizophrenia: a unifying neurobiological mechanism? Current psychiatry reports. 2005;7(4):283–291. doi: 10.1007/s11920-005-0082-8. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Follow-Back when administered by telephone and by computer. Drug and Alcohol Dependence. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Hariri AR. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 2012;123(Suppl 1):S59–S71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Ben-Zeev D, Granholm E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am J Psychiatry. 2011;168(2):202–209. doi: 10.1176/appi.ajp.2010.10030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford J. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychology. 2011;8(18-18):21. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: systematic review. The British journal of psychiatry : the journal of mental science. 2008;193(5):357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
- Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. Journal of clinical psychopharmacology. 2000;20(1):94–98. doi: 10.1097/00004714-200002000-00016. [DOI] [PubMed] [Google Scholar]