Muir and colleagues challenge the view of adipose tissue fibrosis as a contributing factor to type 2 diabetes in human obesity in a paper in the current issue of Obesity. In line with previous observations, they found that adipocyte hypertrophy is more tightly associated with diabetes even when normalized for overall obesity. However in contrast to other studies, they report that diabetic individuals display reduced extracellular matrix (ECM) deposition in their subcutaneous and visceral adipose tissues as judged by Sirius red stain and collagen immunohistochemistry (staining intensity was normalized to tissue area). Furthermore, the adipocytes were smaller in fibrotic areas. There was also an inverse correlation between HbA1c levels and visceral adipose tissue expression of fibrosis genes (such as LOX and Col 6A1) as well as between HbA1c levels and preadipocyte frequency in visceral adipose tissue. Based on these observations, these authors propose that adipose fibrosis is an adaptive feature that preserves adipocyte functionality by restricting hypertrophy (ref to Muir et al). These results stand in sharp contrast to many other studies, e.g. a recently published study by Gugliemi and colleagues reports that omental adipose tissue fibrosis in obese subjects is positively correlated with insulin resistance as judged by glucose clamps (1). The notion of a negative impact of adipose tissue fibrosis on local and systemic parameters is also supported by multiple animal studies that suggest that adipose tissue fibrosis contributes to obesity-related metabolic complications (2, 3, 4, 5, 6).
These discrepancies raise a number of questions: How do we define adipose tissue fibrosis? Does increased deposition of ECM in adipose tissue always imply fibrosis? Is there a causal relationship between ECM deposition and adipocyte hypertrophy or hyperplasia? Are there adipose depot differences? Is a large adipocyte necessarily a dysfunctional adipocyte? These are key questions, and the answers (or lack thereof) highlight the complexity of the adipose tissue response to this (patho)physiological change.
Elegant work from Karine Clément’s team demonstrated that pericellular fibrosis i.e. collagen fibers surrounding individual adipocytes, rather than total ECM deposition is elevated in subcutaneous and visceral adipose tissue of obese subjects. In lean individuals, pericellular fibrosis was rarely present and found only close to fibrous bundles (7) (Fig. 1). This is in line with our recent work showing that lean healthy mice have relatively high amounts of ECM in their adipose tissue. This ECM is organized as fibrous bundles, or so-called “septa” that compartmentalize fat pads into smaller units. These septa disappear however quickly in response to high fat diet feeding. The disappearance of these septa may be the most important step towards enabling adipocytes to grow larger, though this is difficult to test directly in light of any mechanistic insights how these septa arise and disappear. Accordingly, obese mice can display reduced amounts of adipose tissue collagen compared to lean controls (6). Yet, the pericellular fibrosis, which is typically associated with “crown-like structures” (CLSs), increases in obese adipose tissue, reflecting local crosstalk of adipocyte remnants with infiltrating macrophages (5).
Fig. 1.
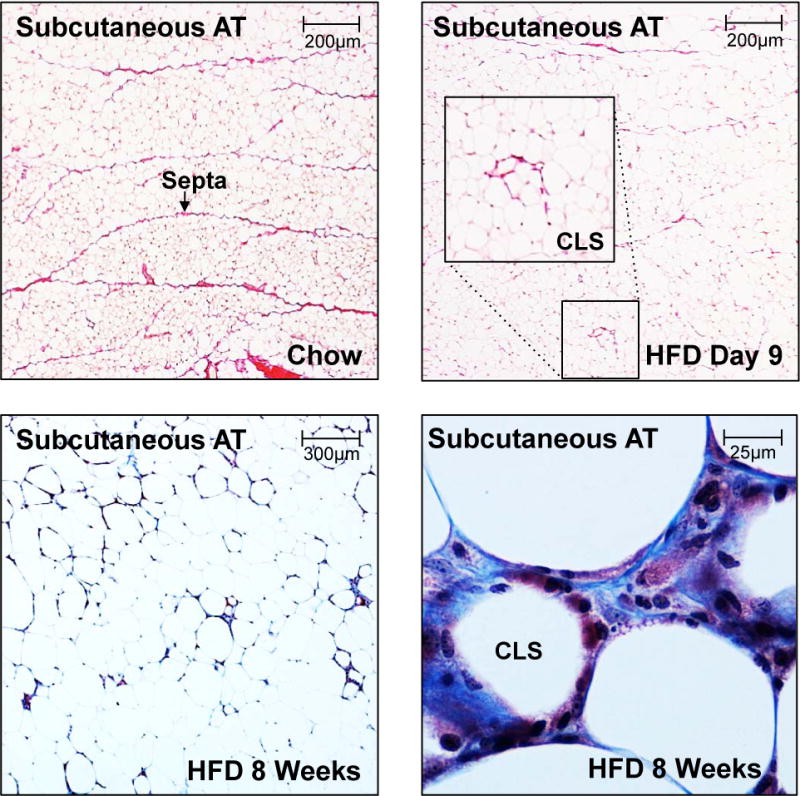
Picrosirius stain (top) and trichrome stain (bottom) highlighting fibrotic areas in subcutaneous fat in a lean animal on chow diet (top left), after 9 days of high fat diet (top right), after 8 weeks on high fat diet at low magnification (bottom left) and high magnification (bottom right).
Thus, the ECM may serve as an important structural component of adipose tissue and higher amounts of ECM do not necessarily define the tissue as fibrotic in a pathological sense. However, there is a strong argument to be made that it is the reduced ability to degrade and remodel the ECM during adipose tissue expansion that is the key pathological event, thereby exerting a barrier against healthy adipose tissue expansion. In line with this hypothesis, Pellegrinelli and colleagues show that human adipocyte function is negatively impacted by mechanical stress (8). Furthermore, while adipocyte hypertrophy is often associated with dysfunctional adipose tissue, diet- or genetically-induced obese mice lacking collagen VI (a key ECM component in adipose tissue) display enlarged adipocytes associated with improved metabolic function. This suggests that a looser ECM may allow for enhanced adipocyte growth and reduced mechanical stress with preserved metabolic function (3). Even though hypoxia (with all its negative consequences) is increasing hand in hand with adipocyte hypertrophy, it is possible that mechanical pressure imposed by the ECM also plays a significant role for the degree of adipocyte dysfunction in the obese state.
Our word of caution in the context of the conclusions provided by Muir and colleagues is that it is critically important to carefully assess adipose tissue fibrosis by detailed histological characterization. An inability to degrade fibrous bundles/septa during adipose tissue expansion may well contribute to metabolic dysfunction, but is likely of a different etiology than the pericellular ECM deposition that increase along with increased formation of CLS over the course of pathological adipose tissue expansion.
Acknowledgments
I.W.A. is supported by the Swedish Research Council (2012-1601), NovoNordisk Excellence Project Award, Åke Wiberg Foundation (18431223 and M14-0105), Diabetesfonden (DIA2014-074), Diabetes Wellness Research Foundation (8349/2014SW) and the Magnus Bergvall foundation (2014-00169). P.E.S. is supported by US National Institutes of Health (NIH) grants R01-DK55758, P01-DK088761 and R01-DK099110 (P.E.S.)
References
- 1.Guglielmi V, Cardellini M, Cinti F, Corgosinho F, Cardolini I, D’Adamo M, et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutrition & diabetes. 2015;5:e175. doi: 10.1038/nutd.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and cellular biology. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nature communications. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nature communications. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 6.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell metabolism. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59:2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrinelli V, Heuvingh J, du Roure O, Rouault C, Devulder A, Klein C, et al. Human adipocyte function is impacted by mechanical cues. The Journal of pathology. 2014;233:183–195. doi: 10.1002/path.4347. [DOI] [PubMed] [Google Scholar]


