Abstract
One of the largest threats to biodiversity is land use change and habitat loss. Hollow oaks (Quercus spp. L.) are well-defined patches that are hotspots for biodiversity and red-listed species, but they are often rare and fragmented in the landscape. We investigated the effect of patch size, habitat quality, and isolation on functional groups and red-listed saproxylic beetles in hollow oaks (n = 40) in Norway. The groups were defined by host tree association, trophic grouping, and red-listed status. Habitat quality, represented by tree form was most important in explaining species richness for most groups. Patch size, represented by circumference and amount of dead branches, was most important in explaining abundance. Isolation, that is single oaks compared with oaks in groups, had a negative effect on the abundance of beetles feeding both on wood and fungi (xylomycethopagous), as well as on species associated with broadleaved trees (oak semi-specialists), but did not affect species richness. This indicates that at this scale and in this landscape, isolated oaks are as species rich and valuable for conservation as other oaks, although some functional groups may be more vulnerable to isolation than others. The red-listed species only responded to patch size, indicating that oaks with large circumference and many dead branches are especially important for red-listed species and for conservation.
Keywords: saproxylic, ancient tree, trophic group, fragmentation, Coleoptera
Currently, species are going extinct a thousand times faster than expected by fossil records (Millennium Ecosystem Assessment 2005) and we may already be entering the sixth mass extinction in the history of Earth (Barnosky et al. 2011). The largest threat to biodiversity and ecosystems is land use change and the subsequent loss of habitat (Sala et al. 2000, Millennium Ecosystem Assessment 2005). Loss of habitat also leads to fragmentation of the remaining patches with varying degrees of isolation (Andrén 1994). The effect of landscape fragmentation on species has been studied by several approaches, of which metapopulation theory and island biogeography (Hanski 1999, Ricketts 2001) are among the most important. Island biogeography, focusing on the effect of island size and isolation on populations and species, has been extended to include habitat patches on land, surrounded by nonhabitat referred to as the matrix (Hanski 1998, Dover and Settele 2009, Fischer et al. 2009, Franzén et al. 2012). Other important factors that can affect species richness of fragmented habitats is the degree of isolation and the size of the habitat patch (Andrén 1994, Benedick et al. 2006), as well as habitat patch quality (Thomas et al. 2001). To successfully conserve species richness, we need a better understanding of the responses to fragmentation. This is particularly true for species-rich groups such as insects, which include 1 million described species (IUCN Red List 2014) and contribute to important ecosystem services (Losey and Vaughan 2006). Understanding the patterns and responses of species in fragmented landscapes are essential for their conservation and should be a prioritized research task.
Several studies have tried to identify the traits that make species vulnerable to fragmented landscapes (Henle et al. 2004). Studies of insects reveal that degree of specialization in habitat and food requirements, dispersal ability, body size, population size, and reproductive capacity are traits that can influence species vulnerability (Henle et al. 2004, Benedick et al. 2006, Cagnolo et al. 2009). Species that are highly specialized with narrow niches are most vulnerable, whereas species that are more general in their habitat and food requirements, breed in ephemeral habitats or have rapid growth and dispersal are more likely to be successful (McKinney and Lockwood 1999, Franzén et al. 2012). Species are categorized according to their extinction risk in international and national red lists (IUCN Red List 2014), but the number of species at risk are likely to be higher (McKinney and Lockwood 1999) and a large number of species are not evaluated due to information deficiency (Nieto et al. 2014). The vulnerability of specialization and the Holt’s hypothesis, which proposed that specialized species on top of the food chain should have a stronger response to fragmentation and habitat loss than generalists (Holt et al. 1999), have been confirmed in several studies (Komonen et al. 2000, Valladares et al. 2006, Cagnolo et al. 2009).
Saproxylic species are species dependent on dead wood habitats or its inhabitants (Speight 1989), and they represent important decomposers with high species richness which is severely affected by land use changes. Modern forestry with clear-cutting and monocultures of planted trees has reduced the volumes of available dead wood and made old-growth forests rare or lacking in much of Europe (Grove 2002, Hanski 2008). Some species are highly specialized to certain host trees, whereas others can use a range of dead wood habitats independent of tree species (Grove 2002, Stokland et al. 2012). Oak (Quercus spp. L.) is a temperate broadleaved tree that can become close to 1,000 years old (Drobyshev and Niklasson 2010). As the tree grows old, the architectural diversity increases and a range of new microhabitats appear, such as dead branches, coarse bark, wood mould, and different types of rot (Alexander 2008). Oaks with hollows are normally older than 200 years (Ranius et al. 2009) and represent biodiversity hotspots (Kennedy and Southwood 1984, Sverdrup-Thygeson 2009, Bouget et al. 2014). The recruitment of such oaks is low. Most old-growth deciduous forests disappeared from Europe centuries ago, but the mature and ancient trees that still remain are threatened in large parts of Europe due to direct removal, intensification in forestry and agricultural landscapes, regrowth creating shade, lack of recruitment and pollution (Ranius et al. 2005, Gibbons et al. 2008, The Directorate for Nature Management 2012, Lindenmayer et al. 2012).
Hollow oaks are well defined ‘patches’ with a large number of rare and red-listed species (Ranius 2002b, Sverdrup-Thygeson 2009, The Directorate for Nature Management 2012). Although areas of high density of ancient hollow oaks in Europe do exist, most hollow oaks are scattered and isolated, either in agricultural landscapes or in woodlands (Gibbons et al. 2008, Ranius et al. 2009). For dispersing insects, the distance between suitable trees is likely to represent a challenge, and some beetles associated with hollow trees are known to be poor dispersers (Ranius 2006). The current spatial distribution and connectivity of hollow oaks are therefore of major importance for the oak-dependent species living in hollow trees.
To categorize the species into functional groups can help to identify if some groups are more vulnerable to fragmentation than others, which can in worst case lead to loss of important ecosystem functions. Therefore, to make sound management decisions it would be an advantage to know the most important aspects for maintaining species richness, rare species and ecosystem functions related to hollow oaks. Further, these aspects should be easily detectable in the field. Often, time or money does not allow expensive and time-consuming insect surveys. Recognizing important structures of the oak, or the surroundings, could provide valuable indication of which trees that are associated with high species richness or high number of rare species (Skarpaas et al. 2011). In our study, we have identified important structural variables related to patch size, habitat quality, and isolation, to test if these aspects affect the species richness and abundances in functional groups and red-listed species. We compared responses of different groups of beetles in highly isolated oaks to clustered hollow oaks (low isolation) while including variation in patch size and quality.
The aim of our study was to evaluate the effect of patch size, habitat quality, and isolation on species richness and abundance of functional groups and red-listed oak species in hollow oaks.
We expected a general positive effect of patch size and habitat quality on abundance and species richness, with open surroundings and low tree crown as proxies of high quality. We expected isolation to have strongest effect on the most specialized and vulnerable groups, such as the oak specialists and the red-listed species.
Materials and Methods
Study Design
The hollow oaks in the study (Fig. 1) were selected from the main oak-distribution area in southern Norway. We defined a hollow oak as an oak with a minimum circumference of 60 cm at breast height, with a visible hollow of at least 5 cm in diameter. The study is related to an ongoing study of hollow oaks under the National Program for Surveying and Monitoring Biodiversity—Threatened Species in Norway (Sverdrup-Thygeson et al. 2011).
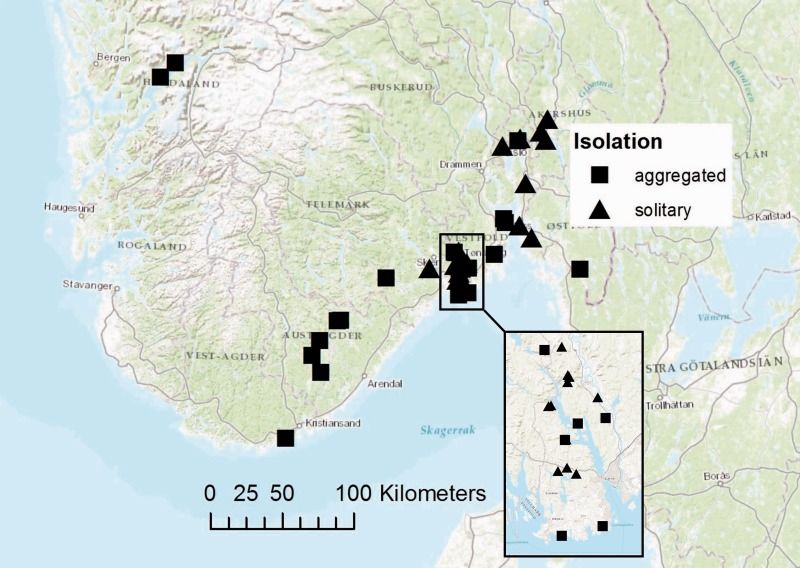
Fig. 1.
Map of southern Norway with the locations of the hollow oaks (n = 40). The symbols show isolation category: solitary (high isolation) and aggregated (low isolation).
Previous studies have indicated that the species composition differs between oaks in forests and parks (Sverdrup-Thygeson et al. 2010). To be able to generalize from our results, half of our hollow oaks were selected from the forest and half from the agricultural landscape. In total, 40 hollow oaks were included. In each of the two landscapes, half of the oaks (n = 20) were situated in areas where we found no or very few other hollow oaks nearby (‘high isolation’), whereas the other half were situated in areas where we found several hollow oaks (‘low isolation’) with close distance (<200 m) to at least four other hollow oaks (Table 1). The ‘high isolation’ trees had a more northerly distribution than the ‘low isolation’ trees (Fig. 1). This could mean that the isolation is even more severe as cold weather might reduce the number of days suitable for flying.
Table 1.
Variables used in the statistical analyses of oak-associated beetles in hollow oaks (n = 40)
| Variable | Cont. or Cat. | Units or levels | Explanation | |
|---|---|---|---|---|
| Isolation | Isolation | Cat. | High | Hollow oaks situated in areas with no or few other hollow oaks nearby (within 200 m) (n = 20) |
| Low | Hollow oaks situated in areas with several hollow oaks and close distance (<200 m) to at least four other hollow oaks (n = 20). | |||
| Patch size | Circumference | Cont. | Cm | Circumference measured in breast height (1.3 m above ground) (min: 60 cm, max: 953 cm, median: 310 cm) |
| Dead branches | Cat. | low, intermediate, high | Amount of dead branches present on the tree was categorized into: low, <50% of branches dead (n = 12), intermediate, 50-80% of branches dead (n = 26), and high, >80% (n = 2) | |
| Habitat quality | Tree form | Cat. | low, middle, high | The shape of the oaks were categorized based on the positon of the tree crown into low (n = 14), middle (n = 20), or high (n = 6) position. The shape of the tree is a combination of past and current growing conditions reflecting whether it has grown in open or closed conditions |
| Openness | Cat. | open, shrub, trees | The hollow oaks were categorized according to presence of woody vegetation within 5 m around the oak creating shade: open (n = 18), surrounded by shrubs (n = 7) or surrounded by trees (n = 15) | |
| Covariate | Landscape | Cat. | cultural | Hollow oaks within agricultural landscapes (n = 20) |
| forest | Hollow oaks in forests (n = 20) | |||
| Random factor | Geographical position | Cont. | UTM32V coordinates | X and Y coordinates from GPS coordinates (WGS84), rounded to nearest 100 m to adjust for uneven spatial distribution. Used in the GLMMs |
| Entomological region | Cat. | regions | Sampling regions commonly used for insects in Norway (University of Oslo 2009) (n = 8). Used in the LMMs |
Cont., continuous, Cat., categorical.
Insect Trapping, Habitat Quality, and Patch Sizes
Beetles were collected from each oak by window traps (flight interception traps) from mid-May until mid-August for 1 year only, during the period 2004–2013. Mean and median number of oak species was similar for all sampled years, and although all the highly isolated hollow oaks were sampled in 2009, there were no differences in the number of oak-associated or red-listed beetle species in 2009 compared with other years (see Birkemoe and Sverdrup-Thygeson 2012). We used two traps per tree, one placed in front of the opening of the hollow and the other one in the canopy (see Sverdrup-Thygeson et al. 2010 for details).
We used circumference and amount of dead branches as proxies of ‘patch size’ for the saproxylic beetles (Table 1). Circumference was measured at breast height 1.3 m above ground, and amount of dead branches present on the tree was categorized into three groups: low, <50% of branches dead, intermediate, 50–80% of branches dead, and high, >80%. We further used tree form and openness around the trees as proxies of ‘habitat quality’. We categorized our oaks’ tree shape based on the positon of the tree crown into low, middle, or high tree form (Table 1). Low tree forms are typically wide-branched and a result of growing in open areas with high sun exposure (Read 2000), believed to create positive thermal conditions for insects. Additionally, low-branched hollow oaks tend to contain a high number of microhabitats, both of which will contribute to increased habitat quality. The openness status was categorized according to presence of woody vegetation around the oak: open, surrounded by shrubs, or surrounded by trees (Table 1). This variable has been found to affect habitat quality in earlier studies (Ranius and Jansson 2000, Widerberg et al. 2012) due to negative effects of shade on both the oak and the beetle diversity within. Landscape type (forest or agricultural) may affect species composition (Sverdrup-Thygeson et al. 2010, Skarpaas et al. 2011) and was included as a covariate in the analysis.
Functional Groups
All beetles were determined to species and categorized to functional groups based on their oak association and trophic guild. Only saproxylic species were included. The classification of oak association was based on Dahlberg and Stokland (2004) and The Saproxylic Database (2014) (Supp 1 [online only]). For oak association the following groups were used: oak specialists, oak semi-specialist, oak generalists, and not oak species. ‘Oak specialists’ were defined as preferring oak, only occurring on oak or only occurring on temperate broadleaved trees in addition to oak. ‘Oak semi-specialists’ were defined as occurring on boreal broadleaved trees in addition to oak, ‘oak generalists’ were defined as occurring on coniferous trees in addition to oak, and ‘not oak’ species were defined as saproxylic species not associated with oak or having unknown host association. The ‘not oak species’ group was not included in the statistical analyses.
Trophic guild information is mainly based on Koehler (2000), supplemented by the BugsCEP database (Buckland and Buckland 2006) and various other resources (including Ehnström and Axelsson 2002, Heliövaara et al. 2004, Lindhe et al. 2010, Norwegian Biodiversity Information Centre 2015, The Swedish Species Information Centre 2015). The following groups were used: ‘xylophage’ for species eating wood, ‘xylomycetophage’ for species dependent on wood and fungi, ‘fungivore’ for species eating fungi, ‘predator’ for predatory species, and ‘mixed feeding group’ for other species, mainly omnivores or species belonging to several of the trophic levels. Species were regarded as xylomycetophagous if they were both listed as xylophage and xylomycetophagous by different authors. When information on larva and imago were different (for example predatory larva and pollenophagous imago), information on the larva was used as the larva for most species is the dominant life stage in dead wood. Similarly, species were categorized as ‘mixed feeding group’ if different authors listed them in different categories (see Supp 1 [online only] for further details on categorization). In addition, red-listed oak species were included as a single group as they are of particular concern for conservation and likely to respond to fragmentation.
Statistics
We used generalized linear mixed effect models (GLMM) with a poisson error distribution and log-link function, using the glmer function in the lme4 package in R to test which predictors that best explained the species richness in the functional groups (trophic levels and host tree association) and red-listed species. Predictor variables were isolation (low and high), landscape (forest or agricultural), dead branches, openness, circumference, and tree form. The variables were checked for collinearity prior to analyses. We used geographical position (in the form of Universal Transverse Mercator (UTM) coordinates) as a random factor to adjust for spatial structure in the data and between site differences. The drop1 function was used to do backward elimination and the optimal models were found by favoring the GLMM model with lowest possible AIC (Akaike Information Criterion) (Zuur et al. 2009). The optimal models were then tested for significance against a null-model in a likelihood ratio test. When AIC values were almost identical for two models, we chose the simplest model. Nonsignificant predictors were included in some of the models to achieve the best model fit (lowest AIC).
One tree had considerably higher species richness than the other oaks and was therefore an outlier in the dataset. To decide whether the outlier should be omitted, the GLMMs were run with and without the outlier to see how much this tree contributed to the optimal models. The outlier had considerable effect on which predictors that were kept in the optimal models, and the outlier was hence excluded in the GLMMs. After exclusion of the outlier, the dead branch variable was used with only two levels (low <50% and intermediate ≥50%) as there were not enough trees with >80% dead branches to keep it as a separate category. The dispersion parameter in the GLMMs ranged from 0.67 to 1.08 and indicated some under-dispersion in some of the models.
For analyses of abundance in each functional group, we used backward elimination in linear mixed models (LMMs) on log-transformed abundances (to achieve homogeneity). There were no outliers after log transformation. In LMMs the random effect has to be categorical, therefore geographic position was grouped into a commonly used categorical variable (‘entomological region’) reflecting sampling region for insects in Norway (University of Oslo 2009). The optimal models were found with the same procedure as for the species. The optimal models were fitted by restricted maximum likelihood, and P-values for the variables were calculated based on a z-distribution as recommended by Zuur et al. (2013). Confidence intervals were checked to ensure that the 95% confidence interval of significant values did not overlap with zero (Zuur et al. 2013).
Results
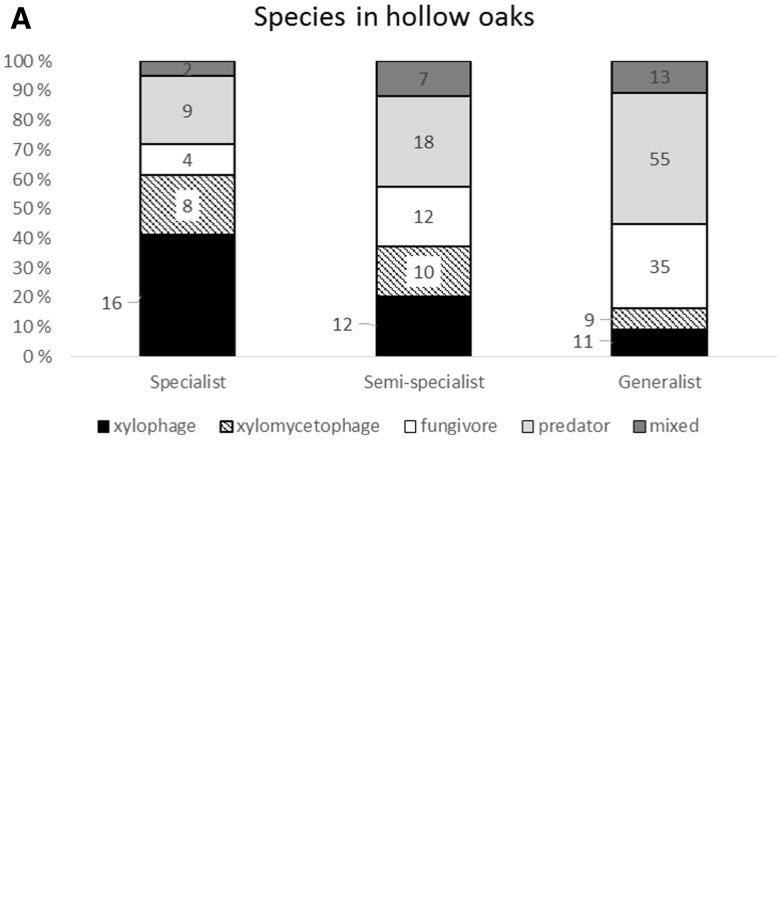
We sampled a total of 350 saproxylic beetle species, of which 221 species and 4,548 individuals were associated with oak. Of the oak-associated species, 40 were on the Norwegian Red-List (Supp. Table 1 [online only]). The different functional groups were heterogeneous in terms of oak association (Fig. 2). Overall the predators had the highest species richness, and both predators and fungivore species had a decreasing trend from the oak generalists to the specialists in species richness and abundance. The xylophagous species dominated among the oak specialist species (41% of the species), whereas predators and fungivores dominated in the oak generalist group with 45% and 28% of the species, respectively (Fig. 2A). This pattern was slightly different for abundance, as the specialists were dominated by xylomycetophages (77% of all species) (Fig. 2B). This was mainly due to two species, Euglenes oculatus Paykull, 1798 and Dorcatoma chrysomelina Sturm, 1837, represented by 784 and 541 individuals. The predators dominated the abundance of oak generalists (65% of the individuals), but only one species was very abundant, Haploglossa villosula Stephens, 1832, with 996 individuals.
Fig. 2.
Percentage of saproxylic beetles in hollow oaks split into specialization and trophic levels. (A) Species richness. (B) Number of individuals. The number of species or individuals in each category is shown.
Effect of Patch Size, Habitat Quality, and Isolation on Functional Groups
Patch size (circumference and amount of dead branches) and isolation did not affect species richness in any of the 10 groups, although it remained in some optimal models (Table 2). Habitat quality (low and intermediate tree crown) on the other hand affected species richness positively in all groups except xylomycetophages, red-listed species, oak specialists, and the mixed feeding group that did not show any response (Table 2, see full details in Supp Table 2 [online only]).
Table 2.
Effect of patch size, habitat quality, and isolation on species richness and abundance of red-listed species and functional groups of oak-associated beetles in hollow oaks
| Patch size |
Habitat quality |
Isolation | |||
|---|---|---|---|---|---|
| Circum. | Dead branches | Tree form | Openness | high versus low | |
| high/inter. versus low | low/inter. versus high | tree/shrub versus open | |||
| Species richness | |||||
| Red-listed species | (+)a | ||||
| All oak species | + | ||||
| Trophic level | |||||
| Xylophage | (+)a | + | |||
| Xylomycetophage | (+)a | (−)a | |||
| Fungivore | + | ||||
| Predator | + | ||||
| Mixed feeding | |||||
| Oak association | |||||
| Specialist | |||||
| Oak semi-specialist | + | ||||
| Generalist | + | ||||
| Abundance | |||||
| Red-listed individuals | + | ||||
| All oak individuals | (+)b | + | (−)b | ||
| Trophic level | |||||
| Xylophage | + | + | + | ||
| Xylomycetophage | + | + | + | – | – |
| Fungivore | + | (+)a | |||
| Predator | + | ||||
| Mixed feeding | + | ||||
| Oak association | |||||
| Specialist | + | ||||
| Oak semi-specialist | + | – | |||
| Generalist | + | (+)a | (-)b | ||
Optimal models of GLMMs were used for species richness and LMMs for log-transformed abundance data. The optimal models were found by backward elimination using the drop1 function in R and AIC as selection criterion. For species, UTM coordinates were used as random effect and entomological region was used as random effect in the LMMs for log-transformed abundance. Only the strongest trend is shown for variables with several levels. Significant effects (P < 0.05) are indicated with a + or − only, whereas the nonsignificant variables (P > 0.05) kept in the optimal models are shown in brackets. For full details, see Supplementary Tables 2 and 3. Explanation: Circum, circumference; Inter., intermediate; a0.1 > P > 0.05; b0.15 > P > 0.1.
Contrary to species richness, the abundance responded to patch size. An increasing patch size increased abundance of 8 of the 10 groups, and only the predators and the mixed feeding group were not affected (Table 2, Supp Table 3 [online only]). The xylomycetophages and oak semi-specialists also responded negatively to isolation. Isolation remained in the optimal model for all oak individuals and oak generalists, but its negative effect was not significant (Table 2). Habitat quality had a mixed effect on the abundances: the xylomycetophages decreased with shrubs and trees whereas the predators and the mixed feeding group increased with shrubs (Supp Table 3 [online only]). The xylomycetophages also increased in abundance with openness. The habitat quality determined by tree form was positive (low and intermediate tree crown) for the abundance of xylomycetophages and xylophages.
Discussion
Our study revealed that beetle species richness in hollow oaks was primarily affected by habitat quality whereas beetle abundance was affected by patch size and isolation. This general pattern emerged despite the varying importance of patch size, habitat quality, and isolation on the different groups.
Habitat Patch Size
Large patch size, represented by circumference and a high proportion of dead branches, was important for the abundance of most functional groups except the predators and the mixed feeding group. Species richness was not affected by patch size, although the xylophage, xylomycetophage and red-listed species had a positive response that was close to significant. In-line with species-area relationships larger patches have room for more species and individuals, and it is likely that decreasing population size occur before extinction in local patches.
Several studies have shown that tree size (measured as circumference) is important for the species richness of beetles (Ranius and Jansson 2000, Sverdrup-Thygeson et al. 2010, Gough et al. 2014). This could be related to larger oaks normally have larger hollows with more wood mould as well as more dead wood overall. Furthermore, the structural heterogeneity in the form of microhabitats also tend to increase with size (Ranius and Jansson 2000, Sverdrup-Thygeson et al. 2010). Large size also provides more stable microclimates, and Ranius and Jansson (2000) suggested that large tree size make it easier for more fungus to establish, thus providing more habitat for fungi-associated beetles. In this study, we show that the proportion of dead wood in the canopy also plays a part, as more of the tree is available for saproxylic insects.
Habitat Quality
Habitat quality was the most important and only significant predictor of species richness for the functional groups. Six of the ten groups, including the all oak group, had a positive response in species richness to low or intermediate tree form. The effect of openness at the other hand was mixed and only abundances were affected. Thus, our results fit party with our expectations; higher species richness in hollow oaks with low tree form and open surroundings. Open surroundings have been found to be important in previous studies (Ranius and Jansson 2000, Widerberg et al. 2012, Gough et al. 2014), and low tree form normally indicates higher structural diversity and also that the tree has been growing in open sunny conditions over time. Our results indicate that abundances of some functional groups (predators and the mixed feeding group) can have a positive response to woody vegetation in the surroundings. Gough et al. (2014) found that oak generalists preferred hollow oaks surrounded by shrubs and trees, and suggested that this could be explained by the amount of forest around these oaks, as tree host diversity would be higher for oak generalists. Sverdrup-Thygeson et al. (2010) also found more dead wood around hollow oaks in forests than in parks, which could increase the habitat patch for certain species. Our study fits well with these results as most of the predators and the mixed feeding species were oak generalists. Still, regrowth of woody vegetation around oaks that are adapted to open conditions creates competition for space and light that can reduce the life span of the oak (Read 2000). It is therefore often advocated to carefully open up around oaks to increase the diversity of insects and to secure the longevity of the oak (Read 2000, Widerberg et al. 2012).
Isolation and Spatial Scales
Isolation only had a negative effect on 2 of 10 groups in the optimal models; on the abundance of xylomycetophages and oak semi-specialists. However, xylomycetophage species richness also showed a negative trend that was close to significant (Table 2). Xylomycetophages are dependent upon the combination of wood and fungi and two-thirds of these species were oak specialists or semi-specialists. This overall high degree of specialization could make them particularly vulnerable to isolation. Overall, we had expected isolation to have an effect on the species richness and abundances of oak specialists and the vulnerable red-listed species. Although these groups had no response to isolation, isolation was always negative whenever it was included in the optimal models, indicating that connectivity is important for several of the functional groups. In our study, we only investigated the immediate surroundings around each oak (within 200 m). Bergman et al. (2012) found that the spatial scales that were most important for different oak beetle species ranged from 52 m to >5 km. Thus, the real scale of response may not have been reached for many species in this study.
Contrary to our expectations, the species richness of oak specialists could not be explained by patch size, habitat quality, or isolation. This could indicate that other factors, not included in our study, may be of importance. As we only investigated the local scale, it is possible that the highly isolated oaks were less isolated at larger scales and available for species with good dispersal abilities. Unfortunately we could not include connectivity on a larger spatial scale in our study as no complete mapping of hollow oaks exists in our study areas. Andrén (1994) demonstrated that fragmentation of a landscape would primarily affect the species in terms of habitat loss, and the additional negative effects of isolation and patch size would mainly occur when the original landscape was truly fragmented and only smaller proportions remained. It is possible that the fragmentation thresholds for some of the functional groups were not reached in our study, especially since several of the groups probably can use other resources than hollow oaks, such as other hollow trees or dead wood in the surroundings. Bergman et al. (2012) also demonstrated that several of the studied species responded to two spatial scales; a very local scale (such as within a 100 m radius) and a scale of several kilometers. Studies on saproxylic beetles have shown that many beetle species associated with hollow trees are considered to be poor dispersers (Hedin et al. 2008). This may be an adaptation to the stability of their habitat, hosting generation upon generation of beetles with limited needs for long-distance dispersal (Ranius 2006, Hedin et al. 2008). Knowledge of which spatial scales that is most important for saproxylic species is limited and difficult to study (Ranius 2006, Sverdrup-Thygeson et al. 2014). Still, the decrease in abundance of the functional groups with isolation in our study indicates that some groups are vulnerable to fragmentation at the very local scale. High connectivity would therefore be advisable to secure survival in the long term.
Red-Listed Species
We found that red-listed species richness and abundance responded to patch size, although the effect on species richness was only close to significant (Table 2). This indicates that large oaks with many dead branches are especially important for the conservation of threatened species. An earlier study of red-listed beetles in hollow oaks in Norway found that species richness was positively related to circumference (patch size), proportion of oaks (low isolation), cavity decay stage, and coarse woody debris (i.e., dead wood) in the surroundings (Sverdrup-Thygeson et al. 2010). They also suggested that coarse woody debris in the surroundings could compensate for small circumference and limited sun exposure. Similarly, Gough et al. (2014) found circumference to be important, and they found a negative effect of regrowth on red-listed species.
We expected red-listed species to be especially vulnerable to isolation, as these species typically are rare or have declining populations. However, no such pattern was found. Nilsson and Baranowski (1997) found an effect of isolation and habitat size on saproxylic red-listed beetles in old-growth beech (Fagus sylvatica L.) forests in Sweden. Ranius (2002a) found that the percentage of trees with presence of certain rare species increased with stand size of hollow oaks. Two of the red-listed species, the rare click beetle Elater ferrugineus Linnaeus, 1758 (Elateridae) and the pseudoscorpion Larca lata H. J. Hansen, 1884, were not present at all in groups of only 1–3 hollow oaks standing <250 m apart. This is comparable to our high isolation sites where there were no or few other hollow oaks within 200 m of the oak. E. ferrugineus needed almost a hundred hollow oaks to be present in a considerable amount of the oaks (Ranius 2002a, 2006), clearly demonstrating that the number of hollow oaks in the immediate surroundings can be important for rare species. The lack of response to isolation in our study could indicate that the most vulnerable species may already have disappeared from our Norwegian sites, and that the difference between high and low isolation was too small. Large hollow oaks with many dead branches (i.e., large patch size) were more important for red-listed species richness and abundance in our study than the type of surroundings or neighbouring hollow oaks. These oaks are therefore especially important to protect to conserve rare species, regardless of whether they stand isolated or not.
Overall habitat quality was most important in explaining species richness for most functional groups, and patch size was most important in explaining abundance. The lack of response in the species richness to high isolation indicates that solitary oaks are as species rich as clustered oaks, but the negative response in the abundance indicates vulnerability to fragmentation in some groups. Hence, it would be advisable to maintain oaks in groups, as lower abundances over time could make species vulnerable to extinctions. The red-listed species only responded to patch size, indicating that hollow oaks with large circumference and high amounts of dead branches are especially important to conserve.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
We are grateful to Oddvar Hanssen and Frode Ødegaard, Norwegian Institute for Nature Research (NINA), for beetle identification, and to Leonie Gough, Imperial College London, for assistance in making the collinearity plots. We are also grateful for valuable comments on the manuscript from two anonymous reviewers. This study was partly carried out under the projects ‘Survey and monitoring of red-listed species’ (ARKO, funded by the Norwegian Environment Agency), and ‘Management of biodiversity and ecosystem services in spatially structured landscapes’ (funded by the Norwegian Research Council, grant 208434/F40).
References Cited
- Alexander K. N. A. 2008. Tree biology and saproxylic Coleoptera: issues of definitions and conservation language. Rev Écol-Terre Vie 10: 9–13. [Google Scholar]
- Andrén H. 1994. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71: 355–366. [Google Scholar]
- Barnosky A. D., Matzke N., Tomiya S., Wogan G. O. U., Swartz B., Quental T. B., Marshall C., McGuire J. L., Lindsey E. L., Maguire K. C., Mersey B., Ferrer E. A. 2011. Has the Earth’s sixth mass extinction already arrived? Nature 471: 51–57. [DOI] [PubMed] [Google Scholar]
- Benedick S., Hill J. K., Mustaffa N., Chey V. K., Maryati M., Searle J. B., Schilthuizen M., Hamer K. C. 2006. Impacts of rain forest fragmentation on butterflies in northern Borneo: species richness, turnover and the value of small fragments. J. Appl. Ecol. 43: 967–977. [Google Scholar]
- Bergman K.-O., Jansson N., Claesson K., Palmer M. W., Milberg P. 2012. How much and at what scale? Multiscale analyses as decision support for conservation of saproxylic oak beetles. Forest Ecol. Manag. 265: 133–141. [Google Scholar]
- Birkemoe T., Sverdrup-Thygeson A. 2012. Importance of long-term surveys for beetle diversity in veteran oaks, pp. 291–296. In Rotherham I., Handley C., Samojolik T., Agnoletti M. (eds.), Trees beyond the wood. Sheffield Hallam University, Sheffield, UK. [Google Scholar]
- Bouget C., Larrieu L., Brin A. 2014. Key features for saproxylic beetle diversity derived from rapid habitat assessment in temperate forests. Ecol. Ind. 36: 656–664. [Google Scholar]
- Buckland P. I., Buckland P. C. 2006. BugsCEP Coleopteran Ecology Package. IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series # 2006-116. NOAA/NCDC Paleoclimatology Program, Boulder CO, USA (http://www.bugscep.com). [Google Scholar]
- Cagnolo L., Valladares G., Salvo A., Cabido M., Zak M. 2009. Habitat fragmentation and species loss across three interacting trophic levels: effects of life-history and food-web traits. Conserv. Biol. 23: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Dahlberg A., Stokland J. 2004. Substrate requirements of wood-inhabiting species - a synthesis and analysis of 3600 species. Skogsstyrelsen. Report 7-04, pp. 75. [Google Scholar]
- Dover J., Settele J. 2009. The influences of landscape structure on butterfly distribution and movement: a review. J. Insect. Conserv. 13: 3–27. [Google Scholar]
- Drobyshev I., Niklasson M. 2010. How old are the largest southern Swedish oaks? A dendrochronological analysis. Ecol. Bull. 53: 155–163. [Google Scholar]
- Ehnström B., Axelsson R. 2002. Insekters gnag i bark och ved. ArtDatabanken, SLU, Uppsala, Sweden: [in Swedish] [Google Scholar]
- Fischer J., Lindenmayer D. B., Hobbs R. J. 2009. pp. 431–437. In Levin S. A. (ed.), The Princeton guide to ecology. Princeton University press, New Jersey. [Google Scholar]
- Franzén M., Schweiger O., Betzholtz P-E. 2012. Species-area relationships are controlled by species traits. PLoS One 7: e37359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons P., Lindenmayer D. B., Fischer J., Manning A. D., Weinberg A., Seddon J., Ryan P., Barrett G. 2008. The future of scattered trees in agricultural landscapes. Conserv. Biol. 22: 1309–1319. [DOI] [PubMed] [Google Scholar]
- Gough L. A., Birkemoe T., Sverdrup-Thygeson A. 2014. Reactive forest management can also be proactive for wood-living beetles in hollow oak trees. Biol. Conserv. 180: 75–83. [Google Scholar]
- Grove S. J. 2002. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 33: 1–23. [Google Scholar]
- Hanski I. 1998. Metapopulation dynamics. Nature 396: 41–49. [Google Scholar]
- Hanski I. 1999. Metapopulation ecology. Oxford University Press, Oxford. [Google Scholar]
- Hanski I. 2008. Insect conservation in boreal forests. J. Insect Conserv. 12: 451–454. [Google Scholar]
- Hedin J., Ranius T., Nilsson S., Smith H. 2008. Restricted dispersal in a flying beetle assessed by telemetry. Biodivers. Conserv. 17: 675–684. [Google Scholar]
- Heliövaara K., Mannerkoski I., Siitonen J. 2004. Suomen Sarvijaarat: Longhorn Beetles of Finland (Coleoptera, Cerambycidae). Tremex, Helsinki, Finland. [Google Scholar]
- Henle K., Davies K. F., Kleyer M., Margules C., Settele J. 2004. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 13: 207–251. [Google Scholar]
- Holt R. D., Lawton J. H., Polis G. A., Martinez N. D. 1999. Trophic rank and the species-area relationship. Ecology 80: 1495–1504. [Google Scholar]
- Kennedy C. E. J., Southwood T. R. E. 1984. The number of species of insects associated with British trees: a re-analysis. J. Anim. Ecol. 53: 455–478. [Google Scholar]
- Koehler F. 2000. Saproxylic beetles in nature forests of the northern Rhineland: comparative studies on the saproxylic beetles of Germany and contributions to German nature forest research. Schrr. LÖBF/LAFAO NRW (Recklinghausen) 18: 1–351. [Google Scholar]
- Komonen A., Penttilä R., Lindgren M., Hanski I. 2000. Forest fragmentation truncates a food chain based on an old-growth forest bracket fungus. Oikos 90: 119–126. [Google Scholar]
- Lindenmayer D. B., Laurance W. F., Franklin J. F. 2012. Global decline in large old trees. Science 338: 1305–1306. [DOI] [PubMed] [Google Scholar]
- Lindhe A., Jeppsson T., Ehnström B. 2010. Longhorn beetles in Sweden – distribution and abundance during two hundred years. Entomologisk Tidskrift 131: 241–510. [Google Scholar]
- IUCN Red List. 2014.Table 1: numbers of threatened species by major groups of organisms (1996–2014) ( http://cmsdocs.s3.amazonaws.com/summarystats/2014_3_Summary_Stats_Page_Documents/2014_3_RL_Stats_Table_1.pdf) (accessed 20 April 2015).
- Losey J. E., Vaughan M. 2006. The economic value of ecological services provided by insects. BioScience 56: 311–323. [Google Scholar]
- McKinney M. L., Lockwood J. L. 1999. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14: 450–453. [DOI] [PubMed] [Google Scholar]
- Millenium Ecosysem Assessment. 2005. Ecosystems and human well-being: synthesis. Island Press, Washington, DC. [Google Scholar]
- Nieto A., Roberts S. P. M., Kemp J., Rasmont P., Kuhlmann M., García Criado M., Biesmeijer J. C., Bogusch P., Dathe H. H., De la Rúa P., et al. 2014. European Red List of bees. Publication Office of the European Union, Luxembourg. [Google Scholar]
- Nilsson S. G., Baranowski R. 1997. Habitat predictability and the occurrence of wood beetles in old-growth beech forests. Ecography 20: 491–498. [Google Scholar]
- Norwegian Biodiversity Information Centre. 2015. Norsk rødliste for arter 2010. Norwegian Biodiversity Information Centre. [in Norwegian] (http://www.artsportalen.artsdatabanken.no/) (accessed 20 February 2015). [Google Scholar]
- Ranius T. 2002a. Influence of stand size and quality of tree hollows on saproxylic beetles in Sweden. Biol. Conserv. 103: 85–91. [Google Scholar]
- Ranius T. 2002b. Osmoderma eremita as an indicator of species richness of beetles in tree hollows. Biodivers. Conserv. 11: 931–941. [Google Scholar]
- Ranius T. 2006. Measuring the dispersal of saproxylic insects: a key characteristic for their conservation. Popul. Ecol. 48:177–188. [Google Scholar]
- Ranius T., Aguado L. O., Antonsson K., Audisio P., Ballerio A., Carpaneto G. M., Chobot K., Gjurasin B., Hanssen O., Huijbregts H. 2005. Osmoderma eremita (Coleoptera, Scarabaeidae, Cetoniinae) in Europe. Anim. Biodiver. Conserv. 28: 1–44. [Google Scholar]
- Ranius T., Jansson N. 2000. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol. Conserv. 95: 85–94. [Google Scholar]
- Ranius T., Niklasson M., Berg N. 2009. Development of tree hollows in pedunculate oak (Quercus robur). Forest. Ecol. Manag. 257: 303–310. [Google Scholar]
- Read H. 2000. Veteran Trees: A guide to good management. English Nature, Peterborough. [Google Scholar]
- Ricketts T. H. 2001. The matrix matters: effective isolation in fragmented landscapes. Am. Nat. 158: 87–99. [DOI] [PubMed] [Google Scholar]
- Sala O. E., Chapin F. S., III, Armesto J. J., Berlow E., Bloomfield J., Dirzo R., Huber-Sanwald E., Huenneke L. F., Jackson R. B., Kinzig A., Leemans R., Lodge D. M., Mooney H. A., Oesterheld M., Poff N. L., Sykes M. T., Walker B. H., Walker M., Wall D. H. 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- Skarpaas O., Diserud O. H., Sverdrup-Thygeson A., Ødegaard F. 2011. Predicting hotspots for red-listed species: multivariate regression models for oak-associated beetles. Insect Conserv. Diver. 4: 53–59. [Google Scholar]
- Speight M. 1989. Saproxylic invertebrates and their conservation. Nature and environment series, vol. 42 Council of Europe, Strasbourg. [Google Scholar]
- Stokland J. N., Siitonen J., Jonsson B. G. 2012. Biodiversity in dead wood. Ecology, biodiversity and conservation. Cambridge University Press, Cambridge. [Google Scholar]
- Sverdrup-Thygeson A. 2009. Oaks in Norway: Hotspots for red-listed beetles (Coleoptera), pp. 13–26. In Buse J., Alexander K.N.A., Ranius T., Assmann T. (eds.), Saproxylic beetles: their role and diversity in European woodland and tree habitats - Proceedings of the 5th symposium and workshop. Pensoft Publishers, Sofia-Moscow. [Google Scholar]
- Sverdrup-Thygeson A., Bratli H., Brandrud T. E., Endrestøl A., Evju M., Hanssen O., Skarpaas O., Stabbetorp O., Ødegaard F. 2011. Hule eiker - et hotspot-habitat: Sluttrapport under ARKO-prosjektets periode II. NINA rapport 710. Norsk institutt for naturforskning, Oslo. [in Norwegian]. [Google Scholar]
- Sverdrup-Thygeson A., Gustafsson L., Kouki J. 2014. Spatial and temporal scales relevant for conservation of dead-wood associated species: current status and perspectives. Biodivers. Conserv. 23: 513–535. [Google Scholar]
- Sverdrup-Thygeson A., Skarpaas O., Ødegaard F. 2010. Hollow oaks and beetle conservation: the significance of the surroundings. Biodivers. Conserv. 19: 837–852. [Google Scholar]
- The Directorate for Nature Management. 2012. Handlingsplan for utvalgt naturtype hule eiker, vol. 1, pp. 80 [in Norwegian] [Google Scholar]
- The Saproxylic Database, 2014. (http://radon.uio.no/WDD/Login.aspx? ReturnUrl=%2fwdd%2fDefault.aspx) [Google Scholar]
- The Swedish Species Information Centre. 2015. Artfakta - Sök rödlistade arter i Sverige 2010. ArtDatabanken - Swedish Species Information Centre. [in Swedish] (http://www.artfakta.se/GetSpecies.aspx?SearchType=Advanced) (accessed 20 February 2015). [Google Scholar]
- Thomas J. A., Bourn N. A. D., Clarke R. T., Stewart K. E., Simcox D. J., Pearman G. S., Curtis R., Goodger B. 2001. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 268: 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares G., Salvo A., Cagnolo L. 2006. Habitat fragmentation effects on trophic processes of insect-plant food webs. Efectos de la Fragmentación del Hábitat Sobre Procesos Tróficos de Redes Alimenticias Insecto-Planta. Conserv. Biol. 20: 212–217. [DOI] [PubMed] [Google Scholar]
- University of Oslo. 2009. Strand-systemet - en inndeling av Norge til bruk ved insektkartlegging. University of Oslo; [in Norwegian] (http://www.nhm.uio.no/fakta/zoologi/insekter/norcol/regioner_lang.html) (accessed 16 January 2015). [Google Scholar]
- Widerberg M. K., Ranius T., Drobyshev I., Nilsson U., Lindbladh M. 2012. Increased openness around retained oaks increases species richness of saproxylic beetles. Biodivers. Conserv. 21: 3035–3059. [Google Scholar]
- Zuur A. F., Hilbe J., Ieno E. N. 2013. A beginner’s guide to GLM and GLMM with R: A frequentist and bayesian perspective for ecologists. Highland Statistics Ltd., Newburgh, UK. [Google Scholar]
- Zuur A. F., Ieno E. N., Walker N. J., Saveliev A. A., Smith G. M. 2009. Mixed effects models and extensions in ecology with R. Statistics for Biology and Health, Springer, New York. [Google Scholar]