Abstract
Diabetes mellitus is one of the major health problems. This study was designed to investigate the effect of biotin to regulate blood glucose level, reduced toxicity and oxidative stress in liver of diabetic mice STZ-induced type 1. Male mice were divided into three groups, the first one served as the control group, the second and the third groups received single ip dose of 150 mg/kg of STZ, the second group served as the untreated diabetic group, the third group received daily oral dose of 15 mg/kg of biotin, livers and liver index showed insignificant difference among groups. Blood glucose level showed a significant decrease in treated diabetic mice compared to untreated diabetic mice. Biochemical analysis showed a significant decrease in liver enzymes AST and ALT compared to the control group. Histopathological examination showed severe changes in untreated diabetic liver tissue manifested by dilated portal vein, leukocytic infiltration, fatty degeneration and moderate to severe histopathological score, whereas, treated diabetic mice with biotin showed reduction in hepatotoxicity represented by appearance of relative healthy hepatocytes and normal histopathological score. Immunohistochemistry of acrolein showed intense immunoreactions in liver section of untreated diabetic mice and faint immunoreactions in treated diabetic mice with biotin as evidence to oxidative stress reduction.
Keywords: Diabetes, Hepatotoxicity, Oxidative stress, Acrolein, Mice
1. Introduction
Diabetes mellitus is a group of metabolic disorders of glucose characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (Nakhaee et al., 2009). Insulin is normally produced in highly specialized cells of the endocrine pancreas. The tissue-specific expression of insulin is tightly regulated at the transcriptional level, and the major regulatory elements are located in the 5′ flanking region of the insulin gene (Ohneda et al., 2000). Insulin expression is specific to β cells in the pancreatic islets, although proinsulin has also been detected in the fetal and postnatal thymus and spleen–lymphoid tissues (Vafiadis et al., 1997) In spite of the introduction of hypoglycemic drugs, diabetes and related complications continue to be a major medical problem (Nammi et al., 2003). In addition, hyper glycemia in diabetic patients is associated with alteration in glucose and lipid metabolism and modification in liver enzyme levels (Jenson and Deckert, 1998).
Hyperglycemia generates reactive oxygen species (ROS), which in turn cause damage to the cells in many ways. Damage to the cells ultimately results in secondary complications in diabetes mellitus. So, oxidative stress is thought to be a major contributor to cardiovascular disease in diabetes mellitus. In fact, it is well documented that diabetes is associated with increased oxidative stress, as evidenced by the increased accumulation of lipid peroxidase in the plasma of diabetes mellitus (Giugliano et al., 1995, Kakkar et al., 1995, Ozdemirler et al., 1995, Hunt et al., 1988, Jaganjac et al., 2013, Tiwari et al., 2013).
Biotin is a hydrosoluble vitamin that acts as a prosthetic group of carboxylases. Unrelated to its role as carboxylase prosthetic group, biotin regulates gene expression (Rodriguez-Melendez and Zempleni, 2003, Dakshinamurti and Chauhan, 1994) and has a wide repertoire of effects on systemic processes such as development (Watanabe and Endo, 1990) and immunity (Báez-Saldaña and Ortega, 2004). The first evidence that biotin affects glucose metabolism was reported by Dakshinamurti et al. (1968) in biotin-deficient rats. They observed that glucose tolerance test curves in biotin-deficient rats were significantly higher than those in nondeficient rats, as well as glucose phosphorylation and incorporation into glycogen in the liver. Romero-Navarro et al. (1999) demonstrated that insulin expression and secretion were increased in response to biotin. On the other hand, biotin deficiency decreased pancreatic glucokinase activity and decreased insulin secretion from pancreas.
2. Material and methods
2.1. Animals
Male mice of the Swiss albino strain weighing 25 ± g were used for the experiment, aged 12 weeks. The animals were acclimated to 22 ± 1 °C and maintained under conditions of 12-h periods of light and dark, with free access to clean water and commercial mice food. The animals were housed in polypropylene cages inside a well-ventilated room. The experiments were approved by the state authorities and followed Saudi Arabian rules of animal protection.
2.2. Induction of diabetes mellitus
All mice were fasted for 20 h before diabetes was induced by Streptozotocin (STZ) injection which was obtained from Sigma Chemicals Co., St. Louis, MO, USA. STZ dissolved in cold 0.01 M citrate buffer, pH 4.5 (always prepared fresh for immediate use within 5 min). STZ single dose injection (150 mg/kg) was given intraperitoneally. The blood glucose concentration was measured after 3 days of STZ injection for diabetes induction confirmation. The blood samples were collected from the tail and deproteinized. The obtained supernatant was immediately used for the determination of blood glucose by glucose oxidase/peroxidase spectrophotometry method (Braham and Tinder, 1972).
2.3. Experimental groups and protocol
The animals were distributed into three experimental groups. Each group consists of 10 mice in the beginning of the study. Animals in group I that served as the control group were injected with equivalent amount of cold citrate buffer (pH 4.5). Animals in group II were intraperitoneally administered single injection of 150 mg/kg of STZ. Animals in group III were intraperitoneally administered single injection of 150 mg/kg of STZ and received 15 mg/kg of biotin orally by stomach gavage daily for 12 days (biotin treatment was carried out after 3 days of STZ injection and glucose test was performed) (Reddi et al., 1988, Aldahmash et al., 2015). All animals were killed by ether at the end of experiment, blood samples and liver samples were collected.
2.4. Liver index
At the end of the experimental period, each mouse was weighed; livers were removed and weighed. Finally, the liver index was calculated by dividing the liver weight by the body weight and then multiplying by 100 and the results were statistically analyzed by SPSS (16.0).
2.5. Biochemical analysis
Blood samples for the measurement of blood chemistry were drawn into prechilled EDTA-containing tubes and immediately placed on ice. Blood samples were centrifuged at 3000 r/m for separation of serums and stored at −8 °C until assay. Serums were used for the estimation of glucose, ALT and AST.
2.6. Histological examination and histopathological score system
Livers were collected and cut into small pieces, fixed in 10% neutral buffered formalin. Following fixation, specimens were dehydrated, embedded in wax, and then sectioned to 5 μm thicknesses. Sections were stained with hematoxylin and eosin.
2.7. Histopathological score system
For the microscopic analysis, the liver fragment slides were stained with hematoxylin and eosin (HE). The scoring system was recommended by the Pathology Committee of NASH Clinical Research Network (Kleiner et al., 2005). Steatosis, normal (<5%) = 0, 5–33% = 1, 33–66% = 2 and >66% = 3, Microvesicular steatosis, none = 0 and present = 1, Inflammation, none = 0, <2 foci per 200× field = 1, 2–4 foci per 200× field = 2, >4 foci per 200× field = 3, Ballooning, none = 0, few balloon cells = 1, many cells = 2, prominent balloon cells = 3, the overall results represented as follows 0 = normal, 1 = mild, 2 = moderate and 3 = severe.
2.8. Immunohistochemical localization of acrolein (ABC method)
Paraffin embedded kidney sections were deparaffinized in xylene and rehydrated in descending grades of alcohol and finally distilled water. Sections then were heated in citrate buffer (pH 6) within microwave for 5 min. After that sections were washed with PBS buffer for 5 min and incubated in peroxidase blocking solution for 10 min. Sections were incubated overnight at 4 °C in diluted primary antibody (anti-acrolein) (mouse monoclonal antibody ab48501) then incubated in biotinylated goat anti-mouse (ab128976) as secondary antibody for 30 min, followed by incubation in avidin–biotin complex for 30 min, then incubated in DAB (ab64238) as chromogenic substrate for ten minutes, The stained sections were counter stained with Mayer’s hematoxylin, and dehydrated within ascending grades of alcohol and cleared with two changes of xylene, mounted with cover slip based on DPX mountant (all reagents from Abcam company). Liver sections were examined under microscope for brown immunoreactivity color and photos at magnification 200×.
2.9. Statistical analysis
Statistical significance of the control and diabetic mice groups was evaluated by SPSS16.0 (One-way T test). Comparison was made between control and diabetic groups in mice weight, liver weight, liver index, glucose levels, ALT and AST, p < 0.05 was considered to be significant.
3. Results
3.1. Weight and liver index
Weight of mice showed no change in the untreated diabetic group compared with the control group, whereas treated diabetic group with biotin showed an insignificant increase in mice weights compared with the control group (Table 1). Weights of livers of untreated and treated diabetic groups showed no change compared with the control group. Liver index showed no change in the untreated diabetic group compared with the control group (Table 1), moreover treated diabetic group with biotin showed an insignificant decrease compared with the control group.
Table 1.
Shows weights of mice, weights of livers and liver index in control, diabetes and diabetes treated with 15 mg/kg of biotin for 12 days after diabetes induction.
| Control | Diabetes | Diabetes and biotin | |
|---|---|---|---|
| Weight of mice | 25.7 ± 0.8 | 25.5 ± 4.3 | 31.5 ± 2.2 |
| Weight of liver | 1.9 ± 0.3 | 1.9 ± 0.1 | 2 ± 0.3 |
| Liver index | 7.6 ± 1.6 | 7.5 ± 0.7 | 6.4 ± 1.1 |
Data = Mean ± SEM (Standard Error of Means), p < 0.05 considered significant difference when diabetic groups compared with control group.
3.2. Biochemical analysis
Glucose levels showed a significant increase in untreated and treated diabetic groups compared with the control group p < 0.05 (Table 2) whereas, glucose level showed a significant decrease p < 0.05 in treated diabetic with biotin group versus untreated diabetic group. AST and ALT levels showed a significant decrease in untreated and treated groups versus control group p < 0.05 (Table 2). Moreover, treated diabetic with biotin group showed a significant decrease versus untreated diabetic group p < 0.05.
Table 2.
Shows glucose, AST and ALT levels in control, untreated diabetes and diabetes treated with 15 mg/kg of biotin for 12 days after diabetes induction.
| Control | Diabetes | Diabetes and biotin | |
|---|---|---|---|
| Glucose | 117 ± 0.8 | 333 ± 46 | 221 ± 82⁎ |
| AST | 354 ± 2.3 | 250 ± 79⁎ | 167 ± 0 |
| ALT | 223 ± 1.7 | 71 ± 27⁎ | 40 ± 10⁎ |
Data = Mean ± SEM (Standard Error of Means).
Significant difference in the group, p < 0.05 considered significant difference when diabetic groups compared with control group.
3.3. Histopathological and immunohistochemistry analysis
Control mice liver section showed normal liver architecture consisting of central vein and anastomose network of healthy hepatocytes arranged in strands, some of them showed double nuclei as a result of regeneration, strands of hepatocytes separated from each other by blood sinusoids, Kupffer cells were abundant in the sinusoids (Fig. 1), all items of histopathological score showed 0 = normal (Table 3).
Figure 1.
Liver of control mice showed normal liver structure. (Hx &E- Mag×400).
Table 3.
Showed liver pathological score.
| Items | Control | Diabetes | Diabetes and biotin |
|---|---|---|---|
| Steatosis | 0 | 2.1 ± 0.08⁎ | 1 ± 0 |
| Microvesicular steatosis | 0 | 1 ± 0.09⁎ | 0 ± 0.08 |
| Inflammation | 0 | 2 ± 0.2⁎ | 1 ± 0.1⁎ |
| Ballooning | 0 | 3 ± 0.06⁎ | 0 ± 0.08 |
Data = Mean ± SEM (Standard Error of Means).
p < 0.05 considered significant difference when diabetic groups compared with control group.
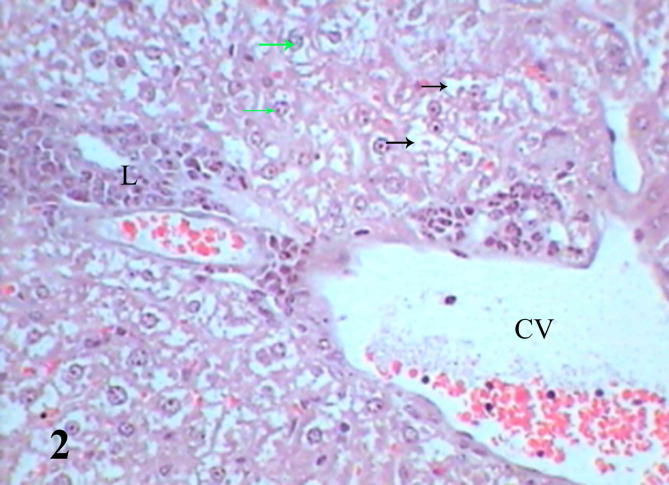
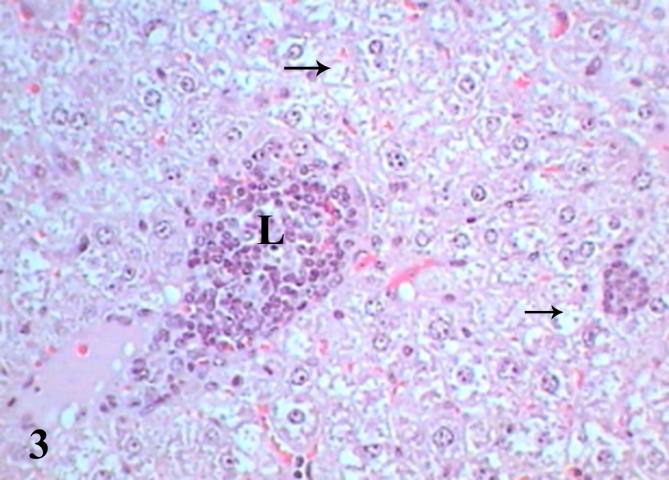
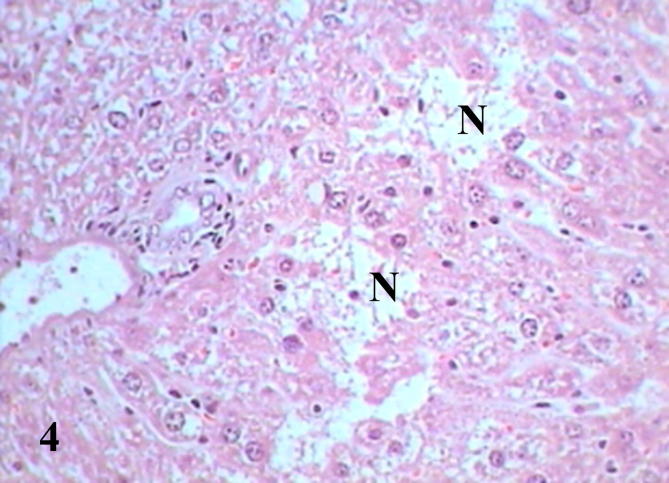
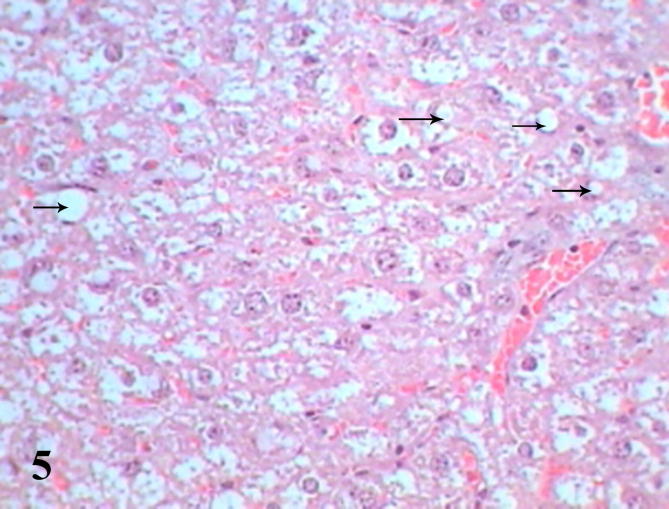
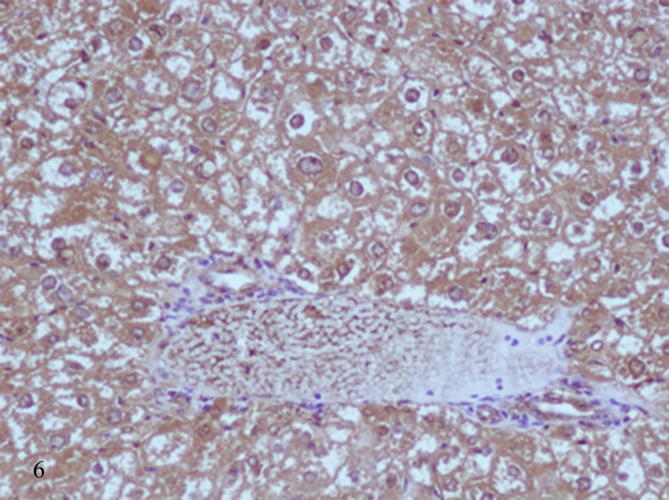
Histopathological examination of diabetic mice liver shows severe pathological alterations manifested by dilated and congested portal vein with destructed wall surrounded by leukocytic infiltration. Hepatocytes suffered from severe cytoplasmic degeneration (Fig. 2), most of cells showed pyknotic nuclei and karyolysed nuclei. Neutrophilic and eosinophilic aggregation was abundant in the tissue besides necrotic areas (Figure 3, Figure 4), some cells showed microvesicular steatosis (fatty degeneration) (Fig. 5), histopathological scoring showed moderate steatosis score (2), positive for microvesicular steatosis, moderate inflammation score (2) and severe ballooning score (3) (Table 3). Testing of diabetic liver section for anti-acrolein gene expression by the ABC method showed intense brownish immune precipitate in the tissue that referred to oxidative stress (acrolein is a main product of lipid peroxidation resulted from oxidative stress), immuoprecipitate accumulated in the cells and around central vein (Fig. 6).
Figure 2.
Liver section of diabetic mice STZ-induced showed dilated central vein (CV), cytoplasmic degeneration (black arrows), leukocytic infiltration (L) and pyknotic nuclei (green arrows). (Hx &E- Mag ×400).
Figure 3.
Liver section of diabetic mice STZ-induced showed aggregations of leukocytes (L) and degenerated cells (black arrows). (Hx &E- Mag ×400).
Figure 4.
Liver section of diabetic mice STZ-induced showed necrotic areas (N) and scattered inflammatory cells. (Hx &E- Mag ×400).
Figure 5.
Liver section of diabetic mice STZ-induced showed microvesicular fatty degeneration (arrow). (Hx &E- Mag ×400).
Figure 6.
Liver section of diabetic mice STZ-induced showed intense brown immunoprecipitate of antiacrolein in the cytoplasm of hepatocytes. (ABC method- Mag ×200).
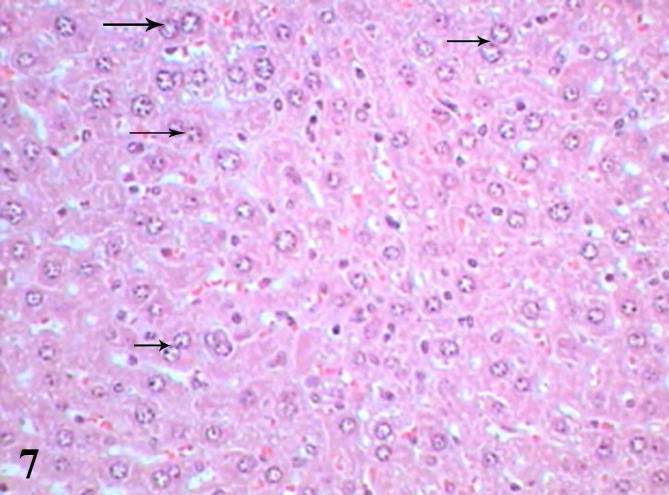
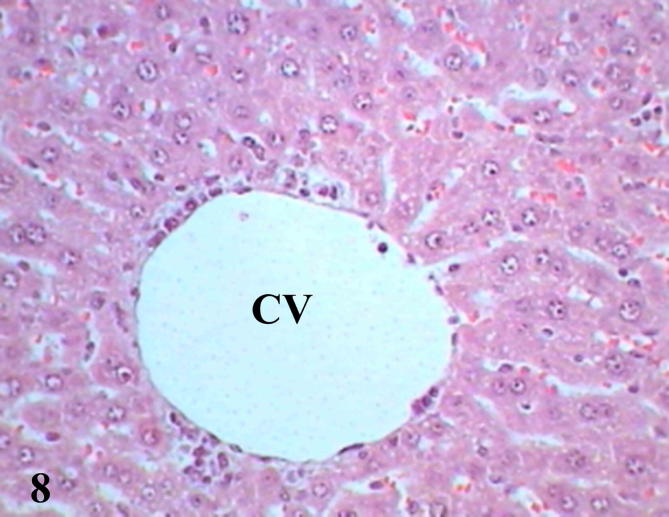
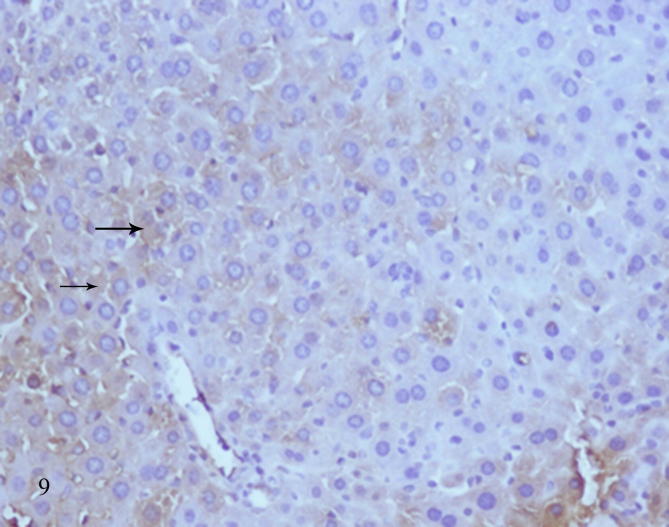
Examination of diabetic mice liver treated with biotin showed to great extent improved sections represented by normal shape of central vein surrounded by a few scattered leukocytes and more healthy hepatocytes, binucleated cells were present as a result of regeneration (Fig. 7), some cells showed pyknotic nuclei (Fig. 8), histopathological scoring system showed mild steatosis score (1), negative to microvesicular steatosis, mild inflammation score (1) and normal score (0) for ballooning (Table 3). Testing of diabetic liver sections treated with biotin to anti-acrolein gene expression showed faintly stained brownish immunoprecipitate compared with untreated diabetic liver section (Fig. 9), that referred to reduction in oxidative stress.
Figure 7.
Liver section of diabetic mice STZ-induced and treated with biotin showed healthy hepatocytes and binucleated cells (arrows). (Hx &E- Mag ×400).
Figure 8.
Liver section of diabetic mice STZ-induced and treated with biotin showed normal central vein (CV) surrounded by a few number of inflammatory cells. (Hx &E- Mag ×400).
Figure 9.
Liver section of diabetic mice STZ-induced and treated with biotin showed faintly stained brown immunoprecipitate of anti acrolein (arrows) due to reduction of oxidative stress. (ABC method- Mag ×200).
4. Discussion
Diabetes is by far the most common of endocrine disorders and a major threat to health care worldwide. It is projected that by 2010, at least 239 million people will be affected by the disease (Mcanuff et al., 2003). Streptozotocin induced diabetes provides a relevant example of endogenous chronic oxidative stress due to the resulting hyperglycemia (Low et al., 1997). STZ is a pancreatic β cell toxin that induces rapid and irreversible necrosis of cells (Arora et al., 2009) whereas a single diabetogenic dose of STZ (70–250 mg/kg, body weight) has been demonstrated to induce complete destruction of β cells in most species within 24 h, multiple sub-diabetogenic doses of STZ partially damage islets, thereby triggering an inflammatory process leading to macrophage and subsequent lymphocyte infiltration, which is followed by the onset of insulin deficiency (Kolb and Kroneke, 1993). The present results run in full agreement with the previous studies that single dose of 150 mg/kg of STZ induced damage for β cells in pancreas resulted in diabetes type 1 in mice after 3 days.
Diabetic albino rats STZ-induced registered significant decrease in weights and significant increase in liver weights at variable time intervals (Zafar and Naqvi, 2010). Moreover, the body weight of diabetic mice was significantly lowered than that of the nondiabetic group and relative weights of liver were significantly higher in diabetic mice compared to nondiabetic control mice (Kim et al., 2014). In contrast to the previous studies, the present results showed no change in diabetic mice weight compared to control mice and insignificant increase in treated diabetic mice with biotin compared to control and diabetic mice whereas, liver weight registered no change among control and diabetic groups. Many studies revealed that mice that received single dose of STZ showed an increase in glucose, ALT and AST levels (Zafar et al., 2009, Zafar and Naqvi, 2010, Ahmed et al., 2012, Kim et al., 2014). The present results agreed with the previous studies that single dose of STZ caused a significant increase in glucose level in diabetic mice compared to control mice, whereas, diabetic mice treated with biotin showed a significant decrease in glucose level compared to untreated diabetic mice that agreed with Carty, 1999, Mejia, 2005 who showed that biotin lowered the fasting glucose of diabetes patients and increased lymphocyte activity that was explained by Romero-Navarro et al. (1999) who proved that biotin stimulates hepatic and pancreatic glucokinase, an enzyme that plays an important role in glucose homeostasis regulating insulin secretion in response to changes in blood glucose concentration and hence insulin expression and secretion were increased in response to biotin. Coggeshall et al. (1985) stated that biotin reduced hyperglycemia in human patients type 1 received 16 mg/day of biotin for 1 week. In contrast to Degirmenchi et al., 2002, Zafar et al., 2009, Ahmed et al., 2012 results that diabetic mice STZ induced showed an increase in ALT and AST, the present study revealed a decrease in ALT and AST in diabetic mice compared to control mice, moreover treated diabetic mice with biotin showed a decrease in ALT and AST compared to untreated diabetic mice that agreed with Dakshinamurti and Li (1994).
In the present study, administration of single dose of STZ caused severe histopathological changes represented by dilated and congested portal vein, leukocytic infiltration, cytoplasmic degeneration and fatty degeneration, moderate steatosis and inflammation, whereas severe ballooning cells were registered in histopathological scoring system, these findings are in agreement with Zafar et al., 2009, Degirmenchi et al., 2002 who showed dilatation of veins, loss of usual arrangement of hepatocytes, lymphocytic infiltration and accumulation of lipid droplets in the cytoplasm of hepatocytes. In the present study administration of 15 mg/kg of biotin for 12 days revealed reduction of hepatotoxicity in diabetic mice STZ-induced manifested by healthy hepatocytes, binucleated cells as an evidence of regeneration and to some extent normal central vein, mild steatosis and inflammation whereas, no ballooning cells were registered in histopathological system score. There is increasing evidence that aldehydes generated endogenously during lipid peroxidation contribute to the pathophysiologic effects associated with oxidative stress in cells and tissues. A number of reactive lipid aldehydes, such as 4-hydroxy-2-alkenals and malondialdehyde, have been implicated as causative agents in cytotoxic processes initiated by the exposure of biologic systems to oxidizing agents. Recently, acrolein (CH2 CH−CHO), a ubiquitous pollutant in the environment, was identified as a product of lipid peroxidation reactions. The identification of acrolein as an endogenous lipid-derived product suggests an examination of the possible role of this aldehyde as a mediator of oxidative damage in a variety of human diseases (Uchida, 1999). Lipid peroxidation is implicated in the pathogenesis of numerous diseases; including atherosclerosis, diabetes, cancer, and rheumatoid arthritis, as well as in drug-associated toxicity, post is chemic reoxygenation injury, and aging. Lipid peroxidation proceeds by a free-radical chain reaction mechanism and yields lipid hydroperoxides as major initial reaction products. Subsequently, decomposition of lipid hydroperoxides generates a number of degradation products that lead to a wide variety of damaging actions (Esterbauer et al., 1991). In the present study, untreated diabetic mice liver showed a positive intense immunoreaction for acrolein, whereas treated diabetic mice with biotin showed faintly immunoreactions or acrolein as evidence that biotin can reduce oxidative stress.
The present study aimed to investigate whether biotin, that is a kind of vitamin B could reduce hyperglycemia, hepatotoxicity and oxidative stress in the liver. The overall results showed that biotin could reduce glucose levels in diabetic mice STZ-induced, moreover, biotin could improve injured liver due to diabetes and reduce oxidative stress in liver.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No. (RG-1435-030).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed O., Mahmoud A., Abdel-Moneim A., Ashour M. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012;41:53–67. [Google Scholar]
- Aldahmash B., El-Nagar D., Ibrahim K., Metwaly M. Biotin amelioration of nephrotoxicity in streptozotocin-induced diabetic mice. Saudi J. Biol. Sci. 2015;22:564–569. doi: 10.1016/j.sjbs.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Ojha S., Vohora D. Characterisation of streptozotocin induced diabetes mellitus in swiss albino mice. Global J. Pharmacol. 2009;3:81–84. [Google Scholar]
- Báez-Saldaña A., Ortega E. Biotin deficiency accelerates thymus involution, blocks thymocyte maturation and decreases nose-rump length in mice. J. Nutr. 2004;134:1979–1987. doi: 10.1093/jn/134.8.1970. [DOI] [PubMed] [Google Scholar]
- Carty M. High-dose biotin, an inducer of glucokinase expression, may synergize with chromium picolinate to enable a definitive nutritional therapy for type II diabetes. Med. Hypotheses. 1999;52:401–406. doi: 10.1054/mehy.1997.0682. [DOI] [PubMed] [Google Scholar]
- Coggeshall C., Heggers P., Robson C., Baker H. Biotin status and plasma glucose levels in diabetics. Ann. N. Y. Acad. Sci. 1985;447:389–392. [Google Scholar]
- Dakshinamurti K., Li W. Transcriptional regulation of liver phosphoenolpyruvate carboxykinase by biotin in diabetic rats. Mol. Cell. Biochem. 1994;132:127–132. doi: 10.1007/BF00926921. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K., Modi V., Mistry P. Some aspects of carbohydrates metabolism in biotin-deficient rats. Proc. Soc. Exp. Biol. Med. 1968;127:396–400. doi: 10.3181/00379727-127-32699. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K., Chauhan J. In: Vitamin Receptors: Vitamins as Ligands in Cell. Dakshinamurti K., editor. Vol. 1. Cambridge University Press; 1994. Biotin-binding proteins; pp. 200–249. [Google Scholar]
- Degirmenchi I., Kalender S., Ustuner C., Kalender Y., Gunes V., Unal N., Basaran A. The effects of acarbose and rumex patientia on liver ultrastructure in streptozotocin-induced diabetic (type-II) rats. Drugs Exp. Clin. Res. 2002;28:229–234. [PubMed] [Google Scholar]
- Esterbauer H., Schaur J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A., Paolisso G. Diabetes mellitus, hypertension and cardiovascular disease: which role for oxidative stress? Metabolism. 1995;44:363–368. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Hunt J., Dean R., Wolff S. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem. J. 1988;256:205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganjac M., Tirosh O., Cohen G., Sasson S., Zarkovic N. Reactive aldehydes—second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013;47:39–48. doi: 10.3109/10715762.2013.789136. [DOI] [PubMed] [Google Scholar]
- Jenson T., Deckert T. Abnormalities in plasma concentration of lipoprotein and fibrinogen in type 1 (insulin dependent) diabetic patients with increased urinary albumin excretion. Diabetologia. 1998;31:142–146. doi: 10.1007/BF00276846. [DOI] [PubMed] [Google Scholar]
- Kakkar R., Kalra J., Mantha S., Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- Kim J., Choi J., Lee M., Kang K., Paik M., Jo S., Jung U., Park H., Yee S. Immunomodulatory and antidiabetic effects of a new herbal preparation (HemoHIM) on streptozotocin-induced diabetic mice. Evid. Based Complementary Alternat. Med. 2014:1–8. doi: 10.1155/2014/461685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Brunt E., Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kroneke D. IDDM: lessons from the low dose streptozotocin model in mice. Diabetes Rev. 1993;1:116–126. [Google Scholar]
- Low A., Nickander K., Tritschler J. The role of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46:38–41. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- Mcanuff M., Omoruyia F., Morrison E., Asemote H. Hepatic function enzymes and lipid peroxidation in STZ-induced diabetic rats fed bitter yam (Dioscorea polygonoides) steroidal sapogenin extract. Diabetol. Croat. 2003;32:17–23. [Google Scholar]
- Mejia C. Pharmacological effects of biotin. J. Nutr. Biochem. 2005;16:424–427. doi: 10.1016/j.jnutbio.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Nakhaee A., Bokaeian M., Saravani M., Farhangi A., Akbarzadeh A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by eucalyptus globules. Indian J. Clin. Biochem. 2009;24:419–425. doi: 10.1007/s12291-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nammi S., Boini K., Lodagala S., Behara S. The juice of fresh leaves of Catharanthus roseus Linn reduces blood glucose in normal and alloxan diabetic rats. BMC Complement. Altern. Med. 2003;3:1–4. doi: 10.1186/1472-6882-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda K., Ee H., German M. Regulation of insulin gene transcription. Semin. Cell Dev. Biol. 2000;11:227–233. doi: 10.1006/scdb.2000.0171. [DOI] [PubMed] [Google Scholar]
- Ozdemirler G., Mehmetcik G., Oztezcan S., Toker G., Sivas A., Uysal M. Peroxidation potential and antioxidant activity of serum inpatients with diabetes mellitus and myocardial infraction. Horm. Metab. Res. 1995;27:194–196. doi: 10.1055/s-2007-979938. [DOI] [PubMed] [Google Scholar]
- Reddi B., De Angelis B., Frank O., Lasker N., Baker H. Biotin supplementation improves glucose and insulin tolerances in genetically diabetic KK mice. Life Sci. 1988;42:1323–1330. doi: 10.1016/0024-3205(88)90226-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Melendez R., Zempleni J. Regulation of gene expression by biotin. J. Nutr. Biochem. 2003;14:680–690. doi: 10.1016/j.jnutbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Romero-Navarro G., Cabrera-Valladares G., German S., Matschinsky M., Wang J., Fernandez-Mejia C. Biotin regulation of pancreatic glucokinase and insulin in primary cultured rat islets and in biotin deficient rats. Endocrinology. 1999;140:4595–4600. doi: 10.1210/endo.140.10.7084. [DOI] [PubMed] [Google Scholar]
- Tiwari B., Pandey K., Abidi A., Rizvi S. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013;2013:1–8. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc. Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Endo A. Teratogenic effects of maternal biotin deficiency on mouse embryos examined at midgestation. Teratology. 1990;42:295–300. doi: 10.1002/tera.1420420313. [DOI] [PubMed] [Google Scholar]
- Vafiadis P., Bennett T., Todd A., Nadeau J., Grabs R., Goodyer G., Wickramasinghe S., Colle E., Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- Zafar M., Naqvi S. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: a comparative study. Int. J. Morphol. 2010;28:135–142. [Google Scholar]
- Zafar M., Naqvi S., Ahmed M., Kaimkhani Z. Altered liver morphology and enzymes in streptozotocin induced diabetic rats. Int. J. Morphol. 2009;27:719–725. [Google Scholar]