Abstract
Cadmium (Cd) exposure can induce acute lethal health-related threats in humans since it has an exceptional ability to accumulate in living organism tissues and cause toxicological effects. Curcumin (Cur) on the other hand has a wide variety of biological activities and several studies have suggested its potential therapeutic or protective effects against several ailments and infections.
To study the effect of Cur on the toxicity of Cd, Swiss–Webster strain male and female mice (sixty each) were divided into 6 groups of ten each at random. Group-1 served as the naïve control and received no treatment. Group-2, 3 and 4 were the experimental controls and were administered once a day with a single oral dose of 50% dimethyl sulfoxide (DMSO), Cur (300 mg/kg) or Cd (100 mg/kg) respectively, for 2 weeks. Group-5 and 6 received Cur and Cd in combination once a day orally for 2 weeks except that Cur in a dose of 150 and 300 mg/kg to group 5 and 6 respectively, was administered one hour before Cd administration to both groups.
After treatment period, the male animals were subjected to social standard opponent test and females were subjected to the tube restraint tests and thereafter, their blood was collected to measure the blood composition indices and level of reproductive hormones. The animals were sacrificed to collect their brain for the estimation of acetylcholinesterase (AChE).
Results indicated that Cd significantly increased nonsocial activities in males and latency to first bite in females, whereas the social activities in males and the number of bites in females were significantly decreased. All measured indices of blood composition and levels of progesterone (female) and testosterone (male) in blood and AChE in their brain tissues were significantly decreased due to Cd treatment.
However, administration of Cur along with Cd had an ameliorating effect on all the behavioral and biochemical parameters studied herein and reduced the toxicity of Cd significantly and dose-dependently. Thus, Cur may be beneficial for general health and for protection from Cd intoxication.
Keywords: Standard opponent test, Tube restraint test, Cadmium, Curcumin, Social behavior
1. Introduction
Cadmium (Cd) represents one of the most toxic and carcinogenic heavy metal (IARC, 1993). Cd is an important industrial and environmental pollutant that currently ranks seventh in the Agency for Toxic Substances and Disease Registry (ATSDR)/EPA list of hazardous substances (Attia et al., 2014). It has been shown to be an environmental toxic pollutant metal from health-related threat point of view to humans as well as to other living organisms (Antonio et al., 1999, Singh et al., 2007, Tarasub et al., 2011). It is invariably present in our society, either as useful products in the form of nickel–cadmium batteries, dyes, plastics, electrochemicals, and paint pigments or in controlled wastes as a major source of pollution in water and as a constituent of food material (Jarup et al., 1998, Ikeda et al., 2000). The main source of Cd is through the diet and smoking (Barregard et al., 2014).
Women often have a higher Cd uptake compared to men due to iron deficiency (causing increased intestinal Cd uptake), which is more common in women. In humans it has been found to produce a wide range of biochemical and physiological dysfunctions as manifested in the forms of various diseases viz. Itai-itai, kidney malfunction, inflammation, Parkinson’s disorder, liver malfunction (Singh et al., 2011, NTP, 1993).
Curcumin (Cur) is a well-known biologically active natural phytochemical phenolic compound (diferuloylmethane) found as a major component in turmeric, a yellow curry spice, extracted from the rhizome of Curcuma longa L. (family Zingiberaceae). Cur is well absorbed in the body system and has exceedingly low toxicity (Rahman et al., 2006). It possesses many beneficial activities in the body and is effective in several disorders including anorexia, coryza, cough, hepatic diseases, and sinusitis (Khanna, 1999, Tirkey et al., 2005). Recent studies provide scientific evidence regarding the potential pharmacological, prophylactic or therapeutic use of Cur, as anti-inflammatory, anticarcinogenic, antiviral, antifungal, antiparasitic, antimutagenic, antiinfectious and antioxidant compound (Chen et al., 2006, Perez-Arriaga et al., 2006). Multiple beneficial effects of Cur have also been elaborated in neurogenesis process which in turn have been reported for their neuroprotective effects in age-related neurodegenerative diseases (Ramsewak et al., 2000). Several studies have shown that Cur exhibits protective effects against oxidative damage and has antioxidant properties exerting powerful oxygen free radical scavenging effects and increased intracellular glutathione concentration, thereby protecting lipid peroxidation (Aggarwal et al., 2007, Cole et al., 2007, Kuhad et al., 2007, Ciftci et al., 2010, Ciftci et al., 2011). Commercial Cur contains 77% curcumin, 17% de-methoxycurcumin and 3% bisdemethoxycurcumin and virtually all these three components in Cur are biologically active and possess protective properties (Ahsan et al., 1999, Jayaprakasha et al., 2006). Numerous reports indicate that the effects of Cd in laboratory animals can be prevented or markedly reduced by the administration of excess Cur (Cole et al., 2007, Jayaprakasha et al., 2006).
In the light of the above information it appears that Cur may prove beneficial in several ways for Cd toxicity and this aspect needs more and more research work. Thus, the present study was undertaken to explore the effects of Cur against the Cd induced social behavioral deficits and biochemical toxicity in male and female mice.
2. Materials and methods
2.1. Experimental animals
Sixty male and sixty female Swiss–Webster strain mice (8–10 weeks old) were housed in opaque plastic cages under hygienic conditions in the animal facility of the Zoology Department, King Saud University, Riyadh, Saudi Arabia. All animals were maintained under reversed lighting conditions with white lights on from 22.00 to 10.00 h local time. The ambient temperature was regulated between 20 °C and 22 °C. Food (Pilsbury’s Diet) and water were available ad libitum, unless otherwise indicated. All procedures were carried out in accordance with the ethical guidelines for care and use of laboratory animals, and all protocols were approved by the local Ethics and Care of Experimental Animals Committee.
All animals were divided into six different groups with ten animals in each. Group I consisted of untreated mice and served as naïve controls. Group II was treated with 50% DMSO (solvent of Cur). Group III was treated with 300 mg/kg Cur dissolved in 50% DMSO. Group IV was treated with Cd (100 mg/kg). Groups V and VI consisted of mice administered with Cur + Cd in combination in doses of 150 + 100 and 300 + 100 mg/kg respectively. All exposures were through oral administration, once a day, for two weeks, except that in groups V and VI, Cur was administered one hour before Cd exposure.
2.2. Cur and Cd administration
Cur of analytical grade, Sigma Chemical Company, USA, was dissolved in 50% DMSO to give a dose of 150 and 300 mg/kg body weight and diluted further with drinking water in 1.0 ml volume and was administered orally once a day. Cd was also administered orally once a day in the form of cadmium chloride (analytical grade, Riedel de Haen, Germany) dissolved in drinking tap water at a dose of 100 mg/kg body weight in 1.0 ml volume. In the fifth and sixth groups of animals where Cur and Cd were administered together orally once a day, Cur was administered one hour before Cd administration. The naïve control group received 1.0 ml plain tap water only. The doses of Cur and Cd used in this study are at par with the effective doses reported in the literature for such studies. The factor for the possibility of presence of Cd traces in food and tap water was not taken into account for calculating the daily Cd intake. However, this factor was minimized by giving the same source of food and tap water to all experimental groups including the controls.
2.3. Behavioral studies
Social behavior in all male animals was measured in the “standard opponent” test and the “tube restraint test” was conducted in female animals.
2.3.1. “Standard opponent” test
10 animals from each male treatment category and control group were individually housed in new cages for 14 days. After this isolation period, these male animals were subjected to “standard opponent” tests under dim red lighting (ca. 8 lux). The docile and age-matched male “standard opponents” were rendered anosmic by applying 25 μl of 4% zinc sulfate solution to the nasal tract under ether anesthesia for three days prior to encounters. The anosmic ‘standard opponent’ intruders were introduced in the home cages of ‘test animals’ and the “standard opponent” test of each ‘test animal’ was observed visually for 500 s.
The opponents were used to assess the selected “elements” of behavior as studied earlier by Brain et al., 1987, Ajarem and Ahmad, 1991, Abu-Taweel and Ajarem, 2008.
2.3.2. Tube restraint test
Sixty treated females were subjected to the ‘tube restraint test’. Ten females from each treated group were used for this test. The apparatus was based on the equipment described by Ajarem and Ahmad, 1992, Abu-Taweel et al., 2006 and consisted of a cylindrical transparent perspex tube 13 cm in length and with an internal diameter of 3.1 cm. One end of the tube was blocked by a perforated perspex wall through which a 2 cm long metal target was attached to a telegraph key/electronic counter arrangement. This enabled recording the number of bites directed by the restrained mouse toward the target. The test was conducted visually as outlined by Ajarem and Ahmad, 1994, Abu-Taweel et al., 2006 for 500 s under normal laboratory white lighting and temperature.
2.4. Blood parameters
After completion of behavioral tests, the blood was collected from the retro-orbital plexus of the animals in heparinized tubes at the end of the experiments. Blood parameters namely, red blood count, packed cell volume, hemoglobin content, total white blood count and blood platelets were measured using the automated parameter hematology analyzer (T 450, USA).
2.5. Estimation of testosterone and progesterone in plasma
The collected blood was centrifuged at 4000 rpm/min for 10 min and plasma was obtained and kept at −30 °C until it was used for hormone estimations. Testosterone and progesterone were estimated using hormone analysis instrument (Hitachi-Eleceys 2010-Roche Diagnostic, USA) by the method of electrochemiluminescence immunoassay.
2.6. Biochemical studies in brain tissue
Brains of animals were removed and gently rinsed in physiological saline (0.9% NaCl), and then blotted on a Whatman filter paper. Their fresh weights were recorded, and organs were then frozen.
2.6.1. Brain homogenate preparation
A 10% (w/v) homogenate of each frozen brain was prepared in a Teflon-glass homogenizer at 4 ± 1 °C, centrifuged at 1000×g for 10 min. to remove cell debris and the supernatant was used for enzyme assays. The brain homogenate was prepared in an ice-cold phosphate buffer, (0.067M, pH7.2) solution.
2.6.2. Estimation of AChE
The AChE activity in the homogenized brain tissue was estimated by the method of Ellman et al. (1961), utilizing acetylthiocholine iodide (ATCI) as substrate. The rate of production of thiocholine is determined by the continuous reaction of thiol with 5,5-dithiobis-2-nitrobenzoate (DTNB) ion to produce the yellow anion of 5-thio-2-nitro-benzoic acid. Spectrophotometric assay of enzyme activity was performed by adding 0.4 ml of the supernatant to a cuvette containing phosphate buffer (2.6 ml, pH 8) and 0.2 ml of DTNB (5,5%-dithio-bis(2-nitrobenzoic acid), Sigma Chem. Co., St. Louis, MO,USA). After adjusting the absorbance to zero, 0.02 ml of the substrate acetylthiocholine iodide (Sigma Chem. Co., St. Louis, MO, USA) was added and change in absorbance over 5 m was recorded. The specific activity of AChE was expressed as μ moles of acetylthiocholine iodide hydrolyzed/min/g of wet tissue.
2.7. Statistical analysis
Data of ‘standard opponent’ and ‘tube restraint’ tests were compared within the experimental groups by the analysis of variance (ANOVA) and subsequently were analyzed using Mann–Whitney U tests of Sokal and Rohlfe (1981). Data of biochemical analyses were compared within the experimental groups by the analysis of variance (ANOVA) using minitab computer program and were subsequently analyzed by Student’s t-test by Yamane (1973).
3. Results
3.1. Social behavior
Results indicate that exposure to Cd led to changes in social behavior of male mice. Data of ‘standard opponent’ and ‘tube restraint tests’ (Table 1A, Table 1B, Table 2 respectively) showed that Cd significantly increased nonsocial investigation, number of wall rears and rears, latency to threat (p < 0.001), attack (p < 0.01) in males and latency to first bite in females (p < 0.05) while it significantly decreased social investigation, time of attack (p < 0.001) and threat (p < 0.05), number of fights, naso-nasal and naso-genital contacts (p < 0.001) in males, number of bites (p < 0.01) in females. Furthermore, the data in these tables show that Cur has protective effects and reverses Cd toxicity.
Table 1A.
Effect of curcumin (Cur) on the cadmium (Cd) intoxicated social behavior of male mice.
| Group | Median number (with ranges) of seconds allocated to behaviors like |
|||||
|---|---|---|---|---|---|---|
| Nonsocial investigation | Social investigation | Defense | Threat | Attack | Displacement | |
| Control | 120.30 (84.30–171.20) | 228.10 (214.10–242.40) | 23.80 (13.40–20.70) | 24.30 (20.10–35.20) | 74.20 (40.40–98.20) | 29.30 (9.50–45.50) |
| DMSO | 132.85## (125.0–140.0) | 215.75## (211.70–221.60) | 25.25 (20.50–30.50) | 23.6# (20.30–26.20) | 79.15## (73.80–82.80) | 23.9 (19.0–27.10) |
| Cur (300 mg/kg) | 162.30## (105.80–190.20) | 222.40## (204.30–240.10) | 22.15 (18.20–25.30) | 16.00 (12.00–35.30) | 50.10 (40.30–77.10) | 32.55 (26.40–36.50) |
| Cd (100 mg/kg) | 307.60∗∗∗ (270.20–370.00) | 102.70∗∗∗ (59.50–159.80) | 22.40 (16.00–40.30) | 12.40∗ (9.50–23.00) | 12.05∗∗∗ (0.00–20.60) | 33.45 (23.10–37.60) |
| Cd + Cur (100 + 150 mg/kg) | 201.0# (200.0–210.0) | 171.0# (164.0–172.0) | 22.50 (22.0–25.0) | 24.0# (22.0–27.0) | 51.95## (43.5–53.20) | 30.65 (30.36–30.80) |
| Cd + Cur (100 + 300 mg/kg) | 170.25## (110.80–190.80) | 201.70## (201.60–240.40) | 22.15 (15.20–42.30) | 22.55# (15.70–27.30) | 49.15## (35.50–77.10) | 34.20 (14.50–36.50) |
∗, ∗∗ and ∗∗∗ significantly different (p < 0.05, p < 0.01 and p < 0.001) respectively from the control group and #, ## significantly different (p < 0.05, p < 0.01) from the cadmium treated group by ANOVA and Mann–Whitney U test.
DMSO is dimethyl sulfoxide.
Table 1B.
Effect of curcumin (Cur) on the cadmium (Cd) intoxicated social behavior of male mice.
| Group | Median number (with ranges) of seconds allocated to behaviors like |
||||||
|---|---|---|---|---|---|---|---|
| Latency to threat (sec) | Latency to attack (sec) | Number of fights | Number of naso-nasal contacts | Number of naso-genital contacts | Wall rears | Rears | |
| Control | 10.00 (3.00–30.00) | 45.00 (10.00–70 .00) | 19.00 (15.00–21.00) | 26.00 (23.00–29.00) | 17.00 (14.00–22.00) | 12.00 (10.00–16.00) | 10.00 (8.00–12.00) |
| DMSO | 15.5### (5.0–30.0) | 37.50## (20.0–90.0) | 16.50## (14.0–20.0) | 19.50## (14.0–21.0) | 10## (10.0–14.0) | 9.50### (7.0–14.0) | 6.0### (4.0–8.0) |
| Cur (560 mg/kg) | 55.00### (10.00–90.00) | 97.00# (37.00–130.00) | 12.00# (10.00–19.00) | 21.00 (15.00–27.00) | 11.00# (9.00–19.00) | 19.00# (13.00–21.00) | 17.00# (14.00–25.00) |
| Cd (100 mg/kg) | 450.00∗∗∗ (290.00–480.00) | 415.00∗∗ (0.00–490.00) | 5.00∗∗∗ (0.00–11.00) | 5.00∗∗∗ (4.00–9.00) | 7.00∗∗∗ (5.00–13.00) | 34.00∗∗∗ (25.00–53.00) | 29.00∗∗∗ (21.00–36.00) |
| Cd + Cur 100 + 150 mg kg | 77.50### (60.0–90.0) | 165.0## (150.0–175.0) | 16.50 (15.0–17.0) | 22.50 (20.0–25.00) | 14.0# (12.0–16.0) | 15.50# (13.0–17.0) | 13.0## (11.0–14.0) |
| Cd + Cur 100 + 300 mg kg | 58.00### (11.00–89.00) | 115.00## (47.00–154.00) | 13.00# (2.00–18.00) | 21.00 (17.00–25.00) | 12.00# (8.00–17.00) | 22.00# (13.00–30.00) | 18.00# (11.00–22.00) |
∗∗ and ∗∗∗ significantly different (p < 0.01, p < 0.001) respectively from the control group and #, ## and ### significantly different (p < 0.05, p < 0.01 and p < 0.001) respectively from the cadmium treated group by ANOVA and Mann–Whitney U test.
DMSO is dimethyl sulfoxide.
Table 2.
Effect of curcumin (Cur) on cadmium(Cd) intoxicated biting behavior of female mice in the tube restraint test.
| Treatment group | Measures (Median values with ranges) |
|
|---|---|---|
| Latency to first bite (sec) | Number of bites | |
| Control | 15.0 (10.0–40.0) | 70.0 (45.0–96.0) |
| DMSO | 45.0 (30.0–60.0) | 82.50 (80.0–90.0) |
| Cur 300 mg/kg | 25.0## (10.0–55.0) | 72.0## (40.0–120.0) |
| Cd 100 mg/kg | 132.0∗ (0.0–180.0) | 13.0∗∗ (0.0–54.0) |
| Cd + Cur (100 + 150 mg/kg) | 75.0## (60.0–90.0) | 37.0# (30.0–45.0) |
| Cd + Cur (100 + 300 mg/kg) | 18.0# (10.0–32.0) | 70.0## (49.0–98.0) |
∗ and ∗∗ significantly different at (p < 0.05 and p < 0.01) respectively from the control groups and #, ##, significantly different at (p < 0.05 and p < 0.01) respectively from the cadmium treated groups by ANOVA and Mann–Whitney U test. DMSO is dimethylsulfoxide.
3.2. Blood parameters
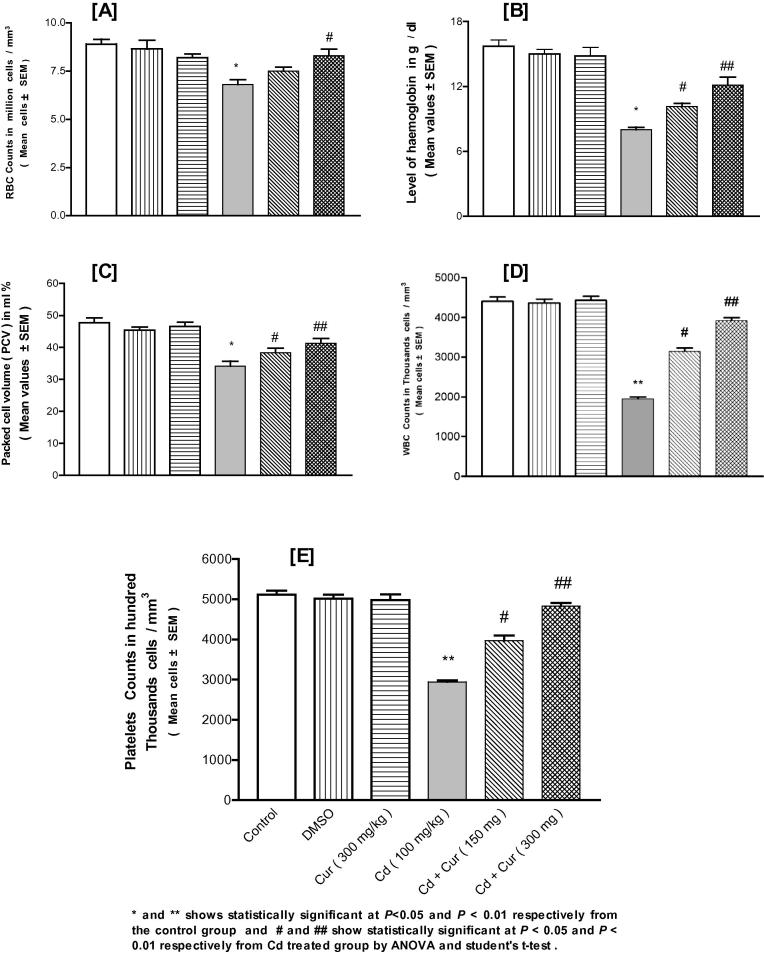
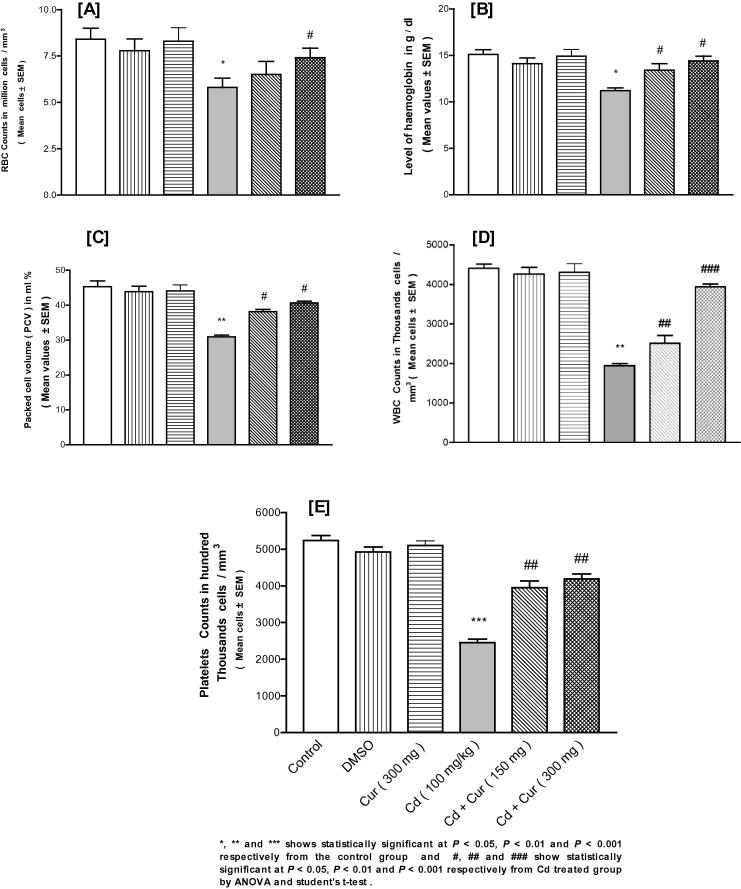
Cd exposure led to significant depletion in some of the observed blood parameters like the red blood cell count, packed cell volume, hemoglobin content, white blood cell count and platelets count in females (Fig. 1) and males (Fig. 2) and Cur ameliorated this depletion significantly and dose-dependent manner (Figure 1, Figure 2).
Figure 1.
Effect of cadmium (Cd) exposure on the depletion of some blood indices in female mice and ameliorating effect of curcumin (Cur) on these indices like red blood cell count (A), packed cell volume (B), hemoglobin content (C), white blood cell count (D) and platelet count (E). DMSO is dimethyl sulfoxide.
Figure 2.
Effect of cadmium (Cd) exposure on the depletion of some blood indices in male mice and ameliorating effect of curcumin (Cur) on these indices like red blood cell count (A), packed cell volume (B), hemoglobin content (C), white blood cell count (D) and platelet count (E). DMSO is dimethyl sulfoxide.
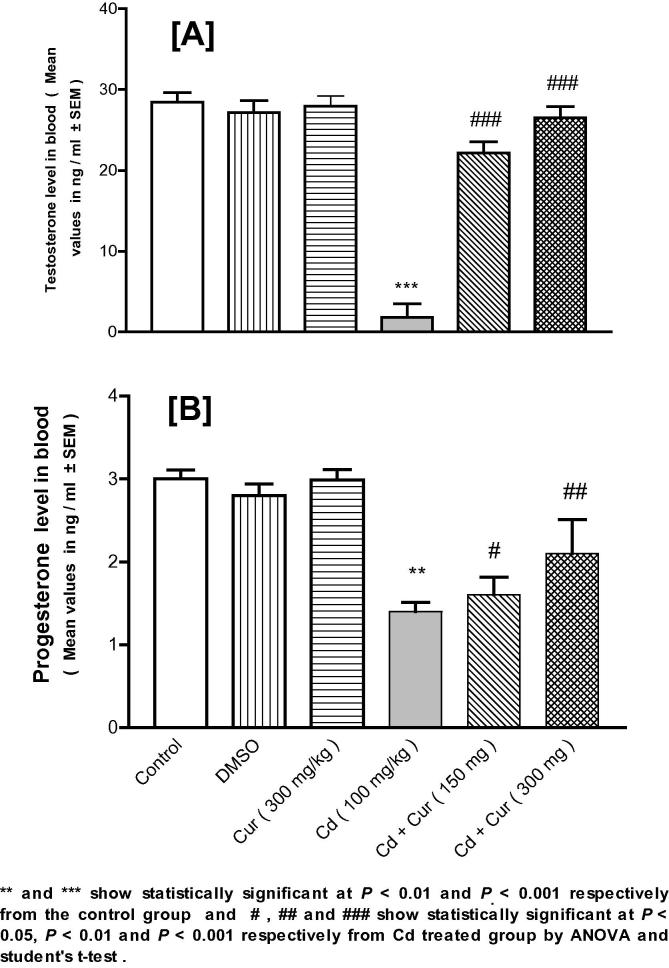
3.3. Testosterone and progesterone
Testosterone in male (Fig. 3A) and progesterone in female (Fig. 3B) mice were depleted significantly due to Cd exposure. This depletion was ameliorated significantly in a dose-dependent manner due to Cur treatment.
Figure 3.
Effect of cadmium (Cd) exposure on the depletion of testosterone and progesterone hormones in plasma of male (A) and female (B) mice respectively and ameliorating effect of curcumin (Cur) significantly and dose-dependently on these hormones like testosterone (A) and progesterone (B). DMSO is dimethyl sulfoxide.
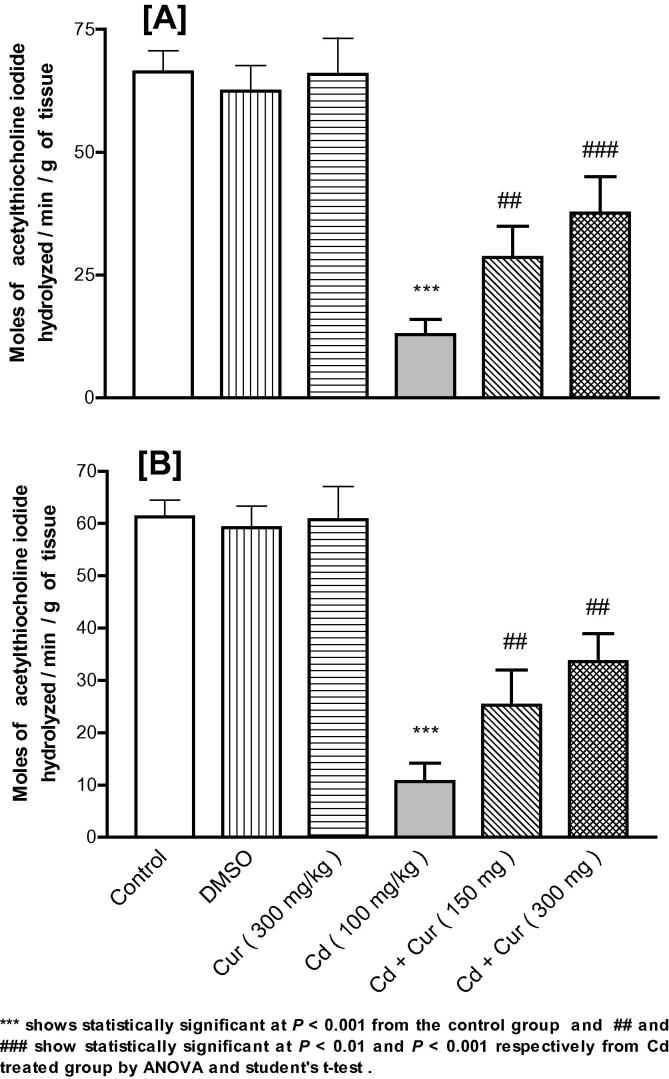
3.4. Acetylcholinesterase enzyme
The present results show that acetylcholinesterase (AChE) enzyme in the brain tissue of male mice (Fig. 4A) and female mice (Fig. 4B) were significantly depleted due to Cd exposure and this effect was ameliorated due to Cur treatment significantly in a dose-dependent manner.
Figure 4.
Effect of cadmium (Cd) exposure on the depletion of acetylcholinesterase (AChE) in the brain tissue of male (A) and female (B) mice respectively and ameliorating effect of curcumin (Cur) significantly and dose-dependently on AChE in male (A) and female (B) mice. DMSO is dimethyl sulfoxide.
4. Discussion
The present results suggest that exposure of mice to Cd is toxic and influences various behavioral activities as well as the blood parameters and levels of AChE activities in the brain tissues of the treated animals. The rodents exposed to Cd in earlier studies also are reported to display lowered body weight (Sorell and Graziano, 1990, Salama and El-Bahr, 2007), impaired behavioral activity and worsened conditioned reflex response (Baranski, 1984, Claverie et al., 2000), impaired neurobehavioral (Viaene et al., 2000) and neurotoxicological (Desi et al., 1998) developments. These earlier studies are in accordance with the present findings and support the present effects of Cd on behavioral activities in mice. The present results suggest that exposure of mice to Cd is toxic and influences various behavioral activities as well as the blood parameters, levels of hormones in the blood and levels of AChE in the brain tissues of the treated animals. Thus, these results clearly emphasize that Cd exposure is extremely teratogenic and influences the social behavior of rodents. The present findings are concordance with earlier studies (Nagymajtenyi et al., 1997, Abu-Taweel, 2007) and in disagreement also with other studies (Tercariol et al., 2011). In fact, Cd is able to cross the blood–brain barrier (BBB) and accumulates in the brain (Abu-Taweel et al., 2013). Kaoud and Mekawy (2011) investigated the effects of cadmium chloride on social behavior of mice and morphology of their brains. They found a significant and dose-dependent increase in the non-social behavior, a significant decline in the social behavior including naso-genital and naso-nasal contact, number of fights, rear, wall rear and displacement activities of the Cd exposed groups.
The major target organs that are reported for the acute oral toxicity of Cd are liver (Ognjanovic et al., 1995) and central nervous tissue (Lehotzky et al., 1990). Previous investigations show that oral intake of Cd induces its accumulation in the red blood cells (Karmakar et al., 2000, Ognjanović et al., 2003, Jelena et al., 2014), heart (Zikic et al., 1998) and skeletal muscle of rats (Pavlovic et al., 2001), which was accompanied by considerable alterations of enzymatic and non-enzymatic component of antioxidant defense system (AOS). At cellular level also it has been reported that, Cd mainly accumulates in the cytosol (70%), followed by the nucleus (15%) and lowest in mitochondria and the endoplasmic reticulum (Stohs et al., 2001). Such accumulation of Cd mainly in cytosol might have lead to variations in the phosphate pool of the animals which ultimately lead to disturbed energy source with consequent disturbance in their metabolism (Wilson, 1973, Waisberg et al., 2003), and this is probably reflected in the form of disturbed behavioral activities.
Testosterone is primarily produced in the testes and it is the main male sex hormone and an anabolic steroid. The brain is an important site of action for testosterone (Rommerts, 2004). It is demonstrated that testicular secretions are necessary for aggressive behavior in roosters. Increased aggression was observed in males exposed to anabolic androgenic steroids, which are independent of treatment age (Abu-Taweel et al., 2011). Research on the neuroendocrinology of aggression has been dominated by the paradigm that the brain receives gonadal hormones, primarily testosterone, which modulate relevant neural circuits (Soma et al., 2008). There is overwhelming evidence that most of the effects of testosterone in mediating aggression occur after aromatization (Mehta and Beer, 2010). Testosterone and progesterone were decreased significantly by Cd treatment in present study and also as reported in earlier findings (Sayed et al., 2014, Siu et al., 2009). This significant decline in these hormones may be associated with the element alternation of the “standard opponent” and “tube restraint” tests.
Blood parameter results, namely red blood cell count, packed cell volume, hemoglobin content, total white blood cell count and blood platelets, were depleted in male and female Cd-intoxicated mice. Our results are in agreement with other studies (Karmakar et al., 2000, Kostić et al., 1993, El-Demerdash et al., 2004, Mladenović et al., 2014). Exposure to Cd can result in toxic effects on a variety of tissues, but the first to be affected is blood, as Cd binds to the membrane of erythrocytes and plasma albumin and is then transported to the liver (Wilson, 1973). Erythrocytes are the most common markers of oxidative stress due to the sensitivity of their cell membranes and antioxidant enzymes to free radicals (Matés, 2000, Halliwell and Gutteridge, 2007). Since increased LPO disrupts the normal function or destroys the membrane of erythrocytes, the final outcome of these events is a decrease in packed cell volume (hematocrit), hemoglobin content total white blood cell count, blood platelets and eventually anemia, and such a phenomenon was also observed in our study (Wilson, 1973).
Cur in the present study had a significant ameliorating effect on the Cd-induced deficits in the social behavior, blood parameters and biochemical analysis. Furthermore, the ineffectiveness of Cur alone to cause any behavioral and biochemical deficits, clearly suggests that Cur alone is non-toxic and further supports the ameliorating effect of Cur on the behavioral and biochemical toxicity induced by Cd. Cur reportedly has potent antioxidant activities (Stajn et al., 1997), anti-inflammatory (Motterlini et al., 2000) and chemoprotective properties (Ray, 2005). The biochemical damage due to Cd and ameliorating effect of Cur in the present study may be due to the fact that Cur has many possible reported benefits, however; full effects are not yet fully understood and more and more research work is needed.
5. Conclusion
It is concluded from the present results that Cur possesses several multifold beneficial effects that may include protection from Cd induced toxicity that may be causing disturbances in the social behavior, enzyme activity in brain and disturbance in blood parameters and hormonal dysfunction in the blood. Thus, inclusion of Cur in our normal diet and its use as a nutritional supplement may have a tremendous potential for protection from Cd intoxication and health improvement.
Conflict of interest
No conflict of interest whatsoever.
Acknowledgements
The author would like to extend sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-VPP-240.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abu-Taweel G., Ajarem J.S., Ahmad M. Effect of prenatal lead in the cross-fostered mice offspring. Saudi J. Biol. Sci. 2006;13:80–90. [Google Scholar]
- Abu-Taweel G.M. Effect of postnatal lead exposure on the social behavior of laboratory mice offspring at adolescent age. Indian J. Appl. Pure Biol. 2007;22:165–170. [Google Scholar]
- Abu-Taweel G., Ajarem J.S. Effect of perinatal lead exposure on the social behaviour of laboratory mice offspring at adolescent age. Saudi J. Biol. Sci. 2008;15:67–72. [Google Scholar]
- Abu-Taweel G.M., Ajarem J.S., Ebaid H. Aluminum-induced testosterone decrease results in physiological and behavioral changes in male mice. Afr. J. Biotechnol. 2011;10:201–208. [Google Scholar]
- Abu-Taweel G., Ajarem J.S., Ahmad M. Protective effect of curcumin on anxiety, learning behavior, neuromuscular activities, brain neurotransmitters and oxidative stress enzymes in cadmium intoxicated mice. J. Behav. Brain Sci. 2013;3:74–84. [Google Scholar]
- Aggarwal B.B., Sundaram C., Malani N., Ichi-kawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Ahsan H., Parveen N., Khan, Hadi S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Ajarem J.S., Ahmad M. Behavioural and biochemical consequences of perinatal exposure of mice to instant coffee: a correlative evaluation. Pharmacol. Biochem. Behav. 1991;40:847–852. doi: 10.1016/0091-3057(91)90096-k. [DOI] [PubMed] [Google Scholar]
- Ajarem J.S., Ahmad M. Effects of perinatal exposure of cardamom (Elettaria cardamomum) on the post-natal development and social behaviour of mice offspring. J. King Saud Univ. Sci. 1992;4:151–162. [Google Scholar]
- Ajarem J.S., Ahmad M. Effects of consumption of fresh kola-nut extract by female mice on the postnatal development and behaviour of their offspring. J. King Saud Univ. Sci. 1994;6:41–50. [Google Scholar]
- Antonio M.T., Corpas I., Leret M.L. Neurochemical changes in newborn rat’s brain after gestational cadmium and lead exposure. Toxicol. Lett. 1999;104:1–9. doi: 10.1016/s0378-4274(98)00125-8. [DOI] [PubMed] [Google Scholar]
- Attia A.M.M., Ibrahim F.A.A., Abdel-Latif N.A., Aziz S.W. Antioxidant effects of curcumin against cadmium chloride-induced oxidative stress in the blood of rats. J. Pharmacognosy Phytother. 2014;6:33–40. [Google Scholar]
- Baranski B. Effect of exposure of pregnant rats to cadmium on prenatal and postnatal development of the young. J. Hyg. Epidemiol. Microbiol. 1984;29:253–262. [PubMed] [Google Scholar]
- Barregard L., Bergström G., Fagerberg G. Cadmium, type 2 diabetes, and kidney damage in a cohort of middle-aged women. Environ. Res. 2014;135:311–316. doi: 10.1016/j.envres.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Brain P.F., Ajarem J.S., Petkov V.V. The utility of ethological assessments of murine agonistic interactions in behavioural teratology: the foetal alcohol syndrome. In: Oliver B., Mos J., Brain P.F., editors. Ethopharmacology of Agonistic Behaviour in Animals and Humans. Martinus Nijhoff Publishers; Dordrecht: 1987. pp. 110–121. [Google Scholar]
- Chen J., Tang X.Q., Zhi J.L., Cui Y., Yu H.M., Tang E.H., Sun S.N., Feng J.Q., Chen P.X. Curcumin protects PC12 cells against 1-Methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- Ciftci O., Tanyildizi S., Godekmerdan A. Protective effect of curcumin on immune system and body weight gain on rats intoxicated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Immunopharmacol. Immunotoxicol. 2010;32:99–104. doi: 10.3109/08923970903164318. [DOI] [PubMed] [Google Scholar]
- Ciftci O., Ozdemir I., Tanyildizi S., Yildiz S., Ogu-zturk H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dio-xin-induced oxidative stress in rat liver. Toxicol. Ind. Health. 2011;27:447–453. doi: 10.1177/0748233710388452. [DOI] [PubMed] [Google Scholar]
- Cole G.M., Teter B., Frautschy S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie C., Corbella R., Martin D., Diaz C. Protective effects of zinc on cadmium toxicity in rodents. Biol. Trace Elem. Res. 2000;75:1–9. doi: 10.1385/BTER:75:1-3:1. [DOI] [PubMed] [Google Scholar]
- Desi I., Nagymajtenyi L., Schulz H. Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J. Appl. Toxicol. 1998;18:63–70. doi: 10.1002/(sici)1099-1263(199801/02)18:1<63::aid-jat475>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ellman G.L., Courtney K.D., Valentino A.J.R., Featherstone R.M. A new and rapid colorimetric determination of Acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium-induced changes in lipid peroxidation, blood haematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and β-carotene. Food Chem. Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. Oxygen is a toxic gas – an introduction to oxygen toxicity and reactive species. In: Halliwell B., Gutteridge J.M.C., editors. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 2007. pp. 1–29. [Google Scholar]
- IARC . IARC Press; Lyon: 1993. International Agency for Research on Cancer Monographs. Cadmium. (pp. 119–238) [Google Scholar]
- Ikeda M., Zang Z.W., Moon C.S., Shimbo S., Watanabe T., Nakatsuka H., Matsudainoguchi N., Higashikawa K. Possible effects of environmental cadmium exposure on kidney function in the Japanese general population. Int. Arch. Occup. Environ. Health. 2000;73:15–25. doi: 10.1007/pl00007933. [DOI] [PubMed] [Google Scholar]
- Jarup L., Alfven T., Persson B., Toss G., Elinder C.G. Cadmium may be a risk factor for osteoporosis. Occup. Environ. Med. 1998;55:435–439. doi: 10.1136/oem.55.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Rao L.J., Sakariah K.K. Anti-oxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. doi: 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- Jelena M., Branka O., Nataša Đ., Miloš M., Veroljub K., Andraš S., Zorica S. Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arh. Hig. Rada. Toksikol. 2014;65:37–46. doi: 10.2478/10004-1254-65-2014-2405. [DOI] [PubMed] [Google Scholar]
- Kaoud H.A., Mekawy M.M. Effect of cadmium pollution on neuromorphology and function of brain in mice offspring. Nat. Sci. 2011;9:28–35. [Google Scholar]
- Karmakar R., Bhattacharya R., Chatterjee M. Biochemical, haematological and histopathological study in relation to time-related cadmium-induced hepatotoxicity in mice. Biometals. 2000;13:231–239. doi: 10.1023/a:1009279803842. [DOI] [PubMed] [Google Scholar]
- Khanna N. Turmeric: nature’s precious gift. Curr. Sci. 1999;76:1351–1356. [Google Scholar]
- Kostić M.M., Ognjanović B.I., Dimitrijević S., Žikić R.V., Štajn A., Rosić G.L., Živković R.V. Cadmium induced changes of antioxidant and metabolic status in red blood cells of rats: in vivo effects. Eur. J. Haematol. 1993;51:86–92. doi: 10.1111/j.1600-0609.1993.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Kuhad A., Pilkhwal S., Sharma S., Tirkey N., Chopra K. Effect of curcumin on inflammation and oxidative stress in displatin-induced experimental nephrotoxicity. J. Agric. Food Chem. 2007;55:10150–10155. doi: 10.1021/jf0723965. [DOI] [PubMed] [Google Scholar]
- Lehotzky K., Ungvary G., Polinak D., Kiss A. Behavioral deficits due to prenatal exposure to cadmium chloride in CFY rat pups. Neurotoxicol. Teratol. 1990;12:169–172. doi: 10.1016/0892-0362(90)90130-5. [DOI] [PubMed] [Google Scholar]
- Matés J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83–104. doi: 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Mehta P.H., Beer J. Neural mechanisms of the testosterone aggression relation: the role of orbitofrontal cortex. J. Cogn. Neurosci. 2010;22:2357–2368. doi: 10.1162/jocn.2009.21389. [DOI] [PubMed] [Google Scholar]
- Mladenović J., Ognjanović B., Đorđević N., Miloš Matić M., Knežević V., Andraš Štajn A., Saičić Z. Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arh. Hig. Rada. Toksikol. 2014;65:37–46. doi: 10.2478/10004-1254-65-2014-2405. [DOI] [PubMed] [Google Scholar]
- Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Nagymajtenyi L., Schulz H., Desi I. Behavioural and functional neurotoxicological change caused by cadmium in a three-generational study in rats. Hum. Exp. Toxicol. 1997;16:691–699. doi: 10.1177/096032719701601201. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, NTP Toxicology and Carcinogenesis in Studies of Turmeric Oleoresin, 1993. (CAS No. 8024-37-1) (Major Component 79–85% Curcumin, CAS No. 458-37-7) in F344/N rats and B6C3F1 mice (Feed Studies). N. Toxicol. Prog. Tech. Repro. Ser. 427, 1–275. [PubMed]
- Ognjanovic B., Zikic R.V., Stajn A., Saicic Z.S., Kostic M.M., Petrovic V.M. The effects of selenium on the antioxidant defense system in the liver of rats exposed to cadmium. Physiol. Res. 1995;44:293–300. [PubMed] [Google Scholar]
- Ognjanović B.I., Pavlović S.Z., Maletić S.D., Žikić R.V., Štajn A.Š., Radojčić R.M., Saičić Z.S., Petrović V.M. Protective influence of vitamin E on antioxidant defence system in the blood of rats treated with cadmium. Physiol. Res. 2003;52:563–570. [PubMed] [Google Scholar]
- Pavlovic S.Z., Ognjanovic B.I., Stajn A.S., Zikic R.V., Saicic Z.S., Petrovic V.M. Antioxidant defense system in skeletal muscle of rats treated with cadmium. A possible protective role of coenzyme Q10. Jugo. Med. Biochem. 2001;20:229–235. [Google Scholar]
- Perez-Arriaga L., Mendoza-Magana M.L., Cortes-Za-rate R., Corona-Rivera A., Bobadilla-Morales L., Troyo- Sanroman R., Ramirez-Herrera M.A. Cytotoxic effect of curcumin on Giardia lamblia trophozoites. Acta Trop. 2006;98:152–161. doi: 10.1016/j.actatropica.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary poly-phenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ramsewak R.K., DeWitt D.L., Nair M.G. Cyto-toxicity, antioxidant and anti-inflammatory activities of curcumin III from Curcuma longa. Phytomedicine. 2000;7:303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Ray A. Cancer preventive role of selected dietary factors. Indian J. Cancer. 2005;42:11–20. doi: 10.4103/0019-509x.15095. [DOI] [PubMed] [Google Scholar]
- Rommerts, F.G., 2004. Testosterone: an overview of biosynthesis, transport, metabolism and non-genomic actions. In: Nieschlag, E., Behre, H.M. (Eds.), Testosterone: Action Deficiency Substitute. pp. 1–37.
- Salama A.F., El-Bahr S.M. Effect of curcumin on cadmium-induced oxidative testicular damage in rats. J. Med. Res. Inst. 2007;28:167–173. [Google Scholar]
- Sayed M.M., Hassanein K.M.A., Senosy W. Protective effects of thymoquinone and l-cysteine on cadmium-induced reproductive toxicity in rats. Toxicol. Rep. 2014;1:612–620. doi: 10.1016/j.toxrep.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Chaudhary S., Patni A., Sankhla V. Effect of cadmium chloride induced genotoxicity in bone marrow chromosomes of Swiss albino mice and subsequent protective effects of Emblica officinalis and vitamin C. J. Herb. Med. Toxicol. 2007;1:67–71. [Google Scholar]
- Singh P., Mogra P., Sankhla V., Deora K. Protective effects of curcumin on cadmium chloride induced colon toxicity in Swiss albino mice. J. Cell. Mol. Biol. 2011;9:31–36. [Google Scholar]
- Siu E.R., Mruk D.D., Porto C.S., Cheng C.Y. Cadmium induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R., Rohlfe F.J. W.H. Freeman; San Francisco, Starling, J.A.: 1981. Biometry: The Principles and Practice of Statistics in Biological Research. 1975. [Google Scholar]
- Soma K.K., Scotti M.A., Newman A.E., Charlier T.D., Demas G.E. Novel mechanisms for neuroendocrine regulation of aggression. Front. Neuroendocrinol. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Sorell T.L., Graziano J.H. Effect of oral cadmium exposure during pregnancy on maternal and fetal zinc metabolism in the rat. Toxicol. Appl. Pharmacol. 1990;102:537–545. doi: 10.1016/0041-008x(90)90048-y. [DOI] [PubMed] [Google Scholar]
- Stajn A., Zikic R.V., Ognjanovic B., Saicic Z.S., Pavlovic S.Z., Kostic M.M., Petrovic V.M. Effect of cadmium and selenium on the antioxidant defense system in rat kidneys. Comp. Biochem. Physiol. 1997;117C:167–172. doi: 10.1016/s0742-8413(97)00063-7. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Bagchi D., Hassoun E., Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001;20:77–88. [PubMed] [Google Scholar]
- Tarasub N., Tarasub C., Ayutthaya W.D.N. Protective role of curcumin on cadmium-induced nephrotoxicity in rats. J. Environ. Chem. Ecotoxicol. 2011;3:17–24. [Google Scholar]
- Tercariol S.G., Almeida Alaor Aparecido, Godinho A.F. Cadmium and exposure to stress increase aggressive behavior. Environ. Toxicol. Pharmacol. 2011;32:40–45. doi: 10.1016/j.etap.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Tirkey N., Kaur G., Vij G., Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005;5:1–15. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene M.K., Masschelein R., Leenders J., De Groof M., Swerts L.J.V.C., Roels H.A. Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Occup. Environ. Med. 2000;57:19–27. doi: 10.1136/oem.57.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisberg M., Joseph P., Hale B., Beyersmann D. Molecular mechanisms of cadmium carcinogenesis: a review. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- Wilson J.G. Mechanisms of teratogenesis. Am. J. Psychiatry. 1973;136:129–132. doi: 10.1002/aja.1001360202. [DOI] [PubMed] [Google Scholar]
- Yamane T. The distribution. In: Yamane T., editor. Statistics, and Introductory Analysis. 3rd ed. Harper and Row Publishers; London: 1973. pp. 647–650. [Google Scholar]
- Zikic R.V., Stajn A.S., Ognjanovic B.I., Saicic Z.S., Kostic M.M., Pavloviv S.Z., Petrovic V.M. The effect of cadmium and selenium on the antioxidant enzyme activities in rat heart. J. Environ. Pathol. Toxicol. Oncol. 1998;17:259–264. [PubMed] [Google Scholar]