Abstract
TH17 cells and their associated signature cytokines, IL-17 and IL-22, are highly elevated in primary Sjögren's syndrome (pSjS). The levels of IL-22 present in sera showed significant correlations with many disease parameters, specifically hyposalivation, anti-SSB, anti-SSA/SSB, hypergammaglobulinemia and rheumatoid factor. The present study aims to examine the biological function of IL-22 on human salivary glands. To accomplish the goal, microarray analysis using the HumanHT-12 v4 Expression BeadChip was utilized to determine the biological function of IL-22. Differential expression analyses were conducted using the LIMMA package from the Bioconductor project. MTT assay, flow cytometry and Western blotting were used to identify the function of IL-22 on human salivary gland cells. Results indicate an extensive effect of IL-22 on many major molecular functions including activation of antimicrobial genes and downregulation of immune-associated pathways. Functional studies performed in-vitro using human salivary gland cells treated with IL-22 indicated a direct effect of IL-22 on cell cycling, specifically reducing cellular proliferation at the G2-M phase by activation of STAT3. These results suggest the important role of IL-22 in the salivary gland function. The present study suggests that IL-22 might be involved in regulating inflammation and controlling the cell proliferation in SjS.
Keywords: IL-22, Cytokine, Sjogren's syndrome, Gene expression, Microarray
1. Introduction
Sjögren's syndrome (SjS) is a complex chronic autoimmune disease that targets the exocrine glands, predominantly the salivary and lacrimal glands, resulting in xerostomia and keratoconjunctivitis sicca. While SjS can be diagnosed as a stand-alone disease, referred to as primary SjS (pSjS), it is often seen in association with other autoimmune diseases, referred to as secondary SjS [1], [2]. The underlying pathogenesis of SjS is still elusive, but is thought to involve abnormal salivary gland homeostasis, neural circuitry malfunction from the presence of autoantibodies, and progressive tissue destruction mediated by infiltrating cells [2]. One of the major criteria in the disease diagnostic is the presence of periductal lymphocytic infiltrations, observed as lymphocytic foci (LF) in the salivary and lacrimal glands [3]. Although, LF are not correlated with the severity of the autoimmunity, they remain a critical element in understanding the etiology of the disease. Our previous studies demonstrated the presence of TH17 cells producing interleukin (IL)-17 and dendritic cells (DC) producing IL-23 in the salivary glands of human and animal models of SjS [4]. While neither IL-17 nor IL-23 correlated significantly with major disease parameters, levels of IL-22 correlated directly with hyposalivation, anti-SSB, anti-SSA/SSB combined, hypergammaglobulinemia and rheumatoid factor (RF) [5]. Furthermore, Ciccia et al. have shown that IL-22 and associated cytokine IL-17 and IL-23 is highly present in the inflamed salivary glands of primary SjS patients [6]. IL-22 is a cytokine belonging to the IL-10 family, along with IL-10, IL-19, IL-20, IL-24, and IL-26 [7]. IL-22 mediates host defenses against invading pathogens. It requires the presence of IL-23 in order to be expressed by immune cells [8]. IL-22 is produced predominantly by CD4+ TH17 cells, but its expression can also be found among other activated T cell subsets, natural killer (NK) cells, DC and innate lymphoid cells (ILCs), although at lower levels [9].
IL-22 receptor (R) complex is a heterodimeric molecule composed of IL-22RA1 and IL-10R2 [10] expressed mainly on non-hematopoietic cells such as keratinocytes, epithelial cells, and pancreas acinar cells [11]. The receptor transduces a signal through phosphorylation of tyrosine kinases JAK1 and TYK2, followed by the activation of STAT3, and to a lesser degree heterodimeric STAT1/3 during a signaling cascade [12]. IL-22 has also been reported to activate signal transductions via the MAPK pathways of ERK1/2, JNK, and p38 for induction of IL-22 related genes [13]. Epithelial cells express high levels of IL-10R2 and IL-22R1, therefore IL-22 can initiate a strong response from epithelial cells which includes production of cytokines, chemokines, acute phase proteins, and a number of anti-microbial molecules such as β-defensin, lipocalins, and S100 calcium binding proteins [7]. It is also involved in tissue repair following exacerbated immune responses and epithelial-barrier functions against bacterial infection [14]. Interestingly, IL-22 has been shown to be pathogenically associated with several autoimmune diseases including rheumatoid arthritis [15], Crohn's disease [16], as well as non-autoimmune diseases such as respiratory-distress syndrome [17] and cystic fibrosis [18].
The association of IL-22 with several major disease parameters is striking [5]. Increased levels of IL-22 were significantly present in the salivary glands of pSjS patients and the major source of IL-22 production was identified to be NKp44(+) NK cells [6]. At the present, however, little is known about its biological and immunological effects on the gland cells. As a result, the focus of this study is to first examine the global downstream biological function of IL-22 on human salivary gland cells, then subsequently determine its specific role in cellular proliferation. Results indicate IL-22 has significant impact on a number of biological and molecular pathways, particularly the cell cycle of gland cells.
2. Materials and methods
2.1. Sample and RNA preparation
Human submandibular gland (HSG) cell line was initially generated at the National Institute of Dental and Craniofacial Research (NIDCR). HSG cells were generously provided by Dr. Seunghee Cha who obtained it from Dr. Joseph Katz at the University of Florida [19]. HSG cells were cultured in complete media and treated with recombinant (r) IL-22 at 100 ng/mL for 45 min. Reactions consisted of replicates of three wells. Total RNA was isolated as instructed by the manufacturer (AM1921, PARIS kit, ThermoFisher). Total RNA was labeled with biotin using Target Amp cDNA synthesis kit (Epicenter). Labeled cRNA was hybridized to the HumanHT-12 v4 Expression BeadChip (Illumina).
2.2. Differential gene expression analysis
Microarray hybridization and analysis were carried out as previously described [20], [21]. In brief, microarray data were normalized via the lumi package in R, using the variance stabilizing transformation of the package and robust spline normalization. Differential expression analyses were conducted using the LIMMA package from the Bioconductor project [22]. We used the false discovery rate (FDR) to adjust for multiple testing [23]. B-statistic (the log of the odds that the gene is differentially expressed) was calculated for each gene. Duplicate genes, when present, were removed and their expression levels averaged across the duplicates. A total of 392 genes (88 upregulated and 304 downregulated) were found with at least 1.5-fold change in expression levels. Gene expression levels of selected genes were confirmed by real-time PCR (data not shown). Gene Ontology analysis was done by the Bioconductor package and “GOstats” was used for testing the enrichment of Gene Ontology terms (biological processes, molecular functions and cellular compartments) in the differentially expressed genes [24]. A p-value based on the hypergeometric test was computed to assess whether the number of genes associated with the term is larger than expected. The p-values obtained were adjusted for multiple testing using the FDR.
2.3. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) viability assay
HSG cells were cultured in DMEM, supplemented with 10% FBS. Cells (2 × 105) were treated with recombinant (r) IL-22 cytokine (R&D) at a concentration of 0, 10, 50, 100 or 500 ng/mL in triplicate for 48 h. MTT solution (Sigma) was added 4 h before the 48 h stimulation. At 48 h, supernatant was discarded and MTT solvent was added to dissolve purple MTT crystals. Contents from each well were transferred completely to an ELISA plate and absorbance values were read using an ELISA plate reader at wavelength 570 nm and with background absorbance at 655 nm subtracted.
2.4. Flow cytometry for cell cycle
HSG cells (2 × 105) cultured in complete media were treated in triplicate with rIL-22 at a concentration of 100 ng/m. Cells were allowed to proliferate for 72 h at 37 °C with 5% CO2 and then treated with DyeCycle Ruby stain according to the manufacturer's instructions (Life Technologies). After 30 min at 37 °C incubation, cells were trypsinized and analyzed using Accuri C6 Flow Cytometer (Accuri). Data analysis was performed using FlowJo (Tree Star).
2.5. Western blotting
HSG cells (2 × 105) were plated overnight in serum-free media. Cells were stimulated in triplicate with different concentration of rIL-22 for 45 min (0, 10, 50, 100 and 500 ng). At a specific concentration, cell lysates were obtained using nonidet-P40 (NP40) buffer (150 mM NaCl, 1.0% NP-40, pH 8.0 50 mM Tris). The lysates were separated on 4–20% linear gradient SDS-PAGE gels and transferred to PVDF membranes. The membranes were probed with primary antibodies, including anti-STAT3 and anti-pSTAT3 at 1:1000 (Cell Signaling) and anti-β-actin at 1:20,000 (Sigma). Secondary IRDye infrared dye antibodies were used (1:20,000 for IRDye 800CW for anti-β-actin and IRDye 650 for anti-STA3 and anti-pSTAT3). The signals were visualized using the Odyssey imager (Li-Cor). Signal intensity was quantified by ImageJ with relative fold difference obtained by normalizing against respective β-actin levels. The experiment was repeated twice for consistency.
2.6. Statistical analysis
Statistical evaluations were determined using the Mann–Whitney U test. A two-tailed p value < 0.05 was considered significant. Data are presented as mean ± SEM. Statistical analyses and graphs were generated by the GraphPad softwares (GraphPad).
3. Results
3.1. Differential gene expressions in HSG cells mediated by IL-22
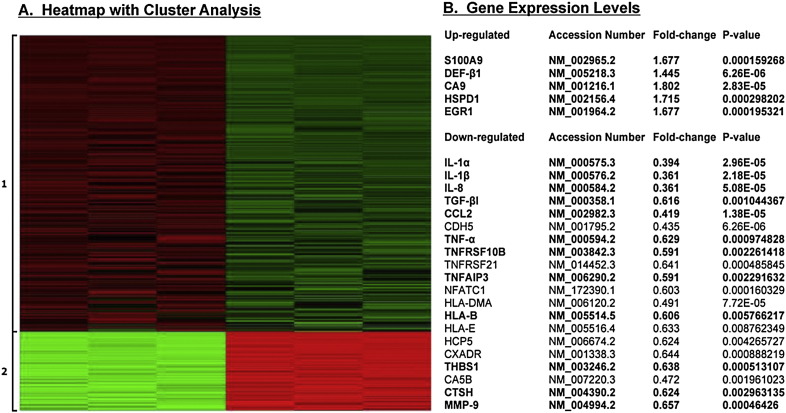
IL-22R (IL-22Rα1 and IL-10R2) are expressed on non-hematopoietic cells, but not on hematopoietic cells, suggesting IL-22 mediates downstream effect via a paracrine system. Moreover, IL-22R is highly expressed in the salivary glands of SjS patients and animal models. However, the biological effect of IL-22 on the salivary gland epithelial cells has not been explored. To address this issue, rIL-22 was used to stimulate HSG expressing both subunits of IL-22R as a means to profile the physiological function(s) IL-22 exerts on salivary gland epithelial cells. HSG cells were stimulated with rIL-22 for 45 min at 100 ng/mL. Total RNAs were isolated from stimulated cells and subjected to transcriptome analysis using microarray. As indicated in Fig. 1, based on HP Cluster analyses, stimulation of rIL-22 resulted in two distinctive differential gene expression (DGE) clusters with the majority of the gene sets down-regulated (specifically a total of 392 genes with at least 1.5-fold change in expression levels, with 304 down-regulated and 88 up-regulated). Examining individual genes (Fig. 1) within the two clusters revealed up-regulation of two antimicrobial proteins, S100A9 and β-defensin-1 (DEF-β1). In addition, IL-22 up-regulated carbonic anhydrase 9 (CA9), heat shock 60 kDa protein 1 (HSPD1), and early growth response 1 (EGR1), each having been shown to be associated with SjS. Of the 304 genes down-regulated, a number of these genes exhibited immune-modulated functions particularly IL-1α, IL-1β, IL-8, TGF-βI, CCL2, tumor necrosis factors associated (TNF-α, TNFRSF10B, TNFRSF21, TNFAIP3, NFATC1), major histocompatibility complexes (HLA-DMA, HLA-B, HLA-E, HCP5), and matrix metalloproteinase 9 (MMP-9). Therefore, DGE data suggest that IL-22 plays a significant role in activating anti-microbial gene products while negatively regulating gene sets associated with inflammation, thereby contributing to the general suppressive function of IL-22.
Fig. 1.
Transcriptome profiles of differentially expressed genes by IL-22 depicted by Heatmap and HP Cluster analyses. Heatmap of differentially expressed genes (n = 392) exhibited by HSG cells stimulated with rIL-22 at 100 ng/mL for 45 min, grouped into two clusters based on expression profiles. Upregulated gene expressions are shown in red, and downregulated gene expressions are shown in green (A). Selected individual genes with gene expression levels. Bold highlighted genes are examples of genes that have been shown to be associated with SjS (B).
3.2. Pathway analysis based on transcriptome profiles of HSG cells stimulated by IL-22
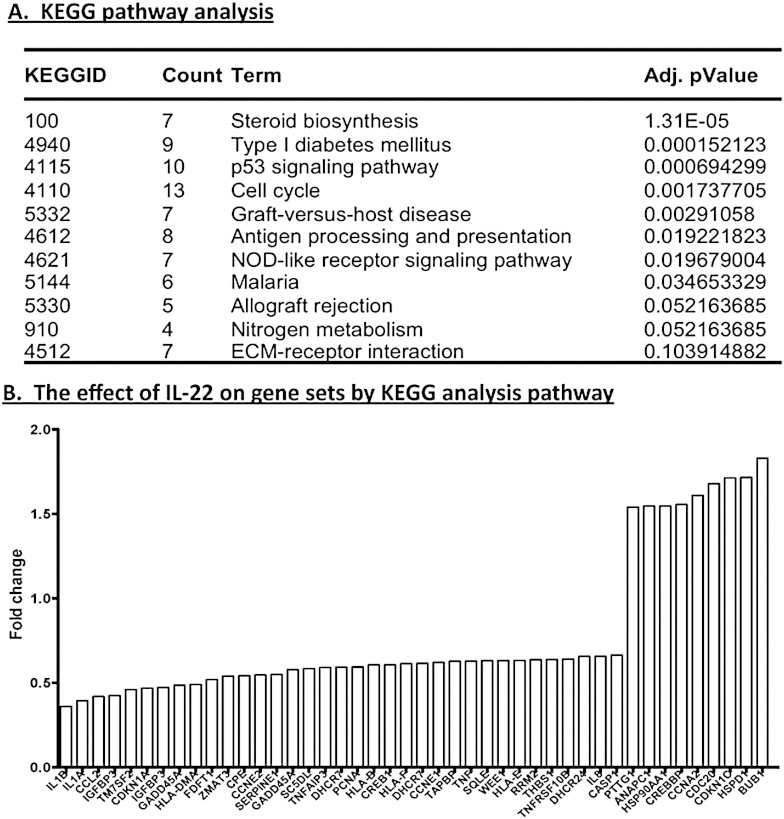
To further understand the role of IL-22, the transcriptome profile was employed to discern general functions of the cytokine on gland cells. Differential expression analyses were performed using the LIMMA package. The Bioconductor package “GOstats” was used to compartmentalize significant associations of the 392 differentially expressed genes with biological processes, molecular functions and cellular compartments. Some 374 biological processes (Supplementary Table 1) proved to be statistically significantly affected by IL-22 stimulation. The 13 most highly significant processes are presented in Table 1. The majority of these biological processes involve glandular development, regulation of steroid hormone stimulation, cell proliferation and apoptosis, processes implicated in SjS of both human and animal models [20], [21], [25]. The molecular function and cellular components that drive these processes are also activated, as presented in Table 1, Table 2. Analysis of the KEGG pathway revealed similar biological processes such as steroid biosynthesis and cell cycle pathway with p53 signaling. In addition, the KEGG pathway analysis showed significant associations with autoimmune responses and inflammation, specifically, antigen processing and presentation, NOD-like receptor signaling pathway, ECM-receptor interaction, type I diabetes mellitus, p53 signaling pathway, graft-versus-host disease, allograft rejection and malaria. Microarray data analysis uncovered a number of important pathways, which are significantly associated with IL-22 exposure (Fig. 2A). One pathway that appears to be consistently present in both biological pathway and KEGG pathway analysis is the cell cycle regulation. Consistently, the differentially expressed genes tend to indicate a strong association with cell cycling whether analyzed by their biological processes, molecular functions or cellular components, defined by KEGG. Examining individual genes revealed that most of the transcripts are down-regulated when exposed to rIL-22 (Fig. 2B), substantiating the potential suppressive nature of IL-22.
Table 1.
Biological and molecular processes.
| GOBPIDa | Count | Term | Adj. p valuea |
|---|---|---|---|
| GO:0048513 | 83 | Organ development | 2.93E − 07 |
| GO:0008283 | 58 | Cell proliferation | 1.00E − 06 |
| GO:0048545 | 22 | Response to steroid hormone stimulus | 1.91E − 06 |
| GO:0051726 | 31 | Regulation of cell cycle | 4.25E − 06 |
| GO:0006950 | 74 | Response to stress | 4.63E − 06 |
| GO:0010033 | 45 | Response to organic substance | 1.38E − 05 |
| GO:0006915 | 52 | Apoptosis | 1.38E − 05 |
| GO:0009719 | 31 | Response to endogenous stimulus | 2.57E − 05 |
| GO:0070482 | 16 | Response to oxygen levels | 2.79E − 05 |
| GO:0048523 | 73 | Negative regulation of cellular process | 2.80E − 05 |
| GO:0048518 | 82 | Positive regulation of biological process | 2.89E − 05 |
| GO:0008285 | 26 | Negative regulation of cell proliferation | 3.19E − 05 |
| GO:0048519 | 77 | Negative regulation of biological process | 3.27E − 05 |
| GOMFIDa | Count | Term | Adj. p value |
| GO:0005515 | 215 | Protein binding | 5.39E − 07 |
| GO:0005488 | 269 | Binding | 0.002692335 |
| GO:0016538 | 5 | Cyclin-dependent protein kinase regulator activity | 0.006754462 |
| GO:0005520 | 5 | Insulin-like growth factor binding | 0.014497184 |
| GO:0005102 | 36 | Receptor binding | 0.015243094 |
| GO:0005198 | 26 | Structural molecule activity | 0.029975379 |
| GO:0008201 | 9 | Heparin binding | 0.029975379 |
| GO:0004666 | 2 | Prostaglandin-endoperoxide synthase activity | 0.029975379 |
| GO:0005153 | 2 | Interleukin-8 receptor binding | 0.029975379 |
| GO:0016404 | 2 | 15-Hydroxyprostaglandin dehydrogenase (NAD +) activity | 0.029975379 |
| GO:0042288 | 3 | MHC class I protein binding | 0.030373016 |
| GO:0016628 | 4 | Oxidoreductase activity | 0.034730469 |
| GO:0005539 | 10 | Glycosaminoglycan binding | 0.042239084 |
| GO:0045236 | 2 | CXCR chemokine receptor binding | 0.049216246 |
| GO:0001786 | 3 | Phosphatidylserine binding | 0.049216246 |
| GO:0004861 | 3 | Cyclin-dependent protein kinase inhibitor activity | 0.049216246 |
| GO:0050840 | 4 | Extracellular matrix binding | 0.049216246 |
| GO:0016491 | 25 | Oxidoreductase activity | 0.049216246 |
| GO:0019887 | 7 | Protein kinase regulator activity | 0.049216246 |
| GO:0001871 | 10 | Pattern binding | 0.049216246 |
| GO:0030247 | 10 | Polysaccharide binding | 0.049216246 |
| GO:0019838 | 8 | Growth factor binding | 0.049216246 |
| GO:0001968 | 3 | Fibronectin binding | 0.049216246 |
| GO:0032393 | 3 | MHC class I receptor activity | 0.049216246 |
| GO:0042287 | 3 | MHC protein binding | 0.049216246 |
| GO:0005178 | 6 | Integrin binding | 0.049939254 |
GOBPID: gene ontology biological process identifier, GOMFID: gene ontology molecular function identifier, Adj. p value: adjusted p value.
Table 2.
Cellular components.
| GOCCIDa | Count | Term | Adj. p valuea |
|---|---|---|---|
| GO:0005737 | 194 | Cytoplasm | 0.000243044 |
| GO:0044421 | 41 | Extracellular region part | 0.000874948 |
| GO:0031012 | 21 | Extracellular matrix | 0.001868771 |
| GO:0005615 | 32 | Extracellular space | 0.002199408 |
| GO:0005829 | 48 | Cytosol | 0.005541339 |
| GO:0005576 | 61 | Extracellular region | 0.007301133 |
| GO:0070557 | 2 | PCNA-p21 complex | 0.023598719 |
| GO:0043228 | 75 | Non-membrane-bounded organelle | 0.031959872 |
| GO:0043232 | 75 | Intracellular non-membrane-bounded organelle | 0.031959872 |
| GO:0044444 | 129 | Cytoplasmic part | 0.036837262 |
| GO:0005578 | 15 | Proteinaceous extracellular matrix | 0.047411834 |
| GO:0009986 | 16 | Cell surface | 0.061747818 |
| GO:0044424 | 235 | Intracellular part | 0.076893351 |
| GO:0000307 | 3 | Cyclin-dependent protein kinase holoenzyme complex | 0.076893351 |
| GO:0005792 | 12 | Microsome | 0.077763342 |
| GO:0042611 | 4 | MHC protein complex | 0.082698152 |
| GO:0043292 | 8 | Contractile fiber | 0.083047799 |
| GO:0042598 | 12 | Vesicular fraction | 0.083047799 |
| GO:0005856 | 42 | Cytoskeleton | 0.086652092 |
| GO:0070161 | 9 | Anchoring junction | 0.104658968 |
| GO:0042612 | 3 | MHC class I protein complex | 0.105664403 |
| GO:0022627 | 4 | Cytosolic small ribosomal subunit | 0.105664403 |
| GO:0005925 | 6 | Focal adhesion | 0.124055094 |
| GO:0005622 | 238 | Intracellular | 0.124055094 |
GOCCID: gene ontology cellular components identifier. Adj. p value: adjusted p value.
Fig. 2.
General suppression of transcripts by IL-22 stimulation in KEGG pathway. KEGG pathway analysis (A). Specific gene transcripts affected by IL-22 (B). Fold change indicates the changes in expression levels of rIL-22 treated and untreated cells.
3.3. IL-22 induces cell cycle arrest at the G2-M phase of the cell cycle
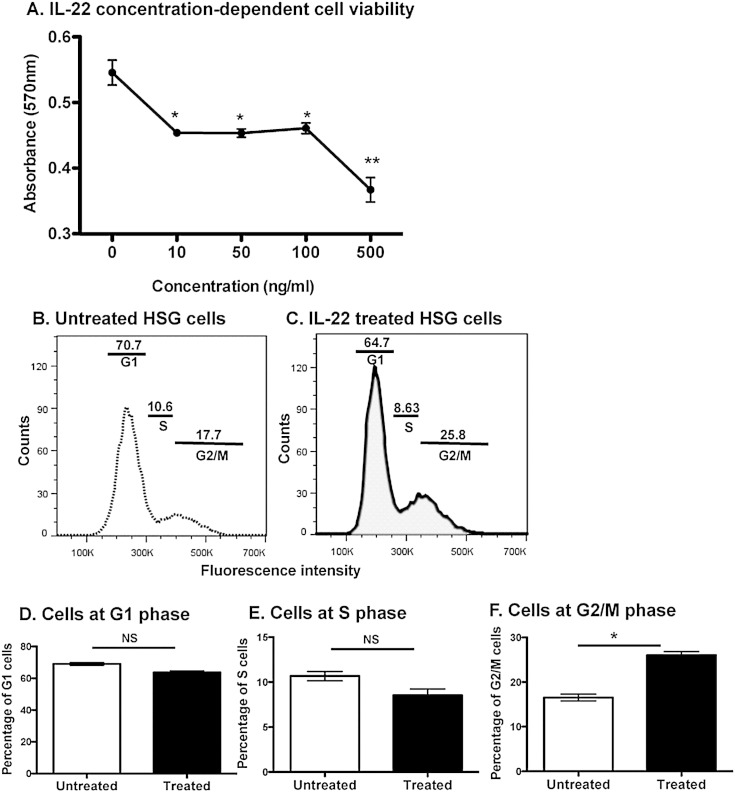
As presented, DGE cluster analysis has indicated that IL-22 exhibited antimicrobial and immune-modulation functions. More importantly, biological function analysis has suggested that IL-22 is involved in cell cycling, although the precise manner or mechanism remains unknown. To examine the effect of IL-22 on cell cycle effects, HSG cells were treated with rIL-22 at concentrations of 10, 50, 100, or 500 ng/mL and absorbance measurements were taken at 48 h after treatment. MTT assays were performed to examine the viability potential of IL-22 on HSG cells. Results presented in Fig. 3A reveal that rIL-22 induced a significant decreased in cell viability when treated at 10 (p = 0.0138), 50 (p = 0.0167), 100 (p = 0.0247), and 500 (p = 0.0092) ng/mL when compared to untreated HSG cells. These results indicate a concentration-dependent activity of IL-22 on cellular viability and proliferation.
Fig. 3.
The effect of IL-22 on cell viability and cell cycle. IL-22 reduces cell viability (A). Cells (2 × 105) were incubated overnight in a 24-well plate. Cells in triplicate wells were treated with 0, 10, 50, 100 or 500 ng/mL of IL-22 for 48 h. At 4 h before time point, MTT solution was added. MTT solvent was added to dissolve purple MTT crystals. Contents from each well were transferred to an ELISA plate and absorbance values were read using an ELISA plate reader at wavelength 570 nm, with background absorbance at 655 nm subtracted. IL-22 induces cell cycle arrest at G2/M phase (B–F). Cells (2 × 105) were either treated with 100 ng/mL of IL-22 for 72 h or left untreated. Analysis was performed upon treatment with Vybrant® DyeCycle™ Ruby stain according to manufacturer's instructions. Representative cell cycle analysis of untreated (B) and (C) is presented. IL-22 treated and untreated cells are shown at G1 (D), S (E) and G2/M (F) phases (n = 3). Each experiment was performed three times for consistency. Statistical significance was determined using Mann–Whitney U test. The two-tailed p value < 0.05 was considered significant. NS: not significant, *p < 0.05.
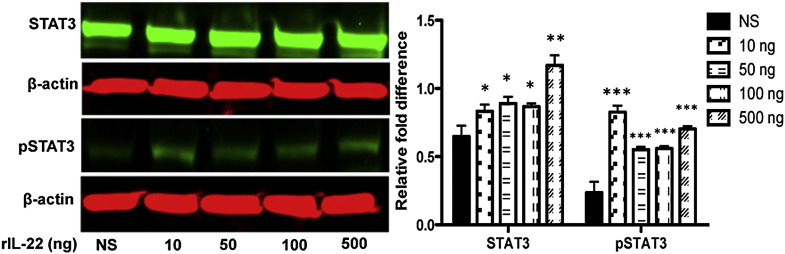
To determine which phase of the cell cycle is affected by IL-22, cells were treated with 100 ng/mL of IL-22, and then analyzed against untreated cells via flow cytometry. Analysis revealed that 17.7% of untreated cells were in the G2-M phase of the cell cycle compared to 25.8% of IL-22 treated cells, whereas no significant changes in the percentage of cells in G1 and S phase were observed between IL-22 treated and untreated cells (Figs. 3B-F). The signal transduction pathway of IL-22 is channeled via the Jak-STAT pathway inducing the phosphorylation of STAT1, STAT3 and STAT5 [12], [13], [26]. As indicated in Fig. 4, stimulation of HSG with rIL-22 activated STAT3 and pSTAT3 in a concentration-dependent manner. No significant changes were observed with STAT1, STAT5 and STAT6 or pSTAT1, pSTAT5 and pSTAT6 (data not shown). These results indicate that treatment of HSG cells with IL-22 induces cell proliferation arrest, which occurs at the G2-M phase of the cell cycle. In addition, IL-22 signal transduction pathway in HSG cells is mediated via the activation of STAT3. Therefore, IL-22 has the potential to reduce viability and proliferation by causing cell cycle arrest mainly at the G2-M phase.
Fig. 4.
Activation of STAT3 and pSTAT3 by IL-22 stimulation. HSG cells (2 × 105) in serum-free media were stimulated without (NS: no stimulation) or with rIL-22 at 10, 50, 100, and 500 ng. Cells lysates were obtained and separated by electrophoresis. The membranes were probed with specific primary antibodies, including anti-STAT3 and their anti-pSTAT3 (Cell Signaling, 1:1000 dilution for anti-STATs and -pSTATs) and anti-β-actin at 1:20,000 (Sigma). Membranes were probed with secondary antibody conjugated with IRDye infrared dye (1:20,000) for 1 h at RT. The signals were visualized using the Odyssey Dual-Mode Imaging System. Blots on each film were quantified by using ImageJ software (http://rsbweb.nih.gov/ij/index.htm) with relative fold difference obtained by normalizing against respective β-actin. Experiments were repeated twice for consistency. Statistical significance was determined using Mann–Whitney U test. The two-tailed p value < 0.05 was considered significant. *p < 0.05, **p < 0.01, and ***p < 0.001.
4. Discussion
Previous studies have demonstrated that IL-22 levels are elevated in the sera of patients with pSjS, and this correlated significantly with lower saliva flow, anti-SSB, both anti-SSA and anti-SSB, RF, and hypergammaglobulinemia. However, at present, the biological function of IL-22 in salivary gland remains unknown. Using transcriptome analysis, we have explored the global transcriptional changes in HSG cells mediated by IL-22. The present study demonstrates a systemic effect of IL-22 on many major molecular functions. KEGG pathway analyses showed that IL-22 contributes specifically to steroid biosynthesis, type I diabetes mellitus, p53 signaling pathway, cell cycle, graft-versus-host disease, antigen processing and presentation, NOD-like receptor signaling pathway and malaria. In addition, functional studies performed in-vitro using HSG cells treated with IL-22 indicated the direct effect of IL-22 on cell cycling, especially cell cycle arrest at the G2-M phase. These results clearly indicate the importance of IL-22 expression in the salivary glands.
IL-22 levels have been associated with many autoimmune and infectious diseases. Production of IL-22 by TH17 and TH22 cells is found in a number of autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus [7]. SjS is characterized by lymphocytic foci in the salivary glands that can be correlated with disease progression and severity [27]. We have found high levels of IL-17 expressed in sera and LSG of pSjS patients; however, there was a lack of correlation with disease parameters [4]. The fact that IL-22 is found at high levels in patents' sera with significant disease phenotype correlations together with its high expression in the exocrine glands implicates its role in the autoimmune process [5].
We have proposed that IL-17 directly contributes to the gross pathology and clinical outcome of SjS, specifically the formation of LF in the salivary glands and the decrease in saliva flow rates in animal models and human patients with SjS [28], [29]. However, until now, there has been limited data presented on whether IL-22 also contributes to the pathology observed in SjS. The current data further reveals a critical role of IL-22 in exerting potential changes on the infiltrating lymphocytic population and epithelial cells to elicit signals that could be pathogenic or protective on the salivary/lacrimal glands depending on the immunological or disease process. One important aspect is the possibility that IL-22 acts as a potential counter-balance to IL-17, providing a possible protective role, as reported for some bacterial/viral infections, inflammatory bowel disease, and hepatitis in experimental animal models [30], [31], [32]. The protective nature of IL-22 is even more evident by examining individual gene expressions by microarray. The up-regulation of antimicrobial products (S100A9, DEF-β1) secreted in saliva is beneficial in maintaining and protecting the oral health, which is often compromised in SjS patients. Increase in HSPD1 or HSP60 upon IL-22 stimulation provides supporting evidence for the protective nature of IL-22 as demonstrated in an elegant study by Delaleu et al. [33] in which the authors determined that immunization with Hsp60 in NOD mice, an animal model of SjS, could retain normal saliva flow, lower focus score and systemic decrease in a majority of pro-inflammatory cytokines. Furthermore, a number of immune-modulating factors were found to be down-regulated in particular Il-1α, Il-1β, Il-8, Tgf-β1, chemokine ligand Ccl2, cadherin Cdh5, TNF-related genes, and MHC complexes, thereby supporting a general suppressive nature of IL-22. MMP-9 is shown to be the major indicator of acinar cell destruction in salivary glands of SjS patients. A significant decrease in Mmp-9 following IL-22 stimulation could implicate the protective role of IL-22 in acinar cell integrity and homeostasis.
Biological and molecular function analyses with KEGG pathway suggest an important role of IL-22 in cellular viability and cell cycle. Using an in-vitro study to support the gene expression analysis, the present study demonstrated that IL-22 has the propensity to arrest cell proliferation, targeting the cell cycle at the G2-M phase. It is likely that this occurs by IL-22 down-regulating phosphorylation of the pro-proliferative signaling mediators ERK1/2 and AKT. Ciccia et al. determined that IL-22R and pSTAT3 are co-localized on mononuclear cells from salivary glands mononuclear cells and peripheral blood cells [34]. Our data indicated that IL-22-IL-22R is signaled via activation of STAT3/pSTAT3 with little change in STAT1, STAT5, and STAT6. Biological pathway analysis supports the anti-proliferative potential of IL-22 evidenced by 57 differentially down-regulated genes and only 11 genes up-regulated. A number of critical genes associated with cellular proliferation and significantly decreased upon IL-22 stimulation includes PCNA, INSIG1, FGFBP1, CYR61, and CTGF. Concomitantly, CEBPB, CDC20, SECURIN, and CDKN1C are up-regulated upon IL-22 activation. CEBPB and CDC20 elevation indicates the inhibition of proliferation [35], [36]. Interestingly, increased CDC20 levels, which are counteracted by the similar increase in anaphase inhibitor SECURIN, a molecule preventing cells from metaphase to anaphase transition [37]. CDKN1C regulates apoptosis, cell invasion and metastasis is also highly increased to promote cellular arrest [38]. Similarly, all the genes involved in p53 pathway are significantly downregulated via the KEGG analysis. P53 is a major pathway that contributes to many cellular processes including cell cycle arrest, apoptosis, DNA repair and damage prevention and inhibition of mTOR pathway [39]. The data suggest that IL-22 might have reduced viable cells in part by activating cell death programs as well as by cell cycle arrest. This observation is critical for the development of SjS since there is a considerable hyperplasia observed in the ducts in LSG of pSjS patients with concomitant loss of acinar cell mass [40]. Moreover, SjS is a hyperproliferative disorder of B cells that can eventually transition to non-Hodgkin's lymphoma [40], [41]. Based on this result, IL-22 might have a role in providing protection, attempting to compensate against cellular hyperplasia and B cell hyperproliferation, especially in early stage disease. Further study is needed to determine if infiltrating immune cells are affected similarly by IL-22 activity.
In conclusion, these data suggest a critical role of IL-22 in modulating the salivary epithelial cells. Limited information has been generated dealing with the role of IL-22 in regard to critical biological processes linked to the development of SjS, including glandular development, regulation of steroid hormone stimulation and apoptosis. While the current studies have addressed some of these issues, and have shown a relationship of IL-22 with cell cycle arrest that might be a counter-balance to the high cell proliferation associated with onset of SjS, additional work is needed to fully elucidate the role of IL-22 in autoimmunity.
The following are the supplementary data related to this article.
Biological process mediated by IL-22 on HSG cells.
Competing interests
CN is a consultant for Boehringer Ingelheim. All other authors have no competing financial interests.
Acknowledgments
This study was supported financially in part by PHS grants DE023433 and DE018958 (CQN) from the National Institutes of Health (NIH), and funds from the University of Florida College of Veterinary Medicine Consolidated Faculty Research Award.
References
- 1.Fox R.I. Sjogren's syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen C.Q., Peck A.B. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. Ocul. Surf. 2009;7:11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H.M., Alexander E.L., Carsons S.E., Daniels T.E., Fox P.C., Fox R.I., Kassan S.S., Pillemer S.R., Talal N., Weisman M.H. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen C.Q., Hu M.H., Li Y., Stewart C., Peck A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie T.N., Stewart C.M., Berg K.M., Li Y., Nguyen C.Q. Expression of interleukin-22 in Sjogren's syndrome: significant correlation with disease parameters. Scand. J. Immunol. 2011;74:377–382. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccia F., Guggino G., Rizzo A., Ferrante A., Raimondo S., Giardina A., Dieli F., Campisi G., Alessandro R., Triolo G. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren's syndrome. Ann. Rheum. Dis. 2012;71:295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W., Kolls J.K., Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 9.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Kotenko S.V., Izotova L.S., Mirochnitchenko O.V., Esterova E., Dickensheets H., Donnelly R.P., Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 2001;276:2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 11.Witte E., Witte K., Warszawska K., Sabat R., Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 2010;21:365–379. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Xie M.H., Aggarwal S., Ho W.H., Foster J., Zhang Z., Stinson J., Wood W.I., Goddard A.D., Gurney A.L. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 13.Lejeune D., Dumoutier L., Constantinescu S., Kruijer W., Schuringa J.J., Renauld J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 14.Vivier E., Spits H., Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 15.Ikeuchi H., Kuroiwa T., Hiramatsu N., Kaneko Y., Hiromura K., Ueki K., Nojima Y. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 16.Brand S., Beigel F., Olszak T., Zitzmann K., Eichhorst S.T., Otte J.M., Diepolder H., Marquardt A., Jagla W., Popp A., Leclair S., Herrmann K., Seiderer J., Ochsenkuhn T., Goke B., Auernhammer C.J., Dambacher J. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 17.Whittington H.A., Armstrong L., Uppington K.M., Millar A.B. Interleukin-22: a potential immunomodulatory molecule in the lung. Am. J. Respir. Cell Mol. Biol. 2004;31:220–226. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 18.Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J.L., Dubin P.J., Pilewski J.M., Myerburg M.M., Mason C.A., Iwakura Y., Kolls J.K. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz J., Blake E., Medrano T.A., Sun Y., Shiverick K.T. Isoflavones and gamma irradiation inhibit cell growth in human salivary gland cells. Cancer Lett. 2008;270:87–94. doi: 10.1016/j.canlet.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen C.Q., Sharma A., Lee B.H., She J.X., McIndoe R.A., Peck A.B. Differential gene expression in the salivary gland during development and onset of xerostomia in Sjogren's syndrome-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Arthritis Res. Ther. 2009;11:R56. doi: 10.1186/ar2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen C.Q., Sharma A., She J.X., McIndoe R.A., Peck A.B. Differential gene expressions in the lacrimal gland during development and onset of keratoconjunctivitis sicca in Sjogren's syndrome (SJS)-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Exp. Eye Res. 2009;88:398–409. doi: 10.1016/j.exer.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dessau R.B., Pipper C.B. ‘‘R"–project for statistical computing. Ugeskr. Laeger. 2008;170:328–330. [PubMed] [Google Scholar]
- 23.Fernando R.L., Nettleton D., Southey B.R., Dekkers J.C., Rothschild M.F., Soller M. Controlling the proportion of false positives in multiple dependent tests. Genetics. 2004;166:611–619. doi: 10.1534/genetics.166.1.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcon S., Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23:257–258. doi: 10.1093/bioinformatics/btl567. [DOI] [PubMed] [Google Scholar]
- 25.Hjelmervik T.O., Petersen K., Jonassen I., Jonsson R., Bolstad A.I. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 26.Nagalakshmi M.L., Rascle A., Zurawski S., Menon S., de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson M.V., Skarstein K., Jonsson R., Brun J.G. Serological implications of germinal center-like structures in primary Sjogren's syndrome. J. Rheumatol. 2007;34:2044–2049. [PubMed] [Google Scholar]
- 28.Nguyen C.Q., Yin H., Lee B.H., Chiorini J.A., Peck A.B. IL17: potential therapeutic target in Sjogren's syndrome using adenovirus-mediated gene transfer. Lab. Investig. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen C.Q., Yin H., Lee B.H., Carcamo W.C., Chiorini J.A., Peck A.B. Pathogenic effect of interleukin-17A in induction of Sjogren's syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res. Ther. 2010;12:R220. doi: 10.1186/ar3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connor W., Jr., Zenewicz L.A., Flavell R.A. The dual nature of T(H)17 cells: shifting the focus to function. Nat. Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 32.Radaeva S., Sun R., Pan H.N., Hong F., Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 33.Delaleu N., Madureira A.C., Immervoll H., Jonsson R. Inhibition of experimental Sjogren's syndrome through immunization with HSP60 and its peptide amino acids 437–460. Arthritis Rheum. 2008;58:2318–2328. doi: 10.1002/art.23656. [DOI] [PubMed] [Google Scholar]
- 34.Ciccia F., Guggino G., Rizzo A., Bombardieri M., Raimondo S., Carubbi F., Cannizzaro A., Sireci G., Dieli F., Campisi G., Giacomelli R., Cipriani P., De Leo G., Alessandro R., Triolo G. Interleukin (IL)-22 receptor 1 is over-expressed in primary Sjogren's syndrome and Sjogren-associated non-Hodgkin Lymphomas and is regulated by IL-18. Clin. Exp. Immunol. 2015 doi: 10.1111/cei.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirayama M., Toth A., Galova M., Nasmyth K. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature. 1999;402:203–207. doi: 10.1038/46080. [DOI] [PubMed] [Google Scholar]
- 36.Gutsch R., Kandemir J.D., Pietsch D., Cappello C., Meyer J., Simanowski K., Huber R., Brand K. CCAAT/enhancer-binding protein beta inhibits proliferation in monocytic cells by affecting the retinoblastoma protein/E2F/cyclin E pathway but is not directly required for macrophage morphology. J. Biol. Chem. 2011;286:22716–22729. doi: 10.1074/jbc.M110.152538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.S., Jeon Y.K., Ha G.H., Park H.Y., Kim Y.J., Shin H.J., Lee C.G., Chung D.H., Lee C.W. Functional interaction between BubR1 and securin in an anaphase-promoting complex/cyclosomeCdc20-independent manner. Cancer Res. 2009;69:27–36. doi: 10.1158/0008-5472.CAN-08-0820. [DOI] [PubMed] [Google Scholar]
- 38.Lee M.H., Reynisdottir I., Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 39.Stiewe T. The p53 family in differentiation and tumorigenesis. Nat. Rev. Cancer. 2007;7:165–168. doi: 10.1038/nrc2072. [DOI] [PubMed] [Google Scholar]
- 40.Voulgarelis M., Moutsopoulos H.M. Lymphoproliferation in autoimmunity and Sjogren's syndrome. Curr. Rheumatol. Rep. 2003;5:317–323. doi: 10.1007/s11926-003-0011-y. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen C.Q., Cha S.R., Peck A.B. Sjögren's syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front. Biosci. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biological process mediated by IL-22 on HSG cells.