Abstract
MicroRNAs (miRNAs) are small, non-coding RNA molecules that bind to the mRNA of the target genes and regulate the expression of the gene at the post-transcriptional level. Zebrafish is an economically important freshwater fish species globally considered as a good predictive model for studying human diseases and development. The present study focused on uncovering known as well as novel miRNAs, target prediction of the novel miRNAs and the differential expression of the known miRNA using the small RNA sequencing data of the brain and pineal gland (dark and light treatments) obtained from NCBI SRA. A total of 165, 151 and 145 known zebrafish miRNAs were found in the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively. Chromosomes 4 and 5 of zebrafish reference assembly GRCz10 were found to contain maximum number of miR genes. The miR-181a and miR-182 were found to be highly expressed in terms of number of reads in the brain and pineal gland, respectively. Other ncRNAs, such as tRNA, rRNA and snoRNA, were curated against Rfam. Using GRCz10 as reference, the subsequent bioinformatic analyses identified 25, 19 and 9 novel miRNAs from the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively. Targets of the novel miRNAs were identified, based on sequence complementarity between miRNAs and mRNA, by searching for antisense hits in the 3′-UTR of reference RNA sequences of the zebrafish. The discovery of novel miRNAs and their targets in the zebrafish genome can be a valuable scientific resource for further functional studies not only in zebrafish but also in other economically important fishes.
Keywords: MiRNA, NcRNAs, Novel miRs, Phylogenetic analysis, Zebrafish
Graphical abstract
1. Introduction
The discovery of the first miRNAs in Caenorhabditis elegans in 1993 paved the way for unearthing of thousands of the mature miRNAs in a wide variety of organisms, including animals, plants and even viruses [1], [2], [3]. In recent years, intensive studies have been carried out on identification of novel miRNAs and their targets, with the addition of newly discovered miRNAs to miRBase and subsequently resulting in its new version release. The current miRBase release 21 has miR information on 9 fish species. MiRNAs are a distinct class of endogenous RNA molecules, which do not code for any protein and are about 22 nucleotides in length [4]. MicroRNAs (miRNAs) are small, non-coding RNA molecules that function as master regulators of the genome. They bind to the mRNA of the target genes; thus, regulating the gene expression at the post-transcriptional level. Many miR-related discoveries have come from zebrafish investigations [5], [6], [7], [8], [9], [10]. After the release of Zv9 (zebrafish genome draft), the zebrafish genome project joined the Genome Reference Consortium (GRC) for further improvement and ongoing maintenance. The GRC has now released a new reference assembly, GRCz10. Correlating the miR functions from a model organism to that of human health largely depends on recognizing true orthologs of human miRs. Thus, for the benefit of the entire miR community, a better understanding of the miRNome is essential [11].
Each miRNA appears to regulate the expression of tens to hundreds of genes to efficiently coordinate multiple cellular pathways. Precursor-miRNAs are usually 60–80 nucleotides in length with a hairpin secondary structure while mature miRNAs are mostly 18–26 nucleotides [12]. Many miRNAs are conserved across vertebrates [13]. Mutation in miRNA genes or the improper miRNA and target gene interaction may become a cause of various genetic diseases. With the advent of high-throughput sequencing technologies, non-conserved or weakly expressed miRNAs, along with species-specific miRNAs can be identified from a wide range of organisms [14], [15], [16], [17], [18]. Recent studies have focused on bioinformatic analysis of the NGS data obtained from small RNA sequencing, where algorithms predict miRNA precursor molecules based on the presence of hairpins and other associated parameters and how they are processed into mature miRNAs. This sort of analysis can lead to the discovery of both novel and evolutionary conserved miRNAs. Kloosterman et al. [19] reported 66 new miRNAs and 11 star sequences corresponding to 116 potential miRNA hairpins in the zebrafish genome by deep sequencing of two small RNA cDNA libraries. Bizuayehu et al. [20] worked on the Atlantic Halibut and the results indicate a wide conservation of miRNA precursors and involvement of miRNA in multiple regulatory pathways. Despite enormous research on zebrafish, the annotation of miR-producing genes remains limited.
Zebrafish is a fish species of freshwater ecosystem and considered popular organism for studying the gene functions of the vertebrate, especially human development and genetic diseases. It is a favored model organism due to their specific features such as virtually transparent embryos, small size, ability to keep them together in large numbers, ease of breeding and easy to maintain/manipulate/observe in the lab experiments. The critical role of miRNAs in gene expression is highly evident from the recent studies in zebrafish. The miRNAs play key roles in zebrafish organ formation and their expression at different time points.
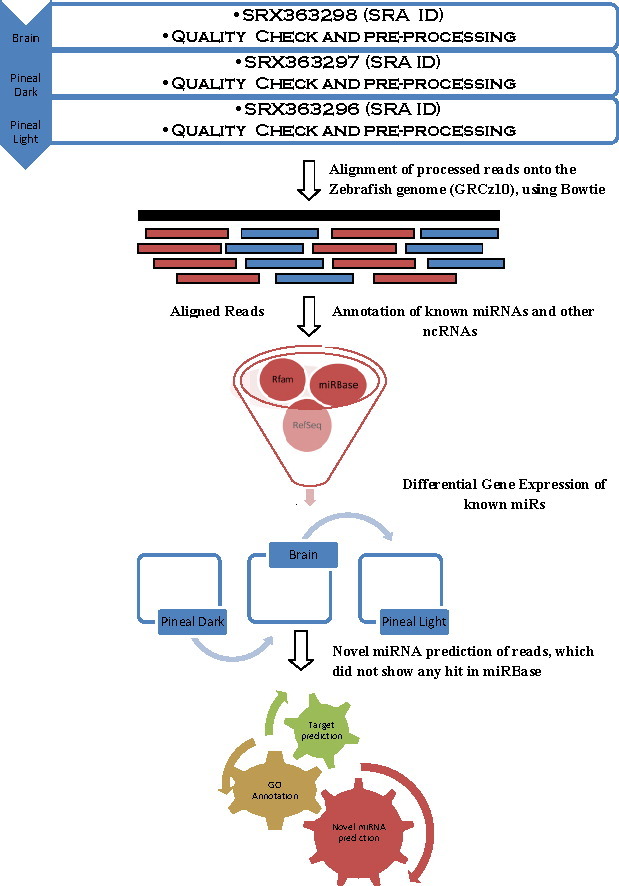
In the present work, we used Illumina HiSeq2000 small RNA sequencing data from the brain and pineal gland (dark and light treatments) of zebrafish from NCBI SRA database. The total number of reads in the data obtained from NCBI SRA was ~ 6 million, ~ 10.4 million and ~ 14.8 million for the pineal light, pineal dark and brain, respectively. An integrative bioinformatic strategy was applied to detect and analyze the whole miRNA transcriptome of zebrafish. The present study led to the discovery of novel miRNAs in the brain and pineal gland of zebrafish, which will contribute for a better understanding of the role miRNAs play in regulating diverse biological processes.
2. Materials and methods
2.1. Raw data retrieval and pre-processing
The small RNA sequencing data from three mature miRNA libraries (pineal light, pineal dark and brain) of zebrafish were downloaded from NCBI SRA (SRX363296, SRX363297 and SRX363298) and were subsequently used for analysis in the present study. The downloaded data was in SRA format, which was subsequently converted to fastq format using sratoolkit (version 2.3.4-2) [30], fastq-dump option. The obtained data was generated on HiSeq2000 using standard Illumina sequencing workflow with the multiplexing option. A custom Perl script was written to remove low quality bases, adaptor sequences, count the number of occurrences of each read, and eliminate reads outside the targeted size range (≥ 16 and ≤ 30).
2.2. Identification of conserved miRNAs and other ncRNAs in zebrafish and other fishes
The filtered reads were further aligned onto the latest released version of zebrafish genome GRCz10, using bowtie [31] with two mismatches and zero gaps. Only the aligned reads were used for the downstream analysis. MiRBase [32], [33] release 21 was used for annotation of known miRNA. A custom based Perl script was written in order to extract only the fish miRNA from the miRBase, along with the preparation of unique fish miRNA database, and its annotation files. Aligned reads were annotated against the unique fish miRNA database, RefSeq database, as well as noncoding RNA sequences of zebrafish from Rfam (version 11) [34]. A custom Perl script was written in order to extract the best read hit, which depicts the fish miRNA, and to segregate the miRNA hits into known zebrafish miRNA and other fish miRNA.
2.3. Differential expression of known miRNAs
The individual read counts of the 3 data sets were fed into a custom based Perl script to prepare the final read count table, which was taken as input for DeSeq [35]. The results were further segregated into up-regulated, down-regulated and neutral miRs based on the log2 fold change value. For the log2 fold change greater than 1, less than − 1 and between 1 and − 1, the miRs were designated as up, down and neutral miRs. A heatmap of few highly regulated miRNAs was drawn using R script.
2.4. Identification of novel miRNA candidates
The reads with no hits in the unique fish miRNA database and Rfam were further used for the prediction of putative new miRs using Mireap (version 0.2). All novel pre-miRNAs were identified based on the presence of a classic hairpin structure [36]. These filtered small RNA reads were aligned with the zebrafish genome using bowtie with strict parameters (number of mismatch; — v = 0). Mireap was used for the detection of novel miRs based on alignment, secondary structure, free energy and location on the precursor arm. The parameters used for Mireap prediction included: i) minimal miRNA length = 18; ii) maximal miRNA length = 26; iii) minimal miRNA reference length = 20; iv) maximal miRNA reference length = 24; v) uniqueness of miRNA = 20; vi) maximal energy allowed for a miRNA precursor = − 18 kcal/mol; vii) minimal and maximal space between the miRNA and miRNA* = 5 and 35 respectively; viii) minimal mature pair = 14; ix) maximal mature bulge = 4; x) maximal duplex asymmetry = 5; and xi) flank sequence length = 100 [37]. It is evident from the previous studies that more than 90% of miRNA precursors have MFEIs greater than 0.85 (tRNAs ~ 0.64, rRNAs ~ 0.59, and mRNAs ~ 0.65) [38]. Therefore, minimal folding free energy index (MFEI) is a new criterion for assaying miRNAs and distinguishing miRNAs from other non-coding and coding RNAs. MFEI is equal to MFE / (precursor length) × 100 / (G + C)%. In the present study, reads which showed MFEI value to be greater than 0.85, were considered as novel miRNAs candidates.
2.5. Target prediction of novel miRs
Target prediction of the novel miRNA was done against the 3′-UTR sequences of zebrafish (www.ensembl.org/biomart) using miRanda (version 3.3a) [39]. The miRanda results were parsed based on score > 150 and energy <− 20. The gene ontology (GO) terms of the predicted targets were taken from UniProt database and were further classified into most abundant GO terms for biological process, cellular component and molecular function.
3. Results and discussion
3.1. Raw data retrieval and pre-processing
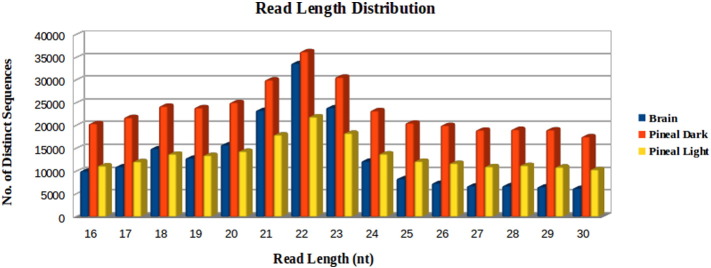
NCBI SRA was the main source of NGS data, from where the small RNA sequencing data of three mature miRNA libraries (pineal light, pineal dark, and brain) of zebrafish (SRX363296, SRX363297 and SRX363298) was downloaded and was subsequently used in the present study. The total numbers of reads were found to be ~ 6 million, ~ 10.4 million and ~ 14.8 million for the pineal light, pineal dark and brain, respectively. A custom Perl script was written to remove low quality sequences, adapter sequences and to count the number of occurrences of each read, with the elimination of reads outside the targeted size range (≥ 16 and ≤ 30). A read length distribution graph (Fig. 1) was prepared to get insight into the number of reads at each read length. The maximum numbers of distinct sequences were found to be of 22 bp and most of the data was falling between 21 and 23 bp in all the 3 data sets. The numbers of distinct sequences were calculated both before and after length filtering. Finally, the total numbers of distinct sequences after length filtering (Table 1), which were considered for downstream analyses were 200,520, 204,696 and 351,638 for the brain, pineal light and pineal dark, respectively.
Fig. 1.
Read length distribution. The figure represents the read length distribution of the 3 data sets after length filtering.
Table 1.
Statistics of the 3 data sets before and after length filtering.
| Parameter | Brain | Pineal gland (light treatment) | Pineal gland (dark treatment) |
|---|---|---|---|
| Total number of sequences | 14,781,569 | 5,933,638 | 10,385,264 |
| Total number of distinct sequences | 259,897 | 265,007 | 463,789 |
| Total number of sequences after length filtering | 14,347,869 | 5,724,361 | 10,008,434 |
| Total number of distinct sequences after length filtering | 200,520 | 204,696 | 351,638 |
3.2. Identification of conserved miRNAs and other ncRNAs in zebrafish and other fishes
The filtered reads were further aligned onto the latest released version of zebrafish genome GRCz10, using bowtie with two mismatches and zero gaps. A total of 88.58%, 60.00% and 61.14% reads aligned to the zebrafish genome GRCz10 for the brain, pineal gland (dark) and pineal gland (light), respectively. Only the aligned reads were used for the downstream analysis. miRBase release 21 was used to determine the known and conserved miRNAs in fishes. MiRNA data of 9 fishes was extracted from miRBase, comprising of 1637 miRNA sequences. Unique fish miRNA database was prepared by eliminating the redundant sequences, which comprised of 1029 sequences. The aligned reads from all the 3 data sets were blast against the unique fish miRNA database. A total of 165, 151 and 145 known zebrafish miRNAs were found in the brain, pineal gland (dark treatment) and pineal gland (light treatment) along with their expression values and were further annotated for their presence in other fishes (Table S1). A total of 221, 196 and 195 distinct reads showed hits with other fish miRNAs in the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively (Table S1).
A comparative study of all the detected fish miRNAs showed 321 miRNAs to be common along all the 3 data sets, with 40, 6 and 6 miR genes to be uniquely expressed in the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively (Fig. 2). The maximum read count was observed for miR-181a and miR-182 in the brain and pineal gland, respectively. The miR-181a is mainly involved in proliferation, and is an active miRNA regulating regeneration process. Rudnicki et al. [21] and Frucht et al. [22] also reported that miR-181a can encourage proliferation in both quiescent and proliferating chick basilar papilla. It is also reported that over-expression of miR-182 results in production of ectopic hair cells [21]. The miR-182 forms a part of miR-183/96/182 cluster, whose expression is considerably enriched in the pineal gland and up-regulated by light [23], [24]. Because of the role of these miRNA in promoting regeneration and mediating the targets and transcriptional factors involved in regeneration, these miRNAs may prove to be promising in therapeutics.
Fig. 2.
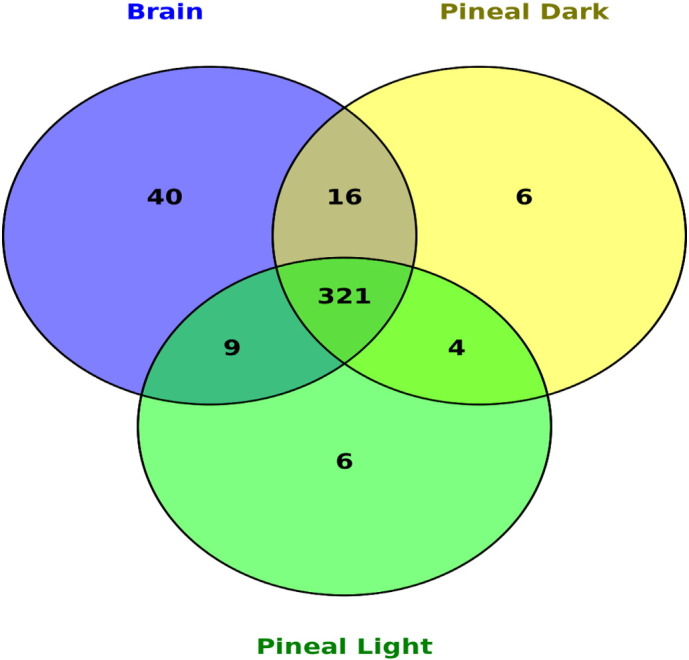
Venn diagram of conserved miRNAs in the brain, pineal gland (dark treatment) and pineal gland (light treatment).
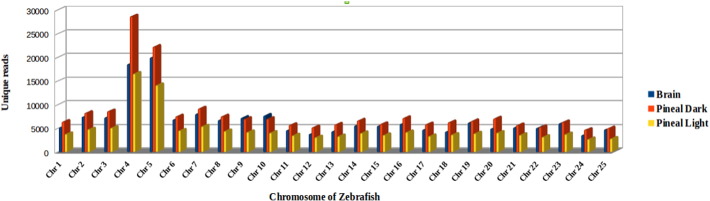
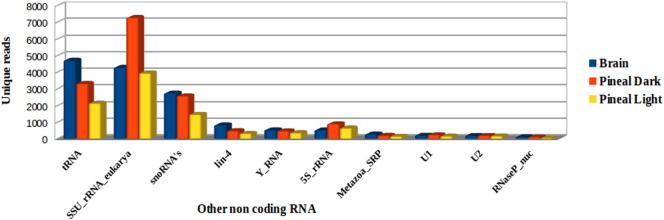
Chromosome wise coverage of the reads aligning to zebrafish genome GRCz10 showed that the maximum numbers of miR genes were coded by chromosomes 4 and 5 (Fig. 3). Thatcher et al. [25] also confirmed the presence of miR-430 family in two large clusters of 10 and 57 genes on chromosome 4. The reads which did not show any hits in miRBase were analyzed by BLAST against the Rfam database (ftp.sanger.-ac.uk/pub/databases/Rfam) and Refseq proteins to annotate rRNA, tRNA, snRNA, mRNA and other ncRNA sequences. The maximum numbers of reads were annotated against eukaryotic small subunit ribosomal RNA (SSU_rRNA_eukarya) and tRNA, followed by other ncRNAs (Fig. 4).
Fig. 3.
Distribution of unique miRNA reads across the zebrafish genome.
Fig. 4.
Unique reads representing other ncRNAs in the data.
3.3. Differential expression of known miRNAs
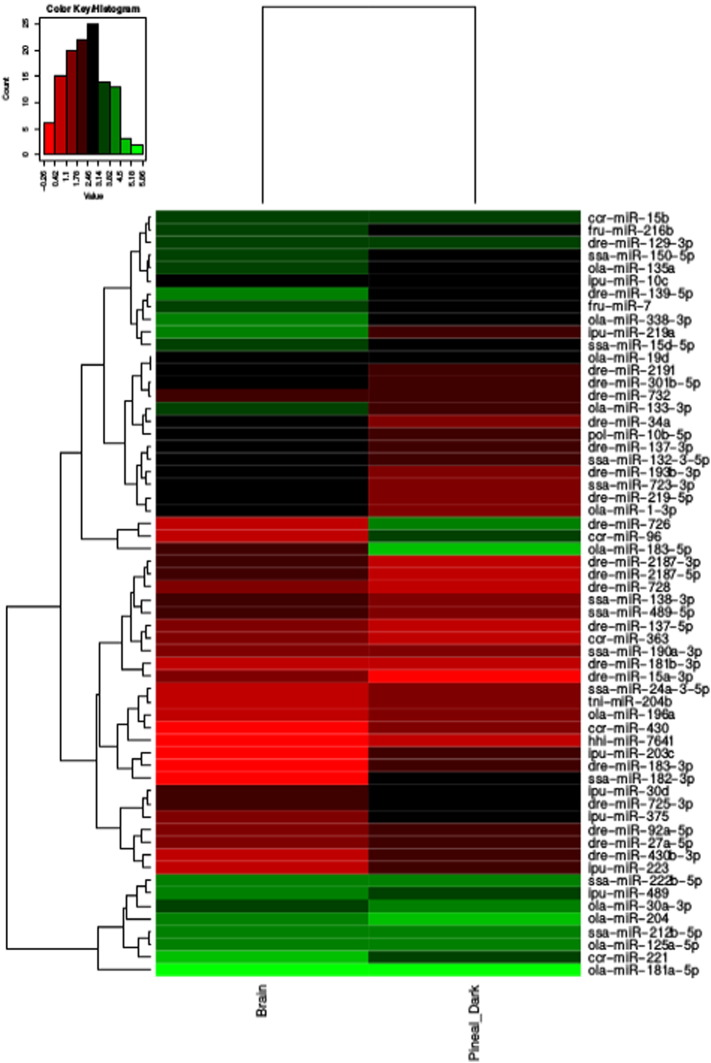
The differential expression of the miRNA was computed as 2 different sets: 1) brain v/s pineal dark, and 2) brain v/s pineal light. For the brain v/s pineal dark, a total of 93, 99 and 145 miRs were found to be up, down and neutrally regulated, with 49 and 10 miRNAs only expressed in the brain and pineal dark, respectively (Table S2). For the brain v/s pineal light, a total of 105, 101 and 124 miRs were found to be up, down and neutrally regulated, with 56 and 10 miRNAs only expressed in the brain and pineal light, respectively (Table S3). Few up-regulated, down-regulated and neutral miRs from the brain v/s pineal dark are depicted as a heatmap (Fig. 5). The results from both the expression analyses showed that the miR-183/96/182 cluster is highly up-regulated, along with miR-726. The expression of miR-183/96/182 cluster is considerably enriched in the pineal gland and up-regulated by light [23], [24]. These findings are consistent with the light-regulation of this cluster in the mouse retina [26]. This cluster also plays a major role in the regulation of circadian rhythm via its targeting of adcy6, a clock-controlled gene that modulates melatonin synthesis [27]. Dre-miR-726 is found to be expressed in the retina of larval and adult zebrafish. From the previous studies it is evident that many miRNAs are transcribed along with their regulating genes, the proximity of miR-726 to SWS2 and LWS opsins suggests that dre-miR-726 could play a vital role in opsin regulation [28].
Fig. 5.
Heatmap of few up-regulated, down-regulated and neutral miRs from the brain and pineal gland.
3.4. Identification of novel miRNA candidates
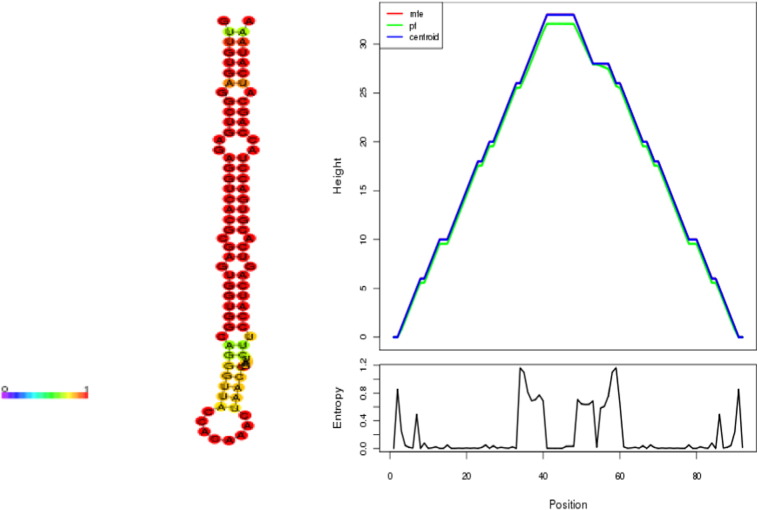
Mireap was used for the prediction of novel miRs, using reads that did not align to the unique fish miRNA database and Rfam. The reads were aligned onto the zebrafish genome using bowtie (with − v 0). A total of 84.40%, 83.68% and 87.72% of reads aligned for the brain, pineal dark and pineal light, respectively. For a small RNA to be considered as a potential miRNA candidate, it should meet the following strict criteria: 1) the miRNA precursor sequence should fold into an appropriate stemloop hairpin secondary structure, 2) the mature miRNA sits in one arm of the hairpin structure, 3) a maximum of 6 mismatches between the predicted mature miRNA sequence and its opposite miRNA* sequence in the secondary structure, 4) there should be no loop or break in the miRNA or miRNA* sequences, and 5) minimal folding free energy index and negative minimal folding free energy of the predicted secondary structure should be higher. The stem-loop hairpin structures with free energy lower than − 18 kcal/mol as per RNAfold were retained. Mireap predicted a total of 50, 34, and 16 novel miRNAs from the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively. All of these novel miRNAs were named temporarily in the form of Dre-mir-novnumber, e.g. Dre-mir-nov1. To increase the authenticity of the predicted novel miRNA, Zhang et al. [29] combined several parameters to form a new criterion called minimal folding free energy index (MFEI). The MFEI value from all the 3 data sets ranged from − 0.42 to − 1.56. A total of 25, 19 and 9 novel miRNA precursors, with a MFEI greater than 0.85, were identified from the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively (Table 2). This indicates that the RNA sequences with MFEI greater than 0.85 are most likely to be miRNAs. Most of the novel miRs were also found to originate from chr 4 and chr 5 of zebrafish. The novel miRs ranged from 21 nt to 24 nt in length, with the precursors ranging from 75 nt to 99 nt in length. The free energy of the precursors ranged from − 28.5 to − 71.5 kcal/mol. The secondary structure of a novel hairpin with the highest read count, i.e. Dre-mir-nov74, is depicted in Fig. 6. Biological experiments can be undertaken to validate the authenticity of the reported novel miRs.
Table 2.
Zebrafish novel miRNA and their characteristics.
| Tissue type | Chromosome | Novel miRNA | Sequence | MiRNA length (nt) | Read count | Precursor position | Strand | Precursor length | % GC | MFEI (− kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Chr 1 | Dre-mir-nov49 | AGAGGCUGUCCGAGUGCUGAU | 21 | 156 | 10303253–10303335 | + | 83 | 49.4 | − 1.56 |
| Chr 20 | Dre-mir-nov8 | GACCUGUAACCAUUGACUUCCU | 22 | 102 | 962031–962117 | + | 87 | 36.78 | − 1.4 | |
| Chr 3 | Dre-mir-nov46 | AAAAGCGUACCAAACUGAACCGU | 23 | 122 | 46496327–46496412 | + | 86 | 48.84 | − 1.3 | |
| Chr 17 | Dre-mir-nov19 | AGACUAGUAGCCAUUGAGAUCUU | 23 | 264 | 20769194–20769271 | − | 78 | 33.33 | − 1.27 | |
| Chr 18 | Dre-mir-nov16 | UGUUUUUUUAGGUUUUGAUUUUU | 23 | 1142 | 45964887–45964967 | + | 81 | 33.33 | − 1.26 | |
| Chr 4 | Dre-mir-nov38 | GAAUAACUCAAACCGGAGGACU | 22 | 106 | 31535442–31535523 | + | 82 | 47.56 | − 1.26 | |
| Chr 4 | Dre-mir-nov42 | AAUAACUCAAACCAGAGGACU | 21 | 126 | 54513045–54513126 | + | 82 | 43.9 | − 1.19 | |
| Chr 18 | Dre-mir-nov15 | UUUCCAGAAAGGUCUGUAUGUGU | 23 | 130 | 26647915–26647993 | + | 79 | 46.84 | − 1.16 | |
| GRCZ10_NA262 | Dre-mir-nov2 | AGACUCUCCAGUACACUGGCCCU | 23 | 862 | 744–825 | − | 82 | 58.54 | − 1.16 | |
| Chr 10 | Dre-mir-nov25 | GAAAACCUGUAACCAUUGACUUCU | 24 | 221 | 29746032–29746122 | + | 91 | 35.16 | − 1.14 | |
| Chr 20 | Dre-mir-nov10 | AAACUUGUAAUCACUGACUUCCU | 23 | 125 | 35014623–35014703 | + | 81 | 33.33 | − 1.12 | |
| Chr 2 | Dre-mir-nov48 | AAUGACUCAAACCCAAGGACUCG | 23 | 1182 | 8746947–8747032 | + | 86 | 40.7 | − 1.1 | |
| Chr 6 | Dre-mir-nov29 | AAACUCUGCAGGACACCAGCUGU | 23 | 268 | 18113026–18113108 | + | 83 | 46.99 | − 1.05 | |
| Chr 17 | Dre-mir-nov18 | AAACUCUGCAGGACACCAGCUG | 22 | 748 | 47039884–47039964 | + | 81 | 46.91 | − 1.02 | |
| Chr 4 | Dre-mir-nov39 | UAAACUCUGCAGGACACCAGCUG | 23 | 1020 | 42571226–42571306 | + | 81 | 46.91 | − 1.02 | |
| Chr 6 | Dre-mir-nov30 | ACUGUACAGACUACUGCCUUGC | 22 | 361 | 41457248–41457346 | + | 99 | 45.45 | − 0.98 | |
| Chr 18 | Dre-mir-nov17 | ACUCUUCACUCGUCUGUGUUCA | 22 | 116 | 50849750–50849835 | − | 86 | 55.81 | − 0.96 | |
| Chr 23 | Dre-mir-nov3 | UCAAAAGGCGUACCAAACUGUAC | 23 | 155 | 18099730–18099807 | − | 78 | 43.59 | − 0.95 | |
| Chr 5 | Dre-mir-nov37 | UCCAUCAGUCACGUGACCUACCA | 23 | 7106 | 35345784–35345875 | − | 92 | 51.09 | − 0.94 | |
| Chr 5 | Dre-mir-nov33 | GGCCCGUCCGGUGCGCUCGGAUCC | 24 | 767 | 824650–824740 | − | 91 | 84.62 | − 0.93 | |
| Chr 5 | Dre-mir-nov31 | UAAACUCUGCAGGACACCAGCUGU | 24 | 158 | 10220904–10221001 | + | 98 | 46.94 | − 0.93 | |
| Chr 5 | Dre-mir-nov32 | GAUGACUCAAACCCAAGGACUCA | 23 | 115 | 15221789–15221886 | + | 98 | 39.8 | − 0.89 | |
| Chr 8 | Dre-mir-nov28 | AAUGACUCAAACCCAAGGACUCGU | 24 | 108 | 2766509–2766596 | + | 88 | 44.32 | − 0.88 | |
| Chr 4 | Dre-mir-nov44 | AGUGAGGUCCUCGGAUCGGCCC | 22 | 1835 | 76320181–76320279 | + | 99 | 68.69 | − 0.87 | |
| Chr 22 | Dre-mir-nov4 | AACGACUCAAGAACCAGAAGACU | 23 | 315 | 18571986–18572067 | + | 82 | 46.34 | − 0.86 | |
| Pineal gland (dark treatment) | Chr 1 | Dre-mir-nov83 | AUCAGCACUCGGACAGCCUCUU | 22 | 173 | 10303251–10303335 | + | 85 | 50.59 | − 1.53 |
| Chr 17 | Dre-mir-nov59 | AGACUAGUAGCCAUUGAGAUCUU | 23 | 101 | 20769194–20769271 | − | 78 | 33.33 | − 1.27 | |
| Chr 18 | Dre-mir-nov58 | UGUUUUUUUAGGUUUUGAUUUUU | 23 | 381 | 45964887–45964967 | + | 81 | 33.33 | − 1.26 | |
| Chr 9 | Dre-mir-nov66 | UACGGGCUGAAUUUAGACAAAUU | 23 | 177 | 5548615–5548705 | + | 91 | 27.47 | − 1.25 | |
| Chr 24 | Dre-mir-nov52 | UAACGUUUCGAGCCCACUGACUG | 23 | 118 | 1825424–1825498 | − | 75 | 46.67 | − 1.19 | |
| GRCZ10_NA262 | Dre-mir-nov51 | AGACUCUCCAGUACACUGGCCCUC | 24 | 997 | 743–826 | − | 84 | 58.33 | − 1.14 | |
| Chr 12 | Dre-mir-nov62 | UUUCCGGAAAGGUCUGUAUGUGC | 23 | 1654 | 44383412–44383489 | + | 78 | 47.44 | − 1.14 | |
| Chr 4 | Dre-mir-nov77 | GAAUAACUCAAACCGGAGGACU | 22 | 103 | 49472235–49472316 | + | 82 | 46.34 | − 1.11 | |
| Chr 6 | Dre-mir-nov72 | UACGGAUAGAAUCAGCGGAGCGA | 23 | 221 | 56909252–56909333 | + | 82 | 60.98 | − 1.11 | |
| Chr 8 | Dre-mir-nov70 | AACCUGUAACCAUUGACUUCCU | 22 | 140 | 10454696–10454780 | + | 85 | 38.82 | − 1.04 | |
| Chr 1 | Dre-mir-nov84 | UAAAGCGUACCAAACUGAACCGU | 23 | 150 | 41154258–41154343 | + | 86 | 46.51 | − 1.00 | |
| Chr 11 | Dre-mir-nov63 | GCUGAAGUCAUUAUUAUUAGGGC | 23 | 116 | 5486604–5486685 | + | 82 | 35.37 | − 0.98 | |
| Chr 6 | Dre-mir-nov71 | ACUGUACAGACUACUGCCUUGC | 22 | 220 | 41457248–41457346 | + | 99 | 45.45 | − 0.98 | |
| Chr 4 | Dre-mir-nov76 | GCAGAGAGAAAUGUCUAUGGCUU | 23 | 1712 | 5403781–5403855 | + | 75 | 48.00 | − 0.97 | |
| Chr 5 | Dre-mir-nov74 | UCCAUCAGUCACGUGACCUACCA | 23 | 15,880 | 35345784–35345875 | − | 92 | 51.09 | − 0.94 | |
| Chr 20 | Dre-mir-nov54 | CUCAUCCCUCUGCUCUAUCCCCU | 23 | 115 | 37928243–37928325 | + | 83 | 50.60 | − 0.93 | |
| Chr 8 | Dre-mir-nov69 | AAUGACUCAAACCCAAGGACUCG | 23 | 224 | 2766510–2766595 | + | 86 | 44.19 | − 0.90 | |
| Chr 12 | Dre-mir-nov61 | AACGACUCAAGAGCCAGAAGACU | 23 | 323 | 3216161–3216240 | + | 80 | 45.00 | − 0.88 | |
| Chr 20 | Dre-mir-nov55 | CAGGGGGUCGGGAAGCACUGCCU | 23 | 4876 | 48676465–48676553 | − | 89 | 58.43 | − 0.85 | |
| Pineal gland (light treatment) | Chr 1 | Dre-mir-nov100 | AUCAGCACUCGGACAGCCUCUU | 22 | 100 | 10303251–10303335 | + | 85 | 50.59 | − 1.53 |
| Chr 9 | Dre-mir-nov91 | UACGGGCUGAAUUUAGACAAAUU | 23 | 101 | 5548615–5548705 | + | 91 | 27.47 | − 1.25 | |
| GRCZ10_NA262 | Dre-mir-nov85 | AGACUCUCCAGUACACUGGCCCUC | 24 | 491 | 743–826 | − | 84 | 58.33 | − 1.14 | |
| Chr 12 | Dre-mir-nov89 | UUUCCGGAAAGGUCUGUAUGUGC | 23 | 1075 | 44383412–44383489 | + | 78 | 47.44 | − 1.14 | |
| Chr 6 | Dre-mir-nov94 | UACGGAUAGAAUCAGCGGAGCGA | 23 | 130 | 56909252–56909333 | + | 82 | 60.98 | − 1.11 | |
| Chr 4 | Dre-mir-nov97 | GCAGAGAGAAAUGUCUAUGGCUU | 23 | 903 | 5403781–5403855 | + | 75 | 48.00 | − 0.97 | |
| Chr 5 | Dre-mir-nov95 | UCCAUCAGUCACGUGACCUACCA | 23 | 8256 | 35345784–35345875 | − | 92 | 51.09 | − 0.94 | |
| Chr 4 | Dre-mir-nov99 | AGUGAGGUCCUCGGAUCGGCCCC | 23 | 144 | 76320181–76320279 | + | 99 | 68.69 | − 0.87 | |
| Chr 20 | Dre-mir-nov88 | CAGGGGGUCGGGAAGCACUGCCU | 23 | 2104 | 48676465–48676553 | − | 89 | 58.43 | − 0.85 |
Fig. 6.
Hairpin structures of Dre-mir-nov74 predicted using RNAfold software.
3.5. Target prediction of novel miRs
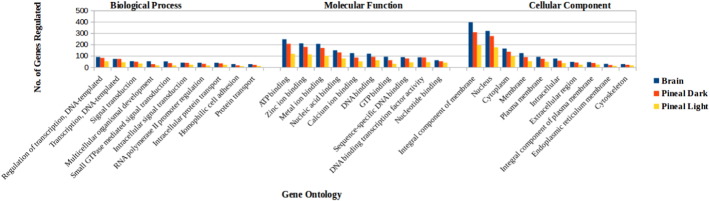
MicroRNAs bind to the mRNA of the target genes, thus regulating the gene expression at the post-transcriptional level. To gain insight into the function of the newly identified miRNAs, the putative target genes of these miRNAs were predicted using miRanda. The 3′-UTR regions of zebrafish mRNAs were downloaded from ensemble biomart and were checked for their complementarity against the novel miRs. Each miRNA was found to target more than one mRNA. The 25, 19 and 9 novel miRs in the 3 data sets were found to regulate 3737, 2527 and 1719 transcripts (Table S4), with the number of targets ranging from 12 to 873 for each miRNA. In order to infer the functional annotation of the novel miRs, GO analysis was done for the predicted Zebrafish targets, which indicated their involvement in the regulation of diverse physiological processes (Fig. 7). The novel miRNAs were found to play a major role in transcription regulation, signal transduction, organism development, RNA polymerase II promoter regulation, protein transport and homophilic cell adhesion. The pathway analysis also revealed the involvement of these novel miRs in amine and polyamine biosynthesis, carbohydrate degradation, iron-sulfur cluster biosynthesis, urea cycle, AMP and XMP biosynthesis via de novo pathway, CTP biosynthesis via salvage pathway and tRNA modification. Our study provided further insight into the novel miRNA-mediated regulation of target genes.
Fig. 7.
Gene ontology of the target genes of novel miRNAs. The graph represents the maximum represented GO in terms of biological process, molecular function and cellular component.
4. Conclusion
In this study, a total of 25, 19 and 9 novel miRNAs were identified from the brain, pineal gland (dark treatment) and pineal gland (light treatment), respectively, using deep sequencing data and in silico bioinformatic analysis. Most of the conserved and novel miRs were found to originate from chr 4 and chr 5 of zebrafish. To gain insight into the function of the newly identified miRNAs, the putative target genes of these miRNAs were predicted using miRanda. Gene ontology analysis was done for the predicted zebrafish targets to infer the functional annotation of the novel miRs, which indicated their involvement in the regulation of diverse physiological processes. The novel miRNAs were found to play a major role in transcription regulation, signal transduction, organism development, RNA polymerase II promoter regulation, protein transport and homophilic cell adhesion. The pathway analysis also revealed the involvement of these novel miRs in amine and polyamine biosyntheses, carbohydrate degradation, iron–sulfur cluster biosynthesis, urea cycle, AMP and XMP biosyntheses via de novo pathway, CTP biosynthesis via salvage pathway and tRNA modification. A total of 165, 151 and 145 known zebrafish miRNAs were found in the brain, pineal gland (dark treatment) and pineal gland (light treatment) along with their expression values and were further annotated for their presence in other fishes. MiR-181a and miR-182 were the highly expressed miRNAs in the brain and pineal gland of zebrafish. Because of the role of these miRNAs in promoting regeneration and mediating the targets and transcriptional factors involved in regeneration, these miRNAs may prove to be promising in therapeutics. The expression analysis of the known miR genes showed that miR-183/96/182 cluster is highly up-regulated, along with miR-726. The expression of miR-183/96/182 cluster is considerably enriched in the pineal gland and up-regulated by light. The authenticity of the reported novel miRs may further be validated by different biological experiments.
Competing interests
The authors have declared that no competing interest exists.
Acknowledgments
The authors are thankful to the Director, NBFGR, Lucknow, for providing necessary laboratory facilities. The financial support provided by Department of Biotechnology, Ministry of Science and Technology, Gov. of India, New Delhi, India vide Sanction Grant No. BT/PR3688/AAQ/3/571/2011 dated 10.09.2013 for the present research works is also duly acknowledged.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gdata.2015.11.013.
Appendix A. Supplementary data
List of known and conserved miRNA in fishes.
Differential expression of known miRNAs of the brain and pineal gland (dark treatment).
Differential expression of known miRNAs of the brain and pineal gland (light treatment).
List of predicted targets for the novel miRNAs using miRanda.
References
- 1.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Li S.C., Chan W.C., Hu L.Y., Lai C.H., Hsu C.N. Identification of homologous microRNAs in 56 animal genomes. Genomics. 2010;96(1):1–9. doi: 10.1016/j.ygeno.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Chen S.J., Chen G.H., Chen Y.H., Liu C.Y., Chang K.P. Characterization of Epstein–Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Flynt A.S., Li N., Thatcher E.J., Solnica-Krezel L., Patton J.G. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat. Genet. 2007;39(2):259–263. doi: 10.1038/ng1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraldez A.J., Cinalli R.M., Glasner M.E., Enright A.J., Thomson J.M., Baskerville S., Hammond S.M., Bartel D.P., Schier A.F. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 7.Giraldez A.J., Mishima Y., Rihel J., Grocock R.J., Dongen S.V., Inoue K., Enright A.J., Schier A.F. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312(5770):75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 8.He X., Eberhart J.K., Postlethwait J.H. MicroRNAs and micromanaging the skeleton in disease, development and evolution. J. Cell. Mol. Med. 2009;13(4):606–618. doi: 10.1111/j.1582-4934.2009.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X., Yan Y.L., DeLaurier A., Postlethwait J.H. Observation of miRNA gene expression in zebrafish embryos by in situ hybridization to microRNA primary transcripts. Zebrafish. 2011;8(1):1–8. doi: 10.1089/zeb.2010.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He X., Yan Y.L., Eberhart J.K., Herpin A., Wagner T.U., Schartl M., Postlethwait J.H. miR-196 regulates axial patterning and pectoral appendage initiation. Dev. Biol. 2011;357(2):463–477. doi: 10.1016/j.ydbio.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desvignes T., Beam M.J., Batzel P., Sydes J., Postlethwait J.H. Expanding the annotation of zebrafish microRNAs based on small RNA sequencing. Gene. 2014;546(2):386–389. doi: 10.1016/j.gene.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mica E., Gianfranceschi L., Pe M.E. Characterization of five microRNA families in maize. J. Exp. Bot. 2006;57(11):2601–2612. doi: 10.1093/jxb/erl013. [DOI] [PubMed] [Google Scholar]
- 13.Barozai M.Y.K. Identification and characterization of the microRNAs and their targets in Salmo salar. Gene. 2012;499(1):163–168. doi: 10.1016/j.gene.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Bar M., Wyman S.K., Fritz B.R., Qi J., Garg K.S. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells. 2008;26(10):2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi W., Tong C., Gan X., He S. Characterization and comparative profiling of MiRNA transcriptomes in bighead carp and silver carp. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y., Shi Z., Wu M., Zhang J., Jia L. Identification and differential expression of microRNAs during metamorphosis of the Japanese flounder (Paralichthys olivaceus) PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan X., Ding L., Li Y., Zhang X., Liang Y. Identification and profiling of microRNAs from skeletal muscle of the common carp. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou J., Meng Q., Li Y., Xiu Y., Du J. Identification and comparative analysis of the Eriocheir sinensis microRNA transcriptome response to Spiroplasma eriocheiris infection using a deep sequencing approach. Fish Shellfish Immunol. 2011;32(2):345–352. doi: 10.1016/j.fsi.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Kloosterman W.P., Steiner F.A., Berezikov E., de Bruijn E., van de Belt J., Verheul M. Cloning and expression of new microRNAs from zebrafish. Nucleic Acids Res. 2006;34(9):2558–2569. doi: 10.1093/nar/gkl278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bizuayehu T.T., Fernandes J.M., Johansen S.D., Babiak I. Characterization of novel precursor miRNAs using next generation sequencing and prediction of miRNA targets in Atlantic halibut. PloS one. 2013;8(4) doi: 10.1371/journal.pone.0061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudnicki A., Avraham K.B. microRNAs: the art of silencing in the ear. EMBO Mol. Med. 2012;4(9):849–859. doi: 10.1002/emmm.201100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frucht C.S., Uduman M., Duke J.L., Kleinstein S.H., Santos-Sacchi J., Navaratnam D.S. Gene expression analysis of forskolin treated basilar papillae identifies microRNA181a as a mediator of proliferation. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Moshe Z., Alon S., Mracek P., Faigenbloom L., Tovin A., Vatine G.D., Gothilf Y. The light-induced transcriptome of the zebrafish pineal gland reveals complex regulation of the circadian clockwork by light. Nucleic Acids Res. 2014;42(6):3750–3767. doi: 10.1093/nar/gkt1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Moshe Z., Foulkes N.S., Gothilf Y. Functional development of the circadian clock in the zebrafish pineal gland. BioMed. Res. Int. 2014 doi: 10.1155/2014/235781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thatcher E.J., Bond J., Paydar I., Patton J.G. Genomic organization of zebrafish microRNAs. BMC Genomics. 2008;9(1):253. doi: 10.1186/1471-2164-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krol J., Busskamp V., Markiewicz I., Stadler M.B., Ribi S., Richter J. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141(4):618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Xu S., Witmer P.D., Lumayag S., Kovacs B., Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J. Biol. Chem. 2007;282(34):25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 28.O'Quin K.E., Smith D., Naseer Z., Schulte J., Engel S.D., Loh Y.H.E. Divergence in cis-regulatory sequences surrounding the opsin gene arrays of African cichlid fishes. BMC Evol. Biol. 2011;11(1):120. doi: 10.1186/1471-2148-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang B.H., Pan X.P., Cox S.B., Cobb G.P., Anderson T.A. Evidence that miRNAs are different from other RNAs. Cell. Mol. Life Sci. 2006;63(2):246–254. doi: 10.1007/s00018-005-5467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leinonen R., Sugawara H., Shumway M. The sequence read archive. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq1019. (gkq1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt1181. (gkt1181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths-Jones S., Grocock R.J., Van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Suppl. 1):D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths-Jones S., Bateman A., Marshall M., Khanna A., Eddy S.R. Rfam: an RNA family database. Nucleic Acids Res. 2003;31(1):439–441. doi: 10.1093/nar/gkg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J., Hao P., Chen H., Hu W., Yan Q., Liu F., Han Z.G. Genome-wide identification of Schistosoma japonicum microRNAs using a deep-sequencing approach. PLoS One. 2009;4(12) doi: 10.1371/journal.pone.0008206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash P., Ghosliya D., Gupta V. Identification of conserved and novel microRNAs in Catharanthus roseus by deep sequencing and computational prediction of their potential targets. Gene. 2015;554(2):181–195. doi: 10.1016/j.gene.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 38.Wang L., Liu H., Li D., Chen H. Identification and characterization of maize microRNAs involved in the very early stage of seed germination. BMC Genomics. 2011;12(1):154. doi: 10.1186/1471-2164-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in Drosophila. Genome Biol. 2004;5(1):R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of known and conserved miRNA in fishes.
Differential expression of known miRNAs of the brain and pineal gland (dark treatment).
Differential expression of known miRNAs of the brain and pineal gland (light treatment).
List of predicted targets for the novel miRNAs using miRanda.