Abstract
Background
In developing countries such as Pakistan, poor training of mid-level cadres of health providers, combined with unregulated availability of labour-inducing medication can carry considerable risk for mother and child during labour. Here, we describe the exposure to labour-inducing medication and its possible risks in a vulnerable population in a conflict-affected region of Pakistan.
Methods
A retrospective cohort study using programme data, compared the outcomes of obstetric risk groups of women treated with unregulated oxytocin, with those of women with regulated treatment.
Results
Of the 6379 women included in the study, 607 (9.5%) received labour-inducing medication prior to reaching the hospital; of these, 528 (87.0%) received unregulated medication. Out of 528 labour-inducing medication administrators, 197 (37.3%) traditional birth attendants (also known as dai) and 157 (29.7%) lady health workers provided unregulated treatment most frequently. Women given unregulated medication who were diagnosed with obstructed/prolonged labour were at risk for uterine rupture (RR 4.1, 95% CI: 1.7–9.9) and severe birth asphyxia (RR 3.9, 95% CI: 2.5–6.1), and those with antepartum haemorrhage were at risk for stillbirth (RR 1.8, 95% CI: 1.0–3.1).
Conclusions
In a conflict-affected region of Pakistan, exposure to unregulated treatment with labour-inducing medication is common, and carries great risk for mother and child. Tighter regulatory control of labour-inducing drugs is needed, and enhanced training of the mid-level cadres of healthcare workers is required.
Keywords: Labour induction, Maternal child health, Obstetrics, Operational research, Oxytocin, Pakistan
Introduction
Globally, reducing maternal and neonatal mortality poses a major challenge, predominantly in developing countries which have a disproportionately high burden of such mortality, in particular within areas transitioning from rural to urban settlements.1 As WHO posits; ‘unavailable, inaccessible, unaffordable or poor quality care … (are) … fundamentally responsible’ for high mortality rates.2 There are various contributing factors, which include local health systems weakened by chronic natural and man-made disasters, and insufficient training and investment for career development of healthcare workers.
Pakistan is such a country, with the second highest maternal mortality ratio (MMR; 401 deaths/100 000 live births in 2013) in South Asia and the seventh highest neonatal mortality (42 deaths per 1000 live births in 2013) in the world.3 The leading causes of maternal mortality in Pakistan include haemorrhage, hypertensive disorders, infections, obstructed labour and unsafe abortions.4 For neonatal mortality, this includes infection, asphyxia as well as prematurity with low birth weight.5
One of the major determinants of maternal and neonatal mortality in Pakistan is the dearth of trained healthcare workers, especially in areas making a rapid transition from rural to urban.1 Poor training for healthcare workers, coupled with unregulated, over-the-counter availability of labour-inducing medications, may lead to the injudicious use of such medication, with a great potential to negatively impact maternal and neonatal outcomes. Labour-inducing medication refers to drugs that stimulate labour and childbirth. Misuse of labour-inducing medication such as oxytocin has been identified in Pakistan and other countries as contributing to maternal and neonatal mortality.6–8
However, causes of perinatal complications and maternal or neonatal death vary according to the specific context, and an enhanced focus on identifying the local determinants of maternal and neonatal health is thus required. There is very limited information on the prevalence of such unregulated use in areas of Pakistan where there is poor regulatory control of medication and over-the-counter purchases are common.9,10 Furthermore, the consequences of such use have not been described among vulnerable populations that are plagued by many additional risk factors for adverse maternal as well as neonatal outcomes. We therefore set out to describe the exposure to labour-inducing medication in this population, and to assess the putative associations between unregulated use of such medication and adverse maternal and neonatal outcomes.
Materials and methods
Study design
This study is a retrospective cohort study which utilizes patient data collected during routine operations of the different departments within the hospital.
Study setting
This study was conducted in the district headquarter hospital (DHQ) of Timurgara, which is a city in Lower Dir district, Khyber Pakhthunkwa (KPK) province, in North West Pakistan, close to the border with Afghanistan. It lies in one of the least developed provinces in Pakistan, and has been the setting of protracted conflict. Access to healthcare in the area is limited, with the public health system suffering from a lack of drugs, equipment and human resources, and the private sector remaining unaffordable for many patients. Access is further compromised by the conflict in the region, as well as by the distances, mountainous terrain and cultural restrictions for women to travel, especially during pregnancy.11
Neonatal and maternal mortality rates in the province of KPK are comparable to those in the rest of the country; however, a paucity of recent, regional data exists to support this, with the latest regional maternal mortality figures dating back to 2006.12 Multiple cadres of women playing a role in pregnancy and labour exist, all having different levels of official accreditation and training. These are categorized as ‘mid-level health providers’.13 The cadres, ranked by training level, include the registered nurse midwife (RNM), the lady health visitor (LHV), community midwife (CMW; also known as skilled birth attendant [SBA]), pupil midwife and midwife, who are trained on the usage of oxytocin for treatment of postpartum haemorrhage, but who are not trained or legally allowed to administer oxytocin for labour induction. These are followed by the lower-level care providers, which are the lady health workers (LHWs), and who are also classified as community health workers (CHWs), who are intended to be a referral and health information link between households/CMWs and higher-trained health providers. They are often assisted by traditional birth attendants (TBAs; also known as ‘dai’)14 but are not meant to administer oxytocin.13 Care during pregnancy and labour in the region is primarily conducted by traditionally trained women providing non-allopathic medication,15,16 with practices during pregnancy and labour being typically dictated by TBAs who fall in the low/basic level care providing cadre, along with the midwives and pupil midwives.15 These women are often preferred and respected in the community for the knowledge and experience they have with regards to pregnancy,15 although no official partnership between TBAs and higher tiers has ever been explored. Patients coming to a district level hospital often do so once traditional and conventional birth practices have failed, or have led to poorly understood and ill-managed complications15 as most TBAs provide services within the patient's house and accept other modes of payment versus officially trained healthcare professionals.
Médecins Sans Frontières' support to the Timurgara Hospital
Médecins Sans Frontières has provided an emergency medical assistance programme in Timurgara District Headquarter Hospital since October 2010. This assistance includes support to the Maternal and Child Health (MCH) department, which is primarily run by the Ministry of Health (MoH) staff, in the form of human resources, provision of medical and logistical supplies, as well as rehabilitation of the emergency operating theatre, recovery room and acute post-operative wards (female ward of 18 beds at the time of the study), and full support to sterilization, hygiene and waste management. The hospital is meant to cater to the inhabitants of Lower Dir (where Timurgara is located), but patients also come from neighbouring districts, with the majority coming from Lower Dir, followed by Upper Dir and others within KPK as well as the Federally Administered Tribal Areas (FATA). All services are provided free of charge.
Admission criteria and obstetric care
Women arrive at the MCH either directly, or via referral from the Emergency Department, other MoH services within the Timurgara hospital or external health care facilities. Once they reach the MCH and meet the admission criteria, they are registered by the staff, and a patient file is opened and completed by the staff team leader of each shift. Whenever the format of the patient files is changed, trainings are conducted for the MCH staff prior to the implementation of the new data collection tools. A patient history is taken either from the patient or the accompanying attendant (due to language barriers) including whether or not they were given labour-inducing medication prior to arrival at the hospital. This is followed by an assessment of the patient condition and severity by either a midwife or a nurse. Comprehensive emergency obstetric and neonatal care17 is offered: after vaginal delivery or caesarean section, 7 out of 10 patients are usually discharged within the first 24 hours, with 50% within the first few hours itself, despite the MSF recommendation of 24 hour admission, post-delivery.
Unregulated use of labour-inducing medication
Currently, oxytocin vials and misoprostol tablets are available over-the-counter for less than US$0.11 (approximately PKR 10.0), and are administered either in combination or on their own. CMWs and other mid-level health providers have been inadequately trained on its administration and associated risks, and are sent out to work in the community without resources, relying on their own judgment and understanding of the drugs. Due to poor regulatory control, LHWs and TBAs also utilize these drugs. Furthermore, the widely available misoprostol tablets are not legally approved either for labour induction or abortion in Pakistan,18 although a country-wide helpline (and website) for women who want to administer self-abortions refers to the use of misoprostol for this purpose. Oxytocin and misoprostol are thus often given in an unregulated manner (outside the regulatory framework), and the quantity and mode of administration are not documented. For the purposes of this study, any administration of oral labour-inducing medication, and any administration of intramuscular (IM) or intravenous (IV) infusion of labour-inducing medication by healthcare workers other than doctors RNMs, LHVs and CMWs/SBAs was considered as unregulated or injudicious, even if its use may have been justified from a medical perspective (i.e., to resolve uterine inertia or to induce labour when this would be in the best interest of mother or child due to maternal or foetal conditions). Conversely, prescription by said professionals was considered regulated (with the exception of misoprostol prescriptions), but may not always have been medically justified.
Study population
All women delivering at the MCH of Timurgara District Headquarter Hospital between the end of December 2013 and mid-October 2014 were included in the study. Women with a known gestational age <27 weeks were excluded, in order to focus the study only on those women who presented in their third trimester. To avoid a selection bias–in which women at higher risk of complications may have been more likely to receive oxytocin from lower cadre healthcare workers–we analysed the risk for adverse outcomes in the following subgroups of patients, who could be considered at uniform risk for specific outcomes: women with prolonged/obstructed labour, at risk for uterine rupture, cervical tears, and severe birth asphyxia (defined as a 5-minute APGAR score ≤3); women with postpartum haemorrhage, at risk for retained placenta and severe haemorrhage (as assessed by the need for blood transfusion as proxy indicator); women with antepartum haemorrhage, at risk for fresh stillbirth; women without any registered complications or risk factors, at risk for fresh stillbirth.
Outcomes of interest were compared within these risk groups between women who were exposed to oxytocin administered by anyone except unauthorized staff (‘unregulated oxytocin’), and women who were not exposed to oxytocin/received oxytocin only from authorized staff (‘regulated oxytocin’), regardless of whether the mid and high level healthcare providers were employed by MSF, public or private sector (i.e., misoprostol use was excluded from this analysis).
Data and analysis
A standardized MCH database was utilized in the MCH department, and data from patient files were single-entered into the database, allowing for the possibility to record up to three exit diagnoses per patient. Ten percent of randomly selected files were checked for accuracy on a weekly basis, and databases as well as data collection tools were regularly checked by the medical data team both in the coordination office (Islamabad) and at headquarters (Brussels). Differences in proportions were compared and associations were tested using a Poisson regression model (generalized linear model). Associations between exposure (unregulated oxytocin) and adverse outcomes were adjusted for patient origin, referral source, parity, gestational age and multiple pregnancies in this model. Differences in risk levels were expressed using relative risks (RR) with their associated 95% CIs. The level of significance was set at p<0.05. Analysis was done using EpiData Analysis version 2.2.2.178 (EpiData Association, Odense, Denmark) and Stata v.11 (StataCorp LP, College Station, TX, USA) for the regression analyses.
Ethics statement
This study has received ethics exemption by the Medical Research Council of Pakistan due to its utilization of routine programmatic data. The study satisfied the Médecins Sans Frontières Ethics Review Board (Geneva, Switzerland) criteria for studies using routinely collected data. As the study used routine programme data that was anonymised and de-identified prior to analysis, the issue of informed consent was not applicable.
Results
Obstetric characteristics of women attending the Timurgara MCH
A total of 8263 women attended the MCH, of whom 1884 were excluded from the analysis: 1781 did not deliver at the MCH and a further 103 presented in the first or second trimester of pregnancy. Of the remaining 6379 who were included in the analysis, 4352 (68.2%) were from Lower Dir while 1402 (22.0%) were from the adjoining district Upper Dir.
The characteristics of the 6379 women delivering at the hospital are shown in Table 1. A wide range in parity was observed (up to 17), with 1716 (26.9%) of all women being primiparous at the time of admission. The majority of women (5304, 83.1%) were self-referred, 787 (12.3%) were referred through health centres and 224 (3.5%) through TBAs.
Table 1.
Characteristics of women delivering at Timurgara district hospital, Pakistan, December 2013 to August 2014
| n | % | |
|---|---|---|
| Total | 6379 | |
| Parity | ||
| Primiparous (parity=0) | 1716 | 26.9 |
| Multiparous (1≤ parity ≤5) | 3655 | 57.3 |
| Grand multiparous (6≤ parity ≤9) | 757 | 11.9 |
| Great grand multiparas (parity ≥10) | 80 | 1.3 |
| Unknown | 171 | 2.7 |
| Referral source | ||
| Self-referral | 5304 | 83.1 |
| Other health structures | 787 | 12.3 |
| Traditional birth attendants | 224 | 3.5 |
| Unknown | 64 | 1.0 |
| Mode of delivery | ||
| Normal vaginal birth | 5317 | 83.4 |
| Instrumental delivery | 306 | 4.8 |
| Caesarean section | 723 | 11.3 |
| Not recorded | 33 | 0.5 |
| Maternal complication | ||
| Uncomplicated | 4266 | 66.9 |
| Direct obstetric complications | 899 | 14.1 |
| Other complications | 836 | 13.1 |
| Unknown | 378 | 5.9 |
| Maternal conditions (top 5) | ||
| Uncomplicated delivery | 4884 | 76.6 |
| Intra-uterine foetal death | 396 | 6.2 |
| Antepartum haemorrhage | 255 | 4.0 |
| Obstructed labour | 252 | 4.0 |
| Previous caesarean sectiona | 245 | 3.8 |
a Women who have either previously undergone a caesarean section more than once or have a previous classical caesarean section incision (scar).
Use of labour-inducing medication
Out of the 6379 women included in the study, 607 (9.5%) received labour-inducing medication prior to reaching the hospital. Characteristics of these women are provided in Table 2; overall, 79 (13.0%) women received regulated labour-inducing medication (non-oral, by authorised staff), while 528 (87.0%) received unregulated medication (any oral medication or IM/IV by non-authorised staff) over the study period, amounting to approximately two cases per day. Among the unregulated treatment administered to 528 women, 16 (3.0%) received oral labour-inducing drugs (likely misoprostol preparations), while 499 (94.5%) received IV or IM injections (likely oxytocin). Unauthorized staff (TBAs and LHWs) was most frequently involved with unregulated administration of labour-inducing medication (Table 2).
Table 2.
Characteristics of women receiving regulated and unregulated administration of labour-inducing medication, among mothers delivering after 26 weeks of gestation in Timurgara district hospital, Pakistan, December 2013 to October 2014
| Regulated administration n (%) | Unregulated administration n (%) | |
|---|---|---|
| Total | 79 | 528 |
| Administered by: | ||
| MSF staff only | 8 (10.1) | 0 ( |
| Doctor (external) | 71 (89.9) | 2 (0.4) |
| Nurse | 0 ( | 14 (2.7) |
| Midwife (RNM) | 0 ( | 32 (6.1) |
| LHV/CMW/SBA/pupil midwives | 0 ( | 11 (2.1) |
| Lady health worker/CHWs | 0 ( | 157 (29.7) |
| Traditional birth attendant (dai) | 0 ( | 197 (37.3) |
| Dispenser (at pharmacy) | 0 ( | 36 (6.8) |
| Other | 0 ( | 79 (15.0) |
| Timing of administration | ||
| Antenatal | 53 (67.1) | 418 (79.2) |
| Perinatal | 3 (3.8) | 27 (5.1) |
| Postnatal | 8 (10.1) | 0 ( |
| Not documented | 15 (19.0) | 83 (15.7) |
| Mode of administration | ||
| Oral | 0 ( | 16 (3.0) |
| Intramuscular | 12 (15.2) | 42 (8.0) |
| Intravenous | 61 (77.2) | 457 (86.6) |
| Not documented | 6 (7.6) | 13 (2.5) |
| Exit diagnosisa | ||
| Delivery without complications | 35 (44.3) | 202 (38.3) |
| Obstructed/prolonged labour | 16 (20.3) | 111 (21.0) |
| Intra-uterine foetal death | 10 (12.7) | 86 (16.3) |
| Postpartum haemorrhage | 3 (3.8) | 32 (6.1) |
| Antepartum haemorrhage | 4 (5.1) | 30 (5.7) |
| Previous caesarean section | 3 (3.8) | 24 (4.5) |
CHW: community health workers also known as lady health workers; CMW: community midwife; LHV: lady health visitor; RNM: registered nurse midwife; SBA: skilled birth attendant.
a Multiple exit diagnoses are possible per patient; proportions do not add up to 100%.
Oxytocin and adverse maternal or neonatal outcomes
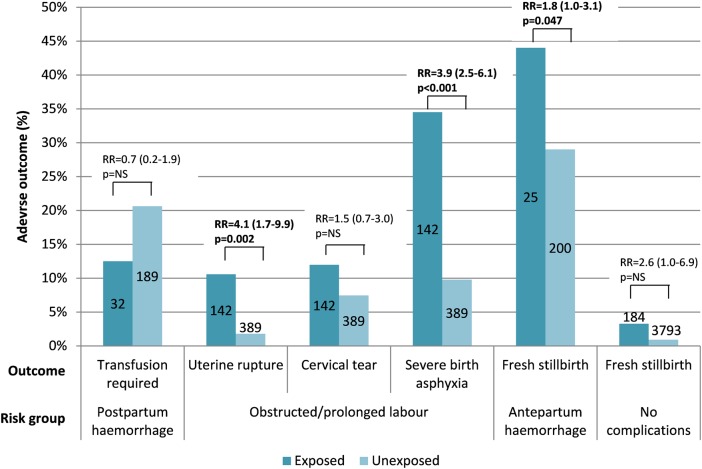
To assess the risk posed by this unregulated use of labour-inducing medication in the community, associations between unregulated use of oxytocin and a number of adverse maternal and neonatal outcomes were evaluated. Subgroups of women considered to be at uniform risk for different adverse outcomes were selected, and within these groups women exposed to unregulated oxytocin treatment (IM/IV) were compared with women without such exposure (Figure 1). Adjustment for potential confounders on which data were available (patient origin, referral source, parity, gestational age, multiple pregnancies) was performed. Statistically significant associations with unregulated oxytocin exposure were seen in the group of women with obstructed or prolonged labour for uterine rupture (RR 4.1, 95% CI: 1.7–9.9) and severe birth asphyxia (RR 3.9, 95% CI: 2.5–6.1), and in the group of women with antepartum haemorrhage for stillbirth (RR 1.8, 95% CI: 1.0–3.1). Over the course of the study, five maternal deaths occurred in the study population: four among the women treated with unregulated treatment and only one among the women receiving regulated treatment (p<0.001).
Figure 1.
Proportional adverse maternal and neonatal outcomes among women in different risk groups, exposed and unexposed to unregulated oxytocin treatment, Timurgara District Hospital, Pakistan, December 2013 to October 2014. p-Values, relative risks and their associated 95% CIs in a Poisson regression model (generalized linear model) are shown. This figure is available in black and white in print and in color at International Health online.
Discussion
We assessed the exposure to the unregulated use of labour-inducing medication and the putative associations between unregulated use of such medication and adverse maternal and neonatal outcomes in a particularly vulnerable population in Pakistan. In a district hospital located in a conflict-affected province of Pakistan, about two women per day presented with self-reported exposure to labour-inducing medication, most typically oxytocin. About 90% of the women exposed to unregulated medication showed significant associations with uterine rupture, severe birth asphyxia and, among women with antepartum haemorrhage, with stillbirth. Additionally, an association with maternal mortality was suggested. Traditional birth attendants and LHWs were most frequently involved with unregulated oxytocin use.
The significant association between unregulated oxytocin use and uterine rupture among women with prolonged or obstructed labour has also been reported in findings from other studies conducted within Pakistan and elsewhere.1,19–24 The incidence was higher in Timurgara and comparable places such as Bannu (also in KPK) than in other settings.24 The widespread misconception that oxytocin can be used to treat obstructed labour, usually associated with abnormal foetal presentation or cephalo-pelvic disproportion and thus not amenable to oxytocin treatment, may contribute to this high usage.19
No associations were found between unregulated oxytocin use and cervical tears, as reported elsewhere,7,25 as this study was not adequately powered to find this association. Additionally, no significant associations were found with retained placenta7—of which no cases were recorded in this study—or severe haemorrhage among patients with postpartum haemorrhage, likely due to the small sample size of these patients (221) and the insensitivity of ‘requiring blood transfusion’ as a proxy indicator for severe haemorrhage. Additionally, as the study was facility-based, such cases may have been selectively missed: since postpartum haemorrhage results in sudden, copious amounts of blood loss as well as rapid death, most patients possibly cannot reach a health facility, as most births are in rural areas in Pakistan and similar countries.26–28
On the neonatal side, unregulated or ‘inappropriate’ oxytocin treatment of women diagnosed with obstructed or prolonged labour was associated with severe birth asphyxia, as also documented in studies in Europe, Nigeria, India and other areas of Pakistan.6,7,22 A higher stillbirth rate was also observed with unregulated oxytocin treatment among women with antepartum haemorrhage as a risk factor for stillbirth. Among women without any recorded complications, this association was not found, though a non-significant trend was observed.
Our study suggests that the ready availability of oxytocin in a region which has been repeatedly subjected to the numerous sequelae of chronic conflict, rendering the population vulnerable (due to lack of investment in health systems, violence leading to destruction of pre-existing infrastructures, internally displaced persons and evacuations leading to mobile populations, stock ruptures in health structures and security risks reducing access) in Pakistan has led to its widespread use for labour induction by all cadres of healthcare workers, especially at midlevel. We advocate for an approach on two levels: 1. A tighter regulatory control of oxytocin, misoprostol and other labour-inducing drugs is needed. Long-term control could focus on accreditation/certification for oxytocin use by the Ministry of National Health Services Regulation and Coordination (NRS) of Pakistan, which is responsible for direct change in policies, and the Drug Regulatory Authority (DRA). Regulation of such measures at a federal/national level could be essential in this.29 Additionally, and more short-term, the economics of the use of oxytocin in the region, including the economic motivations of pharmaceutical companies providing oxytocin, the local pharmacies selling oxytocin over-the-counter, and, to an extent, the prescribers, should be investigated; 2. Enhanced, sustainable training of the mid and lower level cadres of healthcare workers in semi-urban and rural settings is urgently required.30 Focus areas of such training should be clear indications and contra-indications for the use of labour-inducing medication; low-cost approaches to monitoring of patients; and identification of danger signs for obstetric emergencies at an early stage,1 in particular those associated with labour-inducing medication. Quality checks of the care provided, followed by regular training, including refreshing knowledge, are essential components of a long-term strategy.21
Other recommendations include improved referral systems, incorporating the different cadres of healthcare workers (by, for example, provision of mobile communication technologies for rapid access to helplines and requesting of emergency transfers) would also be beneficial in long-term planning.6 Furthermore, dissemination of knowledge in the community among men and women, especially community leaders and mothers-in-law (who are traditionally the decision makers in reproductive health issues), about the link between the usage of labour inducing drugs like oxytocin and complications would be quick and easy to do, and beneficial,20,31 although care must be taken to avoid stigmatisation of specific cadres of health workers.
Our findings and recommendations may be relevant for other drugs that are available without prescription and without quality control or regulatory framework. At the very least, the drugs from the WHO essential drug list32 could be a focus for strengthened regulation and enhanced training.
Strengths of the study were that it was performed under operational conditions, and thus reflects programme reality, and that the overall sample size was large. Limitations of the study included a reliance on self-reporting of medication, though a series of informal interviews with mothers and caretakers suggested that awareness about medication was high, and most women could accurately describe vials of, e.g., oxytocin (referred to as garam teeka). Also, the age of women was not reliably known or recorded; a similar study in Bannu assessed the average age of marriage at 13–14 years, which may represent an additional factor of vulnerability in this population. Details of the person administering labour inducing medication were not systematically recorded, other than the general cadre, and a more qualitative study is recommended to clearly identify the levels of training as well as the range of labour-inducing drugs and practices used (including traditional ones) which could be conducted quite easily. Furthermore, the study was facility-based (due to security constraints), and the district prevalence of unregulated oxytocin treatment could thus not be assessed; additionally, adverse maternal and neonatal outcomes prior to reaching MSF support could not be registered, suggesting the prevalence and consequences of unregulated oxytocin usage documented here may be underestimated. Finally, we could only adjust the analysis for potential confounders on which data was available–additional factors, such as a rural background, poor socio-economic factors, or lack of access to healthcare may have been associated with both unregulated oxytocin exposure and with adverse outcomes, were beyond the scope of the routine data collected and utilized for this study.
Conclusions
In conclusion, use of labour-inducing medication by insufficiently trained cadres of healthcare workers is prevalent in a vulnerable population of Pakistan. The stakes are high, with unregulated usage of such medication resulting in severe consequences for mother and child. Tighter regulatory control measures for the use of labour-inducing medication, as well as better training and awareness among healthcare workers and the community are critical to improve this situation.
Acknowledgments
Authors' contributions: The authors wish it to be known that, in their opinion, SS and RVdB should be regarded as joint first authors. RVdB and JM conceived the study; RVdB, JM, SC, EDP and CVO designed the study protocol; SS, RVdB, JWN, SMD, EDP, NS, AB, GR and TBDK were instrumental in designing and implementing the data tools required for the department and this study and for training staff in their use. JRP, GR, AB and NS did the data collection and cleaning of primary data, whereas RVdB and SS cleaned the database and conducted analysis. SS, RVdB, RA, EDP, SC, RZ and CVO provided interpretation of this data. RVdB an SS drafted the manuscript; SS, RVdB, JRP, GR, AB, NS, RA, SMD, JWN, TBDK, JM, EDP, SC, RZ and CVO all critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. CVO and RZ are guarantors of the paper.
Acknowledgements: We would like to thank the wonderful Maternal Child Health Department staff as well as the data collectors Abdul Basit Khan and Amjad Khan for their excellent work. We are grateful to everyone at the hospital and Timurgara office for all their efforts. Additional thanks go to Renzo Fricke for supporting this study and providing critical feedback, and to the MSF health promotion team of Timurgara consisting of Azia Begum, Mehnaz Begum, Mari Nythun Utheim under the guidance of Yasmine Al Kourdi, to Daphne Lagrou for conducting a pilot analysis on the data, and Maryiam Khwaja as country pharmacist for providing crucial, timely feedback.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Ezechi OC, Mabayoje P, Obiesie LO. Ruptured uterus in South Western Nigeria: a reappraisal. Singapore Med J 2004;45:113–6. [PubMed] [Google Scholar]
- 2.WHO. Why do so many women still die in pregnancy or childbirth? Geneva: World Health Organization; 2014. http://www.who.int/features/qa/12/en/ [accessed 5 January 2015]. [Google Scholar]
- 3.The World Bank. Mortality rate, neonatal (per 1,000 live births). Washington, DC: World Bank; 2015. http://data.worldbank.org/indicator/SH.DYN.NMRT [accessed 5 January 2015]. [Google Scholar]
- 4.Ali R, Khawar A, Kausar S. Maternal Mortality: An Ice Berg One Year Review at DHQ Hospital, Faisalabad. A P M C 2012;6:180–5. [Google Scholar]
- 5.Tinker A, Parker R, Lord D, Grear K. Advancing newborn health: The Saving Newborn Lives initiative. Glob Public Health 2010;5:28–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrier G, Oluyide B, Keramarou M, Grais R. High maternal and neonatal mortality rates in northern Nigeria: an 8-month observational study. Int J Women's Health 2013;5:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffery P, Das A, Dasgupta J, Jeffery R. Unmonitored intrapartum oxytocin use in home deliveries: evidence from Uttar Pradesh, India. Reprod Health Matters 2007;15:172–8. [DOI] [PubMed] [Google Scholar]
- 8.Khaskheli MN, Baloch S, Sheeba A. Iatrogenic risks and maternal health: Issues and outcomes. Pak J Med Sci 2014;30:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabbani F, Cheema FH, Talati N et al. . Behind the counter: pharmacies and dispensing patterns of pharmacy attendants in Karachi. J Pak Med Assoc 2001;51:149–53. [PubMed] [Google Scholar]
- 10.Jeffery P, Brhlikova P. Use and abuse of oxytocin: Millennium Development Goals 4 and 5 in South Asia. Edinburgh, UK: Tracing Pharmaceuticals Dissemination Workshop; 2009. [Google Scholar]
- 11.Fisher J, Cabral de Mello M, Patel V et al. . Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012;90:139G–49G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Research and Advocacy Fund. Provincial & Regional Stakeholders Consultation Meetings Report, Khyber Pakhtunkhwa & FATA. Islamabad: Research and Advocacy Fund Pakistan; 2011. [Google Scholar]
- 13.WHO, Global Health Workforce Alliance. Mid-level health providers: a promising resource to achieve the health Millennium Development Goals. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.Ipas. Expanding the Provider Base for Abortion-Related Care: Findings and Recommendations from an Assessment of Pre-Service Training Needs and Opportunities in Pakistan. Pakistan: Ipas Health Access Rights; 2013. [Google Scholar]
- 15.The Population Council. Unwanted pregnancy and post-abortion complications in Pakistan: findings from a national study. Islamabad, Pakistan: The Population Council; 2004. [Google Scholar]
- 16.Fikree FF, Ali TS, Durocher JM, Rahbar MH. Newborn care practices in low socioeconomic settlements of Karachi, Pakistan. Soc Sci Med 2005;60:911–21. [DOI] [PubMed] [Google Scholar]
- 17.UNICEF, WHO, UNFPA. Guidelines for monitoring the availability and use of obstetric services. New York: United Nations Children's Fund; 1997. [Google Scholar]
- 18.Women on Waves. How can I get Misoprostol? Amsterdam: Women on Waves; 2014. http://www.womenonwaves.org/en/page/961/how-can-i-get-misoprostol [accessed 15 January 2015]. [Google Scholar]
- 19.Shaikh NB, Shaikh S, Shaikh F, Raishem. Uterine rupture: an ongoing tragedy of motherhood. Med Channel 2013;19:24–8. [Google Scholar]
- 20.Ara J, Naheed K, Kazmi F, Sial SS. Uterine rupture : a catastrophic complication. J Rawalpindi Med Coll 2010;14:36–9. [Google Scholar]
- 21.Hassan N, Sirichand P, Zaheen Z, Shaikh F. Uterine rupture at LUMHS: a review of 85 cases. J Liaquat Uni Med Health Sci 2009;8:165–8. [Google Scholar]
- 22.Jonsson M. Use and misuse of oxytocin during delivery. Uppsala: Uppsala University; 2009. [Google Scholar]
- 23.Nisar N, Sohoo NA. Emergency peripartum hysterectomy: frequency, indications and maternal outcome. J Ayub Med Coll Abbottabad 2009;21:48–51. [PubMed] [Google Scholar]
- 24.Qazi Q, Akhtar Z, Khan K, Khan AH. Woman health; uterus rupture, its complications and management in teaching hospital bannu, pakistan. Maedica (Buchar) 2012;7:49–53. [PMC free article] [PubMed] [Google Scholar]
- 25.Brohi ZP, Sadaf A, Zohra N, Perveen U. Frequency and severity of perineal tears in Countess Lady Duffrin Fund Hospital, Hyderabad. J Pak Med Assoc 2012;62:803–6. [PubMed] [Google Scholar]
- 26.Beltman JJ, van den Akker T, Bwirire D et al. . Local health workers' perceptions of substandard care in the management of obstetric hemorrhage in rural Malawi. BMC Pregnancy Childbirth 2013;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igberase GO, Ebeigbe PN. Maternal mortality in a rural referral hospital in the Niger Delta, Nigeria. J Obstet Gynaecol 2007;27:275–8. [DOI] [PubMed] [Google Scholar]
- 28.Mahbuba, Alam IP. Uterine rupture - experience of 30 cases at Faridpur Medical College Hospital. Faridpur Med Coll J 2012;7:79–81. [Google Scholar]
- 29.Nishtar S. Health and the 18th amendment: retaining national functions in devolution. Islamabad, Pakistan: Heartfile; 2015. [Google Scholar]
- 30.Bradley S, McAuliffe E. Mid-level providers in emergency obstetric and newborn health care: factors affecting their performance and retention within the Malawian health system. Hum Resour Health 2009;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty A, Burt R, Rice P, Templeton A. Women's perceptions, expectations and satisfaction with induced labour–a questionnaire-based study. Eur J Obstet Gynecol Reprod Biol 2005;123:56–61. [DOI] [PubMed] [Google Scholar]
- 32.WHO. 19th WHO model list of essential medicines. Geneva: World Health Organization; 2015. [Google Scholar]