Abstract
The recognition of pathogen associated molecular patterns (PAMPs) by pattern recognition receptors (PRR) during viral infection initiates the induction of antiviral signaling pathways, including activation of the Interferon Regulator Factor 3 (IRF3). We identified small molecule compounds that activate IRF3 through MAVS, thereby inhibiting infection by viruses of the families Flaviviridae (West Nile virus, dengue virus and hepatitis C virus), Filoviridae (Ebola virus), Orthomyxoviridae (influenza A virus), Arenaviridae (Lassa virus) and Paramyxoviridae (respiratory syncytial virus, Nipah virus) (1). In this study, we tested a lead compound along with medicinal chemistry-derived analogs to compare the gene transcriptional profiles induced by these molecules to that of other known MAVS-dependent IRF3 agonists. Transcriptional analysis of these small molecules revealed the induction of specific antiviral genes and identified a novel module of host driven immune regulated genes that suppress infection of a range of RNA viruses. Microarray data can be found in Gene Expression Omnibus (GSE74047).
Keywords: RIG-I, Innate, Immunity, Anti-viral, IRF-3
| Specifications | |
|---|---|
| Organism/cell line/tissue | Human, PMA-differentiated THP-1 (human macrophage-like) cell line |
| Sex | N/A |
| Sequencer or array type | Human Agilent Microarray, Agilent-039,494 SurePrint G3 Human GE v2 8x60K Microarray 039,381 (Feature Number version) |
| Data format | Raw and Normalized matrix provided |
| Experimental factors | DMSO, Sendai virus, IFNβ, compounds (KIN1400, KIN1408, KIN1409) |
| Experimental features | This analysis verified a novel class of host-directed immune modulatory molecules that produce antiviral responses through IRF3 and can suppress infection by RNA viruses of distinct genera. |
| Consent | Allowed for reuse. Please contact authors first before reuse |
| Sample source location | Seattle, WA, USA |
1. Direct link to deposited data
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74047
http://ciiid.washington.edu/r_markdown/data_in_brief_012916/
2. Experimental Design, Materials and Methods
2.1. Design
THP-1 cells were differentiated into macrophages in 40 nM phorbol 12-myristate 13-acetate (PMA) for 30 h and treated with the following controls for 20 h in complete RPMI (cRPMI) supplemented with 0.5% (v/v) DMSO: 0.5% DMSO alone, Sendai virus (SeV) (100 HAU/mL) or interferon β (IFNβ; 100 IU/mL). Alternatively, cells were treated in the same manner with the compounds KIN1400, KIN1408, or KIN1409 at doses of 10 μM, 2.5 μM, and 0.625 μM. As an additional control, another set of cells were transfected with 2 μg/mL RIG-I agonist hepatitis C virus (HCV) polyU/UC RNA (pU/UC) or control HCV RNA XRNA in cRPMI without DMSO.
2.2. Compounds
Compounds were synthesized by Life Chemicals Inc. and KINETA, Inc. and were solubilized in 100% DMSO and kept frozen as 10 mM stocks. The compounds were stored in small aliquots to prevent multiple freeze-thaws and were step-wise diluted to reach the desired concentration in 0.5% DMSO for all treatments [2].
2.3. Cell lines and viruses
THP-1 (human monocytic) cell line was cultured in complete RPMI (Invitrogen) supplemented with 10% heat-inactivated FBS. THP-1 cells were differentiated into macrophages in complete RPMI supplemented with 40 nM PMA for 24 h prior to their use in experiments.
2.4. Microarray analysis
Differentiated THP-1 cells treated with complete RPMI supplemented with 0.5% (v/v) DMSO with small molecule (KIN1400, KIN1408 and KIN1409 at 10, 2.5 or 0.625 μM), IFNβ 100 U/mL, SeV (Sendai virus) 100 HAU/mL or 0.5% DMSO alone. Additional cells were transfected with HCV polyU/UC RNA (2.
μg/ml) or XRNA (2 μg/mL) using the TransIT®-mRNA Transfection reagent (Mirus Bio LLC). Cells were harvested 20 h later in RLT buffer. RNA was purified using Qiagen RNeasy kits and submitted to Labcorp, Seattle, WA for microarray analysis using Agilent SurePrint G3 Human Genome Microarrays (version 2).
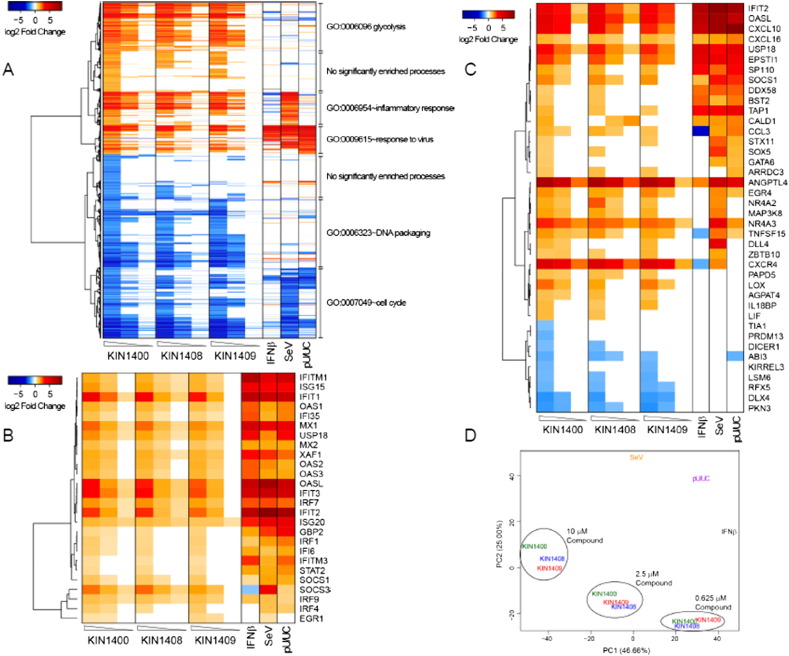
Microarray data has been uploaded to Gene Expression Omnibus (GSE74047) [3]. All downstream analysis was performed by the Gale lab using R (version 3.2.1)/Bioconductor (version 3.1) and R studio (Version 0.99.486). The analysis was captured in a reproducible data report using RMarkdown [4] and can be found at http://ciiid.washington.edu/r_markdown/data_in_brief_012916/. Raw data was downloaded, background corrected and quantile normalized. Controls and low expressing probes were filtered and then applied to a linear model using the limma package (version 3.24.15) [5], [6]. Genes with significant expression changes following treatment were defined by those with a > 2-fold increase or decrease over DMSO (for small molecule compounds, IFNβ and SeV) or XRNA (for pU/UC PAMP RNA) controls with a Benjamini-Hochberg corrected (adjusted p-value) < 0.01. The gene expression heat map (Fig. 1) was clustered in R using Spearman correlation distances and Gene Ontology Biological Processes enriched in lists of genes mapping to clusters by DAVID 6.7 [7], [8]. Genes with predicted IRF7 binding sites according to the UCSC Genome Browser database were identified using Enrichr [9]. Finally, genes mapping to the Reactome Homo sapiens interferon alpha/beta signaling pathway (R-HAS-909,733) were identified using InnateDb [10].
Fig. 1.
Genomics analysis of KIN1400, KIN1408 and KIN1409 treatment. PMA differentiated THP-1 cells were treated with 10, 2.5 and 0.625 μM of compounds KIN1400, KIN1408 and KIN1409 for 20 h. (A) Heat map of the union of differentially expressed genes across treatments. Differential gene expression was defined as at least a 2-fold change in expression and a Benjamini-Hochberg corrected p-value < 0.01 as compared to the appropriate negative control (XRNA for pU/UC RNA and DMSO for all other samples). Expression levels not meeting the cutoff thresholds were set to zero for visual identification of differential expression. Gene clusters identified by hierarchical clustering using spearman correlation as a distance measure and then classified by the most highly enriched gene ontology biological process meeting a Benjamini-Hochberg corrected p-value < 0.05. (B) Heat map of genes whose promoters are predicted by the UCSC Genome Browser database to contain IRF7 binding sites. (C) Heatmap of genes mapping to the Reactome interferon alpha/beta signaling pathway (R-852 HAS-909,733). (D) Two-dimensional principle components analysis shows patterns among gene expression profiles across SeV, IFNβ, HCV pU/UC RNA, KIN1400, KIN1408 and KIN1409 treatments.
3. Conclusion
Transcriptional analysis of the parental small molecule KIN1400 compared to its medicinal chemistry-derived analogs showed that all three family members induced comparable gene expression profiles. The 1408 and 1409 analogs are, if anything, slightly more selective in their induction and repression of specific gene sets while retaining antiviral potency [1], suggesting that medicinal chemical modifications may lead to the design of small molecules with fewer off-target effects. We also compared our IRF3 activating molecules to several control compounds. SeV infection is recognized by the cytoplasmic pathogen recognition receptor RIG-I, leading to activation of IRF-3 through MAVS. We included transcriptional analysis of THP-1 cells infected with SeV to identify the gene sets triggered during a natural infection. We also transfected the cells with the HCV polyU/UC RNA, which is known to directly engage RIG-I to signal to IRF-3 specifically through MAVS [11], and compared the result to transfection with signaling-deficient HCV XRNA as a control. Finally, IFNβ was added to the cells to identify genes induced through JAK/STAT signaling by the type I interferon (IFN) receptor. We found that our small molecules were able to activate IRF7-dependent genes (whose binding site highly overlaps with that of IRF3) and type-I IFN signaling molecules in similar overall patterns to that of the controls. Additionally, our small molecules induced new modules of genes that are distinct from the traditional IRF3-activating controls, indicating that there may be additional factors involved. Further studies will be conducted to identify the mechanisms underlying these signatures.
The demand for broad-spectrum antivirals increases as new RNA viruses emerge or re-emerge to cause disease and impact public health. Our transcriptional analysis of the hydroxyquinoline family of compounds showed that it activates IRF signaling and promotes cellular antiviral responses. Additional analysis of these gene signatures could aid in the development of new antiviral therapies and further refine the mechanisms that enhance host-driven antiviral immune responses against a variety of RNA viruses.
References
- 1.Pattabhi S., Wilkins C.R., Dong R., Knoll M.L., Posakony J., Kaiser S., Mire C.E., Wang M.L., Ireton R.C., Geisbert T.W., Bedard K.M., Iadonado S.P., Loo Y.M., Gale M., Jr. Targeting innate immunity for antiviral therapy through small molecule agonists of the RLR pathway. J. Virol. Dec 16 2015 doi: 10.1128/JVI.02202-15. (pii: JVI.02202–15. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K.M., Wang M.L., Proll S.C., Loo Y.M., Katze M.G., Gale M., Jr., Iadonato S.P. Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J. Virol. 2012;86:7334–7344. doi: 10.1128/JVI.06867-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loraine A.E1., Blakley I.C., Jagadeesan S., Harper J., Miller G., Firon N. Analysis and visualization of RNA-Seq expression data using RStudio, Bioconductor, and Integrated Genome Browser. Methods Mol. Biol. 2015;1284:481–501. doi: 10.1007/978-1-4939-2444-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A.J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J.Y., Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth G.K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3 doi: 10.2202/1544-6115.1027. (Article3) [DOI] [PubMed] [Google Scholar]
- 7.Huang D.W., Sherman B.T., Tan Q., Collins J.R., Alvord W.G., Roayaei J., Stephens R., Baseler M.W., Lane H.C., Lempicki R.A. The DAVID Gene Functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang D.W., Sherman B.T., Tan Q., Kir J., Liu D., Bryant D., Guo Y., Stephens R., Baseler M.W., Lane H.C., Lempicki R.A. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen E.Y., Tan C.M., Kou Y., Duan Q., Wang Z., Meirelles G.V., Clark N.R., Ma'ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinforma. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuer K., Foroushani A.K., Laird M.R., Chen C., Sribnaia A., Lo R., Winsor G.L., Hancock R.E., Brinkman F.S., Lynn D.J. InnateDB: systems biology of innate immunity and beyond–recent updates and continuing curation. Nucleic Acids Res. 2013;41:D1228–D1233. doi: 10.1093/nar/gks1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito T., Owen D.M., Jiang F., Marcotrigiano J., Gale M., Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]