Abstract
TIA-1 related protein (TIAR) is a RNA-binding protein involved in several steps of gene expression such as RNA splicing Aznarez et al. (2008) [1] and translation Piecyk et al. (2000) [2]. TIAR contains three RNA recognition motifs (RRMs) allowing its interaction with specific sequences localized in the untranslated regions (UTRs) of several mRNAs. In myeloid cells, TIAR has been shown to bind and regulate the translation and stability of various mRNA-encoding proteins important for the inflammatory response, such as TNFα Piecyk et al. (2000), Gueydan et al. (1999) [2], [3], Cox-2 Cok et al. (2003) [4] or IL-8 Suswam et al. (2005) [5]. Here, we generated two macrophage-like RAW 264.7 cell lines expressing either a tagged full-length TIAR protein or a RRM2-truncated mutant unable to bind RNA with high affinity Dember et al. (1996), Kim et al. (2013) . By a combination of RNA-IP and microarray analysis (RIP-chip), we identified mRNAs specifically bound by the full-length protein both in basal conditions and in response to LPS (GSE77577).
Keywords: RIP-chip, AU-rich element, TIAR, Translation repression, LPS
| Specifications [standardized info for the reader] | |
|---|---|
| Organism/cell line/tissue | Murine RAW 264.7 cells |
| Sex | None |
| Sequencer or array type | MEEBO Mouse array |
| Data format | Raw and analyzed |
| Experimental factors | Microarray hybridization was performed on TIAR-FLAG-associated mRNAs or TIARΔRRM2-FLAG-associated mRNAs in unstimulated and LPS-stimulated RAW264.7 cells. |
| Experimental features | Enrichment of TIAR-FLAG-associated mRNAs and TIARΔRRM2-FLAG-associated mRNAS were normalized to total transcriptome of TIAR-FLAG and TIARΔRRM2-FLAG expressing-RAW264.7 cells respectively. After amplification and labelling, sample pairs were hybridized onto Mouse Exonic Evidence Based Oligonucleotide (MEEBO) arrays containing on average 38,784 mouse 70mer oligonucleotide probes (Stanford University, US). Hybridizations were replicated with dye swap. |
| Consent | Level of consent allowed for reuse if applicable |
| Sample source location | 1Laboratoire de Biologie Moléculaire du Gène, Faculté des Sciences, Université Libre de Bruxelles (ULB) |
1. Direct link to deposited data [provide URL below]
2. Experimental design, materials and methods
2.1. Generation of RAW264.7 cells expressing tagged full-length or RRM2-truncated TIAR proteins
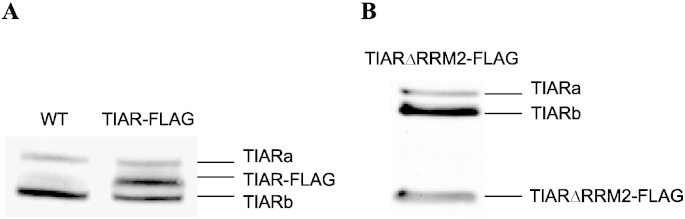
Full-length murine TIARb [8] (Genbank accession number U55861) or a truncated version lacking RRM2 were FLAG-tagged at the 3′ end and cloned in a modified version of the lentiviral plasmid pHR'trip CMV-eGFP where the CMV promoter was replaced by the chicken β-actin promoter (kind gift of Fabrice Bureau, Université de Liège). Lentiviral particles were produced as previously described [9]. Subsequently, 4 × 106 RAW cells were seeded in 96-well plates and infected with the lentiviral particles for 48 h. Cells were washed with PBS and the colony expanded. Subsequently, single cell clones were isolated by limit dilution and amplified. Expression of TIAR-FLAG and TIARΔRRM2-FLAG was verified by Western blot using FLAG M2 mouse monoclonal antibody (Sigma) as described elsewhere [10]. To avoid binding artifacts due to overexpression of TIAR-FLAG proteins, we selected clones expressing TIAR-FLAG or TIARΔRRM2-FLAG at levels similar to the endogenous TIAR protein (Fig. 1).
Fig. 1.
Stable expression of TIAR-FLAG and TIARΔRRM2-FFLAG in RAW 264.7 macrophages.
The expression of TIAR-FLAG and TIARΔRRM2-FLAG was measured by Western blot as described in materials and methods.
2.2. Cell culture and LPS treatment
RAW 264.7 cell clones were cultured in Glutamax Dubelcco Modified Eagle Medium (Gibco-BRL®) complemented with 5% fetal bovine serum, 1 mM Na pyruvate and 1% penicillin–streptomycin. 2 × 106 cells were seeded and were either left untreated or treated for 2 h with 100 ng/ml LPS from Escherichia coli (0127:B8; Sigma®).
2.3. Immunoprecipitation of RNA-protein complexes
We used the RIP-chip method [11] to identify mRNAs bound by TIAR. Briefly, RAW 264.7 cells expressing TIAR-FLAG or TIARΔRRM2-FLAG were lysed in polysome lysis buffer containing 10 mM HEPES pH 7.0, 100 mM KCl, 25 mM EDTA, 2 mM DTT, 5 mM MgCl2, 0.5% NP40 and 2 μl RNAse OUT™ (Invitrogen®). TIAR-FLAG or TIARΔRRM2-FLAG was then immunoprecipitated overnight with anti-FLAG M2 agarose beads (Sigma®) according to the manufacturer's instructions and then eluted in a buffer containing 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM MgCl2, 2 μl de RNase OUT™ (Invitrogen®) and the FLAG peptide (Sigma®) at a final concentration of 250 μg/ml. mRNAs were then isolated by Trizol (Invitrogen®) extraction according to the manufacturer's protocol.
2.4. Microarray, quality control and data processing
Total or immunoprecipitated RNA was purified on RNeasy kit columns (Qiagen). Double-stranded cDNA was synthesized from 1 μg of total RNA, followed by production of antisense RNA containing the modified nucleotide 5-(3-aminoallyl)-uracil triphosphateP using the Amino Allyl MessageAmp™ II aRNA Amplification kit (Ambion, Texas). After labelling with Cy3 or Cy5 (GE Healthcare Bio-Sciences, NJ), samples were hybridized on the Mouse Exonic Evidence Based Oligonucleotide array (Stanford Functional Genomics Facility, CA). The oligonucleotide set consists of 38,784 70-mer probes that were designed using a transcriptome-based annotation of exonic structure for genomic loci. Hybridizations were replicated with dye swap.
Slides were scanned using a Molecular Devices 4000B laser scanner and expression levels were quantified using GenePix Pro 6.1 image analysis software (Axon Instruments, CA). Image acquisitions were performed with automatic photomultiplier gain adjustment. Artefact-associated spots were eliminated by both visual and software-guided flags, as well as spots with a signal/background fluorescence ratio less than 2. The fluorescence values were imported into Acuity 4.0 software package (Molecular Devices, Union City, CA). A non-linear locally weighted scatter plot normalization method applied to each individual block (print-tip option) was carried out. The resulting data files were used for further data analysis. In order to identify differentially expressed genes, normalized log2 ratio obtained from the individual hybridization experiments was selected by a threshold of absolute log2 value > 1.
Enrichment of TIAR-FLAG or TIARΔRRM2-FLAG-associated mRNAs was evaluated by comparison to the total mRNA input used for immunoprecipitation.
The importance of RRM2 for high-affinity binding of TIAR to RNA ligands has been documented in two independent studies [6], [7]. Therefore, to identify high-affinity RNA ligands of TIAR, we discarded from the analysis all the mRNAs that co-precipitated both with TIAR-FLAG and the TIARΔRRM2-FLAG mutants.
2.5. Consolidation of microarray data by qPCR
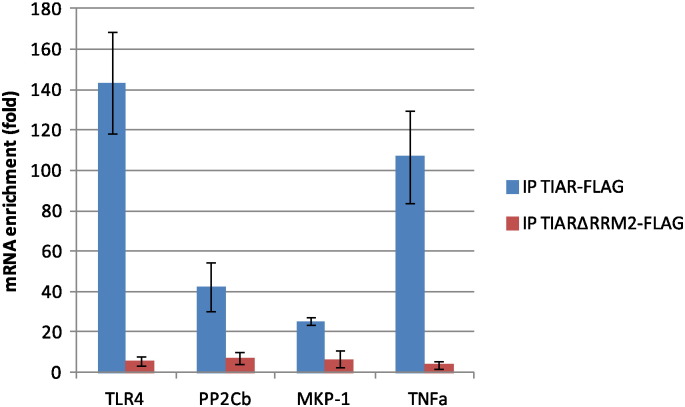
To validate the microarray data, we analyzed by RT-qPCR on independent RIP samples prepared from LPS-stimulated cells the binding of two already known targets of TIAR, TNFα [3] and MKP-1 [12] and two new targets, TLR4 and PP2Cb. As shown in Fig. 2, this assay confirmed that all four tested mRNAs co-precipitate with TIAR-FLAG with high efficiency as compared to TIARΔRRM2-FLAG.
Fig. 2.
TIAR binds to mRNAs encoding TLR4, PP2Cb, MKP-1 and TNFα in response to LPS.
3. Results
3.1. Comparative analysis of TIAR RNA ligands in unstimulated and LPS-stimulated RAW 264.7 macrophages
Our data revealed that 351 transcripts co-precipitated with TIAR in untreated cells and 779 transcripts were found in the RIP-chip performed with LPS-stimulated cells. Of these, 343 transcripts were precipitated in both conditions, 436 exclusively in LPS-treated cells and only 8 exclusively in untreated cells (Fig. 3), showing that the repertoire of mRNAs bound by TIAR increases importantly in response to LPS.
Fig. 3.
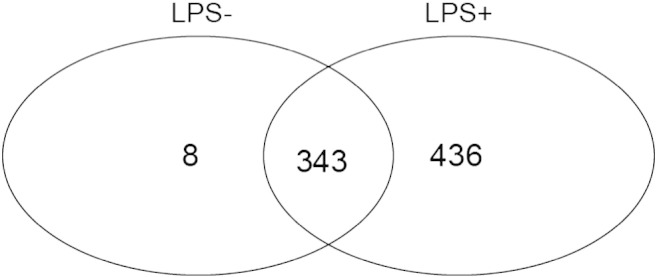
Number of transcripts bound by TIAR in untreated cells (LPS −), LPS-stimulated cells (LPS +) or in both conditions.
Analysis of gene ontology terms or KEGG pathway annotations using DAVID bioinformatics resources [13] shows that in both conditions, TIAR binds to mRNAs important for the inflammatory response, cell proliferation, cell death or metabolism. Terms such as “protein catabolic process”, “cell cycle”, “regulation of apoptosis” or “chemokine signaling pathway” are significantly enriched both in unstimulated and LPS-stimulated samples. Strikingly, the term “positive regulation of I-kappaB kinase/NF-kappaB cascade” was exclusively enriched among the mRNAs bound in response to LPS (Table 1).
Table 1.
Gene ontology and Kegg pathway annotation analysis.
| GO terms and KEGG pathway annotations analysis | |||||
|---|---|---|---|---|---|
| LPS − |
LPS + |
||||
| Terms and annotations | Fold enrichment | P value | Terms and annotations | Fold enrichment | P value |
| GO:0007049 ~ cell cycle | 3.30998363338788 | 7.64364276611717E-09 | GO:0007049 ~ cell cycle | 2.48725127056594 | 4.16348480120036E-09 |
| GO:0042981 ~ regulation of apoptosis | 2.4 | 0.000469749323842693 | GO:0042981 ~ regulation of apoptosis | 2.20927318295739 | 3.81140296380238E-06 |
| GO:0030163 ~ protein catabolic process | 2.38705035971223 | 0.000503035587333267 | GO:0030163 ~ protein catabolic process | 2.62610437965417 | 1.53376399873786E-09 |
| mmu04062:Chemokine signaling pathway | 2.57367122673245 | 0.0336856452640473 | mmu04062:Chemokine signaling pathway | 2.153095684803 | 0.0124230454179306 |
| GO:0043123 ~ positive regulation of I-kappaB kinase/NF-kappaB cascade | 4.51488569909622 | 0.0235639962712211 | |||
3.2. Binding of TIAR to mRNAs containing AU-rich elements (AREs)
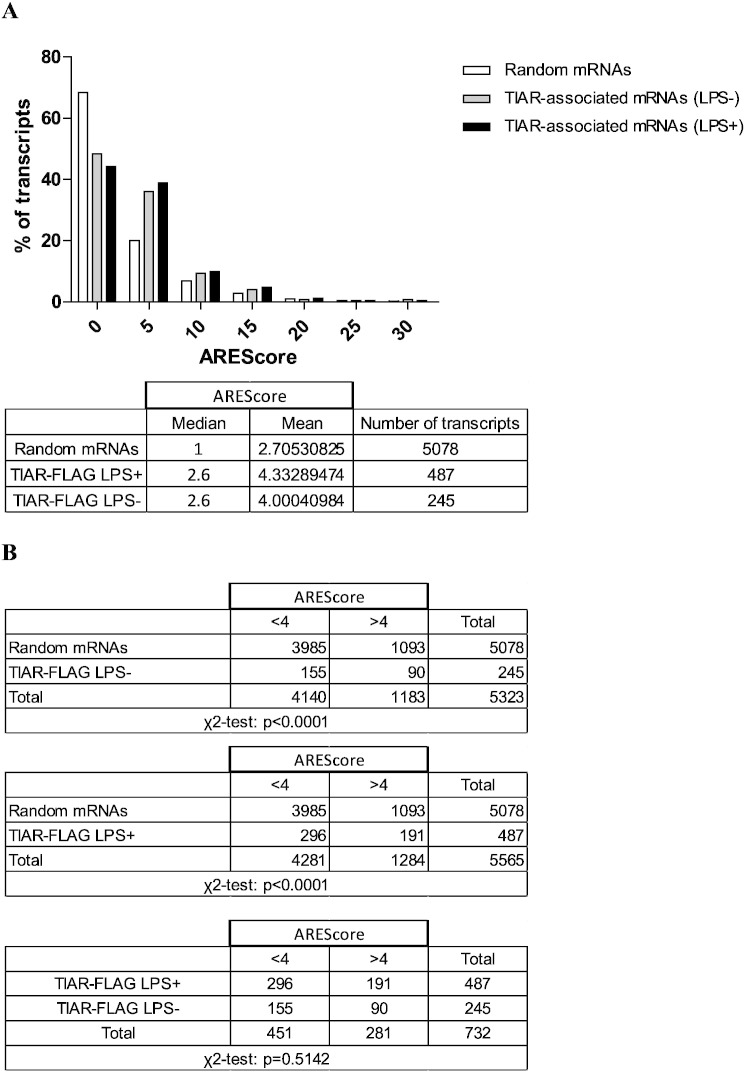
AU-rich elements (AREs) correspond to a family of cis-acting regulatory elements present in the 3'UTR of several mRNAs. These elements are identified by their content in the pentamer AUUUA and have been classified in distinct groups based on the number of pentamers and their U-rich environment [14], [15]. We compared the list of mRNAs identified as TIAR ligands in our RIP-chip with the ARED database. We identified 59 ARE-containing mRNAs present in the ARED database out of the 351 TIAR-binding transcripts from unstimulated cells and 130 out of 779 from LPS-stimulated cells. Besides one transcript encoding Fibroblast growth factor receptor substrate 3 (Frs3) whose association to TIAR is observed in untreated cells only, all other 58 ARE-containing mRNAs were found in the RIP-chip from LPS-stimulated cells.
However, as this database is not exhaustive, we proceeded to an additional analysis of ARE content in TIAR-immunoprecipitated transcripts as described by Spacic et al. [16]. We used the algorithm called AREScore that allows to estimate the number of AUUUA pentamers, their proximity and the surrounding AU-rich regions. This algorithm then assigns a score to each RNA according to these parameters. Fig. 4A shows the AREScore frequency distributions of 5078 randomly selected mRNAs from the total mouse transcriptome and of TIAR-associated mRNA fractions both in unstimulated or LPS-stimulated conditions. We observed more than a two-fold increase in AREScore of TIAR-FLAG-associated mRNAs in both unstimulated and LPS-stimulated conditions (median 2.6 for both conditions) as compared to the score of randomly selected mRNAs (median 1), thereby reflecting preferential binding of TIAR to ARE-containing mRNAs. However, this score was lower than the one obtained for tristretraprolin (TTP), another ARE-binding protein, in a RIP-chip analysis performed with the same cell line (median 7.8) [17]. This confirms the capacity of TIAR to bind other RNA motifs [18]. In order to know if the AREScore distribution of TIAR-FLAG-associated mRNAs was statistically different from randomly selected mRNAs, we proceeded as in [16]. Briefly, we compared the frequency of mRNAs with an AREScore < 4 and > 4 in 2 × 2 contingency tables and we calculated the P-values by χ2-test. The AREScore distributions for both conditions (unstimulated or LPS-stimulated) were significantly different than for the randomly selected mRNAs (p < 0.0001). We then compared the AREScore distributions of TIAR-associated mRNAs in unstimulated and LPS-stimulated conditions and found no difference (p = 0.51) (Fig. 4B). Altogether, these results suggest that TIAR has a preferential affinity to ARE although weaker than TTP and that this affinity is not modulated by LPS.
Fig. 4.
A) AREScore frequency distributions of randomly selected mRNAs and TIAR-binding transcripts either in untreated cells (LPS −) or LPS-treated cells (LPS +). Means and medians of frequency distributions are indicated in the table below. B) χ2-test showing that the AREScore frequency distributions of TIAR-associated transcripts are significantly different from randomly selected mRNAs in both conditions (LPS −- and LPS +). No difference was observed between TIAR-associated mRNAs in untreated or LPS-treated cells.
4. Discussion
We report here a dataset of mRNAs bound by TIAR in the macrophage-like RAW264.7 cell line both in basal (unstimulated) conditions and in response to LPS using the RIP-chip method [11]. Our data show that TIAR binds a large array of mRNAs involved in the inflammatory response in both conditions. The important increase in mRNAs associated to TIAR-FLAG in response to LPS, as compared to unstimulated cells, suggests that its binding activity might be directly modulated by LPS (Fig. 3). However, this process would not be exclusively ARE-dependent (Fig. 4) and therefore might be endorsed by other motifs bound by TIAR and not yet identified. TIAR has been shown to post-transcriptionally control gene expression, either at the RNA splicing step [1] or by repressing mRNA translation [19], [20]. Its binding to mRNAs encoding pro-inflammatory cytokines and chemokines or signaling proteins involved in inflammation [2], [3], [4], [5] might represent a mechanism allowing the cell to rapidly shut down an inflammatory response that otherwise might be harmful for the host. Additional studies are needed to further understand the exact role of TIAR in the metabolism of the mRNAs found in this dataset and to decipher how these mRNAs participate to the inflammatory response.
Acknowledgments
This work was supported by the Fund for Medical Scientific Research (Belgium, Grant 2.4556.08), the Fonds Brachet, the Fonds Van Buuren, and the “Actions de Recherches Concertées” (Grant AV.06/11-345).
References
- 1.Aznarez I., Barash Y., Shai O., He D., Zielenski J., Tsui L.C., Parkinson J., Frey B.J., Rommens J.M., Blencowe B.J. A systematic analysis of intronic sequences downstream of 5' splice sites reveals a widespread role for U-rich motifs and TIA1/TIAL1 proteins in alternative splicing regulation. Genome Res. 2008;18:1247–1258. doi: 10.1101/gr.073155.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piecyk M., Wax S., Beck A.R., Kedersha N., Gupta M., Maritim B., Chen S., Gueydan C., Kruys V., Streuli M., Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gueydan C., Droogmans L., Chalon P., Huez G., Caput D., Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- 4.Cok S.J., Acton S.J., Morrison A.R. The proximal region of the 3'-untranslated region of cyclooxygenase-2 is recognized by a multimeric protein complex containing HuR, TIA-1, TIAR, and the heterogeneous nuclear ribonucleoprotein U. J. Biol. Chem. 2003;278:36157–36162. doi: 10.1074/jbc.M302547200. [DOI] [PubMed] [Google Scholar]
- 5.Suswam E.A., Nabors L.B., Huang Y., Yang X., King P.H. IL-1beta induces stabilization of IL-8 mRNA in malignant breast cancer cells via the 3' untranslated region: involvement of divergent RNA-binding factors HuR, KSRP and TIAR. Int. J. Cancer. 2005;113:911–919. doi: 10.1002/ijc.20675. [DOI] [PubMed] [Google Scholar]
- 6.Dember L.M., Kim N.D., Liu K.Q., Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J. Biol. Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.S., Headey S.J., Yoga Y.M., Scanlon M.J., Gorospe M., Wilce M.C., Wilce J.A. Distinct binding properties of TIAR RRMs and linker region. RNA Biol. 2013;10:579–589. doi: 10.4161/rna.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck A.R., Medley Q.G., O'Brien S., Anderson P., Streuli M. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res. 1996;24:3829–3835. doi: 10.1093/nar/24.19.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiscornia G., Singer O., Verma I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 10.Kharraz Y., Salmand P.A., Camus A., Auriol J., Gueydan C., Kruys V., Morello D. Impaired embryonic development in mice overexpressing the RNA-binding protein TIAR. PLoS One. 2010;5:e11352. doi: 10.1371/journal.pone.0011352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baroni T.E., Chittur S.V., George A.D., Tenenbaum S.A. Advances in RIP-chip analysis: RNA-binding protein immunoprecipitation-microarray profiling. Methods Mol. Biol. 2008;419:93–108. doi: 10.1007/978-1-59745-033-1_6. [DOI] [PubMed] [Google Scholar]
- 12.Kuwano Y., Kim H.H., Abdelmohsen K., Pullmann R., Jr., Martindale J.L., Yang X., Gorospe M. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol. Cell. Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Bakheet T., Frevel M., Williams B.R., Greer W., Khabar K.S. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246–254. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakheet T., Williams B.R., Khabar K.S. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34:D111–D114. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spasic M., Friedel C.C., Schott J., Kreth J., Leppek K., Hofmann S., Ozgur S., Stoecklin G. Genome-wide assessment of AU-rich elements by the AREScore algorithm. PLoS Genet. 2012;80:e1002433. doi: 10.1371/journal.pgen.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoecklin G., Tenenbaum S.A., Mayo T., Chittur S.V., George A.D., Baroni T.E., Blackshear P.J., Anderson P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J. Biol. Chem. 2008;283:11689–11699. doi: 10.1074/jbc.M709657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.S., Kuwano Y., Zhan M., Pullmann R., Jr., Mazan-Mamczarz K., Li H., Kedersha N., Anderson P., Wilce M.C., Gorospe M., Wilce J.A. Elucidation of a C-rich signature motif in target mRNAs of RNA-binding protein TIAR. Mol. Cell. Biol. 2007;27:6806–6817. doi: 10.1128/MCB.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao B., Hu Y., Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat. Struct. Mol. Biol. 2007;14:511–518. doi: 10.1038/nsmb1249. [DOI] [PubMed] [Google Scholar]
- 20.Mazan-Mamczarz K., Lal A., Martindale J.L., Kawai T., Gorospe M. Translational repression by RNA-binding protein TIAR. Mol. Cell. Biol. 2006;26:2716–2727. doi: 10.1128/MCB.26.7.2716-2727.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]