Abstract
This review describes development of currently available pneumococcal vaccines, provides summary tables of current pneumococcal vaccine recommendations in children and adults, and describes new potential vaccine antigens in the pipeline. Streptococcus pneumoniae, the bacteria responsible for pneumonia, otitis media, meningitis and bacteremia, remains a cause of morbidity and mortality in both children and adults. Introductions of unconjugated and conjugated pneumococcal polysaccharide vaccines have each reduced the rate of pneumococcal infections caused by the organism S. pneumoniae. The first vaccine developed, the 23-valent pneumococcal polysaccharide vaccine (PPSV23), protected adults and children older than 2 years of age against invasive disease caused by the 23 capsular serotypes contained in the vaccine. Because PPSV23 did not elicit a protective immune response in children younger than 2 years of age, the 7-valent pneumococcal conjugate vaccine (PCV7) containing seven of the most common serotypes from PPSV23 in pediatric invasive disease was developed for use in children younger than 2 years of age. The last vaccine to be developed, the 13-valent pneumococcal conjugate vaccine (PCV13), contains the seven serotypes in PCV7, five additional serotypes from PPSV23, and a new serotype not contained in PPSV23 or PCV7. Serotype replacement with virulent strains that are not contained in the polysaccharide vaccines has been observed after vaccine implementation and stresses the need for continued research into novel vaccine antigens. We describe eight potential protein antigens that are in the pipeline for new pneumococcal vaccines.
INDEX TERMS: 13-valent pneumococcal polysaccharide conjugate vaccine, 23-valent pneumococcal polysaccharide vaccine, pneumococcal vaccine protein antigens, Streptococcus pneumoniae
INTRODUCTION
The gram-positive cocci Streptococcus pneumoniae causes pneumonia, otitis media, meningitis, and bacteremia in pediatric, elderly, and immunocompromised populations.1 Pneumococcal infection is the leading cause of pneumonia in children, worldwide.2 Pneumococcal infections also occur frequently in at-risk populations including individuals with diabetes, asthma, chronic obstructive pulmonary disease, cardiovascular disease, human immunodeficiency virus (HIV), and sickle cell disease. In developed countries, pneumococcal infection is responsible for approximately 30% of all adult pneumonia cases and has a mortality rate of 11% to 40%.1
Due to this organism's impact on both morbidity and mortality in adults and children, health care efforts have relied on vaccines to reduce the rate of pneumococcal disease over the past 30 years. Vaccine research has focused on using immunogenic proteins and carbohydrates found on the pneumococcal surface as antigens.1 The goal of this review is to describe the evolution of current pneumococcal vaccines; the current guideline recommendations for their use in children, adults and special populations; and the new protein antigens in the pipeline, needed because of serotype replacement after introduction of polysaccharide vaccines.
POLYSACCHARIDE VACCINES
Capsular Polysaccharide
Pneumococci express chains of 1 of 92 immunologically distinct polysaccharide subunits anchored to the cell wall surface. These polysaccharide chains protect pneumococci from complement-mediated opsonophagocytosis, the main mechanism of clearance of pneumococci from the lung. Production of this polysaccharide chain is essential for pneumococcal colonization and virulence, making it an obvious early target antigen.3 These polysaccharide subunits are highly immunogenic, producing antibodies that react with the homologous serotype. The prevalence of particular capsular serotypes can vary greatly among continents, providing differing levels of protection for vaccinated individuals depending on their location. Therefore, capsular serotypes have been monitored internationally since the beginning of their use as vaccine antigens.4
Polysaccharide Vaccines
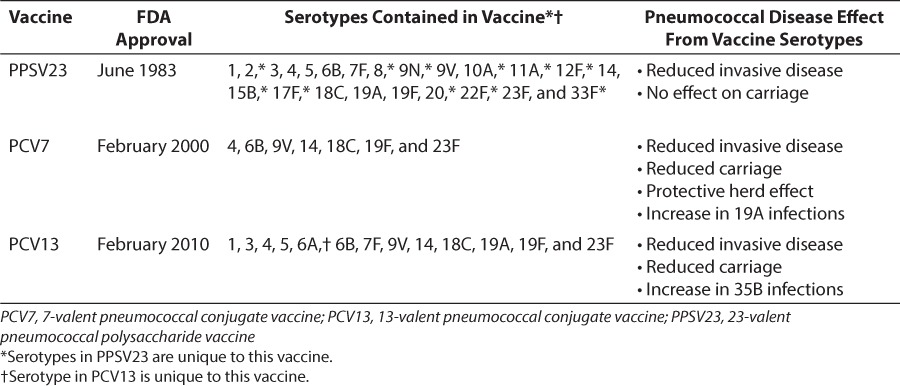
Early vaccines developed against pneumococci contained purified polysaccharide capsules as antigens. Table 1 lists the serotypes contained in each vaccine, the US Food and Drug Administration (FDA) approval dates of the vaccine and each vaccine's overall effect on pneumococcal disease. The first vaccines developed contained 14 capsular polysaccharide serotypes and protected against pneumococcal disease. A 23-valent pneumococcal polysaccharide vaccine (PPSV23) was developed later, in 1983, to provide protection against 80% to 90% of the pneumococcal capsular serotypes causing disease. The current PPSV23 formulation contains the following capsular serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F (Table 1). It is supplied as either a single-dose of 0.5 mL or a multidose 5.0-mL vial to be administered either intramuscularly or subcutaneously into the deltoid muscle or lateral mid-thigh. Common side effects include mild site injection reactions (e.g., swelling), headache, fatigue, and myalgia.5
Table 1.
Pneumococcal Vaccine Approval Dates, Serotypes, and General Effect on Pneumococcal Disease
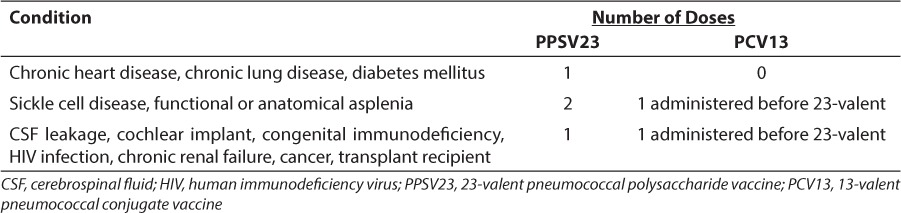
It is recommended that all adults 65 years of age and older receive one dose of PPSV23. Before 65 years of age, a single vaccination with PPSV23 is recommended for at-risk adults with some indications having recommendations for two doses of PPSV23 separated by at least 5 years. Recommendations for vaccination of at-risk children and adults 5 to 64 years of age are described in Table 2. It is important to note that cigarette-smoking patients alone require immunization with PPSV23, and immunization should be included as part of smoking cessation treatment. Additionally, if 1 or 2 doses before age 65 were received due to another indication, a final dose at or after age 65 should be administered, with at least 5 years between doses.6 Adults should not receive more than two doses of PPSV23 before 65 years of age, and the maximum number of lifetime doses is three.
Table 2.
PPSV23 and PCV13 Vaccination Recommendations for Children and Adults 5 to 64 Years of Age With Medical Conditions
This vaccine's efficacy against pneumococcal infections with serotypes contained in the vaccine in immunocompetent persons was 65%.7 Although the rate of invasive pneumococcal disease was reduced in PPSV23-immunized adults, the overall pneumococcal carriage rate was not reduced.8 Unfortunately, the vaccine did not generate an immune response in the group with the highest rate of pneumococcal disease burden, children younger than 2 years of age. The antibody response generated by PPSV23 is T-cell independent due to the fact that the repeating subunits of the capsular polysaccharide can stimulate an immune response in B-cells independent of T-cell help.9 The theory is that their inability to mount a T-cell–independent immune response until this age prevented the vaccine from generating protective antibodies.1
Polysaccharide Conjugate Vaccines
In order to elicit a protective immune response in children under the age of 2, vaccines with the capsular polysaccharide conjugated to diphtheria toxin were developed. These conjugated antigens generated a T-cell–dependent antibody response that was effective in children under 2 years of age. They have also proven effective at generating higher antibody titers in high-risk individuals immunized with the unconjugated vaccine.
The first 7-valent pneumococcal polysaccharide conjugate vaccine (PCV7) developed in 2002 greatly reduced the rate of infections in children under 2 years of age and in unimmunized individuals in the same community through the herd effect.10–12 In the decade after the introduction of PCV7, hospitalizations due to pneumonia have decreased significantly in both immunized children and the eldery.13 PCV7 vaccination has also reduced pneumococcal carriage rates of serotypes covered by the vaccine for both vaccinated children and household members among whom a child was vaccinated.14 Interestingly, vaccination has not reduced the rate of otitis media in vaccinated communities.15 The second conjugate vaccine that was developed, the 13-valent pneumococcal conjugate vaccine (PCV13), contained the 7 serotypes in PCV7, 5 serotypes found in PPSV23, and 1 unique serotype found in neither PPSV23 nor PCV7, serotype 6A. Its increased coverage provided broader protective benefit against pneumococcal infection.16 After PCV13 implementation, invasive and noninvasive pneumococcal infection rates from serotypes covered in the PCV7 vaccine and the six serotypes added to PCV13 again dropped.12,16
Serotype Replacement
After the introduction of PCV7, serotype replacement of pneumococcal infections from serotypes not contained in PCV7 was noted only 5 years after vaccine implementation. Additionally, pneumococcal infection rates returned to pre-vaccine levels in groups at high risk for pneumococcal disease.17 Of particular concern is the observation that levels of antibiotic resistance increased in non-vaccine isolates responsible for infections after the vaccine was introduced.18 PCV13 was introduced to cover six of the most prevalent serotypes that were not included in PCV7. Since the introduction of PCV7 and PCV13, a longitudinal surveillance program of 43 medical centers in the United States has observed that the only serotype that has increased in infection rates is one not covered in any current vaccine, 35B.12 Currently, the long-term effects of PCV13 on serotype replacement of isolates causing pneumococcal disease remain unclear. Given the rise of infections from serotype 35B, it could be hypothesized, however, that infections from serotype replacement strains will continue to occur as observed after the introduction of PCV7.
IMPACT ON PEDIATRIC DISEASE
PCV13 has provided substantial benefits since its introduction in 2010. To date, these benefits have been primarily in reduction of the incidence of invasive pneumococcal disease. Multisite population-based surveillance analyses revealed an overall reduction of 64% in invasive pneumococcal disease in children younger than 5 years of age. Additionally, a reduction in invasive pneumococcal disease was found to be 93% when researchers removed serotypes that were not contained in PCV7 from the analysis.19 The introduction of and subsequent vaccination in children with PCV13 resulted in a spillover effect as reductions in invasive pneumococcal disease were also seen in adults.19 The most recent Cochrane review of the effects of pneumococcal conjugate vaccines for preventing otitis media found modest beneficial effects in healthy infants given PCV7. This review encompassed 1995 to 2013 and reported that there were several ongoing randomized clinical trials studying the newly licensed PCV13 to establish its effects on acute otitis media.20
GUIDELINE RECOMMENDATIONS
PCV13 Vaccination
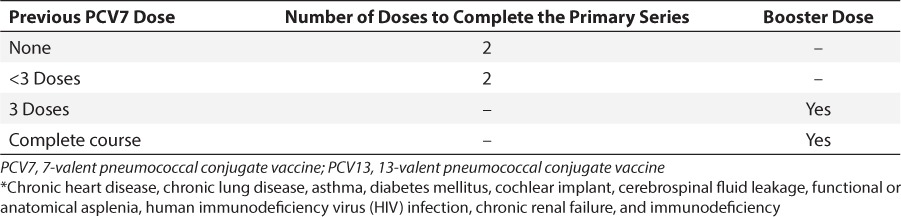
The recommendation for PCV13 vaccination of children and adults 5 to 64 years of age with medical diagnoses and healthy children under 5 years of age are described in Tables 2 and 3. Currently, the Centers for Disease Control and Prevention (CDC) recommend that all children younger than 2 years of age undergo a series of PCV13 immunizations. If the child is less than 6 months old, 4 doses of the vaccine are recommended, with the doses 8 weeks apart (minimum interval of 4 weeks). The fourth dose is considered a booster and should be given between 12 and 15 months of age. Catch-up schedules for children older than 6 months who have not received PCV13 and children who previously received PCV7 are described in Table 3.21 For children 2 to 5 years of age, vaccination with PCV13 is also recommended for those with certain medical diagnoses (Table 4). 21
Table 3.
PCV13 Vaccination Catch-up Dose Recommendations for Healthy Children Under 5 Years of Age *
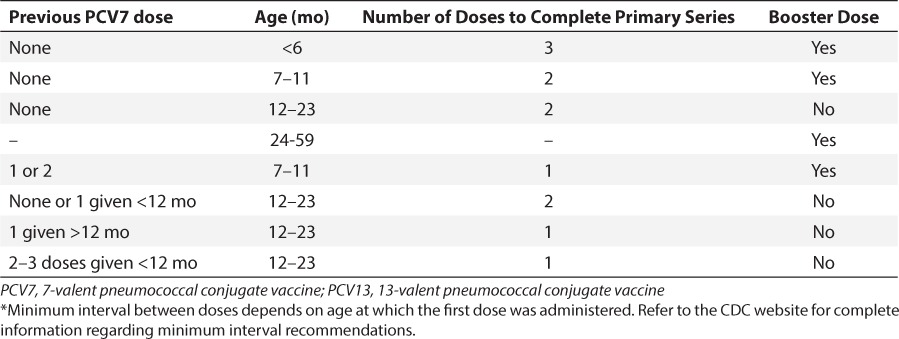
Table 4.
PCV13 Vaccination Recommendations for Children <72 Months of Age With Medical Conditions *
PCV13 is supplied as a prefilled single-dose 0.5-mL syringe for intramuscular injection in the anterolateral aspect of the thigh in infants and in the deltoid muscle of the upper arm in infants, children, and adults. The most common side effects reported include irritability, injection site reactions, decreased appetite and sleep, fever, fatigue, headache, muscle and joint pain, chills, or rash.22
PPSV23 Vaccination in At-Risk Children
It is recommended that at-risk children receive immunization with PPSV23 after they finish an immunization series with conjugated vaccines. In sickle cell pediatric patients, higher titers of the 7 serotypes contained in PCV7 were observed in patients receiving immunization with PCV7 series followed by PPSV23 compared to patients who received the PCV7 series alone.23 In HIV-positive pediatric patients receiving highly active antiretroviral therapy, a series of two PCV7 vaccinations followed by a PPSV23 vaccination increased antibody titers.24 Due to increased titers from PPVS23 vaccination, children who are immunocompromised should receive a single immunization with PPSV23 after the PCV13 vaccination series. For children who have sickle cell disease and/or functional or anatomical asplenia, two doses of PPSV23 are recommended. The first dose is recommended 8 weeks after finishing the PCV13 vaccine series. The second dose is recommended 3 to 5 years after the first dose according to the 2002 National Heart Lung and Blood Institutes Management of Sickle Cell Disease guidelines or 5 years after the first dose according to the 2010 Advisory Committee on Immunization Practices (ACIP) guidelines.21,25 Decreased duration between revaccination with PPSV23 has led to increased occurrences of mild vaccine-related adverse events in adults (and sickle cell pediatric patients) and should be considered when deciding PPSV23 revaccination scheduling in pediatric sickle cell patients.26–28
NEW VACCINE ANTIGENS
The incidence of serotype replacement in both carriage and infection isolates after implementation of conjugate vaccines and the increase in antibiotic resistance among serotype replacement strains stress the need for pneumococcal vaccines with broader coverage against pneumococci. Due to the large diverse pool of capsular polysaccharide serotypes that pneumococci can express, research has focused on finding a more conserved protein-based antigen that could offer protection against pneumococcal disease.4 The following section describes research for potential protein antigens for pneumococcal vaccines.
Pneumococcal Surface Protein A
Pneumococcal surface protein A (PspA) is an antibody-accessible surface protein attached non-covalently via binding to choline residues in lipoteichoic acid and cell wall teichoic acid on the pneumococcal surface.29 PspA has been found on all clinical isolates and is necessary for pneumococcal virulence.30 It aids pneumococcus in escaping complement-dependent immune phagocytosis both by inhibiting the deposition of complement on the surface of the bacterium and by inhibiting the killing of pneumococcus by the innate immune molecule lactoferrin.31,32 The N-terminal coiled-coil region of the protein can be divided into seven immunologically unique families.29 This region has been studied extensively for its ability to elicit an antibody-mediated, protective immune response against pneumococcal infection in mice.33 Immunization with this protein in humans elicits antibodies that are capable of passively protecting mice against infection.34 Vaccine antigen research has focused on this portion of the protein because it is farthest from the C-terminal choline-binding repeats that anchor the protein and was thus hypothesized to be the most accessible to antibodies.
Another conserved region of PspA that is more closely located to the C terminus has also been studied for its ability to elicit a protective immune response. Between the choline-binding repeat anchors and the N-terminal coiled-coil region, is a conserved proline-rich region found in all PspAs. It consists of a series of repeat units with a proline amino acid found every 3 or 4 residues. The proline repeats can be interrupted by a highly conserved sequence of amino acids lacking proline residues termed the “non-proline block.” Approximately 50% of PspAs contain a non-proline block within their proline-rich region.29 This antigen was shown to elicit antibody-mediated protection in mice against invasive pneumococcal disease.35 Its conservation in PspAs suggests it could increase protection when used as an antigen in a protein-based vaccine. Recently, a vaccine delivering the PspA antigen via an attenuated, orally administered Salmonella vector has demonstrated safety in phase I clinical trials in healthy adults.36
Pneumolysin
Another potential protein vaccine antigen is pneumolysin, a thiol-activated toxin expressed by almost all clinical isolates of pneumococci. Pneumolysin interferes with neutrophil function during the immune response and inhibits proliferation and antibody-production by immune cells, ciliary pulmonary cell beat, and toxicity to alveolar epithelial cells.37 Due to its wide array of effects on both pulmonary tract cells and immune cells, the protein is too toxic to be used in human vaccines in its native form. However, site-directed mutagenesis has been used to create variants that are not toxic but are still immunogenic. Vaccination with the variant protects mice against infection.38 Additionally, inclusion of this antigen along with PspA has shown to be protective against pneumococcal infection, suggesting again that a protein-based vaccine with multiple antigen targets may be an effective future vaccine.39 A phase I clinical trial completed in Switzerland has demonstrated immunogenicity, safety and efficacy of human vaccination with the inactivated pneumolysin antigen.40
Table 5.
Protein Vaccine Antigens in the Pipeline
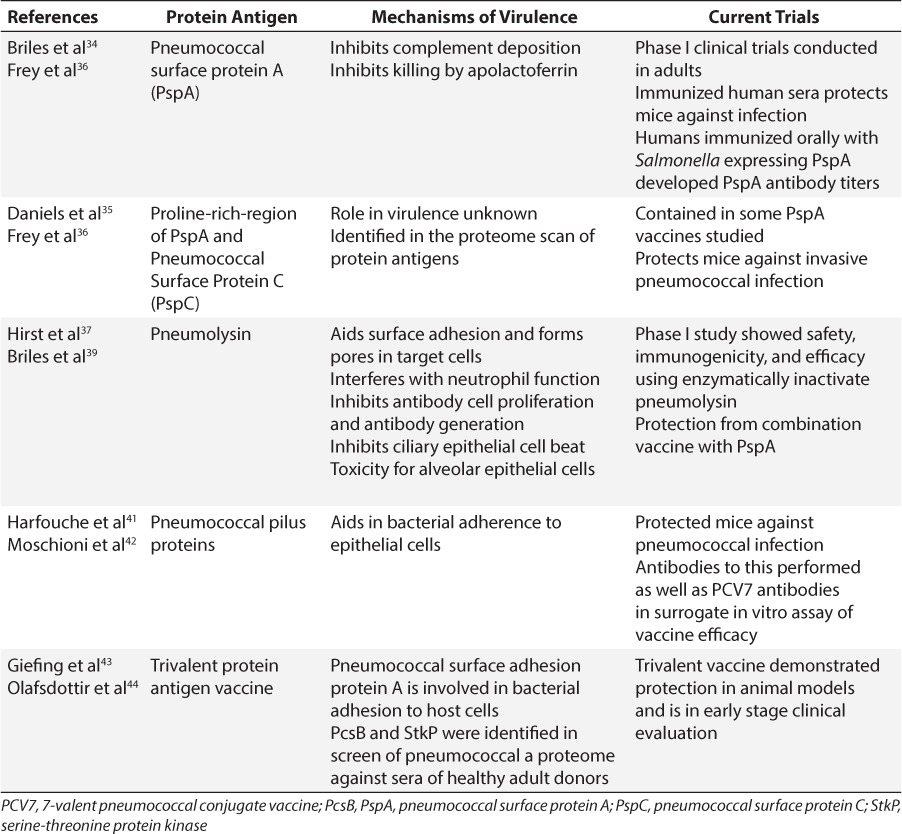
Fusion pneumococcal pilus proteins
Pneumococcal pilus 1 is an epithelial cell adhesion virulence factor composed of three separate proteins. This pilus structure, which has been associated with increased adherence to epithelial cells and plays a role in lung infection, is present on the surface of half of pneumococcal isolates. A recombinant protein formed by the fusion of the three subunits elicited antibodies that were capable of binding to the pneumococcal surface and protecting mice against pneumococcal isolates containing a pilus.41 This protection was observed against strains that had both high and low levels of pilus expression.42 Additionally, sera generated from the fusion antigen displayed efficacy equal to that of sera from PCV7 immunization in the established in vitro antisera-dependent, complement-mediated opsonization phagocytosis assay.41
Protein antigen combination vaccines
In order to determine novel protein antigens, a proteome of pneumococcal proteins was screened using sera from individuals with pneumococcal exposure who lacked an active pneumococcal infection. This proteome was generated by adhering small amino acid fragments of pneumococcal proteins on a slide to represent all potential proteins pneumococcus can make. This array was then screened against the collected sera by determining which amino acid fragments on the array bound antibodies in the sera. The parent proteins of the amino acid fragments identified represent potential vaccine antigens. This screen identified two surface proteins as potential antigens, a protein required for cell wall separation (PcsB) and a serine-threonine protein kinase (StkP). These 2 proteins were highly conserved among clinical isolates, and vaccination with these 2 proteins protected mice against infection with multiple, lethal strains of pneumococci.43 Additionally, they have been included with the protein pneumococcal surface adhesion protein A (PsaA) in a trivalent protein vaccine that has demonstrated protection in animal models and is in early stage clinical evaluation.44
CONCLUSIONS
Polysaccharide pneumococcal vaccines have evolved over the past 20 years, using both unconjugated polysaccharides and polysaccharides conjugated to toxins to elicit a protective immune response in groups at risk for pneumococcal infection. Current CDC immunization guidelines for these vaccines have reduced the rates of pneumococcal infections within immunized communities. However, the rise of antibiotic resistance in serotype replacement strains observed after implementation of current vaccines stresses the need for research into new, non-polysaccharide vaccines with broader coverage. Surface proteins expressed on pneumococci offer hope for potential new vaccines that can provide further protection against pneumococcal infections.
Acknowledgment
At the time of manuscript preparation, Dr Daniels was a PharmD candidate in the College of Pharmacy, University of Tennessee Health Science Center, Memphis, TN.
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control and Prevention
- CSF
cerebrospinal fluid
- FDA
Food and Drug Administration
- HIV
human immunodeficiency virus
- PPSV23
23-valent pneumococcal polysaccharide vaccine
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PspA
pneumococcal surface protein A
Footnotes
Disclosure The author(s) declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy. 2005;25(9):1193–1212. doi: 10.1592/phco.2005.25.9.1193. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw T, White Johansson E, Hodge M. Pneumonia: The forgotten killer of children. New York: United Nations Children's Fund/World Health Organization; 2006. [Google Scholar]
- 3.Kamerling JP. Pneumococcal polysaccharides: a chemical view. In: Tomasz A, editor. Streptococcus pneumoniae: Molecular Biology and Mechanisms of Disease. New Rochelle, NY: Mary Ann Liebert, Inc; 2000. pp. 81–114. (ed) [Google Scholar]
- 4.Bogaert D, Hermans PW, Adrian PV et al. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22(17–18):2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Pneumovax23 (pneumococcal vaccine polyvalent) [package insert] Whitehouse Station, NJ: Merck & Co., Inc.; 2011. [Google Scholar]
- 6.Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb Mortal Wkly Rep. 2010;59(34):1102–1106. [PubMed] [Google Scholar]
- 7.Shapiro ED, Berg AT, Austrian R et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325(21):1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 8.Hamborsky J, Kroger A, Wolfe S, editors. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington DC: Public Health Foundation; 2015. [Google Scholar]
- 9.Heilmann C. Human B and T lymphocyte responses to vaccination with pneumococcal polysaccharides. APMIS Suppl. 1990;15:1–23. [PubMed] [Google Scholar]
- 10.Hammitt LL, Bruden DL, Butler JC et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006;193(11):1487–1494. doi: 10.1086/503805. [DOI] [PubMed] [Google Scholar]
- 11.Whitney CG, Farley MM, Hadler J et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 12.Richter SS, Heilmann KP, Dohrn CL et al. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011. Emerg Infect Dis. 2013;19(7):1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin MR, Zhu Y, Moore MR et al. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369(2):155–163. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber M, Barskey A, Baughman W et al. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine. 2007;25(29):5390–5398. doi: 10.1016/j.vaccine.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 15.Fedson DS, Musher DM. Pneumococcal polysaccharide vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia: WB Saunders; 2004. pp. 529–588. [Google Scholar]
- 16.Bryant KA, Block SL, Baker SA et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125(5):866–875. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 17.Singleton RJ, Hennessy TW, Bulkow LR et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HE, Shutt KA, Moore MR et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360(3):244–256. doi: 10.1056/NEJMoa0800836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore MR, Link-Gelles R, Schaffner W et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortanier AC, Venekamp RP, Boonacker CW et al. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev. 2014;4:CD001480. doi: 10.1002/14651858.CD001480.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Moratl Wkly Rep. 2010;59(RR11):1–18. [Google Scholar]
- 22.Prevnar 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) [package insert] Philadelphia, PA: Pfizer, Inc.; 2013. [Google Scholar]
- 23.Vernacchio L, Neufeld EJ, MacDonald K et al. Combined schedule of 7-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal vaccine in children and young adults with sickle cell disease. J Pediatr. 1998;133(2):275–278. doi: 10.1016/s0022-3476(98)70235-5. [DOI] [PubMed] [Google Scholar]
- 24.Abzug MJ, Pelton SI, Song LY et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25(10):920–929. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health National Heart, Lung, and Blood Institute, Division of Blood Diseases and Resources. The Management of Sickle Cell Disease. 4th ed. Bethesda, MD: US National Insitutues of Health; 2002. [Google Scholar]
- 26.Daniels CC, Shelton CM, Bass PJ et al. Limb swelling in a pediatric sickle cell patient after revaccination with pneumococcal vaccine. Int J Clin Pharm. 2014;36(2):261–263. doi: 10.1007/s11096-013-9888-3. [DOI] [PubMed] [Google Scholar]
- 27.Jackson LA, Benson P, Sneller VP et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA. 1999;281(3):243–8. doi: 10.1001/jama.281.3.243. [DOI] [PubMed] [Google Scholar]
- 28.Jackson LA, Nelson JC, Whitney CG et al. Assessment of the safety of a third dose of pneumococcal polysaccharide vaccine in the Vaccine Safety Datalink population. Vaccine. 2006;24(12):151–6. doi: 10.1016/j.vaccine.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 29.Hollingshead SK, Becker R, Briles DE. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000;68(10):5889–5900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crain MJ, Waltman WD, II, Turner JS et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58(10):3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren B, Szalai AJ, Thomas O et al. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun. 2013;71(1):75–85. doi: 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaper M, Hollingshead SK, Benjamin WH, Jr, Briles DE. PspA protects Streptococcus pneumoniae from killing apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected] Infect Immun. 2004;72(9):5031–5040. doi: 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briles DE, King JD, Gray MA et al. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14(9):858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 34.Briles DE, Hollingshead SK, King J et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182(6):1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 35.Daniels CC, Coan P, King J et al. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun. 2010;78(5):2163–2172. doi: 10.1128/IAI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey SE, Lottenbach KR, Hill H et al. A Phase I, dose-escalation trial in adults of three recombinant attenuated Salmonella Typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine. 2013;31(42):4874–4880. doi: 10.1016/j.vaccine.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Hirst RA, Kadioglu A, O'Callaghan C, Andrew PW. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol. 2004;138(2):195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander JE, Lock RA, Peeters CC et al. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62(12):5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briles DE, Hollingshead SK, Paton JC et al. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J Infect Dis. 2003;188(3):339–348. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- 40.Kamtchoua T, Bologa M, Hopfer R et al. Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults. Vaccine. 2013;31(2):327–333. doi: 10.1016/j.vaccine.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Harfouche C, Filippini S, Gianfaldoni C et al. RrgB321, a fusion protein of the three variants of the pneumococcal pilus backbone RrgB, is protective in vivo and elicits opsonic antibodies. Infect Immun. 2012;80(1):451–460. doi: 10.1128/IAI.05780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moschioni M, De Angelis G, Harfouche C et al. Immunization with the RrgB321 fusion protein protects mice against both high and low pilus-expressing Streptococcus pneumoniae populations. Vaccine. 2012;30(7):1349–1356. doi: 10.1016/j.vaccine.2011.12.080. [DOI] [PubMed] [Google Scholar]
- 43.Giefing C, Meinke AL, Hanner M et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205(1):117–131. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. Novel protein-based pneumococcal disease in neonatal mice. Infect Immun. 2011;80(1):461–468. doi: 10.1128/IAI.05801-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


