Abstract
OBJECTIVES: Gaps in pediatric therapeutics often result in off-label use and specifically, novel uses for existing medications, termed “drug repurposing.” Drug Information (DI) queries to a Pediatric Medication Resource Center of a large metropolitan pediatric hospital in New York and inherent difficulties in retrieving evidence-based information prompted a review of current medication repurposing for pediatric patients. The objective included characterization of innovative off-label use of medications Food and Drug Administration (FDA)-approved for 1 or more indications to treat a totally different disorder or indication in pediatric patients.
METHODS: A systematic literature review was conducted to retrieve publications describing repurposed medications in pediatric patients. Excluded was FDA-approved indications used off-label in pediatric patients (e.g., different dose), preclinical data, adult use only, and experimental use. Evidence quality was classified using a modified American Academy of Neurology Level of Evidence. Results were analyzed using χ2 at p < 0.05.
RESULTS: Over 2000 references were retrieved and reviewed. A total of 101 medications repurposed for novel off-label uses for pediatric patients were identified: 38 for neonates, 74 for children, and 52 for adolescents. Neonates and infants were least likely to receive a medication for a repurposed use. Strong or intermediate evidence existed in 80.2% of cases. The evidence was weak in 19.8%. No significant relationship was observed between the pediatric age group and strength of the literature. Most repurposed uses pertained to generic or widely used medications. Less than 5% of medications were first marketed after 2011.
CONCLUSIONS: While not exhaustive, the present study represents the most comprehensive listing of novel uses exclusive to pediatric patients. Further research is needed to identify the frequency of repurposed uses. The valuable DI role of pharmacists in assessing repurposed uses is of expanding and increasing importance to ensure such uses are evidence-based.
INDEX TERMS: drug information services, drug repositioning, drug utilization, off-label use, pediatrics, unlabeled indication
INTRODUCTION
In 1963, Dr Harry C. Shirkey stated, “By an odd twist of fate, infants and children are becoming therapeutic or pharmaceutical orphans.”1 More than half a century later, most medications used in pediatric patients are not Food and Drug Administration (FDA) approved for use in this patient population. That is, they are used off-label. Off-label use is the mainstay of therapy in pediatric patients. Several studies have shown that between 39% and 79% of children admitted to pediatric hospitals receive 1 or more courses of off-label therapy.2,3
Legislative efforts to improve pediatric drug therapy include The Best Pharmaceuticals for Children Act (BPCA)4 and the Pediatric Research Equity Act (PREA).5 Table 1 lists similarities and differences between BPCA and PREA. Both became permanent in 2012 under the FDA Safety and Innovation Act and have resulted in about 500 pediatric labeling changes.6 Despite this success, less than half of all products are labeled with pediatric information.7
Table 1.
Provisions of the BPCA and PREA
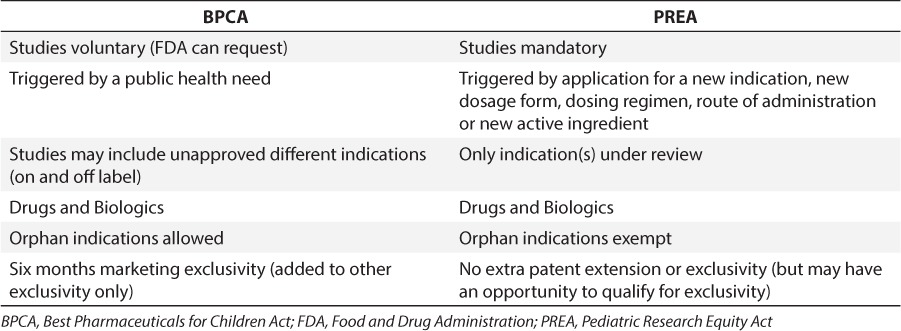
The FDA does not mandate pediatric ages to be tested, and medications have received exclusivity by testing in 12- to 17-year-olds, extending use to adolescent but not pediatric patients. A recent study of 192 medications that were granted pediatric exclusivity by the FDA reported that studies in support of exclusivity are often not designed to meet pediatric patient needs. For example, hypertension and high cholesterol are the therapeutic category most frequently granted pediatric exclusivity.8 Additionally, exclusivity is currently not conditioned upon studies in pediatric patients being either successful or resulting in FDA approval.
No legislation exists to incentivize pediatric research of FDA-approved medications that have no existing patent life, especially where generic versions exist. For such medications or where the medication is inexpensive, manufacturers have little financial incentive to invest in costly pediatric clinical trials. Likewise, there is no incentive to submit Supplemental New Drug Applications (SNDAs), which when approved will allow a company to make changes in a product that already has an approved new drug application (NDA) (e.g., change to FDA-approved indications). Rarely are SNDAs used to obtain FDA approval for repurposed or innovative indications. However, for high cost medications, the incentive to conduct such trials may exist. Regardless, nothing mandates manufacturers to seek FDA approval for new indications as they become established. Even for NDAs, pharmaceutical companies are reluctant to include extra indications that might further complicate their application's approval.9
Contributing factors for the extensive lack of pediatric clinical trials include, for example, difficulty recruiting pediatric patients, especially where the risk:benefit ratio is unclear or there is limited pediatric prevalence for the condition. Even with Orphan Drug Act10 drug development incentives, it still may be financially unattractive to conduct research in the pediatric population where contradictory evidence may be found and the manufacturer is already profiting from a medication's recognized off-label use. For monoclonal antibodies, for example, twice as many off-label uses as FDA-approved indications exist. Under the Federal Food, Drug, and Cosmetic Act, manufacturers are prohibited from directly marketing a medication for a use other than FDA-approved indications.11 However, the FDA does not have the legal authority to regulate the practice of medicine, and prescribers may prescribe a medication off-label.
Off-label use can be classified into 2 broad categories: 1) using an FDA-approved medication for 1 or more indications to treat an entirely different disorder or indication, or 2) prescribing a medication for an indication it was FDA-approved for, but outside certain specifications (age, weight, route of administration, doses, or patient populations).12 In the first instance, the off-label use can be classified as innovative or novel where, for example, the benefit was serendipitously clinically observed. In other cases, these uses have been discovered through mining chemical structure or pharmacogenomics databases, clinical research, or trial and error.
While studies have been conducted in hospitalized pediatric populations to determine incidence of off-label use,13–15 and to investigate pediatric off-label use of unapproved medications,6,16 few studies have investigated either the incidence or characterization of repurposed or innovative off-label medication use in pediatric patients. The focus of this article is innovative off-label use of medications approved for 1 or more indications in adult and/or pediatric patients to treat a totally different disorder or indication in pediatric patients.
METHODOLOGY
A literature search was performed to retrieve all publications describing repurposed medications in pediatric patients. Patient ages were categorized as: infant (< 1 year), child (1 to < 12 years), and adolescent (12–18 years). This classification closely follows FDA age categories for exclusivity studies. The search was conducted in PubMed (1966 through January 2015), EMBASE, Ovid, The Cochrane Library, and Google Scholar using the search terms “pediatric,” “off-label,” “repurposed medications,” “repositioning medications,” “drug repositioning,” “drug rediscovery,” and “drug repurposing.” No language or date restrictions were considered. The FDA “Pediatric Labeling Database,”17 FDA “Rare Disease Repurposing Database,”18 Off-Label Drug Facts,19 and Harriet Lane Handbook were also reviewed.20 To ensure the latest prescribing information was reviewed, the FDA Web site (Center for Drug Evaluation and Research) listing of marketed medications was used.
Off-label was defined as an indication not listed in the current prescribing information (i.e., package insert). For the purposes of this study, repurposed uses were defined as those involving different categories of diseases (e.g., asthma and epilepsy), rather than merely different diseases in the same category (i.e., eczema and boils). Both prescription and over-the-counter (OTC) products were included.
Clinical observations of innovative uses from a large metropolitan pediatric hospital were included, and further literature searches were conducted to obtain publications reporting the novel use. Additionally, included were Drug Information requests received at a Pediatric Medication Resource Center pertaining to the need for information about dosing, supporting literature, and administration for innovative uses.21
An effort was made to locate the best and most recent evidence (e.g., randomized clinical trials versus anecdotal case reports). Included were randomized controlled trials, cohort and case control studies, professional guidelines or recommendations, case reports, case series, and studies conducted on small numbers of pediatric patients. Preclinical and chemical screening studies, review articles (other than meta-analysis or systematic reviews), letters to the editor, editorials, and commentaries were excluded. Both positive and negative evidence were included. For each repurposed use identified, a second search was conducted both in the databases above as well as by reviewing the bibliography of each article to locate the best evidence possible.
To identify the strength of the evidence and evaluate evidence quality, a modified American Academy of Neurology (AAN) Level of Evidence classification for therapeutic intervention was employed.22 Our modified classification involved a 3-tiered system (i.e., strong, intermediate, weak). Strong evidence involved prospective randomized controlled trials or prospective matched group cohort studies directly relevant yielding positive findings or inclusion in a Pediatric Association Guideline. Intermediate evidence involved conflicting data in randomized clinical trials or cohort studies, case-control studies (including well-defined natural history controls or patients serving as their own controls), some evidence in the form of small or pilot (preliminary) trials or case series, or consensus recommendation in the absence of relevant clinical trials and better evidence than case reports. Weak evidence involved isolated or anecdotal case reports, expert opinion, or where the results of strong or intermediate evidence indicated the medication was not clinically useful for the repurposed usage. Only a few key citations were included in the citation column even where multiple existed. Finally, we reported out the specific ages of the pediatric patient population or subpopulation (e.g., adolescents) for which the novel use was either most likely to be used or was reported as used. Results were analyzed using χ2 and significance was set at p < 0.05.
Excluded were FDA-approved indications used off-label in pediatric patients; innovative uses which were used in both adult and pediatric patients but primarily in adult patients or where the literature pertained solely to adult patients; where the use was only described in adults and the literature pertained only to adults; and where no literature support was located or where the only available literature regarding the novel off-label use pertained to preclinical studies. In such cases, the use would be experimentation requiring informed consent or perhaps submission of an Investigational New Drug Application (IND) to the FDA.
RESULTS
The search retrieved over 2000 references, many of which were excluded as either not pertinent or related to pediatric off-label, but not necessarily repurposed, use of medication FDA approved for the indication in adults. No single source contained all the repurposed indications, but Off Label Drug Facts identified the most. Tables 2 to 4 detail repurposed uses of medications for pediatric patients, and provides the medication name, the repurposed indication, patient age ranges, strength of evidence, and reference(s). Table 2 represents medications for which strong published evidence was retrieved based on a modified AAN Level of Evidence.23–78 Tables 3 and 4 represent intermediate79–129 and weak130–156 evidence, respectively. A total of 101 medications used in a repurposed manner in pediatric patients were identified; 38 medications for neonates and/or infants; 74 for children; and 52 for adolescents. The majority (i.e., 58) involved multiple age categories; thus the total adds up to 164. Medications repurposed for multiple pediatric categories were as follows: 11 infants and children; 42 adolescents and children; and 5 infants, children, and adolescents. Neonates and infants were least likely to receive a medication for an innovative off-label use.
Table 2.
Repurposed Medications for Pediatric Patients With Strong Evidence for Use
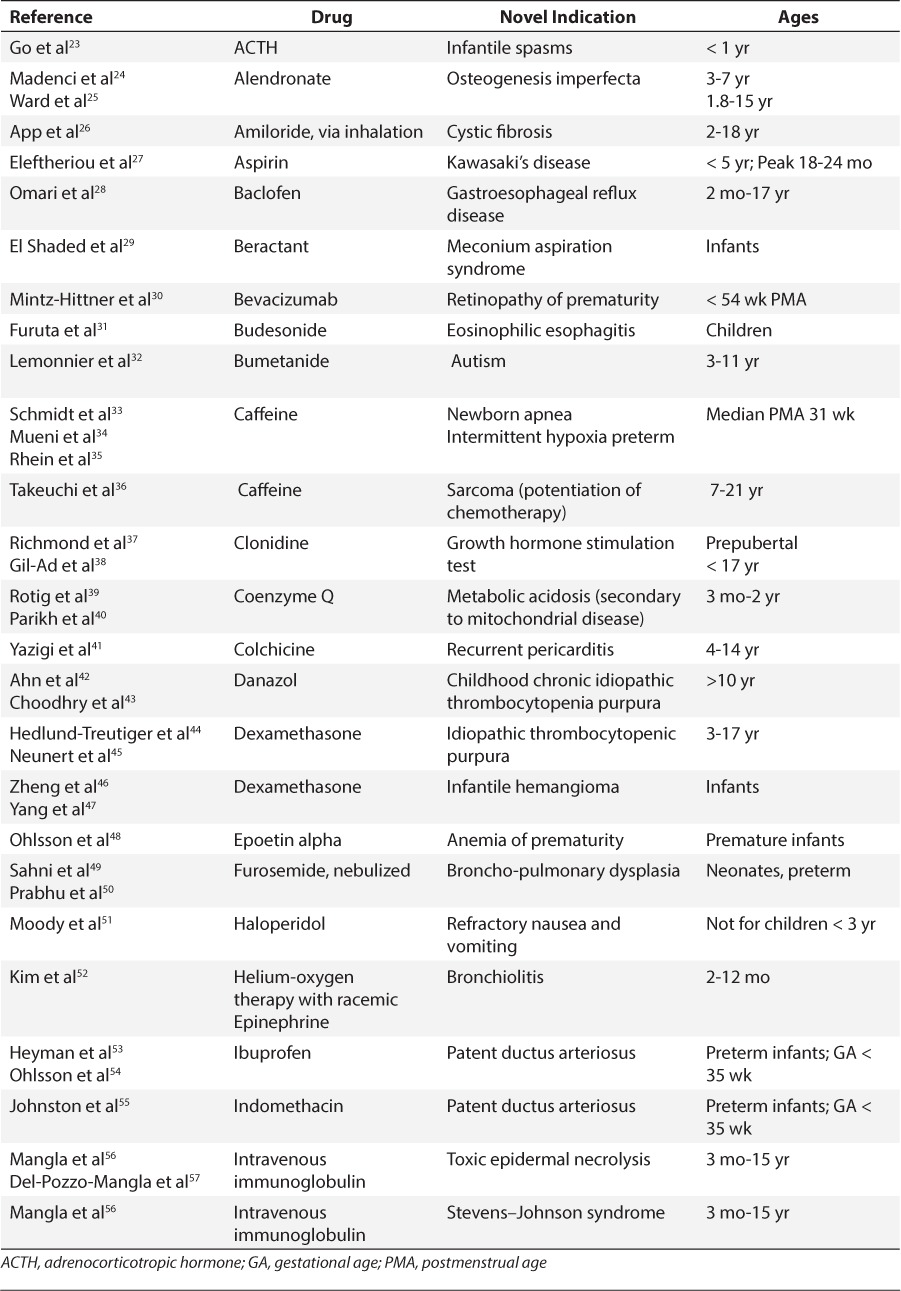
Table 3.
Repurposed Medications for Pediatric Patients With Intermediate Evidence for Use
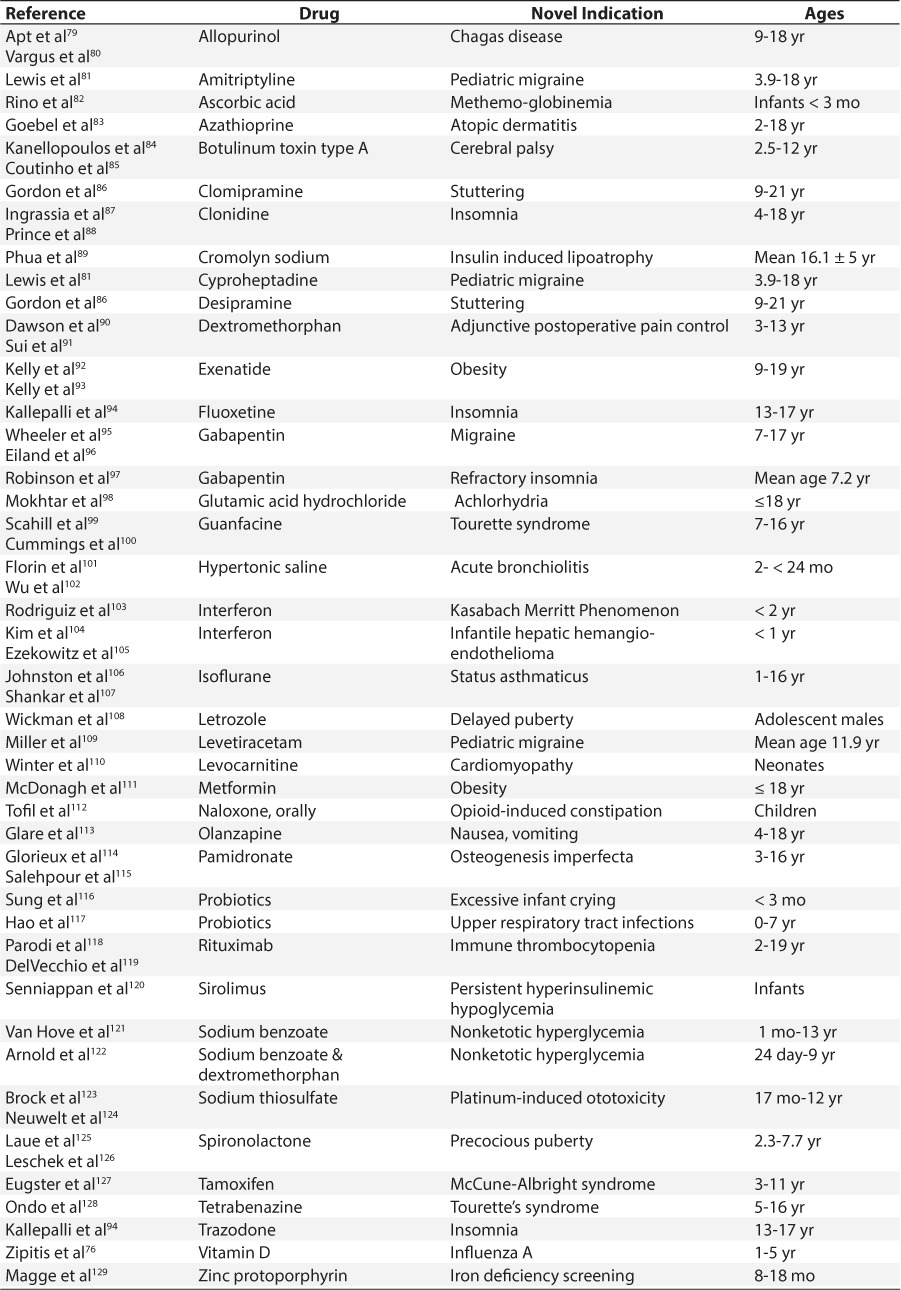
Table 4.
Repurposed Medications for Pediatric Patients With Weak Evidence for Use
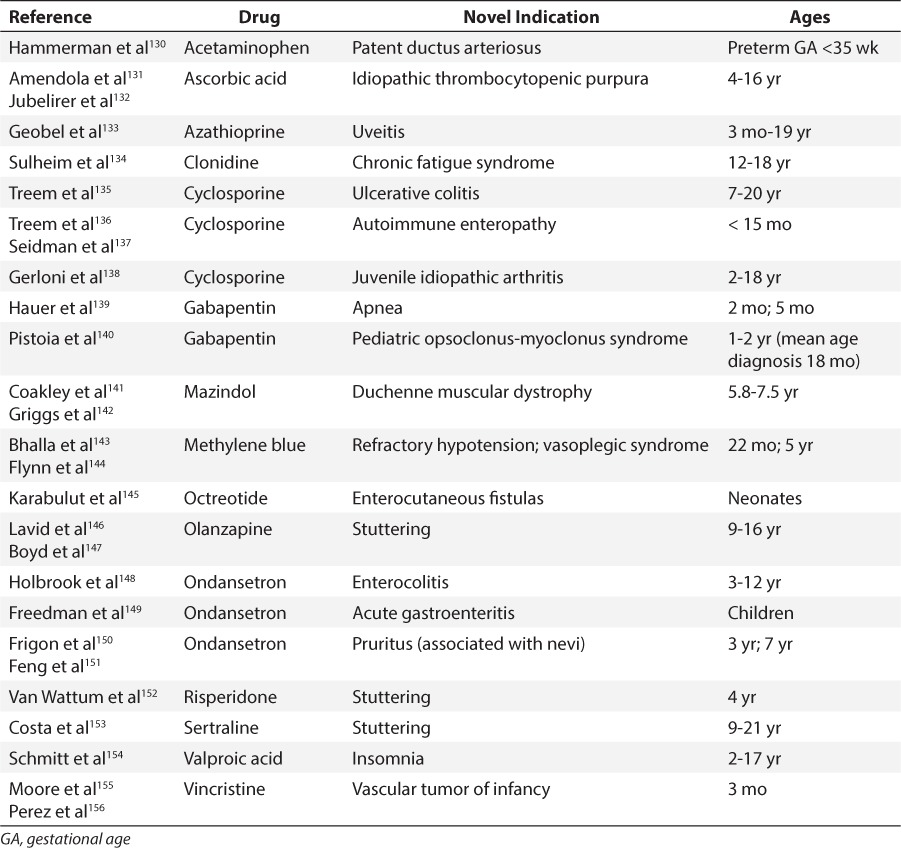
Table 2.
Repurposed Medications for Pediatric Patients With Strong Evidence for Use (cont.)
Strong or intermediate evidence existed in a majority of cases (81/101) (80.2%); that is, the use was supported by the published literature. The evidence was strong in 40/101 instances (39.6%); intermediate in 41/101 instances (40.6%); and weak in only 20/101 (19.8%). While only about 20% of the evidence was considered weak, this may have been a result of failure to identify every repurposed usage. Table 5 identifies the strength of the evidence by age group. Using χ2 analysis, no significant relationship was observed between the pediatric age group and strength of the literature.
Table 5.
Summary of Strength of the Evidence for the Age Categories

Most references provided the dosages used; most often weight-based, but sometimes fixed dosing was used. In a number of cases, the doses differed for the same repurposed indication. Dosing information was not included in Tables 2 to 4, as it was felt very important for clinicians to retrieve and review the latest primary literature.
Our research also revealed the fact that, in almost every instance, the medical record did not indicate that patients or their guardians/parents were informed that a drug was being prescribed for an unlabeled indication.
DISCUSSION
Due to resource constraints, we did not attempt to produce the most exhaustive list possible. Rather, our list represented the most extensive list of repurposed medications in pediatric patients published to date. A recent study by Blatt and Corey157 identified 63 repurposed medications used in pediatric patients with emphasis on pediatric hematology/oncology. The authors looked for medications having at least 1 pediatric indication for which a newer use was in hematology/oncology. The present study differed in that it did not focus on hematology/oncology. Additionally, repurposed medications were excluded if the use was also found in adult patients (e.g., aspirin for colon cancer prevention).
Some studies have shown that off-label use is extensive in hospital as well as outpatient settings.13–15 The present study was limited to the literature and hospital setting and did not seek to answer the question of frequency of off-label prescribing in either inpatients or outpatients. Further research is needed to determine the extent of novel off-label use of medications in pediatric populations.
A recent study revealed that few drug-labeling changes made under pediatric legislation include neonates.158 Previous research has revealed that hospitalized neonates and infants receive the greatest proportion of off-label use of medications.12 Although this may be true for all off-label use (e.g., age, dose, weight, and route of administration), the present study reveals that this pediatric subpopulation is actually least likely to receive an off-label use for a repurposed indication. Our results reflect other literature that has found neonates to be less likely to receive medications off-label and attributed this to the scarcity of reliable dosing information, as well as a more conservative approach in this pediatric subpopulation.159
Results presented in this paper validate that the majority of the literature pertained to studies in small numbers of children and that several therapeutic categories had multiple repurposed medications. For example, medications were repurposed more than several times for cerebral palsy, muscular dystrophy, insomnia, cystic fibrosis, migraine, apnea, and stuttering. Our data also revealed that research may be needed on older medications. Most innovative uses pertained to medications that had been on the market for years; that is, older generic medications or widely used medications for which little evidence exists of harm to children. Few new medications were used for innovative uses. In fact, depending upon the definition of a new medication, less than 5% of the medications in Tables 2 to 4 were first marketed after 2011 and could be considered new medications (i.e., exenatide, letrozole, and tocilizumab). Possible reasons for this may include lack of experience on the part of clinicians even in adult patients to use new medications, fear of liability, and/or the fact that novel uses often emerge from postmarketing experience.
In some cases, promising novel uses reported by clinicians to the manufacturer may prompt a clinical trial with subsequent FDA approval. For example, while conducting this research, propranolol, used for pediatric hemangioma, received FDA approval for this indication. Similarly, imipramine is now FDA-approved for nocturnal enuresis in pediatric patients and does not appear in Tables 2 to 4. However, in other cases uses identified decades ago in the literature were not followed up upon and more recent studies could not be located. In a number of cases, the early literature was strong but FDA approval was never sought nor were later clinical studies conducted.
This paper highlights the difficulty in locating information on medications repurposed for pediatric patients. There are few resources that enable a clinician to locate drug information on prescribing, dosing, and dispensing of approved medications for unlabeled repurposed pediatric indications. Such uses can no longer be discussed at symposia or professional meetings. Most of the uses noted in Tables 2 to 4 did not appear in the Harriet Lane Handbook or other pediatric textbooks (e.g., Nelson's Textbook of Pediatrics). Prescribers frequently consult pharmacists regarding complex pediatric pharmacotherapies and the role of pharmacists as drug information experts in facilitating evidence-based prescribing for unlabeled pediatric use of medications is of increasing importance.160 Pediatric Drug Information practice today entails more and more requests for information about off-label uses. In a recent study of drug information queries to a Pediatric Medication Resource Center conducted by the author, safety and efficacy of off–label uses, dosages, and regimens constituted a large number of requests.21 Pharmacy clinicians need to be knowledgeable as to where to locate information. Table 6 provides a listing of drug information resources that include unlabeled pediatric indications.
Table 6.
Drug Information Resources Which Include Unlabeled Pediatric Indications

This study underscores the need for clinical trials. A number of specific repurposed medications did not appear on the FDA list of medications requiring further study in children. It is hoped that our results will assist in further prioritizing certain medications for additional pediatric study. Where evidence is weak, perhaps IND applications would be needed or clinicians could not obtain institutional review board approval to conduct a study in the first place. Repositioned medications do not require the typical 7 to 9 years required for new drug development but go directly to preclinical testing and clinical trials thus reducing risks and costs.161
The BPCA and PREA have increased the study of drugs in children. Prior to these laws, more than 80% of drugs approved for adult use were being used in children, even though safety and efficacy had not been established in children. The FDA estimates that today that number is about 50%. The FDA often requests that manufacturers conduct pediatric studies and it gives the manufacturers a deadline for them. In some cases (e.g., prostate cancer drug), the FDA will waive pediatric study requirements. In other cases, if the manufacturer does not perform the studies, the FDA can grant extensions. But, if a company does not conduct the study or ask for extensions, since August 2013, the FDA has published the non-compliance letters it issues together with the company's response on its Web site…a type of public humiliation.
The BPCA Priority List of Needs in Pediatric Therapeutics is an important initiative to promote pediatric research. The BPCA requires that the National Institutes of Health, and specifically the National Institute of Child Health and Human Development (NICHD), identify drug needs in pediatric therapeutics. The NICHD sponsors relevant clinical trials and is responsible for submission of resulting clinical trial data to the FDA for pediatric labeling changes. Although progress has been made, revisions to the BPCA and the PREA are essential for these laws to achieve their original intent. Clinical studies in pediatric patients should actually be required to include pediatric patients (as opposed to adolescents), be meaningful, and successful to result in exclusivity.
Our results indicate that despite evidence of the benefits of off-label usage, many off-label indications lack scientific support or literature of efficacy or safety. Some have only anecdotal or case report data. Although the vast majority of re-purposed indications were for older medications, research for safety and efficacy is, nevertheless, required. Prescribers are often unaware that the medication does not have FDA approval for a use when they prescribe it. Even worse, off-label use can have no therapeutic effect resulting in wasteful medication use and worst-case scenario the off-label use can harm the patient (Figure). For example, codeine for postoperative analgesia after pediatric tonsillectomy and/or adenoidectomy was used for years before it was discovered that cytochrome P4502D6 ultrarapid metabolizers were at risk of life-threatening or fatal adverse effects from normal doses. Similarly, promethazine, commonly used previously in pediatric patients, received a black box warning in 2005 for children less than 2 years due to respiratory depression and death. Therefore, a procedure that yields information on safety and pharmacovigilance on off-label uses remains imperative to avoid therapeutic roulette.
Figure.
Benefit-to-risk spectrum for off-label use: patient benefit or therapeutic roulette.
Moreover, legal implications of prescribing and dispensing a medication for non-approved uses will be minimized if the prescriber and pharmacist, in the exercise of sound professional judgment conclude the use is rationale, safe, and reasonable.162
Some hospitals have policies and procedures for innovative use of medications. Table 7 provides an example of possible elements to include in such a policy. When the use of a medication is experimental, then the patient (or guardian) should be informed of its experimental status. According to the American Academy of Pediatrics Policy Statement, off-label use is neither incorrect nor investigational if based on sound scientific evidence, expert medical judgment, or published literature; when use is truly investigational, or when the prescriber proposes to treat a group of patients rather than a single patient, the use should be performed in conjunction with well-controlled clinical trials.163
Table 7.
Policy Title: Innovative Use of Medications

Guidelines for appropriate off-label prescribing would help to inform clinical practitioners. In February 2013, the FDA issued a guidance entitled “Pediatric Information Incorporated Into Human Prescription Drug and Biological Products Labeling,” which provides recommendations for placement and content of pediatric information in prescription drug labeling when available data support a pediatric indication and when data do not support a pediatric indication (i.e., data are negative or inconclusive). This is a step in the right direction but does not address repurposed uses.
The fact remains that there is no central repository for comprehensive data on pediatric off-label uses for policy makers, regulators, payers, and clinicians. Recently, it has been suggested that an online forum be developed to share novel uses of medications for pediatric patients.
It is apparent that an enormous research agenda exists that begs to be addressed to permit more repurposed uses with strong evidence on the product label. Further research should also focus on determining the circumstances of off-label prescribing and its appropriateness. The FDA has perceived its role with regard to identification of important supplemental indications to be somewhat passive. There is a growing recognition that the FDA should adopt a much more active role, facilitating research, evaluation, and labeling revisions for off-label uses. Perhaps preliminarily assessing the available data or providing some other regulatory mechanism for accelerated approval for pediatric indications should be considered. The use of an application similar to an Orphan Drug Act (ODA) request or actually providing ODA designation for all pediatric SN-DAs might be beneficial to accelerate approval for pediatric indications. Ideally, an FDA-mandated efficacy assessment of innovative off-label uses could be established to collect data on clinical effectiveness and adverse events (AEs).164 This would involve the FDA systematically collecting postmarketing data to quantify the risk:benefit of innovative off-label uses; synthesizing evidence regarding these uses and disseminating requests. Grant funding would assure proper study and monitoring. Such a model has been applied to clinical/surgical registries, AE reporting, and medical device failures. Alternatively, manufacturers could be made responsible for collecting efficacy data and develop pharmacovigilance plans to detect and report AE associated with innovative off-label use. Importantly, however, all this must be accomplished without compromising rigorous efficacy or safety standards.
The use of medications for repurposed uses plays an important role in pediatric pharmacotherapy. When package inserts lag behind clinical practice, clinicians must critically evaluate the entire body of evidence available, become familiar with the strength of the literature on the proposed use, and carefully weigh the potential therapeutic benefits against the risks. Rigorous literature evaluation by pharmacists is of increasing importance to ensure that innovative uses are evidence-based. The valuable role of pediatric clinical pharmacists in assessing novel off-label uses continues to expand and is established in most of the approximately 175 US children's hospitals.
By describing drug repurposing in pediatric patients, the hope is clinicians, industry, academia, and government will continue to cooperate so that Dr Shirkey's phrase pediatric “therapeutic orphan” becomes a colloquialism.
Acknowledgment
The author acknowledges the work of Jennifer Lee and Beatrice Popovitz, PharmD candidates, St John's University, New York, in helping to compile Tables 2, 3, and 4. A poster presentation was presented at the 2015 American Pharmacists Association Meeting (APPM) in San Diego, California. The poster received the APPM Research Merit Reward.
Footnotes
Disclosure The author declares no conflict or financial interest in any product or service mentioned in the manuscript including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1. Statement of Harry C. Shirkey, MD at the Conference of Professional and Scientific Societies, Commission on Drug Safety. 1963.
- 2.Shah SS, Hall M, Goodman DM et al. Off-label drug use in hospitalized children. Arch Peds Adol Med. 2007;161(3):282–290. doi: 10.1001/archpedi.161.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Turner S, Longworth A, Nunn AJ et al. Unlicensed and off label drug use in paediatric wards: prospective study. Br Med J. 1998;316(7128):343–345. doi: 10.1136/bmj.316.7128.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Best Pharmaceuticals for Children Act. P.L. 107-109 (2002)
- 5. Pediatric Research Equity Act. P.L. 108-155 (2003)
- 6. Food and Drug Administration Safety and Innovation Act. P.L. 112-144 (2012)
- 7.Sachs AN, Avant D, Lee CS et al. Pediatric information in drug labeling. JAMA. 2012;307(18):1914–1915. doi: 10.1001/jama.2012.3435. [DOI] [PubMed] [Google Scholar]
- 8.Rivera DR, Hartzema AG. Pediatric exclusivity: evolving legislation and novel complexities within pediatric therapeutic development. Ann Pharmacother. 2014;48(3):369–379. doi: 10.1177/1060028013514031. [DOI] [PubMed] [Google Scholar]
- 9.Jin G, Wong ST. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discovery Today. 2014 doi: 10.1016/j.drudis.2013.11.005. http://dx.doi.org/10.1016/j.drudis.2013.11.005. Accessed July 22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orphan Drug Act. P.L. 97-414 (1983)
- 11. Food, Drug Cosmetic Act. U.S.C. 21 §§301-97 (1938)
- 12.Kimland E, Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91(5):796–801. doi: 10.1038/clpt.2012.26. [DOI] [PubMed] [Google Scholar]
- 13.‘T Jong GW, Vulto AG, deHoog M. A survey of the use of off-label and unlicensed drugs in a Dutch children's hospital. Pediatrics. 2001;108(5):1089–1093. doi: 10.1542/peds.108.5.1089. [DOI] [PubMed] [Google Scholar]
- 14.Turner S, Gill A, Nunn T, Hewitt B, Choonara I. Use of “off-label” and unlicensed drugs in a pediatric intensive care unit. Lancet. 1996;347(9000):549–550. [PubMed] [Google Scholar]
- 15.Craig JS, Henderson CR, Magee FA. The extent of unlicensed and off-label drug use in the paediatric ward of a district general hospital in Northern Ireland. Ir Med J. 2001;94(8):237–240. [PubMed] [Google Scholar]
- 16.McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child. 2000;83(6):498–501. doi: 10.1136/adc.83.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. New Pediatric Labeling Information Database. http://www.accessdata.fda.gov/scripts/sda/sdnavigation.cfm?sd=labelingdatabase.pdf. Accessed July 22, 2015.
- 18.US Food and Drug Administration. Rare Disease Repurposing Database. http://www.fda.gov/ForIndustry/Developing-ProductsforRareDiseasesConditions/. Accessed July 23, 2015.
- 19.Generali JA, Cada DJ, editors. Off-Label Drug Facts. Facts & Comparisons Publishing Group, Wolters Kluwer Health; 2014. [Google Scholar]
- 20.Custer J, Rau R, editors. The Johns Hopkins Hospital Harriet Lane Handbook. 20th ed. Philadelphia, PA: Elsevier Mosby; 2014. [Google Scholar]
- 21.Houst B, Rumore MM. Development of a Pediatric Specific Medication Resource Center at a Metropolitan Children's Hospital. Poster Presentation. American Pharmacist's Association. March 1–4, 2013, Los Angeles, CA. J Amer Pharm Assoc. 2013;53(2):78. [Google Scholar]
- 22.Lohr KN. Rating the strength of scientific evidence: relevance for quality improvement programs. Int J Qual Health Care. 2004;16(1):9–18. doi: 10.1093/intqhc/mzh005. [DOI] [PubMed] [Google Scholar]
- 23.Go CY, Mackay MT, Weiss SK et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78(24):1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madenci E, Yilmazk K, Yilmazk M et al. Alendronate treatment in osteogenesis imperfecta. J Clin Rheumatol. 2006;12(2):53–56. doi: 10.1097/01.rhu.0000208490.22492.09. [DOI] [PubMed] [Google Scholar]
- 25.Ward LM, Raunch F, Whyte MP et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96(2):355–364. doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 26.App EM, King M, Helfesrieder R et al. Acute and long term amiloride inhalation in cystic fibrosis lung disease: a rational approach to cystic fibrosis therapy. Amer Rev Resp Dis. 1990;141(3):605–612. doi: 10.1164/ajrccm/141.3.605. [DOI] [PubMed] [Google Scholar]
- 27.Eleftheriou D, Levin M, Shingadia D et al. Management of Kawasaki's disease. Arch Dis Child. 2014;99(1):74–83. doi: 10.1136/archdischild-2012-302841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omari TI, Benninga MA, Sansom L et al. Effect of baclofen on esophagogastric motility and gastrointestinal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. J Pediatr. 2006;149(4):468–474. doi: 10.1016/j.jpeds.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 29.El Shaded A, Dargarville P, Ohlsson A, Soll RF. Surfactant for meconium aspiration syndrome in full term infants. Cochrane Database Syst Rev. 2007;3:CD002054. doi: 10.1002/14651858.CD002054. [DOI] [PubMed] [Google Scholar]
- 30.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta GT, Liacouras CA, Collins MH et al. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterol. 2007;133(4):1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Lemonnier E, Degrez C, Phelep M et al. A randomized controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry. 2012(2):e202. doi: 10.1038/tp.2012.124. http://www.nature.com/tp/journal/v2/n12/pdf/tp2012124a.pdf. Accessed July 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt B, Roberts RS, Davis P et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 34.Mueni E, Opiyo N, English M. Caffeine for the management of apnea in preterm infants. Int Health. 2009;1(2):190–195. doi: 10.1016/j.inhe.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhein L, Dobson NR, Darnell RA et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014;168(3):250–257. doi: 10.1001/jamapediatrics.2013.4371. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi A, Tsuchiya H, Yamamoto N et al. Caffeine-potentiated chemotherapy for patients with high-grade soft tissue sarcoma: long-term clinical outcomes. Anticancer Res. 2007;27(5B):3489–3496. [PubMed] [Google Scholar]
- 37.Richmond EJ, Rogol AD. Growth hormone deficiency in children. J Pituitary Soc. 2008;11(2):115–120. doi: 10.1007/s11102-008-0105-7. [DOI] [PubMed] [Google Scholar]
- 38.Gil-Ad E, Topper E, Laron Z. Oral clonidine as a growth hormone stimulation test. Lancet. 1979;314(8137):278–280. doi: 10.1016/s0140-6736(79)90293-9. [DOI] [PubMed] [Google Scholar]
- 39.Rotig A, Mollet J, Rio M, Munnich A. Infantile and pediatric deficiency diseases. Mitochondrion. 2007;7(suppl):S112–S121. doi: 10.1016/j.mito.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Parikh S, Saneto R, Falk MJ et al. A modern approach to the treatment of mitochondrial disease. Curr Treat Options Neurol. 2009;11(6):414–430. doi: 10.1007/s11940-009-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazigi A, Abou-Charaf LC. Colchicine for recurrent pericarditis in children. Acta Paediatr. 1998;87(5):603–604. doi: 10.1080/08035259850158399. [DOI] [PubMed] [Google Scholar]
- 42.Ahn YS, Harrington WJ, Simon SR, Mylvaganam R, Pall LM, So AG. Danazol for the treatment of idiopathic thrombocytopenic purpura. N Engl J Med. 1983;308(23):1396–1399. doi: 10.1056/NEJM198306093082306. [DOI] [PubMed] [Google Scholar]
- 43.Choodhry VP, Ahlawat S, Pati HP, Kashyap R. Vinblastine and danazol therapy in steroid resistant childhood chronic idiopathic thrombocytopenic purpura. Internat J Hematol. 1995;61(3):157–162. doi: 10.1016/0925-5710(95)00357-x. [DOI] [PubMed] [Google Scholar]
- 44.Hedlund-Treutiger I, Henter JI, Elinder G. Randomized study of IVIG and high dose dexamethasone therapy for children with chronic idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2003;25(2):139–144. doi: 10.1097/00043426-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Neunert C, Lim W, Crowther M et al. American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 46.Zheng JW, Zhou Q, Yang XJ et al. Treatment guideline for hemangiomas and vascular malformations of the head and neck. Head Neck. 2010;32(8):1088–1098. doi: 10.1002/hed.21274. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Sun M, Cheng X et al. Bleomycin A5 plus dexamethasone for control of growth in infantile parotid hemangiomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(1):62–69. doi: 10.1016/j.tripleo.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;5:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Sahni J, Phelps SJ. Nebulized furosemide in the treatment of bronchopulmonary dysplasia in preterm infants. J Pediatr Pharmacol Ther. 2011;16(1):14–22. [PMC free article] [PubMed] [Google Scholar]
- 50.Prabhu VG, Keszler M, Dhanireddy R. Pulmonary function changes after nebulized and intravenous furosemide in ventilated premature infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(1):32–35. doi: 10.1136/fn.77.1.f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moody K, Siegal L, Scharbach K et al. Pediatric palliative care. Prim Care Clin Office Pract. 2011;38:327–361. doi: 10.1016/j.pop.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Kim IK, Phrampus E, Sikes K et al. Helium-oxygen therapy for infants with bronchiolitis. Arch Pediatr Adolesc Med. 2011;165(12):1115–1122. doi: 10.1001/archpediatrics.2011.605. [DOI] [PubMed] [Google Scholar]
- 53.Heyman E, Morag I, Batash D, Keidar R et al. Closure of patent ductus arteriosus with oral ibuprofen suspension in premature newborns: a pilot study. Pediatr. 2003;112:354–358. doi: 10.1542/peds.112.5.e354. [DOI] [PubMed] [Google Scholar]
- 54.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Library. 2013;4:1–153. doi: 10.1002/14651858.CD003481.pub5. http://www.thecochranelibrary.com. Accessed July 23, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Johnston P, Gillam-Krakauer M et al. Evidence-based use of indomethacin and ibuprofen in the neonatal intensive care unit. Clinics Perinatol. 2012;39(1):111–136. doi: 10.1016/j.clp.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low-dose intravenous immunoglobulins in children with toxic epidermal necrolysis: an open uncontrolled study. Indian J Dermatol Venereal Leprol. 2005;71(6):398–400. doi: 10.4103/0378-6323.18943. [DOI] [PubMed] [Google Scholar]
- 57.Del Pozzo-Magana BR, Lazo-Langner A, Carleton B et al. A systematic review of treatment of drug-induced Stevens-Johnson Syndrome and toxic epidermal necrolysis in children. J Popul Ther Clin Pharmacol. 2011;18(1):121–133. [PubMed] [Google Scholar]
- 58.Pinto N, Cipkala DA, Ladd PE, Pu Y, Cohn SL. Treatment of two cases with refractory, metastatic intermediate-risk neuroblastoma with isotretenoin alone or observation. Pediatr Blood Cancer. 2014;61(6):1104–1106. doi: 10.1002/pbc.24889. [DOI] [PubMed] [Google Scholar]
- 59.Pope E, Krafchik BR, Macarthur C et al. Oral versus high-dose pulse corticosteroids for problematic infantile hemangiomas: a randomized, controlled trial. Pediatrics. 2007;119(6):1239–1247. doi: 10.1542/peds.2006-2962. [DOI] [PubMed] [Google Scholar]
- 60.Chen S, Lu K, Sun H. Randomized placebo-controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2–6 yr. Pediatr Allergy Immunol. 2006;17(1):49–54. doi: 10.1111/j.1399-3038.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 61.Ballard RA, Truog WE, Cnaan A et al. NO CLD Study Group. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355(4):343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 62.Mercier JC, Hummier H, Durrmeyer X et al. EUNO Study Group. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies: a randomized controlled trial. Lancet. 2010;376(9738):346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 63.Fenichel GM, Griggs RC, Kissel J et al. A randomized efficacy and safety trial of oxandrolone in the treatment of Duchenne dystrophy. Neurology. 2001;56(8):1075–1079. doi: 10.1212/wnl.56.8.1075. [DOI] [PubMed] [Google Scholar]
- 64.Brousseau DC, Duffy SJ, Anderson AC, Linakis JG. Treatment of pediatric migraine headaches: a randomized, double-blind trial of prochlorperazine vs ketorolac. Ann Emerg Med. 2004;43(2):256–262. doi: 10.1016/s0196-0644(03)00716-9. [DOI] [PubMed] [Google Scholar]
- 65.Hagman J, Gralla J, Siegal E et al. A double-blind, placebo-controlled study of risperidone for the treatment of adolescents and young adults with anorexia nervosa. J Am Acad Child Adol Psy. 2011;50(9):915–924. doi: 10.1016/j.jaac.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joung KH, Cho SC. The effect of sucrose on infants during a painful procedure. Korean J Pediatr. 2010;53(8):790–794. doi: 10.3345/kjp.2010.53.8.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anand KJ. International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adoles Med. 2001;155(2):173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 68.Lazzerini M, Martelossi S, Magazzu G et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease. A randomized clinical trial. JAMA. 2013;310(20):2164–2173. doi: 10.1001/jama.2013.280777. [DOI] [PubMed] [Google Scholar]
- 69.Felipez LM, Gokhale R, Tierney MP, Kirschner BS. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J Pediatr Gastroenterol Nutr. 2012;54(4):28–33. doi: 10.1097/MPG.0b013e318228349e. [DOI] [PubMed] [Google Scholar]
- 70.Galeotti C, Boucheron A, Guillaume S, Kone-Paut I. Sustained remission of multicentric Castleman disease in children treated with tocilizumab, an anti-interleukin-6 receptor antibody. Mol Cancer Ther. 2012;11(8):1623–1626. doi: 10.1158/1535-7163.MCT-11-0972. [DOI] [PubMed] [Google Scholar]
- 71.Winner P, Pearlman EM, Linder SL et al. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45(10):1304–1312. doi: 10.1111/j.1526-4610.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 72.Lakshmi CU, Singhi P, Malhi P, Ray MJ. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo controlled trial. Child Neurol. 2007;22(7):829–835. doi: 10.1177/0883073807304201. [DOI] [PubMed] [Google Scholar]
- 73.Rumore MM. Vitamin A as an immunomodulating agent. Clin Pharm. 1993;12(7):506–514. [PubMed] [Google Scholar]
- 74.American Academy of Pediatrics Committee on Infectious Diseases: Vitamin A treatment of measles. Pediatrics. 1993;91(5):1014–1015. [PubMed] [Google Scholar]
- 75.Gabbay MA, Sato MN, Finazzo C et al. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and the decline of residual B-cell function in new onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2012;166(7):601–607. doi: 10.1001/archpediatrics.2012.164. [DOI] [PubMed] [Google Scholar]
- 76.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systemic review and meta-analysis. Arch Dis Child. 2008;43(9):1093–1098. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 77.Marchisio P, Consonni D, Baggi E et al. Vitamin D supplementation reduces the risk of otitis media in otitis-prone children. Ped Infect Dis J. 2013;32(10):1055–1060. doi: 10.1097/INF.0b013e31829be0b0. [DOI] [PubMed] [Google Scholar]
- 78.Milgrom P, Ly KA, Tut OK et al. Xylitol pediatric topical oral syrup to prevent dental caries. Arch Pediatr Adolesc Med. 2009;163(7):601–607. doi: 10.1001/archpediatrics.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apt W, Aquilera X, Arribada A et al. Treatment of chronic Chagas' disease with itraconazole and allopurinol. Amer J Trop Med. 1998;59(1):133–138. doi: 10.4269/ajtmh.1998.59.133. [DOI] [PubMed] [Google Scholar]
- 80.Vargus SL, Rodriguiz J. Itraconazole or allopurinol in the treatment of chronic American trypanosomiasis: the regression and presentation of electrocardiographic abnormalities during 9 years of follow-up. Amer J Trop Parasitol. 2003;97(1):23–29. doi: 10.1179/000349803125002751. [DOI] [PubMed] [Google Scholar]
- 81.Lewis DW, Diamond S, Scott D, Jones V. Prophylactic treatment of pediatric migraine. Headache. 2004;44(3):230–237. doi: 10.1111/j.1526-4610.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 82.Rino PB, Scolnik D, Fustinana A, Mitelpunkt A, Glatstein M. Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Amer J Ther. 2014 doi: 10.1097/MJT.0000000000000028. Published ahead of print. PMID:24914501. http://www.ncbi.nlm.nih.gov/pubmed/24914501. Accessed July 22, 2015. [DOI] [PubMed] [Google Scholar]
- 83.Goebel JC, Roesel M, Heinz C et al. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Brit J Ophthalmol. 2011;95(2):209–213. doi: 10.1136/bjo.2009.173542. [DOI] [PubMed] [Google Scholar]
- 84.Kanellopoulos A, Mavrogenis AF, Mitsiokapa EA et al. Long lasting benefits following the combination of static night upper extremity splinting with botulinum toxin A injections in cerebral palsy children. Eur J Phys Rehabil Med. 2009;45(4):501–506. [PubMed] [Google Scholar]
- 85.Coutinho dos Santos LH, Rodrigues DC, Simoes de Assis TR, Bruck I. Effective results with botulinum toxin in cerebral palsy. Pediatr Neurol. 2011;44(5):357–363. doi: 10.1016/j.pediatrneurol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Gordon CT, Cotelingam GM, Stager S. A double-blind comparison of clomipramine and desipramine in the treatment of developmental stuttering. J Clin Psy. 1995;56(6):238–242. [PubMed] [Google Scholar]
- 87.Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopment disorders. Eur Child Adolesc Psychiatry. 2005;14(1):34–40. doi: 10.1007/s00787-005-0424-4. [DOI] [PubMed] [Google Scholar]
- 88.Prince JB, Wilens TE, Biederman J et al. Clonidine for sleep disturbances associated with attention-deficit hyperactivity disorder: a systematic chart review of 62 cases. J Am Acad Child Adolesc Psy. 1996;35(5):599–605. doi: 10.1097/00004583-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Phua E, Lopez X, Ramus J, Goldfine AB. Cromolyn Sodium for insulin-induced lipoatrophy: old drug, new use. Diabetes Care. 2013;36(12):e204–e205. doi: 10.2337/dc13-1123. http://care.diabetesjournals.org/content/36/12/e204.full.pdf+html. Accessed July 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dawson GS, Seidman P, Ramadan HH. Improved postoperative pain control in pediatric adenotonsillectomy with dextromethorphan. Laryngoscope. 2001;111(7):1223–1226. doi: 10.1097/00005537-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 91.Sui A, Drachtman R. Dextromethorphan: a review of N-Methyl D-Aspartate receptor antagonist in the management of pain. CNS Drug Rev. 2007;13(1):96–106. doi: 10.1111/j.1527-3458.2007.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelly AS, Rudser KD, Nathan BM et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity. JAMA Pediatr. 2013;167(4):355–360. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kelly AS, Metziq AM, Rudser KD et al. Exenatide as a weight-loss therapy in extreme pediatric obesity: a randomized, controlled pilot study. Obesity. 2012;20(2):364–370. doi: 10.1038/oby.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kallepalli BJ, Bhatara VS, Fogas BS et al. Trazodone is only slightly faster than fluoxetine in relieving insomnia in adolescents with depressive disorders. J Child Adol Psychopharm. 1997;7(2):97–107. doi: 10.1089/cap.1997.7.97. [DOI] [PubMed] [Google Scholar]
- 95.Wheeler DS, Vaux KK, Tam DA. Use of gabapentin in the treatment of childhood refiex sympathetic dystrophy. Pediatr Neurol. 2000;22(3):220–221. doi: 10.1016/s0887-8994(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 96.Eiland LS, Jenkins LS, Durham SH. Pediatric migraine: pharmacologic agents for prophylaxis. Ann Pharmacother. 2007;41(7):1181–1190. doi: 10.1345/aph.1K049. [DOI] [PubMed] [Google Scholar]
- 97.Robinson AA, Malow BA. Gabapentin shows promise in treating refractory insomnia in children. J Child Neurol. 2013;28(12):1618–1621. doi: 10.1177/0883073812463069. [DOI] [PubMed] [Google Scholar]
- 98.Mokhtar GM, Shaaban SY, Elbarbary NS, Fasyed WA. A trial to access the efficacy of glutamic acid in prevention of vincristine-induced neurotoxicity in pediatric malignancies: a pilot study. J Pediatr Hem/Onc. 2010;32(8):594–600. doi: 10.1097/MPH.0b013e3181e9038d. [DOI] [PubMed] [Google Scholar]
- 99.Scahill L, Chappell PB, Kim YS et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 100.Cummings DD, Singer HS, Krieger M, Miller TL, Mahone EM. Neuropsychiatric effects of guanfacine in children with mild Tourette syndrome: a pilot study. Clin Neuropharmacol. 2002;25(6):325–332. doi: 10.1097/00002826-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Florin TA, Shaw KN, Kittick M et al. Nebulized hypertonic saline for bronchiolitis in the emergency department. A randomized clinical trial. JAMA Pediatr. 2014;168(7):664–670. doi: 10.1001/jamapediatrics.2013.5306. [DOI] [PubMed] [Google Scholar]
- 102.Wu S, Baker C, Lang ME et al. Nebulized hypertonic saline for bronchiolitis. A randomized clinical trial. JAMA Pediatr. 2014;168(7):657–663. doi: 10.1001/jamapediatrics.2014.301. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguiz V, Lee A, Witman PM, Anderson PA. Kasabach-Merritt phenomenon: case series and retrospective review of the Mayo Clinic experience. J Pediatr Hematol Oncol. 2009;31(7):522–526. doi: 10.1097/MPH.0b013e3181a71830. [DOI] [PubMed] [Google Scholar]
- 104.Kim EH, Kyung NK, Park M et al. Clinical features of infantile hepatic hemangioendothelioma. Korean J Pediatr. 2011;54(6):260–266. doi: 10.3345/kjp.2011.54.6.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326(22):1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 106.Johnston RG, Noseworthy TW, Friesen EG et al. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698–701. doi: 10.1378/chest.97.3.698. [DOI] [PubMed] [Google Scholar]
- 107.Shankar V, Churchill KB, Deshpande JK. Isoflurane therapy for severe refractory status asthmaticus in children. Intensive Care Med. 2006;32(6):927–933. doi: 10.1007/s00134-006-0163-0. [DOI] [PubMed] [Google Scholar]
- 108.Wickman S, Siplia I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomized controlled trial. Lancet. 2001;357(9270):1743–1748. doi: 10.1016/S0140-6736(00)04895-9. [DOI] [PubMed] [Google Scholar]
- 109.Miller GS. Efficacy and safety of levetiracetam in pediatric migraine. Headache. 2004;44(3):238–243. doi: 10.1111/j.1526-4610.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 110.Winter SC. Plasma carnitine deficiency. Amer J Dis Child. 1987;141(6):660–665. doi: 10.1001/archpedi.1987.04460060076039. [DOI] [PubMed] [Google Scholar]
- 111.McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168(2):178–184. doi: 10.1001/jamapediatrics.2013.4200. [DOI] [PubMed] [Google Scholar]
- 112.Tofil NM, Benner KW, Faro SJ, Winkler MK. The use of enteral naloxone to treat opioid-induced constipation in a pediatric intensive care unit. Ped Crit Care Med. 2008;7(3):252–254. doi: 10.1097/01.PCC.0000216421.72002.09. [DOI] [PubMed] [Google Scholar]
- 113.Glare PA, Dunwoodie D, Clark K et al. Treatment of nausea and vomiting in terminally ill cancer patients. Drugs. 2008;68(18):2575–2590. doi: 10.2165/0003495-200868180-00004. [DOI] [PubMed] [Google Scholar]
- 114.Glorieux FH, Bishop NJ, Plotkin H et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 115.Salehpour S, Tauakkoli S. Cyclic pamidronate therapy in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2010;23(1–2):73–80. doi: 10.1515/jpem.2010.23.1-2.73. [DOI] [PubMed] [Google Scholar]
- 116.Sung V, Collett S, deGooyer T et al. Probiotics to treat or prevent excessive infant crying: systemic review and meta-analysis. JAMA Pediatr. 2013;167(12):1150–1157. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 117.Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- 118.Parodi E, Rivetti E, Amenlola G et al. Long-term follow-up analysis after rituximab therapy in children with refractory symptomatic ITP: identification of factors predictive of a sustained response. Brit J Hematol. 2009;144(4):552–558. doi: 10.1111/j.1365-2141.2008.07487.x. [DOI] [PubMed] [Google Scholar]
- 119.DelVecchio G, DeSantis A, Accettura L et al. Chronic immune thrombocytopenia in childhood. Blood Coag Fibrolysis. 2014;25(4):297–299. doi: 10.1097/MBC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 120.Senniappan S, Alexandrescu S, Tatevian N et al. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med. 2014;370(12):1131–1137. doi: 10.1056/NEJMoa1310967. [DOI] [PubMed] [Google Scholar]
- 121.Van Hove JK, Kerckhove KV, Hennermann JB et al. Benzoate treatment and the glycine index in nonketotic hyperglycinaemia. J Inherit Metab Dis. 2005;28(5):651–663. doi: 10.1007/s10545-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 122.Arnold GL, Griebel ML, Valentine JL, Koroma DM, Kearns GL. Dextromethorphan in nonketotic hyperglycemia: metabolic variation confounds the dose-response relationship. J Inherit Metab Dis. 1997;20(1):28–38. doi: 10.1023/A:1005301321635. [DOI] [PubMed] [Google Scholar]
- 123.Brock PR, Knight KR, Freyer DR. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology Boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Neuwelt EA, Gilmer-Knight K, Lacy C et al. Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr Blood Cancer. 2006;47(2):174–182. doi: 10.1002/pbc.20529. [DOI] [PubMed] [Google Scholar]
- 125.Laue L, Jones J, Barnes KM, Cutler GB. Treatment of familial male precocious puberty with spironolactone, testosterone, and deslorelin. J Clin Endocrinol Metab. 1993;76(1):151–155. doi: 10.1210/jcem.76.1.8421081. [DOI] [PubMed] [Google Scholar]
- 126.Leschek EW, Jones J, Barnes KM, Hill SC, Cutler GB. Six-year results of spironolactone and testolactone treatment of familial male-limited precocious puberty with the addition of deslorelin after central puberty onset. J Clin Endocrinol Metab. 1999;84(1):175–178. doi: 10.1210/jcem.84.1.5413. [DOI] [PubMed] [Google Scholar]
- 127.Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH. McCune-Albright Study Group. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr. 2003;143(1):60–66. doi: 10.1016/S0022-3476(03)00128-8. [DOI] [PubMed] [Google Scholar]
- 128.Ondo WG, Jong D, Davis A. Comparison of weight gain in treatments for Tourette syndrome: tetrabenazine versus neuroleptic drugs. J Child Neurol. 2008;23(4):435–437. doi: 10.1177/0883073807307108. [DOI] [PubMed] [Google Scholar]
- 129.Magge H, Sprinz P, Adams WG et al. Zinc protoporphyrin and iron deficiency screening trends and therapeutic response in urban pediatric center. JAMA Pediatr. 2013;167(4):361–367. doi: 10.1001/jamapediatrics.2013.751. [DOI] [PubMed] [Google Scholar]
- 130.Hammerman C, Bin-Nun A, Markovitch E et al. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128(6):1618–1621. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 131.Amendola G, Cirillio G, Spieze M, DiConcillo R, Rolando P. Treatment of childhood chronic idiopathic thrombocytopenia purpura with ascorbate. Clin Pediatr. 1995;34(5):268–370. doi: 10.1177/000992289503400508. [DOI] [PubMed] [Google Scholar]
- 132.Jubelirer SJ. Pilot study of ascorbic acid for the treatment of refractory immune thrombocytopenic purpura. Am J Hematol. 1993;43(1):44–46. doi: 10.1002/ajh.2830430110. [DOI] [PubMed] [Google Scholar]
- 133.Geobel JC, Roesel M, Heinz C et al. Azathioprine as a treatment option for uveitis in patients with juvenile idiopathic arthritis. Brit J Ophthalmol. 2011;95(2):209–213. doi: 10.1136/bjo.2009.173542. [DOI] [PubMed] [Google Scholar]
- 134.Sulheim D, Fagermoen E, Winger A et al. Disease mechanisms and clonidine treatment in adolescent chronic fatigue syndrome. JAMA Pediatr. 2014;168(4):351–360. doi: 10.1001/jamapediatrics.2013.4647. [DOI] [PubMed] [Google Scholar]
- 135.Treem WR, Hyams JS. Cyclosporine therapy for gastrointestinal disease. J Ped Gastroent Nutr. 1994;18(3):270–278. doi: 10.1097/00005176-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 136.Treem WR, Cohen J, Davis PM et al. Cyclosporine for the treatment of fulminant ulcerative colitis in children. Immediate response, long-term results, and impact on surgery. Dis Colon Rectum. 1999;38(5):474–479. doi: 10.1007/BF02148846. [DOI] [PubMed] [Google Scholar]
- 137.Seidman EG, Lacaille F, Russo P, Galeano N. Successful treatment of autoimmune enteropathy with cyclosporine. J Pediatr. 1999;117(6):925–932. doi: 10.1016/s0022-3476(05)80140-4. [DOI] [PubMed] [Google Scholar]
- 138.Gerloni V, Cimaz R, Gattinara M et al. Efficacy and safety profile of cyclosporine A in the treatment of juvenile chronic (idiopathic) arthritis. Results of a 10-year prospective study. Rheumatol. 2001;40(8):907–913. doi: 10.1093/rheumatology/40.8.907. [DOI] [PubMed] [Google Scholar]
- 139.Hauer J, Mackey D. Treatment of gabapentin associated with resolution of apnea in two infants with neurological impairment. J Palliat Med. 2013;16(4):455–458. doi: 10.1089/jpm.2012.0103. [DOI] [PubMed] [Google Scholar]
- 140.Pistoia F, Conson M, Sara M. Opsoclonus-Myoclonus Syndrome in patients with locked-in syndrome: a therapeutic port-hole with gabapentin. Mayo Clin Proc. 2001;85(6):527–531. doi: 10.4065/mcp.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coakley JH, Moorcraft J, Hipkin LJ, Smith CS, Griffiths RD, Edwards RT. The effect of mazindol on growth hormone secretion in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psy. 1988;51(12):1551–1557. doi: 10.1136/jnnp.51.12.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Griggs RC, Moxley RT, Mendell JR et al. Randomized, double-blind trial of mazindol in Duchenne dystrophy. Muscle Nerve. 1990;13(2):1169–1173. doi: 10.1002/mus.880131212. [DOI] [PubMed] [Google Scholar]
- 143.Bhalla T, Sawardekar A, Russell H, Tobias JD. The role of methylene blue in the pediatric patient with vasoplegic syndrome. World J Ped Congenital Heart Surg. 2011;2(4):652–655. doi: 10.1177/2150135111410992. [DOI] [PubMed] [Google Scholar]
- 144.Flynn BC, Sladen RN. The use of methylene blue for vasodilatory shock in pediatric lung transplant patient. J Cardiothor Vasc Anesth. 2009;23(4):529–530. doi: 10.1053/j.jvca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 145.Karabulut R, Karakus C, Hirfanoglu L et al. Treatment of postoperative enterocutaneous fistulas with octreotide in two neonates. Eur J Pediatr Surg. 2008;18(1):56–58. doi: 10.1055/s-2007-965787. [DOI] [PubMed] [Google Scholar]
- 146.Lavid N, Franklin DL, Maguire GA. Management of child and adolescent stuttering with olanzapine: three case reports. Ann Clin Psychol. 1999;11(4):233–236. doi: 10.1023/a:1022365513865. [DOI] [PubMed] [Google Scholar]
- 147.Boyd A, Dworzynski K, Howell P. Pharmacological agents for developmental stuttering in children and adolescents: a systematic review. J Clin Psychopharmacol. 2011;31(6):740–744. doi: 10.1097/JCP.0b013e318234ee3b. [DOI] [PubMed] [Google Scholar]
- 148.Holbrook T, Keet C, Frischmeyer-Guerrerio PA et al. Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2013;132(5):1219–1220. doi: 10.1016/j.jaci.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 149.Freedman SB, Hall M, Shah SS et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168(4):321–329. doi: 10.1001/jamapediatrics.2013.4906. [DOI] [PubMed] [Google Scholar]
- 150.Frigon C, Desparmet J. Ondansetron treatment in a child presenting with chronic intractable pruritus. Pain Res Manag. 2006;11(4):245–247. doi: 10.1155/2006/873870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Feng J, Sethi A, Reyes-Mugica M, Antaya R. Life-threatening blood loss from scratching provoked by pruritus in the bulky perineal nevocytoma variant of giant congenital melanocytic nevus in child. J Am Acad Dermatol. 2005;53(2 suppl 1):S139–S142. doi: 10.1016/j.jaad.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 152.Van Wattum PJ. Stuttering improved with risperidone. J Amer Acad Child Adol Psychiat. 2006;45(2):133. doi: 10.1097/01.chi.0000190465.55329.b0. [DOI] [PubMed] [Google Scholar]
- 153.Costa AD, Kroll RM. Sertraline in stuttering. J Clin Psychopharm. 1995;15(6):443–444. doi: 10.1097/00004714-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 154.Schmitt B, Martin F, Critelli H, Molinari L, Jenni O. Effects of valproic acid on sleep in children with epilepsy. Epilepsia. 2009;50(8):1860–1867. doi: 10.1111/j.1528-1167.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 155.Moore J, Lee M, Garzon M et al. Effective therapy of a vascular tumor of infancy with vincristine. J Pediatr Surg. 2001;36(8):1273–1276. doi: 10.1053/jpsu.2001.25793. [DOI] [PubMed] [Google Scholar]
- 156.Perez Payarols J, Pardo MJ, Gomez BC. Treatment of life-threatening infantile hemangiomas with vincristine. N Engl J Med. 1995;333(1):69. doi: 10.1056/nejm199507063330120. [DOI] [PubMed] [Google Scholar]
- 157.Blatt J, Corey SJ. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Dis Today. 2013;18(1–2):4–10. doi: 10.1016/j.drudis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 158.Laughon MM, Avant D, Tripathi N et al. Drug labeling and exposure in neonates. JAMA Pediatr. 2014;168(2):130–136. doi: 10.1001/jamapediatrics.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shah SS, Hall M, Goodman DM et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161:282–290. doi: 10.1001/archpedi.161.3.282. [DOI] [PubMed] [Google Scholar]
- 160.Macaulay TE, Cook AM, Fink JL, Rapp RP, Vincent WR. Pharmacist's role in facilitating evidence-based prescribing for unlabeled use of medications. Am J Health Syst Pharm. 2009;66(19):1735–1739. doi: 10.2146/ajhp080352. [DOI] [PubMed] [Google Scholar]
- 161.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 162.Podell LB. Legal implications of preparing and dispensing approved drugs for unlabeled indications. Am J Health Syst Pharm. 1983;40(1):111–113. [PubMed] [Google Scholar]
- 163.American Academy of Pediatrics. Policy statement. Off-label use of drugs in children. Pediatrics. 2014;133(3):563–567. doi: 10.1542/peds.2013-4060. [DOI] [PubMed] [Google Scholar]
- 164.Liang BA, Mackey T. Reforming off-label promotion to enhance orphan disease treatment. Science. 2010;327(5963):273–274. doi: 10.1126/science.1181567. [DOI] [PubMed] [Google Scholar]


