Abstract
OBJECTIVES: The US Food and Drug Administration industry guidelines for manufacturers of oral, over-the-counter, liquid medications recommend that these products be packaged with dosage-delivery devices. This study describes the prevalence of these devices and instructions packaged with prescription, oral, liquid medications.
METHODS: This was a descriptive study of prescription oral-liquid medications dispensed during a 6-month period at a community pharmacy. Product information was obtained from the National Library of Medicine's DailyMed database and from the products themselves. Endpoints included provision of a measuring device, the type of device, the maximum dose measurable and intervals on the provided device, and inclusion of instructions to the pharmacist.
RESULTS: A total of 382 liquid prescription medications were included in the study. Forty-nine of the 382 products (12.8%) were packaged with a measuring device. The most commonly provided device was a calibrated dropper (n = 18; 36.7%), followed by an oral syringe with a bottle adaptor (n = 9, 18.4%). Specific instructions on proper use of the provided measuring device were included with 20 products (40.8%). Among the products that did not provide a measuring device, only 70 of the 333 package inserts (21%) included instructions to the pharmacist regarding counseling the patient on proper administration.
CONCLUSIONS: Packaging of prescription oral-liquid medications is inconsistent and leaves room for vast variability in patient or parent administration practices. In the future, patterns of actual dispensing practices among pharmacies and pharmacists would help determine the true incidence of dispensing of measuring devices.
INDEX TERMS: administration, medication errors, oral, pediatrics
INTRODUCTION
The method by which parents and caregivers measure liquid medications for children has long been identified as potentially problematic. In previous studies, measuring devices used to administer liquid medications have included a variety of implements, including household teaspoons, dosing cups, droppers, cylindrical spoons, and oral syringes.1 The frequency of use of household teaspoons to measure liquid medications has been reported to be as high as 73% of participants2 to 16.7% of participants3 more recently. It has been shown previously that household teaspoons do not accurately measure the 5-mL volume that is intended in dosing medications.4
Manufacturers of over-the-counter (OTC) medications commonly include measuring devices in the packaging of OTC liquid medications, which should assist parents in measuring medications accurately. In November 2009 and again in May 2011, the US Food and Drug Administration (FDA)5,6 released a Guidance for Industry that provided a guideline for the measuring devices packaged with OTC liquid medications. This guidance stated that dosage-delivery devices should be included for all oral, liquid OTC products and that the units of measure should correspond to the units of measure on the labeled dosage directions on the carton labeling, with no extraneous measurement markings. The Consumer Healthcare Products Association (CHPA) has released a similar guideline.7 A study assessing compliance of OTC liquid products with these guidances showed a glaring need for improvements in dosing devices for these products, with 98.6% of products having a discrepancy between the dose on the label and the measuring device in the package.8 A more recent study reported that 91% of dosing directions and 62% of dosing devices adhered to “top tier” recommendations in the guidances, indicating an improvement in adherence.9
Although provision of a measuring device is now common with OTC medications, the same is not true for prescription medications. Unlike OTC liquid medications, prescription liquid medications may or may not be dispensed by the pharmacy in the original container; original containers may be used to dispense more than one prescription. However, dispensing in original containers is common, and provision of a measuring device is at the discretion of the manufacturer and the dispensing pharmacist. Advocacy for the provision of measuring devices for prescription oral liquid medications began many years ago10 and continues today.11
In April 2013, the FDA12 released a Draft Guidance for Industry, which outlines proper design and information for container labels and carton labeling for prescription medications. It states that those devices included with drug products should be appropriate for the dosages that would be measured with that drug and that measurement markings should be in milliliter increments. The guidance does not recommend that all prescription oral-liquid medications include measuring devices.
In March 2014 the National Council for Prescription Drug Programs (NCPDP)13 published a white paper with recommendations and guidance for standardization of labels on prescription containers for oral liquid medications and states “dosing devices with numeric graduations and units that correspond to the container labeling should be made easily and universally available such as including a device each time oral liquid prescription medications are dispensed.”
In an effort to assess the consistency with which measuring devices are packaged with prescription oral-liquid medications, we systematically assessed the availability of these devices in the packaging of the products as well as the doses they were designed to measure.
MATERIALS AND METHODS
Selection of Prescription Liquid Medication Products
A list of medications dispensed between November 1, 2012, and April 30, 2013, was generated from a chain community retail pharmacy in suburban New Jersey. We presumed that this 6-month period would include a fair sampling of medications. From this list, medications were included if they were liquids, intended for oral use, and available by prescription only. Medications that were available OTC were excluded because literature examining the provision of measuring devices already exists for OTC medications, and there are separate guidance documents for OTC oral, liquid medications.5–9
Data regarding how medications were packaged were collected from package inserts, which were gathered from the National Library of Medicine's DailyMed database,14 the product itself if it was able to be obtained, or a medical information request to the manufacturer. If no data were available, the medication was excluded.
Variables for Analysis
Each product's unique NDC (National Drug Code) number was used to search for the labeling in the DailyMed database. DailyMed, the official database for FDA-approved drug-label information (package inserts), is provided by the National Library of Medicine. The information is the most recently submitted information to the FDA.
If no information was available on the DailyMed website, an attempt was made to obtain the product, and if that could not be done, the manufacturer was contacted. Information collected included manufacturer, concentration, and provision of a measuring device. If a measuring device was included in the packaging, information on the device itself was collected, including type of device (as defined by the package insert), maximum volume (in milliliters) of liquid measurable with the provided device, interval of markings on the device (in milliliters), and whether instructions on how to use the device were provided to the user. For products with no information in the labeling, the products were obtained, and the information was taken from the actual measuring device. If no measuring device was included in the packaging, the labeling was then evaluated to determine whether the package insert provided instructions to the pharmacist to counsel the patient or caregiver on proper dosing of the liquid medication with a separate dosing device. Information was evaluated by both investigators.
Each product was categorized into 1 of 10 specific therapeutic areas: analgesic, anti-infective, cardiovascular, central nervous system (CNS), endocrine, gastrointestinal, immunosuppressant, respiratory, a combination, or other. Products in the “combination” category were those that had multiple ingredients that treat different indications. A product designated in the respiratory category included anything related to the treatment of asthma as well as antihistamine products. The immunosuppressant category included antirejection medications and corticosteroids. Any product designated in the “other” category did not fit into the remaining 9 categories.
As this study was purely descriptive in nature, no formal statistics were done. In addition, institutional review board approval was not pursued because there were no human subjects involved.
RESULTS
Initially, 13,073 medications were assessed for eligibility: 12,625 products were not included because they were not liquid products, leaving 448 products that were eligible for initial data collection. Of those, 66 products were excluded: 39 were available OTC, 20 did not have data available, 5 were not labeled for oral administration, and 2 products were individual granule packets for suspension. A total of 382 prescription oral, liquid drug products were included in the final evaluation. Of the 382, information for 4 products was gathered from the product itself because of the lack of information on those products in the DailyMed database.
Of the total products, 12.8% (49 of 382) were packaged with a dosing device. When no device was provided, 70 of the 333 package inserts (21%) included instructions to the pharmacist about counseling patients on the proper dosing of the medication. Instructions provided varied by product.
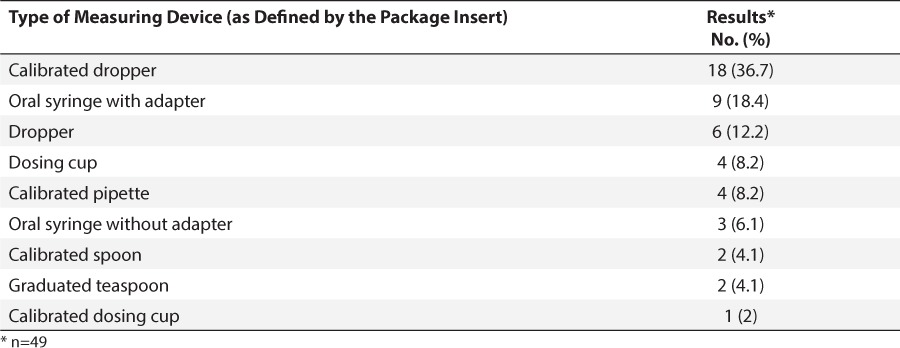
The 49 dosing devices found in this study were then evaluated individually. The most common form of device provided was a calibrated dropper (36.7%; 18 of 49), followed by a syringe with a bottle adapter (18.4%; 9 of 49). A full breakdown of the types of devices provided can be found in Table 1. Measurement intervals on the devices were highly variable, ranging from 0.001 mL to 5 mL, with some devices marking only specific doses (e.g., 2, 3, 4, 5, and 10 mL). The maximum measurable volume from these products ranged from 0.5 mL to 39.7 mL. Two products with an associated, calibrated dropper had markings in milligrams rather than the standard measurement of milliliters. One product measured a total volume equivalent to nearly twice the recommended daily dosage. Specific instructions on the proper use of the provided dosing devices were included in 40.8% (20 of 49) of the products reviewed.
Table 1.
Types of Measuring Devices Packaged with Products
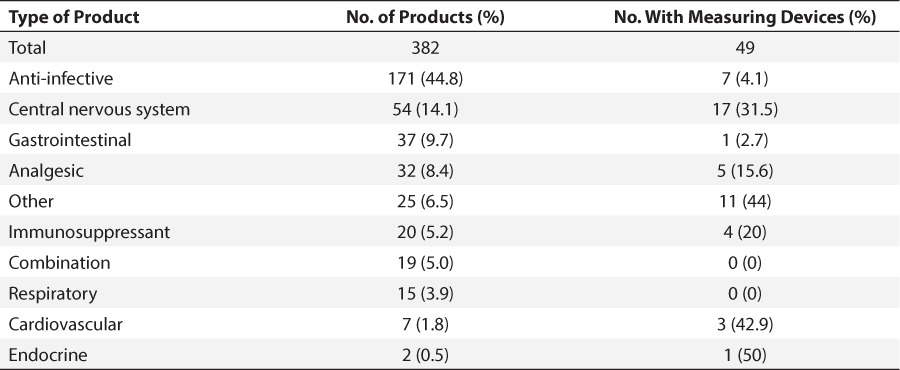
The most common category of products evaluated were anti-infectives (44.8%; 171 of 382). This category included antibiotics, antifungals, antivirals, and antiretrovirals. The CNS products were the second most commonly evaluated (14%; 54 of 382) and included antidepressants and antiepileptic drugs. Measuring devices were included with CNS agents more than they were with any other category of medication. Table 2 shows the full breakdown of data by category.
Table 2.
Categories of Products Studied
DISCUSSION
Our study revealed that most prescription oral, liquid products (87%) were not packaged with a measuring device. In addition, of those that did not include a device, only 21% included instructions in the package insert on how to appropriately administer the medication.
The study by Yin et al,8 done before the publication of the FDA's guidance, showed that 74% of OTC medications were packaged with measuring devices, more than 3 times the rate measured for prescription oral, liquid medications in our study. Since the publication of the FDA's Guidance for Industry and the CHPA's guidelines,5–7 OTC products have made an improvement from baseline. A recent study9 demonstrated that 100% of OTC products studied were compliant with the FDA recommendation that dosing devices be included in the packaging.
The discrepancy between OTC products and prescription products is clear. The recent release of the white paper from NCPDP13 should provide incentive to expedite changes for prescription products. Whether the changes come in the form of devices provided by the manufacturer or by dispensing pharmacies has yet to be seen.
Although this study investigated the provision of a measuring device within product packaging, many prescription oral, liquid medications are packaged with enough volume to fill multiple prescriptions, and pharmacies transfer contents to separate, usually amber, bottles. In this case, even if the product was packaged with an appropriate measuring device, the pharmacist may use that bottle for multiple prescriptions and would thus have to provide the additional measuring devices for those patients.
In addition, of those products in our study that were not packaged with a measuring device, the dispensing pharmacy may have provided one. Of those products that were packaged with a measuring device, the dispensing pharmacy may not have included that device when dispensing the medication to the patient. Dispensing practices of community pharmacies are quite variable, with some providing measuring devices, and some not providing them. One study reported that 20 of 61 pharmacies (33%) did not dispense a measuring device for a prescription of liquid penicillin potassium.15 Some pharmacies dispense oral syringes with liquid medications without the corresponding bottle caps that fit the syringe. Without the cap, the caregiver must pour the medication into a small container and draw the liquid into the syringe, which creates air bubbles and can be difficult and messy. This could lead parents to use household teaspoons or tablespoons out of frustration. Although this study addressed how medications are packaged, how they are actually dispensed is an area for future research.
Other limitations of our study include the variability in how data were extracted and that medications included were limited to those dispensed in a 6-month period from one pharmacy.
The NCPDP white paper recommends that the “milliliter (mL) should be used on prescription container labels for oral liquid medications” in place of the commonly used teaspoon or tablespoon.13 This recommendation is aimed at ending the practice of using teaspoons and tablespoons as measuring devices for liquid medications. However, household teaspoons and tablespoons may be the only option for patients and caregivers who do not have access to appropriate measuring devices.
Household teaspoons and tablespoons are commonly used measuring devices for liquid medications.2 This practice is only reinforced by prescriptions whose doses are written in teaspoons or tablespoons. Pediatricians have been encouraged to only prescribe liquid medications in milliliters since the 1970s.10,11 In a recent study, 39.4% of parents made an error in measuring doses, and those who used teaspoons or tablespoons were twice as likely to measure incorrectly compared with those who measured using a milliliter-only device.3 Multiple studies have proven the ability of parents to accurately measure medication doses with oral syringes.1,16,17 Given the variability of potential doses in prescription oral, liquid products, such as antibiotics, an oral syringe may be the most reasonable measuring device to be included with these products.
Most medication is used in the outpatient setting, and although much attention has been given to OTC liquid medications and their measuring devices, prescription liquid medications deserve equal consideration. Efforts to improve outpatient drug safety should include education as well as passive strategies that do not require patients (or caregivers) to modify their behavior.18 Inclusion of dosing devices with prescription oral medications can help patients and caregivers use liquid medications more safely.
The results of our study indicate that there is tremendous variability in the way that manufacturers package prescription oral, liquid products and that a dosing device is only rarely provided. More-consistent provision of appropriate measuring devices is needed.
Acknowledgment
This work was presented as a poster at the American Society of Health-System Pharmacists Mid-year Clinical Meeting, Orlando, Florida, December 2013; and as an encore presentation at the Pediatric Pharmacy Association Group Annual Meeting in Minneapolis, Minnesota, May 2015.
Abbreviations:
- CHPA
Consumer Healthcare Products Association
- CNS
central nervous system
- FDA
US Food and Drug Administration
- NCPDP
National Council for Prescription Drug Programs
- NDC
National Drug Code
- OTC
over-the-counter
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Both Dr Meyers and Dr Johnson had full access to all data in the study and take responsibility for its integrity and the accuracy of the data analysis.
REFERENCES
- 1.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42(1):46–52. doi: 10.1345/aph.1K420. [DOI] [PubMed] [Google Scholar]
- 2.Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract. 2000;49(8):741–744. [PubMed] [Google Scholar]
- 3.Yin HS, Dreyer BP, Ugboaja DC et al. Unit of measurement used and parent medication dosing errors. Pediatrics. 2014;134(2):e354–e361. doi: 10.1542/peds.2014-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falagas ME, Vouloumanou EK, Plessa E et al. Inaccuracies in dosing drugs with teaspoons and tablespoons. Int J Clin Pract. 2010;64(9):1185–1189. doi: 10.1111/j.1742-1241.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Draft guidance for industry on dosage delivery devices for over-the-counter liquid drug products; availability. Fed Regist. 2009;74(213):57319. [Google Scholar]
- 6.US Food and Drug Administration. Guidance for industry: dosage delivery devices for orally ingested OTC liquid drug products. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory-Information/Guidances/UCM188992.pdf. Published May 2011. Accessed October, 20, 2015.
- 7.Consumer Healthcare Products Association. Volumetric measures for dosing of over-the-counter oral liquid drug products for children ≤ 12 years of age. http://www.chpa.org/VolCodesGuidelines.aspx. Adopted 1934. Revised 1944, 1951, 1955, 1966, 2009, 2015. Accessed October, 20, 2015.
- 8.Yin HS, Wolf MS, Dreyer BP et al. Evaluation of consistency in dosing directions and measuring devices for pediatric non-prescription liquid medications. JAMA. 2010;304(23):2595–2602. doi: 10.1001/jama.2010.1797. [DOI] [PubMed] [Google Scholar]
- 9.Budnitz DS, Lovegrove MC, Rose KO. Adherence to label and device recommendations for over-the-counter pediatric liquid medications. Pediatrics. 2014;133(2):e283–e290. doi: 10.1542/peds.2013-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe SJ, Bierman CW, Cann HM et al. Inaccuracies in administering liquid medication. Pediatrics. 1975;56(2):327–328. [PubMed] [Google Scholar]
- 11.Paul IM, Yin HS. Out with teaspoons, in with metric units. AAP News. 2012;33(3) [Google Scholar]
- 12.US Food and Drug Administration. Draft guidance for industry: safety considerations for container labels and carton labeling design to minimize medication errors. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm349009.pdf. Published April 2013. Accessed October 20, 2015.
- 13.National Council for Prescription Drug Programs. NCPDP recommendations and guidance for standardizing the dosing designations on prescription container labels of oral liquid medications, version 1.0. http://www.ncpdp.org/education/whitepaper. Published March 2014. Accessed October 20, 2015. [DOI] [PMC free article] [PubMed]
- 14.US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed/services/. Accessed May 2013–November 2013. [DOI] [PubMed]
- 15.Dusdieker LB, Murph JR, Milavetz G. How much antibiotic suspension is enough? Pediatrics. 2000;106(1):E10. doi: 10.1542/peds.106.1.e10. [DOI] [PubMed] [Google Scholar]
- 16.Beckett VL, Tyson LD, Carroll D, Gooding NM et al. Accurately administering oral medication to children isn't child's play. Arch Dis Child. 2012;97(9):838–841. doi: 10.1136/archdischild-2012-301850. [DOI] [PubMed] [Google Scholar]
- 17.Yin HS, Mendelsohn AL, Wolf MS, Parker RM et al. Parents' medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164(2):181–186. doi: 10.1001/archpediatrics.2009.269. [DOI] [PubMed] [Google Scholar]
- 18.Budnitz DS, Layde PM. Outpatient drug safety: new steps in an old direction. Pharmacoepidemiol Drug Saf. 2007;16(2):160–165. doi: 10.1002/pds.1242. [DOI] [PubMed] [Google Scholar]


