Abstract
The neocortex is the part of the brain responsible for the execution of higher-order brain functions, including cognition, sensory perception and sophisticated motor control. During evolution, the neocortex has developed an unparalleled neuronal diversity, which still remains partly unclassified and unmapped at the functional level. Here, we first broadly review the structural blueprint of the neocortex and discuss the current classification of its neuronal diversity. We then cover the principles and mechanisms that build neuronal diversity during cortical development and consider the impact of neuronal class-specific identity in shaping cortical connectivity and function.
INTRODUCTION
The neocortex is the crowning achievement of brain evolution, containing unparalleled cellular diversity, which has evolved to support complex behaviors. The diversity of neocortical cell types, the sophisticated local and long distance cortical circuits, and the remarkable functional capacities of the neocortex, have made the study of cortical development, evolution and function a topic of very high interest over a span of decades, marked by continuing discoveries. An additional motivation for this research is that dysfunctional cortical networks or abnormal cortical development often translate into prominent neurodevelopmental and neuropsychiatric diseases, which remain poorly understood and largely untreated.
The basic structural and functional features of neocortical organization have been identified many years ago, but much remained to be clarified regarding the cellular composition of the neocortex and its relationship to cortical function. Several questions are still at center stage in the field regarding the classification of cortical neurons and the strategies that build neuronal diversity during developmental corticogenesis. For example, when during the progression from progenitors to neurons are lineage bifurcation decisions controlled, and what is the regulatory logic that allows the development of so many neuronal classes? How, mechanistically, do these large numbers of neurons maintain their class-specific features unchanged for the life of the organism? Finally, how do so many neurons integrate during development of the local cortical circuit to guarantee balanced cortical activity and function?
This review first covers key concepts of the structural and functional organization of the cerebral cortex, highlighting selcted discoveries that led to our current understanding of cortical organization. It then reviews the cellular composition of the cortex, focusing on neuronal diversity and the developmental strategies that build it during development. Finally, it considers how neuronal class-specific identity informs connectivity choices, and integration into local circuit, and the behavior of glia.
EVOLVING UNDERSTANDING OF TISSUE ORGANIZATION AND NEURONAL COMPOSITION OF THE NEOCORTEX
Basic Principles of Cortical Organization: Areas, Layers and Columns
At the beginning of the 19th century neuroscientists were already aware that regions with specialized functions could be identified on the surface of the cerebral cortex. This led to the theory of “localization of functions”, which preceded the identification of the first functional cortical area in 1865 by neuroanatomist Paul Broca, who demonstrated that speech was localized in a specific region of the frontal lobe (Broca 1865). It is now well established that the neocortex is tangentially parcellated into many functional areas that process specific sensory modalities (vision, hearing, touch, etc..). Cortical areas have defined tangential boundaries, different cytoarchitectonic features, and specialized patterns of afferent-efferent connectivity. For example, areas devoted to processing visual information are located in the caudal neocortex (the occipital cortex) and largely receive input from the lateral geniculate nucleus (LGN) of the thalamus, which in turn is the target of afferent input from the retina (Figure 1a). In contrast, areas that process auditory stimuli are located in the temporal cortex, rostro-laterally to the visual cortex and receive input from a different thalamic nucleus, the medial geniculate nucleus (MGN) (Figure 1a). In the mouse neocortex there are four primary areas: somatosensory cortex (which processes sensory modalities, such as input from the vibrissae), auditory cortex (which processes sound); visual cortex (which processes the sense of sight) and motor cortex (which outputs information to control fine motor behavior) (Figure 1a). For excellent reviews covering the development and structure of cortical areas, we refer the readers to (Rash & Grove 2006).
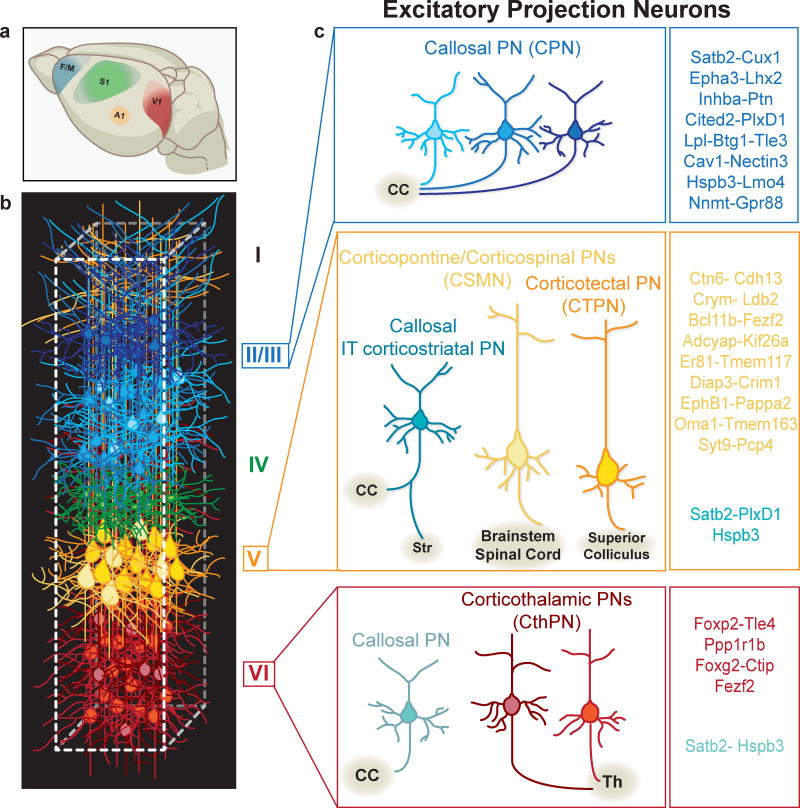
Figure 1.
The neocortex is organized into areas, layers, and columns populated by a great diversity of excitatory and inhibitory neuronal subtypes. (a) Schematic representation of primary neocortical areas dedicated to processing distinct sensory modalities and governing fine motor control. F/M, frontal/motor cortex; S1, somatosensory cortex; A1, auditory cortex; V1, visual cortex. (b) Cortical columns contain horizontally-arranged layers with very diverse neuronal compositions. Only select examples are depicted here. (c) Layer II/III contains different classes of commissural neurons, primarily of distinct CPN identities. Layer V contains CPNs, often maintaining distinctive collaterals to the striatum (IT type of corticostriatal PNs), and different classes of subcerebral PNs that connect to the brainstem, spinal cord, and superior colliculus. Layer VI has different classes of CThPNs, connecting to separate thalamic nuclei and CPNs that connect through the CC.
Cortical PN subtypes express unique gene signatures that in specific combinations identify each class (listed on right).
Abbreviations: CC, corpus callosum; Th, thalamus; CPN, callosal projection neuron; CTPN, corticotectal projection neuron; CThPN, corticothalamic; IT, intratelencephalic; PN, projection neuron, IN, interneurons. Roman numerals refer to the six cortical layers.
The cortex displays unique cytoarchitectural characteristics, which arise from the organization and composition of the constituent cell types and circuits, and which vary in an area-specific manner. One distinctive feature of the neocortex is the organization of neurons into six horizontal layers, historically defined as supragranular (Layer II/III), granular (Layer IV) and infragranular (Layers V and VI) (Figure 1b). Layers contain different classes of neurons and vary in thickness and tissue architecture depending on their areal identity (reviewed in Greig et al. 2013).
From a functional perspective, neurons connect horizontally within and across cortical areas, but also radially within functional columns that contain neurons from different layers connected in a highly stereotyped fashion (da Costa & Martin 2010). Functional columns were defined in the cortex for the first time by Vernon Mountcastle, who proposed the “columnar hypothesis”, which states that the cortex is composed of discrete, modular columns of neurons, characterized by a consistent connectivity profile (Mountcastle 1957). This discovery, originally built on data from electrophysiological recordings in the somatosensory cortex of monkeys, was a turning point in the understanding of neocortical organization. The columnar theory has been prominent in the field for over fifty years, yet much investigation is still ongoing to determine whether this columnar organization applies across the entirety of the neocortex (see Blue Brain Project initiative http://bluebrain.epfl.ch/page-52063.html and (Markram 2006)), and if, as the original formulation of the columnar hypothesis proposes, these columns are repetitive, modular and canonical.
Despite some outstanding questions, there are several well-established examples of the columnar organization of cortical circuit. The rat somatosensory cortex (or “barrel cortex”) illustrates this concept well (Petersen & Sakmann 2001). In rodents, sensory information travels from the sensory neurons in the whisker follicles through the brainstem to the thalamus, which then relays this information to the somatosensory cortex. Thalamic axons carrying information from individual whiskers form clusters of synapses, called barrels, in Layer IV of somatosensory cortex, whose spatial organization delineates a topographic map in the cortex corresponding to the arrangement of the whiskers on the animal’s face (Frostig 2006). From Layer IV, the whisker-specific signal spreads mostly vertically within a column to pyramidal neurons located in Layer II/III, where the signal is processed and integrated by horizontal transmission to neighboring neurons, before reaching output neurons in layer V, which “conclude” the columnar processing of sensory information from the whiskers (Petersen & Sakmann 2001).
Neurons of the neocortex
How many classes of neurons does the neocortex contain? Even ignoring the fact that the answer is probably different in different species, it is fair to say that the current classification of cortical neuron diversity is at best incomplete. The fact that the cortex contains many types of cells (including a variety of neurons) has been appreciated since early neuroanatomists, most prominently Ramon y Cajal, first began to investigate its cellular components. By employing a staining technique invented by Camillo Golgi (the “reazione nera”; today better known as the Golgi method), Cajal was able to generate extremely detailed morphological depictions of individual cells in the cerebral cortex, uncovering great cellular diversity (Cajal 1909). One conclusion that has emerged from a now large body of work is that the neocortex has evolved an extreme heterogenity of neuronal subtypes that are still only partly classified, and whose function is not fully mapped.
Beyond classically defined categories of neurons, recent single-cell transcriptional profiles of adult cerebral cortex suggest the existence of molecularly distinct clusters of cells that appear to represent new types, as they cannot be molecularly assigned to previously known populations (Johnson et al. 2015, Zeisel et al. 2015). Much more work is required to establish whether these are genuinely new neuronal types that were previously unappreciated, and whether these new populations have any distinct functional roles, but it is likely that new neuronal groups await discovery and classification. As famously observed by Cajal, “unfortunately, nature seems unaware of our intellectual need for convenience and unity, and very often takes delight in complication and diversity”, holds true (Cajal 1906).
Below we review the current classification and developmental origin of both excitatory pyramidal (or projection) neurons (PNs) and inhibitory local cortical interneurons (INs).
Cortical projection neurons: the “principal cells”
Projection neurons are excitatory, glutamatergic neurons that connect the cerebral cortex to the entirety of its distal intracortical, subcortical and subcerebral targets. These cells make up the vast majority of the neurons of the cortex (approximately 70–80%) and are stereotypically distributed within the layers. The nomenclature in use today to distinguish projection neuron subtypes is still mostly based on hodology, i.e. long-distance connectivity, as originally proposed by Cajal. However, projection neuron subtype-specific identity is nowadays defined by the intersection of several molecular, electrophysiological, morphological and connectivity traits (Figure 1c).
At a gross level, projection neurons can be broadly classified into intracortical and corticofugal neurons. Intracortical PNs are present in all six layers, but predominantly represented in the upper Layer II/III. They can be further divided into associative and commissural PNs. PNs that project their axons either to targets in the same hemisphere, or to different layers of the same area or column are called associative PNs (Molyneaux et al. 2007). In contrast, commissural projection neurons (CoPNs) project their axons to targets located in the opposite hemisphere, usually in a topographic manner, through one of two major fiber commissures, the corpus callosum (CC) and the anterior commissure (AC). The anterior commissure represents the most evolutionary conserved commissure of the brain, and in non-placental mammals, like marsupials, which lack the corpus callosum, it is the only route by which bilateral information can be exchanged between homologous areas of the cortex. In rodents and in primates, including humans, the majority of commissural neurons connect through the corpus callosum (callosal projection neurons; CPNs), while only a small number of neurons, mainly located in the lateral cortex project through the anterior commissure (Aboitiz et al. 2003) (Figure 1c).
The other main class of projection neurons, corticofugal projection neurons (CFuPNs), are mainly located in the deep layers and send their axons to distal targets outside of the cortex; they can be further classified into corticothalamic projection neurons (CThPNs) and subcerebral projection neurons (ScPNs). CthPNs are a heterogeneous population of projection neurons located in layer VI, which project to different nuclei of the thalamus to modulate incoming sensory information. ScPNs reside in Layer Vb across multiple areas and project their axons to distinct targets below the brain, predominantly to the pons and other nuclei of the brainstem (cortico-pontine projection neurons), to the superior colliculus (corticotectal projection neurons), and to the spinal cord (corticospinal motor neurons, CSMNs) (Molyneaux et al. 2007) (Figure 1).
Some classes of PNs send axons to multiple targets and do not easily fit into any of the classes described above. These include the subset of subcerebral projection neurons that have backward projections and extend axons to both subcerebral targets and the ipsilateral caudal cortex (Cederquist et al. 2013), and the intratelencephalic type of the corticostriatal PNs (CStrPN IT-type), which are present in layers II–VI and project to the ipsilateral and contralateral striatum and also innervate the contralateral cortex (Shepherd 2013) (Figure 1c).
This canonical system for PN nomenclature has been integrated with other classification criteria that consider, for example, electrophysiological and molecular properties. It is known that projection neurons with distinct morphologies and patterns of long distance connectivity have distinctive electrophysiological properties, including modes of firing of action potentials and intrinsic membrane properties. Although a fine-grained characterization of the intrinsic electrophysiological properties of specific classes across a range of pyramidal neuron subtypes is not available, this suggests that distinct subtypes of pyramidal neurons process incoming information in ways that match their function, and, possibly, that the molecular composition of each neuron affects the ultimate choice of electrophysiological behavior.
Over the past 10 years, several studies have tackled the difficult problem of isolating and molecularly profiling classically-defined PN populations. Labeling approaches have included retrograde tracing of distinct neuron types (Arlotta et al. 2005, Catapano et al. 2008, LaVail et al. 1973, Molyneaux et al. 2009), immunopanning (Dugas et al. 2008), and, more recently, genetic labeling (Huang & Zeng 2013) and intranuclear immunostaining with antibodies (Molyneaux et al. 2015) to permit fluorescence-activated cell sorting (FACS) of differentially labeled neuronal subtypes and subsequent molecular profiling. These studies collectively provided the field with the first sets of projection neuron class-specific “signature” genes, which can be used to both molecularly identify distinct classes and to investigate mechanisms of PN subtype-specific development and function (Figure 1c) (Greig et al. 2013-b; Molyneaux et al. 2015; Lodato and Arlotta, personal communication).
Callosal PNs and CSMNs are at the moment among the best characterized subtypes at the molecular level. For these classes, distinct combinations of genes have been identified that can uniquely separate them. Examples of these molecular identifiers are Fezf2, Cntn6, Cad13, Bcl11b, Cry-mu, and Ldb2 for CSMNs (Arlotta et al. 2005, Lodato et al. 2014a) and conversely, Cux2, Inhba, Btg1, Lpl, Cited2 and PlexinD1 for CPNs (Molyneaux et al. 2009, 2015) (Figure 1c). Molecular differences also allow to distinguish between more closely related classes of corticofugal projection neurons, such as CSMNs and CthPNs, and between distinct classes of ScPNs, such as CSMNs and corticotectal projection neurons (CTPNs) (ref). For example, Diap3 labels CSMNs but not CTPNs, Tle4 marks CthPNs but not CSMNs, while CSMNs uniquely express Er81, for which CthPNs are negative. For a searchable database of transcripts differentially expressed in developing CPNs, CthPNs, and ScPNs, we refer the readers to DeCoN (at http://decon.fas.harvard.edu).
A few lessons can be learned from these molecular studies. First, the laminar “coordinates” of a neuron do not fully define its class-specific identity. For example, layer V contains many different subtypes of PNs, of both commissural and subcerebral identity. In addition, different classes of PNs populate layer V in separate areas; ScPNs in layer V of motor cortex subserve control of motor behavior, while ScPNs in layer V of visual cortex process vision-related movements.
Second, molecular profiling suggests the presence of a higher degree of heterogeneity within PN subtypes than is apparent from their long distance connectivity. For example, while CPNs across multiple layers share some common “callosal genes”, such as Satb2 and Hspb3, that distinguish them from the corticofugal classes, there are genes that are only expressed in subpopulations of CPNs, exposing an additional level of parcellation that so far had gone unrecognized (e.g. Cux2 labels CPNs of Layers II–IV, Ptn labels CPNs located in the deepest part of Layer II/III, and EphA3 and Nnmt are only expressed in CPNs of the most superficial part of Layer II/III)(Molyneaux et al. 2009, 2015) (Figure 1c).
Indeed, first-generation, unbiased molecular profiling of single cells isolated from the adult cerebral cortex supports the existence of molecularly distinct neuron populations that cannot be easily assigned to current categories (Zeisel et al. 2015). Third, class-specific profiles of gene expression are temporally dynamic, changing dramatically as neurons undergo lineage bifurcation and mature. For example, EphB1 is specifically expressed in CFuPNs during development but stops being expressed by P7 (Lodato et al. 2014a). Finally, molecular definition of PN classes requires consideration of multiple genes and their expression levels; classes cannot be defined by single markers. For instance, both CthPNs and CSMNs express Bcl11b; however, CSMNs express high levels of Bcl11b in combination with Ldb2, while CthPNs express low levels of Bcl11b and are Ldb2 negative.
Cortical interneurons: the “short-axon” neurons
Cortical interneurons represent approximately 20–30% of the total number of cortical neurons and make local connections within the cortex, which may span multiple layers (Lodato et al. 2014b). INs of the cortex are thought to be extremely diverse, and their classification has been work in progress for many years. Traditionally, interneurons have been subdivided into two broad classes: spiny pyramidal cells and aspiny (or sparsely spiny) non-pyramidal cells. All the spiny interneurons are excitatory glutamatergic neurons located in Layer IV that receive sensory input from the thalamus; the majority of the aspiny cortical interneurons are inhibitory GABAergic neurons located in all layers of the cortex. Aspiny INs are the main inhibitory component of the neocortical circuits, finely modulating projection neurons activity by regulating both synaptic function and the timing of action potential generation (Kepecs & Fishell 2014). Cortical GABAergic interneurons are very diverse; they contain subtypes that differ in morphology, molecular identity, firing properties and patterns of local connectivity, and although tremendous progress has been made in their classification, to date there is no accepted integrated taxonomy (Markram et al. 2004).
Three comprehensive and non-overlapping groups of interneurons can be distinguished in the neocortex based on the expression of distinct molecular markers: Parvalbumin (PV), Somatostatin (SST) or the ionotropic serotonin receptor 5HT3a (5HT3aR) (Kepecs & Fishell 2014, Marín et al. 2012). PV- and SST-positive interneurons are primarily found in the deep layers of the cortex, and 5HT3aR-positive interneurons preferentially populate the upper layers (Lee et al. 2010) (Figure 1c). However, compared to PNs, the laminar distribution of the molecularly distinct IN groups is much less precise.
Within these three classes many other subtypes can be identified based on soma morphology, the shape of their axonal and dendritic arbor, and their electrophysiological properties. PV-positive cells include Fast-Spiking (FS) INs belonging to two main morphological classes: large basket cells (which make synapses on the proximal dendrites and the somas of target pyramidal neurons) and chandelier cells (which target the initial axonal segment of pyramidal neurons). Nest basket cells are also PV-positive but exhibit a variety of firing properties (accommodating, non-accommodating and FS). SST-positive INs include the small basket cells, which are not fast-spiking but target the soma and the proximal dendrites of pyramidal cells, and the Martinotti cells that are burst spiking (BS), co-express calretinin (CR) and target the distal dendrites of pyramidal neurons in Layer I (Ascoli et al. 2008, Markram et al. 2004, Vitalis & Rossier 2011). The 5HT3aR-positive cells include VIP- and CR-positive interneurons with bipolar morphology, targeting the proximal dendrites and firing in burst or adapting mode, and the double bouquet cells that mostly synapse onto other INs. Also included in the 5HT3aR-positive category are NPY and reelin-positive INs (VIP-negative), located in the upper layers, which show multipolar or neurogliaform morphology and contact the dendritic shaft and the blood vessels, respectively (Vitalis & Rossier 2011). It is clear that INs are an extremely heterogenous group of neurons, and their classification has proved very complex and remains incomplete.
DEVELOPMENTAL GENERATION OF CORTICAL NEURONAL SUBTYPES
All neurons of the mammalian cerebral cortex are generated only during a limited period of embryonic development whose length is species-specific. In humans, cortical neurogenesis starts at gestational week (GW) 5 and ends around GW20 (Bystron et al. 2008). In rodents, neurogenic intervals are shorter, as shown by classic [3H] thymidine labeling studies; cortical neurogenesis spans from E11 to E19 in mice (Angevine & Sidman 1961, Caviness 1982, Takahashi et al. 1995), and from E13 to E21 in rats (Bayer & Altman 1991, Berry & Rogers 1965). These birthdate population studies, confirmed by more recent genetic studies (reviewed in Kohwi & Doe 2013), have shown that generation of cortical neurons proceeds in a precise temporal sequence, such that neurons of the deep layers are generated first, followed by those of the upper layers (Angevine & Sidman 1961). Although the initial experiments did not distinguish between PNs and INs, it is now firmly established that all classes of PNs and the majority of the early-born INs populate the cortex following an in an inside-out pattern of migration (Greig et al. 2013, Kohwi & Doe 2013). Interestingly though, a few classes of late born- INs do not obey this rule and despite being born late populate the upper layers (Miyoshi et al. 2010).
The molecular regulatory logic that builds this neuronal diversity during corticogenesis is a likely to involve a plethora of distinct regulatory events. Here, we will focus on the most recent findings on the mechanisms employed at both the progenitors and the post-mitotic neurons stages to generate this unparalleled neuronal complexity.
Birth of excitatory projection neuron diversity: decoding progenitor strategies
All pyramidal neurons of the neocortex are generated from neural progenitors located in the dorsal telencephalon, within the anterior part of the neural tube. Before the onset of neurogenesis (~E9–E10 in the mouse), neural progenitors are neuroepithelial (NE) cells, which divide symmetrically to expand the early progenitor pool. As neuronal production begins, NE cells give rise to more committed progenitors, termed radial glial cells (RGC). RGCs expand in the ventricular zone (VZ) lining the ventricle, through multiple rounds of cell division before undergoing a terminal asymmetric, neurogenic division responsible for the generation of cortical PNs (Figure 2) (Malatesta et al. 2000, Miyata et al. 2001, Noctor et al. 2001). This process generates directly only 10–20% of the total number of excitatory PNs (Kowalczyk et al. 2009). The majority of excitatory PNs are instead derived from an additional type of cortical progenitor, intermediate precursor cells (IPCs), which generate from RGCs via symmetrical, proliferative divisions (Haubensak et al. 2004, Miyata et al. 2001, Noctor et al. 2004). IPCs undergo mitosis away from the ventricle and over time generate a new germinal zone called the subventrcular zone (SVZ), where IPCs divide symmetrically to generate neurons (Figure 2) (Fietz & Huttner 2011, Hansen et al. 2010, Haubensak et al. 2004, Taverna et al. 2014). IPCs generate a substantial fraction of the cortical neuronal population, up to ~80% of all excitatory PNs across all cortical layers (Kowalczyk et al. 2009, Vasistha et al. 2014).
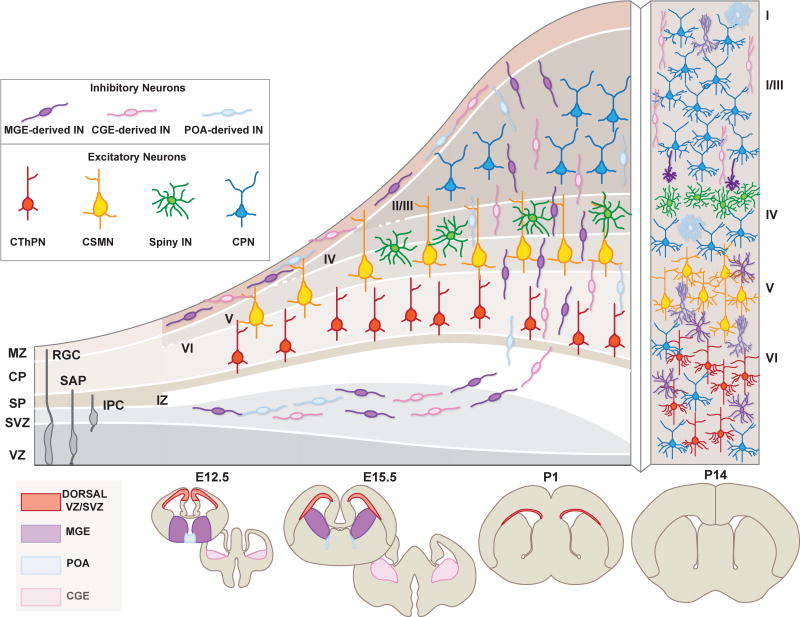
Figure 2.
Neocortical interneurons are characterized by their short-range projections, and can be broadly classified into excitatory and inhibitory interneurons. Here, we depict a schematic representation of the distinct excitatory and inhibitory interneuron classes within the six cortical. Excitatory spiny interneurons display mainly stellate and pyramidal morphology and are primarily located in the intragranular layer IV of the somatosenosry cortex (barrel cortex, shown in yellow boxes). In contrast, each cortical layer contains different types of inhibitory interneurons which display a wide array of morphologies and molecular identities. Both classes can be furher classified into subtypes which express distinctive combinations of molecular markers (listed on right).
In addition to NE cells, RGCs and IPCs, the cortex also contains other progenitor classes, such as the subapical progenitors (SAPs), which also contribute to the expansion of the progenitor pools during neurogenesis (Pilz et al. 2013; for an extensive review of cortical progenitors see Taverna et al. 2014) (Figure 2). The existence of such a variety of cortical progenitor types and their dynamic, yet distinct contributions to the generation of projection neurons suggest that progenitors fated to distinct pyramidal neuron lineages may already be present within the germinal zones, something that is still hotly debated.
At the core of the problem lies the longstanding question of whether the stereotyped production of neurons is due to (i) a “progressive competence restriction” mechanism, in which progenitors progressively and predictably restrict the potential neuronal outcomes that they can generate, and/or to (ii) a “predetermined fate-restriction” model, by which progenitors are pre-committed to generate distinct classes of PNs.
In the early ‘90’s, milestone experiments by Susan McConnell’s group began to address these questions by interrogating the fate potential of cortical progenitors in heterochronic transplantation paradigms (Desai & McConnell 2000, Frantz & McConnell 1996, McConnell & Kaznowski 1991). These studies demonstrated that early cortical progenitors are multipotent, while late cortical progenitors, even when exposed to a “younger” environment, are unable to produce the earlier PN fates (Frantz & McConnell 1996). The work provided clear evidence for a temporal, progressive restriction of progenitor fate potential. This was confirmed by elegant lineage fate-mapping analysis using sparse retroviral infection of VZ progenitors, which showed that when a single progenitor is labeled early in corticogenesis it can give rise to neurons of all layers (Luskin et al. 1988, Walsh & Cepko 1993).
Ex vivo studies by the group of Sally Temple further supported this model by showing that cultured multipotent progenitors sequentially give rise to early-born deep-layer neurons and later-born upper-layer neurons, although observing the birth of all lineages in vitro from the same single progenitor has been challenging (Shen et al. 2006). Thus, considerable evidence has accumulated in the past 15 years for a model in which cortical progenitors undergo changes in fate potential over time, probably mediated by a change in the length of the cell cycle and number of divisions each progenitor undergoes before terminal differentiation (Calegari & Huttner 2003, Calegari et al. 2005, Pilaz et al. 2009).
More recent genetic fate-mapping studies have further probed the fate potential of cortical progenitors to determine whether progenitors fate-committed to produce distinct classes of PNs do exist. Results have been mixed. One study shows that progenitors expressing the transcription factor Cux2 (known to be a marker of CPNs and selected INs of Layer II/III) largely produce CPNs of Layer 2/3. In this study the authors used a Cux2-CreERT2 knock-in line to fate-map cortical progenitors of the early VZ, and found that a large proportion of these progenitors give rise to upper-layer PNs. Cux2-Cre-positive progenitors were present in the VZ as early as embryonic (E) day E10.5, and they divide, rather than differentiate when deep layer CFuPNs are normally produced. Notably, when forced to differentiate during the window of production of deep-layer neurons, such progenitors still generated upper-layer neurons, suggesting that their fate commitment is intrinsically determined and independent of temporal restrictions (Franco et al. 2012).
These results provided evidence that the existence of progenitors subpopulations pre-fated to generate specific subtypes of PNs may be a component of the logic that builds PN diversity during corticogenesis. Although this may be an exciting additional strategy to diversity progenitor behavior and regulate complex lineage determination decisions, two subsequent studies failed to identify fate-restricted cortical progenitors. Guo and colleagues lineage fate-mapped progenitors using the same Cux2-CreERT2 reporter line used by Franco and colleagues (Franco et al. 2012) and, in parallel work, a transgenic bacterial artificial chromosome (BAC) line driving CreERT2 from the Fezf2 promoter (Fezf2-CreERT2), to conclude the existence of multipotent progenitors able to generate not only different classes of PNs but also glia, as detected in P1 cortex (Guo et al. 2013). It is possible that part of these results are confounded by the fact that BAC transgenic lines often do not precisely reproduce the temporally and spatially regulated expression of the endogenous locus, and may not be precise enough for this type of lineage-fate mapping. In addition, it is important to further characterize the identity of the neurons produced by the progenitors marked in older brains, rather than perinatally, when PN neuron migration is incomplete and distinct PN classes do not yet clearly express the molecular signatures that delineate their class-specific identity at later ages.
Support for the progressively restricted generation of cortical PN subtypes from multipotent progenitors also emerged from an elegant Mosaic Analysis with Double Markers (MADM) study of neocortical neurogenesis (Gao et al. 2014). By using the Emx1-CreERT2 and Nestin-CreERT2 lines, independently, in the MADM system, the authors provided quantitative clonal analysis of RGC fate potential with single-cell resolution. The data indicate that clonally-related neurons derived from the same RGC during early neurogenesis (from E10 to E13) span all cortical layers. Clones that generate neurons of a single layer were only found when RGCs were labeled at late stages of neurogenis. Although these data strongly point towards a model of progressive restriction of progenitor fate it is of course possible that more rare progenitors pre-fated to form only selected types of PNs exist, but were not detected. It is worth noting that the two models are not necessarily mutually exclusive as the cortical VZ may be a mosaic of progenitors with different fate potentials.
Integrated approaches of mosaic clonal analysis (using modified retroviruses or the MADM system) and molecular fate-mapping strategies employing multiple knock-in Cre lines (e.g. Cux2-CreERT2, Fezf2-CreERT2) should in the near future help clarify the complexity of regulatory strategies used by progenitors to generate PN diversity.
Birth of cortical interneurons: shared strategies and main differences
Contrary to progenitors of PNs, which are adjacent to the developing cortex, progenitors of cortical INs are spatially segregated in distinct regions of the ventral telencephalon distally to the cortex. It is currently unclear whether distinct types of progenitor cells of the GEs possess distinct lineage potential, and therefore strategies to generate IN diversity are a matter of intense investigation.
In contrast to PNs, cortical INs are generated outside of the developing cortex, from ventrally located progenitors within the medial and caudal ganglionic eminences (MGE and CGE) (Anderson et al. 1997, Tamamaki et al. 1997); and the preoptic area (POA) (Gelman et al. 2009) (Figure 2). From these ventral structures, INs migrate extensively to reach the developing cortex. Here, they first migrate tangentially through the SVZ and the dorsally-located marginal zone (MZ), before invading the cortical plate (Figure 2) (review in Guo & Anton 2014).
Much like dorsally-located progenitors for PNs, IN progenitors can be classified in several subtypes: RGC-like cells, IPCs and SAPs (reviewed in Taverna et al. 2014). Contrary to progenitors of PNs, which are adjacent to the developing cortex, progenitors of cortical INs are spatially segregated in distinct regions of the ventral telencephalon distally to the cortex. In addition, unlike dorsal progenitors, which produce only neurons of the neocortex, progenitors of the ventral germinal zones also produce classes of neurons destined to other brain regions (e.g. the striatum, the hippocampus) and subsets of oligodendrocytes that do not reach the cortex (Qi et al. 2002). These data provide a first level of evidence that these progenitors are likely extremely heterogeneous.
It is unclear whether distinct types of progenitor cells exist in the ventral telencephalon that possess distinct lineage potential, and the strategies used to generate IN diversity are a matter of investigation. Genetic fate-mapping and transplantation studies have so far supported a model in which the anatomical location of progenitors and the timing of neurogenesis have predictive value for the type of interneuron produced (Xu et al. 2004). Specifically, PV-positive fast-spiking chandelier cells and basket cells, SST-positive non-spiking INs, and SST/CR-positive Martinotti cells (Marín et al. 2012; Vitalis & Rossier 2011) are born from progenitors of the MGE during early neurogenesis (between E10–E13). The CGE, instead, produces CR- and VIP- expressing bipolar and double-bouquet INs, and rapidly adapting Reelin-positive INs with multipolar morphology (Rudy et al. 2011). Finally, the POA, which accounts for only 10% of all cortical INs, gives rise to a small but highly diverse group of INs, including multipolar NPY-, basket PV-, and SST-expressing INs (Gelman et al. 2009).
It is known that the MGE, CGE and POA germinal zones express specific codes of transcription factors that collectively define them molecularly (Flames et al. 2007). These transcription factors are not bare markers but, rather, they play key roles in controlling the balanced production of specific IN pools. For example, conditional inactivation of Nkx2.1 (which mostly labels progenitors in the MGE), between E9.5 and E12.5, compromises the generation of fast-spiking PV and SST-expressing INs, and increases the generation of adapting and late-spiking CR- and VIP-expressing INs (Butt et al. 2008). Molecular markers are also known that define subregions within the three main subdivisions. Within the MGE, Nkx6.2 specifically labels the ventral MGE and lineage fate-mapping studies show that this region preferentially produces Martinotti SST/CR-expressing INs (Fogarty et al. 2007, Sousa et al. 2009). The data roots in molecular terms the idea that progenitors are diverse.
Distinct germinal zones give rise to INs of distinct identities, however fate-mapping experiments have also shown that the migratory behavior within the cortex of INs produced in different germinal zones is distinct. MGE-derived IN subtypes migrate to Layers II-VI, following the same inside-out pattern of laminar distribution as PNs (Marín et al. 2012, Xu et al. 2004). Specifically, in agreement with their early development, PV- and SST- INs preferentially occupy the deep cortical layers (Xu et al. 2008). In contrast, CGE-derived INs preferentially populate the most superficial layers, independently from their time of birth (Miyoshi et al. 2010). These data suggest that the intrinsic mechanisms controlling spatial distribution of INs in the cortex and their integration within the columnar microcircuits do not universally apply to every subtype, and these decisions might be intimately dependent on IN class-specific identity, rather than simply their day of birth.
Interestingly, even INs generated within the same germinal zone behave differently. The POA contains at least two small progenitor domains, one expressing Nkx5.1 and the second Dbx1 (Gelman et al. 2009, 2011). Genetic tracing of cells derived from Nkx5.1+ progenitors indicate that they are distributed mainly in the superficial layers, predominantly express the neuropeptide NPY and show adapting firing properties (Gelman et al. 2009). In contrast, the Dbx1 domain generates a wider spectrum of INs that populate the deep layers and are distinct based on a large variety of molecular and electrophysiological traits (Gelman et al. 2011). It is unlikely, but conceivable, that this small pool of progenitors is heterogenous and contains multiple progenitors with distinct pre-fated identities; alternatively, it is possible that the same progenitors could give rise to multiple classes of cortical INs, possibly following a temporal sequence.
Several questions remain unanswered. Do individual progenitors gives rise to multiple IN subtypes? Do INs that are lineage related behave similarly? Are there pre-specified subtypes of progenitors committed to specific populations?
Recent studies have investigated the clonal relationship between INs generated from the same progenitor (Brown et al. 2011, Ciceri et al. 2013). Although using slightly different experimental approaches, both studies found that clonally-related MGE-derived INs tend to cluster in the cortex and their horizontal distribution is non-random (Brown et al. 2011, Ciceri et al. 2013). However, in one study, clones of neurons derived from Nkx2.1+ progenitors labeled at E12.5 were found both in the deep and superficial cortical layers, with most clusters containing INs of different identities. These data are consistent with a model of “progressive fate-restriction” of progenitors such that a single progenitor progressively gives rise, as development proceeds, to different types of INs that adopt distinct laminar addresses. In contrast, the second study, found that marking of Nkx2.1+ progenitors at E11.5 and E14.5 labels clusters of INs largely segregated within either the deep or the superficial cortical layers, respectively (Brown et al. 2011, Ciceri et al. 2013). These data argue for the existence of pre-fated progenitors that produce specific IN lineages, which in turn acquire defined laminar distributions. Like progenitors of PNs, it is possible that these two models (fate-committed and temporally-restricted progenitors) co-exist but apply to different progenitor pools.
To complement and clarify these initial clonal studies, it will be necessary to perform systematic clonal analysis of single interneuron progenitors with exquisite spatial and temporal resolution. The recently developed MADM technique is likely to provide a powerful genetic approach to mark clonally related progeny of single progenitors. This could be combined with the use of viruses that “tag” each progenitor (and their progeny) with a unique identifying sequence, virtually eliminating the risk of analyzing mixed clones.
Although much work remains to be done, the current data point at a need for great diversification of progenitor fate during IN development. In this regard, it is interesting that IN germinal zones are located away from the cerebral cortex, and that molecularly distinct domains that preferentially generate defined IN subclasses are present. This could be a strategy to enable finer, differential control of distinct progenitor pools, given that they are spatially separated. This strategy may have been necessary also because INs destined to other brain regions are also produced within the same domains that generates INs of the neocortex. This is a very distinct strategy from that of the dorsal progenitors, which maintain a spatially confined relationship to the neurons they generate. For both groups of neurons however, the regulatory strategies used by progenitors to produce neuronal diversity require additional investigation.
Postmitotic control of neuronal diversity
It is unquestionable that mechanistically key lineage determination decisions for the establishment of PN and IN diversity occur at the progenitor stage. Compelling emerging evidence though indicates that regulatory events restricted to post-mitotic early stages of development also contribute to establish class-specific identities for both PNs and INs.
The discovery of several neuron subtype-specific transcription factors led to the observation that many of these genes are specifically induced early post-mitotically in neurons as they migrate away from the germinal zone. Mechanistically, reciprocal regulation between arrays of post-mitotically expressed TFs is known to progressively refine neuronal subtype identity during generation of some PN classes (Greig et al. 2013, Srinivasan et al. 2012). The TF Bcl11b, first discovered as a marker of ScPNs controlling axon fasciculation and extension in these neurons (Arlotta et al. 2005), is specifically repressed by Satb2, a chromatin remodeling protein restricted to post-mitotic CPNs (Alcamo et al. 2008, Britanova et al. 2008), and by its partner Ski (Baranek et al. 2012)Baranek:2012bj}. In the absence of either Satb2 or Ski, Bclb11 is ectopically expressed in CPNs, which in turn fail to develop connections through the corpus callosum (a key trait of CPNs) and, instead, extend axons ipsilaterally to subcortical target(Alcamo et al. 2008, Baranek et al. 2012). In addition, several subtype-specific molecular markers of CPNs are not expressed in the absence of Satb2 (Alcamo et al. 2008, Britanova et al. 2008).
Another example is provided by the TF Tbr1, which is expressed post-mitotically in subplate neurons, CthPNs and CPNs; in the absence of Tbr1, the subplate is no longer morphologically discernible and fails to express its specific markers, while CthPNs neurons aberrantly express high levels of Fezf2 and Bcl11b, causing development of ectopic connectivity to subcerebral targets, rather than the thalamus (Han et al. 2011, McKenna et al. 2011).
Notably, post-mitotic decisions can also contribute to the establishment of appropriate cortical architecture and connectivity, by influencing both the temporal dynamics of PN generation and the regional (areal) distribution of specific classes of PNs. The TF Sox5 is required for the correct temporal sequence of generation of both subplate and CFuPNs. In the absence of Sox5, subplate neurons acquire molecular features of ScPNs (a fate normally generated more than 2 days later), and CthPN identity is compromised (Lai et al. 2008).
Other noteworthy TFs are Bhlhb5 and Lmo4 (both expressed only post-mitotically), which regulate area-specific differentiation of CSMNs. In the absence of Bhlhb5, CSMNs from caudal motor cortex are not properly specified and fail to connect to the spinal cord, while in the absence of Lmo4, CSMNs in the rostral motor cortex lack backward-projecting collaterals (Cederquist et al. 2013, Joshi et al. 2008).
Similar post-mitotic specification strategies seem to also be in place during cortical IN differentiation. For example, the LIM-homeodomain protein Lhx6, which is specifically expressed early postmitotically in migratory INs derived from the MGE (Nkx2.1 domain), has been shown to be critical for multiple aspects of the development of all MGE-derived cortical interneurons, including the migration, differentiation and maturation of PV- and SST- expressing INs (Liodis et al. 2007, Neves et al. 2013). Interestingly, Lhx6 is upstream of two other TFs, Sox6 and Satb1 that also act post-mitotically to control the differentiation of MGE-derived INs (Azim et al. 2009, Batista-Brito et al. 2009, Close et al. 2012, Denaxa et al. 2012). In the absence of Sox6, there is a dramatic loss of PV and SST expression and a concomitant increase in NPY expression, accompanied by major physiological and behavioral abnormalities, such as severe epileptic encephalopathy (Azim et al. 2009, Batista-Brito et al. 2009). In the case of Satb1, postnatal conditional inactivation (at P1) affects the maturation of SST-expressing INs, compromising their connectivity and integration into cortical circuits (Close et al. 2012, Denaxa et al. 2012).
The growing evidence that aspects of class specific neuronal identity is controlled postmitotically and that expression of some key transcription factors is maintained suggests the possibilities that TFs actively control at least in part the maintenance of neuronal class-specific identity. This hypothesis remains untested; however, it is interesting that the class-specific identity of young PNs, despite neurons being post-mitotic can be changed, via direct lineage reprogramming. Forced, ecoptic expression of a single transcription factor, the selector gene Fezf2 (Lodato et al. 2014a), can convert CPNs of Layer II/III into neurons with molecular, electrophysiological and connectivity features of CFuPNs (Rouaux & Arlotta 2013). Similarly, stellate excitatory INs of Layer IV change their electrophysiological features and connectivity in response to Fezf2 (la Rossa et al. 2013). Interestingly, the capacity of Fezf2 to instruct CFuPN identity declines dramatically with the age of the neurons, as shown by the fact that targeted CPNs loose their ability to respond to Fezf2 and to reprogram by P21(Rouaux & Arlotta 2013). The data support the hypothesis that at least within a critical early window of “nuclear” plasticity neurons of the cortex maintain their identity through mechanisms that are not irreversible and are independent from the post-mitotic nature of the cell.
Orchestrated assembly of the cortical local circuit
Despite many years of research and much progress in understanding aspects of the functional organization of the neocortex, little is currently known regarding the principles and mechanisms that wire its outstanding diversity of PN and IN classes into a stereotyped local microcircuit.
Although intuitively one might expect that the number of elements in a given system, in this case the number of neurons present in the neocortex, reflects the complexity of the computational functions performed, it is unclear whether beyond numbers, different classes of neurons play critical roles. From a computational standpoint, the neocortex is capable of innumerable nuances of behavior created by only two “opposing forces”, excitation and inhibition. It is possible that one strategy to achieve such complexity from this simplicity involves the generation of a circuit were synaptic contacts are not generated randomly but rather PN and IN sub-classes connect with each other following specific rules.
Understanding the principle driving connectivity of PN and IN diversity in the neocortex requires technologies that can simultaneously provide sufficiently fine resolution of synaptic connectivity, while also allowing for class-specific identification of the neurons present in the circuit. The advent of improved methods to map synaptic connections with second-generation viral tracers combined with genetically modified mouse lines that restrict labeling to defined classes of INs and PNs (Huang & Zeng 2013) holds promise for the generation of a precise connectivity map of the local microcircuit.
Although this fine map is not yet available, the neuron-type specificity of excitatory and inhibitory connections has been interrogated in several electrophysiological studies, some of which also took advantage of optogenetic tools to directly monitor in vivo neuronal activity and the real-time effect of circuit manipulation on specific behaviors (Cardin et al. 2010).
Pioneer electrophysiological studies investigating the computational role of PNs within excitatory circuits (i.e., PN-PN) have shown that different PN classes have highly selective synaptic interconnectivity even within the same local circuits (Brown & Hestrin 2009). The pattern of connectivity shown by different classes of neighboring PNs reflects the identity of both the pre- and postsynaptic cell types, as demonstrated by simultaneous whole-cell recording of multiple PN types within Layer V(Morishima et al. 2011). In the visual cortex, for example, cortico-cortical neurons show a significantly higher preference for connections with their neighboring corticotectal projection neurons than with each other (Brown & Hestrin 2009). Paired recording of retrogradely labeled ScPNs (i.e., corticopontine neurons) also showed preferential connectivity in the frontal cortex, where these neurons make more numerous excitatory inputs onto cells that share the same long-range axonal target than onto those that project ipsilaterally (Morishima et al. 2011). Together these results support a model by which the specific identity of PNs influences the nature of the local excitatory subnetworks.
The circuit organization of inhibitory networks in the neocortex (i.e., PN-IN) is not well mapped, and, despite the great degree of specificity shown by INs in targeting subcellular components of their pyramidal neuron partners (soma versus dendrites or axon initial segment), the general principles underlying IN synaptic connectivity are still elusive. A general model of promiscuous inhibitory connectivity (a “blanket of inhibition”) has been proposed (Fino & Yuste 2011), but many studies also support a rather fine-scale specificity of synaptic connectivity for cortical INs. For example, by paired intracellular recording and photostimulation-evoked synaptic currents analyses, fast-spiking INs of Layer II/III were found to preferentially connect with excitatory PNs of the same layers that establish reciprocal synapses, rather than with PNs that are not reciprocally connected (Yoshimura & Callaway 2005).
Similarly, whole-cell recording from Layer V inhibitory INs showed that they form synapses with neighboring PNs, and participate in intralaminar and interlaminar subnetworks, in a PN-subtype-dependent manner (Otsuka & Kawaguchi 2009). Studies in the prefrontal and enthorinal cortex have also supported the theory that the choice of postsynaptic PN target by inhibitory INs and the properties of their synaptic connections depend on the identity of the PN partners (Lee et al. 2014, Varga et al. 2010).
Interestingly, recent studies have suggested that synaptic interconnectivity between different classes of INs (e.g. IN-IN) also exhibits a high degree of specificity. For example, employing optogenetic manipulation in combination with single cell recording, two independent groups have shown that VIP-expressing INs primarily suppress SST-INs and a small fraction of the PV-expressing INs, which in turn directly inhibit the inputs and outputs of PNs. Therefore, VIP-INs provide disinhibitory regulated control of PN activity (Lee et al. 2013, Pi et al. 2013).
Although the selectivity of connections within neuronal circuits seems to be intimately linked to the identity of the pre- and post-synaptic partners, the cellular and molecular mechanisms underlying pairing selectivity are largely unknown. Some lines of evidence seem to suggest that the final organization of the cortical microcircuits is controlled by events occurring well before completion of neuronal differentiation, during development. For example, PN identity has been shown to affect the radial distribution of cortical INs during development; misplaced PNs lead to aberrant radial migration of INs and thus dysfunctional local circuits. Importantly, this effect is dependent on the class-specific identity of the PNs involved (Lodato et al. 2011).
In line with this finding, recent data on the lamination of CGE-derived INs is compatible with the idea that the class-specific identity of the future PN synaptic partner is crucial in determining IN final laminar location and positioning into circuit. MGE- and CGE-derived IN subtypes display distinct migratory behaviors once they have reached the cortex. MGE-derived IN subtypes are found in layer II–VI, following the same inside-out pattern of laminar distribution as PNs. Specifically, PV- and SST-INs preferentially occupy the deep layers in agreement with their early time of generation (Xu et al. 2008), while CGE-derived INs are more abundant in layer II–IV, and, instead, preferentially populate the most superficial layers, independently from their time of generation (Miyoshi et al. 2010). These results cannot fit a model in which time of birth determines position of INs into layers, and highlights the existence of more finely modulated mechanisms, possibly related to the class-specific identity of the PNs present in each layer.
Along these lines, there is emerging data suggesting that the identity of PNs may also have an impact on glia. PNs in different layers display distinct profiles of myelin distribution along their axons, suggesting an effect of PN identity on the behavior of oligodendrocytes (Tomassy et al. 2014). A novel pattern of myelination, termed ‘intermittent myelin’ is for example found along the axons of PNs in Layer II/III, but not on PNs of layer V and VI, which are both more heavily myelinated and display canonical profiles of myelin distribution. This may reflect idiosyncratic interactions between different types of PNs and oligodendrocytes. Recent years have seen a surge of examples supporting an emerging model in which lineage decisions of developing PNs act upon the behavior of other cells in the cortex, both neurons and glia, to ultimately shape working circuits, allow cortical diversification, and sustain complex behavior.
Closing Remarks
The study of cortical development and function has fascinated neuroscientists for centuries. In particular, as appreciation grew over the years for the unparalleled diversity of neuronal subtypes that populate the cortex, questions have surfaced on the principles that shape this cellular diversity during development, the mechanisms that maintain this landscape of neurons unchanged for the life of the organism, and the rules that wire distinct neurons within complex circuits that subserve higher—order functions. So far, great progress has been made investigating individual aspects of the development, function and plasticity of the neocortex. This has led to great insights into the tissue organization, cytoarchitecture, cellular composition, circuit assembly and function of neocortex. However, the emergence of new technologies today enables implementation of a somewhat new conceptual, experimental approach to understand cortical composition and function. Among others, methods to label, purify and molecularly profile distinct neuronal populations have expanded our understanding of the molecular events that accompany the establishment of neuronal diversity, and will continue to generate molecular insights in higher throughput mode. Single-cell transcriptomics is generating interesting data on the diversity of cortical neurons, raising the possible existence of new neuronal classes (Zeisel et al. 2015). Next-generation viral tracing and optogenetic manipulation of precisely-defined neurons and circuits now provide a new level of resolution in studying the structure-function relationship that drive cortical functions (Cardin et al. 2010, Osakada & Callaway 2013). Finally, new 3D imaging techniques allow for a global view of neuronal diversity and connectivity that was not previously feasible (Chen et al. 2015, Renier et al. 2014). We think that these advancements now enable an integrated, system-level approach that considers multiple aspects of the biology of neurons and circuit (from molecules, to high-throughput maps of connectivity, neuron class-specific manipulation, large scale functional recording and imaging) to decode neocortical neuron diversity and function in development and disease.
Figure 3.
Developmental origin and distribution in the neocortex of projection neuron (PN) and interneuron (IN) subtypes. Excitatory neurons originate from progenitors in the dorsal telencephalon, and cortical inhibitory INs derive from progenitors in the ventral telencephalon [mainly the medial and ganglionic eminences (MGE), the caudal ganglionic eminence (CGE), and the preoptic area (POA)]. Over time, these germinal zones give rise to a diversity of neuronal subtypes, both PNs and INs, that acquire distinct laminar addresses in the neocortex. After reaching the cortex, INs migrate tangentially in streams located above [marginal zone (MZ)] and below [subventricular zone (SVZ)] the cortical plate (CP), before switching to a mode of radial migration to invade the CP. By the end of neurogenesis, PN and IN classes coexist at specific locations in the cortical layers and begin to wire into the local cortical microcircuit.
Abbreviations: CPN, callosal projection neuron; CThPN, corticothalamic; CSMN, corticospinal motor neuron; RGC, radial glial cell; SAP, subapical progenitor; IPC, intermediate precursor cell; VZ, ventricular zone; SVZ, subventricular zone; SP, subplate; IZ, intermediate zone; CP, cortical plate; MZ, marginal zone. E, embryonic; P, postnatal. Roman numerals refer to the six cortical layers.
Acknowledgments
We would like to thank Juliana Brown for her insightful comments and critical reading of the manuscript. We are also very grateful to Dennis Sun for realizing all the illustrations. Work in P.A. lab is supported by grants from the US National Institute of Health, the New York Stem Cell Foundation, Target ALS and the Harvard Stem Cell Institute to P.A.; P.A. is a New York Stem Cell Foundation-Robertson Investigator.
References
- Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav Brain Sci. 2003;26(5):535–52. doi: 10.1017/s0140525x03000128. discussion 552–85. [DOI] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57(3):364–77. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278(5337):474–76. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Angevine JBJ, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in mouse. Nature. 1961;192:766–68. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–21. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12(10):1238–47. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek C, Dittrich M, Parthasarathy S, Bonnon CG, Britanova O, et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc Natl Acad Sci USA. 2012;109(9):3546–51. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, et al. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63(4):466–81. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development. New York: Raven Press; 1991. [Google Scholar]
- Berry M, Rogers AW. The migration of neuroblasts in the developing cerebral cortex. J Anat. 1965;99(4):691–709. [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57(3):378–92. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Broca P. Sur le siege de la faculte du langage articule. Bulletin de la Societe d’anthropologie. 1865;6:337–93. [Google Scholar]
- Brown KN, Chen S, Han Z, Lu C-H, Tan X, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334(6055):480–86. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457(7233):1133–36. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59(5):722–32. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9(2):110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Ramón S. Cajal Histologie du Système Nerveux de l’Homme et des Vértébres A. Maloine. 1909 [Google Scholar]
- Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25(28):6533–38. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116(Pt 24):4947–55. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5(2):247–54. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapano LA, Magavi SS, Macklis JD. Neuroanatomical tracing of neuronal projections with Fluoro-Gold. Methods Mol Biol. 2008;438:353–59. doi: 10.1007/978-1-59745-133-8_27. [DOI] [PubMed] [Google Scholar]
- Caviness VS. Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256(3):293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Cederquist GY, Azim E, Shnider SJ, Padmanabhan H, Macklis JD. Lmo4 establishes rostral motor cortex projection neuron subtype diversity. J Neurosci. 2013;33(15):6321–32. doi: 10.1523/JNEUROSCI.5140-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tillberg PW, Boyden ES. Optical imaging. Expansion microscopy. Science. 2015;347(6221):543–48. doi: 10.1126/science.1260088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marín O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16(9):1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, et al. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32(49):17690–705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa NM, Martin KAC. Whose Cortical Column Would that Be? Front Neuroanat. 2010;4:16. doi: 10.3389/fnana.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, et al. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2012;2(5):1351–62. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127(13):2863–72. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28(33):8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol. 2011;21(1):23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69(6):1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JLR, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27(36):9682–95. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27(41):10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, et al. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337(6095):746–49. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17(1):55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- Frostig RD. Functional organization and plasticity in the adult rat barrel cortex: moving out-of-the-box. Curr Opin Neurobiol. 2006;16(4):445–50. doi: 10.1016/j.conb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell. 2014;159(4):775–88. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, et al. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31(46):16570–80. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29(29):9380–89. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14(11):755–69. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JLR, Chen B. Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron. 2013;80(5):1167–74. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Anton ES. Decision making during interneuron migration in the developing cerebral cortex. Trends Cell Biol. 2014;24(6):342–51. doi: 10.1016/j.tcb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Kwan KY, Shim S, Lam MMS, Shin Y, et al. TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. Proc Natl Acad Sci USA. 2011;108(7):3041–46. doi: 10.1073/pnas.1016723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PRL, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464(7288):554–61. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101(9):3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Wang PP, Atabay KD, Murphy EA, Doan RN, et al. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci. 2015 doi: 10.1038/nn.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molyneaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II–V of the developing neocortex. Neuron. 2008;60(2):258–72. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505(7483):318–26. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci. 2013;14(12):823–38. doi: 10.1038/nrn3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RAM, Bedogni F, et al. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19(10):2439–50. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Rossa De A, Bellone C, Golding B, Vitali I, Moss J, et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat Neurosci. 2013;16(2):193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, et al. SOX5 Controls the Sequential Generation of Distinct Corticofugal Neuron Subtypes. Neuron. 2008;57(2):232–47. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Winston KR, Tish A. A method based on retrograde intraaxonal transport of protein for identification of cell bodies of origin of axons terminating within the CNS. Brain Res. 1973;58(2):470–77. doi: 10.1016/0006-8993(73)90016-4. [DOI] [PubMed] [Google Scholar]
- Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL, Sohal VS. Pyramidal neurons in prefrontal cortex receive subtype-specific forms of excitation and inhibition. Neuron. 2014;81(1):61–68. doi: 10.1016/j.neuron.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30(50):16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16(11):1662–70. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27(12):3078–89. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Molyneaux BJ, Zuccaro E, Goff LA, Chen H-H, et al. Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nat Neurosci. 2014a;17(8):1046–54. doi: 10.1038/nn.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Rouaux C, Quast KB, Jantrachotechatchawan C, Studer M, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69(4):763–79. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Shetty AS, Arlotta P. Cerebral cortex assembly: generating and reprogramming projection neuron diversity. Trends Neurosci. 2014b doi: 10.1016/j.tins.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1(8):635–47. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127(24):5253–63. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marín O, Noebels JL, Avoli M, Rogawski MA, Olsen RW, et al. The Generation of Cortical Interneurons. Nat Rev Neurosci. 2012;13(2):107–20. [Google Scholar]
- Markram H. The blue brain project. Nat Rev Neurosci. 2006;7(2):153–60. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254(5029):282–85. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, et al. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 2011;31(2):549–64. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31(5):727–41. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJB, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30(5):1582–94. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29(39):12343–54. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8(6):427–37. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Goff LA, Brettler AC, Chen H-H, Brown JR, et al. DeCoN: genome-wide analysis of in vivo transcriptional dynamics during pyramidal neuron fate selection in neocortex. Neuron. 2015;85(2):275–88. doi: 10.1016/j.neuron.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima M, Morita K, Kubota Y, Kawaguchi Y. Highly differentiated projection-specific cortical subnetworks. J Neurosci. 2011;31(28):10380–91. doi: 10.1523/JNEUROSCI.0772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUNTCASTLE VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20(4):408–34. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Neves G, Shah MM, Liodis P, Achimastou A, Denaxa M, et al. The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex. Cereb Cortex. 2013;23(8):1811–23. doi: 10.1093/cercor/bhs159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7(2):136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Osakada F, Callaway EM. Design and generation of recombinant rabies virus vectors. Nat Protoc. 2013;8(8):1583–1601. doi: 10.1038/nprot.2013.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29(34):10533–40. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging. J Neurosci. 2001;21(21):8435–46. doi: 10.1523/JNEUROSCI.21-21-08435.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503(7477):521–24. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz L-J, Patti D, Marcy G, Ollier E, Pfister S, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci USA. 2009;106(51):21924–29. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz G-A, Shitamukai A, Reillo I, Pacary E, Schwausch J, et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat Commun. 2013;4:2125. doi: 10.1038/ncomms3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Stapp D, Qiu M. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci. 2002;25(5):223–25. doi: 10.1016/s0166-2236(02)02145-8. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16(1):25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159(4):896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15(2):214–21. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9(6):743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14(4):278–91. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–i10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Leone DP, Bateson RK, Dobreva G, Kohwi Y, et al. A network of genetic repression and derepression specifies projection fates in the developing neocortex. Proc Natl Acad Sci USA. 2012;109(47):19071–78. doi: 10.1073/pnas.1216793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VSJ. Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15(9):6058–68. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17(21):8313–23. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E, Götz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344(6181):319–24. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci. 2010;13(7):822–24. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasistha NA, García-Moreno F, Arora S, Cheung AFP, Arnold SJ, et al. Cortical and Clonal Contribution of Tbr2 Expressing Progenitors in the Developing Mouse Brain. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Rossier J. New insights into cortical interneurons development and classification: contribution of developmental studies. Dev Neurobiol. 2011;71(1):34–44. doi: 10.1002/dneu.20810. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature. 1993;362(6421):632–35. doi: 10.1038/362632a0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, La Cruz De E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24(11):2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506(1):16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8(11):1552–59. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347(6226):1138–42. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]