Abstract
Background
Treatment of pulmonary exacerbations (PEx) in cystic fibrosis (CF) varies widely with no consensus on management practices or best indicators of therapeutic success. To design trials evaluating PEx treatment factors, we characterize the heterogeneity of PEx care in adults and pediatrics, and correlate it with measures of clinical response including short and long term lung function changes, change in symptom severity score, and time to next intravenous (IV) antibiotic therapy.
Methods
Data were used from a prospective observational study of CF patients ≥10 years of age enrolled at six sites between 2007 and 2010. All were started on IV antibiotics for a clinically diagnosed PEx. ANOVA, logistic and Cox regression were used to examine the association of treatment factors with short and long term clinical response.
Results
Of 123 CF patients (60% female, aged 23.1±10.2 years), 33% experienced <10% relative improvement in FEV1 during treatment which was associated with failing to recover baseline lung function three months after treatment (OR=7.8, 95% CI=(1.9, 31.6), p=0.004) and a longer time to next IV antibiotic (HR=0.48, 95% CI=(0.27, 0.85), p=0.011). Symptom improvement was observed but was not associated with subsequent lung function or time to next antibiotic therapy which had a median recurrence time of 143 days.
Conclusions
Immediate symptomatic or respiratory response to PEx treatment did not have a clear relationship with subsequent outcomes such as lung function or IV antibiotic-free interval. These results can inform future research of treatment regimens for PEx in terms of interventions and outcome measures.
Keywords: cystic fibrosis, pulmonary exacerbation, outcomes
INTRODUCTION
Infection, inflammation, and structural damage of the lung are characteristic of cystic fibrosis (CF). Daily symptoms of chronic infection are punctuated with acute episodes of increased cough, fatigue, physical findings, and other signs including rapid drop in lung function called a pulmonary exacerbation (PEx). Such events are associated with mortality,1,2 reduced quality of life,3 unrecoverable loss of lung function,2,4 and increased health care costs.5 There were over 15,000 PEx recorded in the CF Foundation (CFF) National Patient Registry (NPR) in 2012, affecting nearly 40% of CF patients at least once.6 Treatment for PEx varies across patients and centers,7 but often includes a combination of intravenous (IV), inhaled, or oral antibiotics8 in conjunction with increased airway clearance therapy, inhaled mucolytics, or corticosteroids. Location (inpatient or outpatient) and duration of therapy may differ by factors such as patient gender, 9,10 age,11 PEx history, disease severity, microbiology, physician goals and patient preferences.12
Recent guidelines cite scarce evidence to support common treatment regimens for PEx management,13 highlighting the need for research in this area. While studies have examined different short14-16 and long term4,17,18 outcomes of PEx, and factors associated with them, there is no consensus on the most relevant or useful measure of PEx management success -or failure.
Physicians generally treat to alleviate signs and symptoms or recover lost lung function19 but little is understood about the implications of failing to achieve those immediate goals in CF patients. To inform clinical care and research aimed at improving PEx treatment regimens, we analyzed data from a multi-center, prospective study of PEx to characterize the heterogeneity of treatment factors and correlate them with measures of clinical response, including symptom severity, short and long term lung function, and time to next PEx. Specifically, we aimed to determine the association between immediate symptom and lung function response with subsequent need for IV antibiotics and ultimate pulmonary function recovery.
MATERIALS AND METHODS
Patients and Study Design
CF patients 10 years and older being started on two or more IV antibiotics for a clinically diagnosed PEx using criteria outlined by a CFF Consensus Conference20 were eligible to enroll in the observational study conducted at 6 adult and pediatric CF centers between 2007 and 2010.21 Study protocol encouraged at least ten days of antibiotic administration and mucus clearance in accordance with CF Care Guidelines,13 but all other treating factors, including location, concomitant administration of inhaled or oral antibiotics or systemic steroids, were at physician's discretion. Spirometry22 and sputum cultures were performed at time of enrollment (within 24 hours of IV initiation) and approximately 10-14 days later. Forced expiratory volume in 1 second (FEV1) percent predicted of normal was calculated.23,24 Participants completed the CF Respiratory Symptom Diary (CFRSD)25 for each day up to 21 days. The study was reviewed and approved by each site's Institutional Review Board, and participant/guardian informed consent/assent was obtained.
Variables and outcomes
The diagnosis of PEx was made by clinician but verified with a scoring algorithm (score excluding change in FEV1 at admission ≥2.6). 26 Short term outcomes are those that occur during treatment of the PEx or within 30 days of completion: lung function non-response to treatment was categorized as <10% relative improvement in FEV1 (L) from enrollment visit (IV antibiotic initiation) to follow-up visit 10-14 days later –within 10 days of end of IV therapy. This represents ‘immediate response’11 regardless of a patient's ‘baseline’ or ‘sick decline’, and is calculated as a relative change to account for FEV1 at the time of admission. The Chronic Respiratory Infection Symptom Score (CRISS) was applied to the CFRSD to calculate a severity score from 0-100 (100 being most severe).27 Change in symptom score was calculated from enrollment visit to end of IV therapy or last completed CFRSD (up to 21 days after IV initiation), whichever came first.
Study data were merged with CFFNPR to determine other clinical factors including: CF related diabetes (CFRD), respiratory microbiology, best FEV1 percent predicted in the preceding 6 months, and history of IV antibiotic episodes in the preceding 6 months. Interim clinical outcomes (those occurring within 3 months of PEx treatment completion) and long term outcomes (those occurring more than 3 months after IV treatment) were also obtained from the CFFNPR. Best FEV1 percent predicted in the 3 months following study PEx treatment was ascertained to determine 90% recovery of best FEV1 from 6 months prior4 and represents ‘baseline recovery’. Time to next IV antibiotic episode (initiated ≥7 days after end of IV therapy for study PEx) was calculated, and censored at the time of last encounter.
Statistical Analyses
Summaries including mean and standard deviation (SD) are reported for treatment factors and clinical outcomes by age group (<18 or ≥18 years), t-tests and Fisher's exact test compare age groups. Analysis of variance, logistic and Cox regression were used to explore the association of clinical characteristics and treatment factors with symptom response and FEV1 change <10% during IV antibiotic treatment, recovery of pre-PEx FEV1,4 and time to next IV antibiotic episode. Backward selection with significance level 0.30 to remain in model was applied to eliminate covariates from the full model which included: female, age ≥ 18 years, CFRD, Pseudomonas aeruginosa (Pa) positive, methicillin-resistant Staphylococcus aureus (MRSA) positive, FEV1 percent predicted <50% at PEx start, 10% drop in FEV1 percent predicted at initiation of study PEx treatment (‘sick decline’), steroid use, oral or inhaled antibiotic use for PEx treatment, MRSA active drug for PEx treatment (doxycycline, linezolid, minocycline, rifampin, tigecycline, vancomycin or trimethoprim/sulfamethoxazole), duration of IV antibiotics (<10 days, 10-14 days, or >14 days), and history of IV antibiotics in the preceding 6 months. A relaxed 0.30 criteria was used to allow non-significant covariates in the model which stabilize estimates of other parameters.28 Immediate lung function non-response (<10% relative improvement in FEV1 (L) during treatment) and change in symptom score27 were then added to the models for (1) failure to recover baseline FEV1(2) time to next IV antibiotic. Model coefficient estimates including odds ratios (OR) and hazard ratios (HR) are reported with 95% confidence intervals (CIs). Reported p-values are two sided and considered exploratory; no adjustments were made for multiple comparisons. No sample size estimates were performed. Analyses were performed using SAS (version 9.2, SAS Institute Inc., Cary, NC, 2009), and R (version 2.15, The R Foundation for Statistical Computing, Vienna, Austria, 2012).
RESULTS
A total of 123 CF patients (60.2% female) were treated with IV antibiotics for a diagnosed PEx (Table 1). Average age was 23.1 years (SD=10.2) and FEV1 percent predicted (%) at admission was 55.8% (SD=20.9). Pediatric patients (10 -17 years old) made up 35% (n=43) of the cohort, and best FEV1 in 6 months prior was on average (SD) 83.1% (18.3) in children and 53.2% (19.7) in adults. Pa was more prevalent in adults (75.0% versus 44.2%, p=0.001), while MSSA (28.8% versus 27.9%, p=0.9) and MRSA (28.8% versus 39.5%, p=0.23) were similar in adults and children.
Table 1.
Demographics and Baseline Characteristics
| Study Participants (N=123) | ||
|---|---|---|
| N | % | |
| Sex -Female | 74 | 60.2 |
| Race | ||
| Caucasian | 114 | 92.7 |
| Hispanic | 3 | 2.4 |
| African-American | 4 | 3.3 |
| Asian | 1 | 0.8 |
| Pacific Islander | 1 | 0.8 |
| Genotype | ||
| Delta F508 Homozygous | 67 | 54.5 |
| Delta F508 Heterozygous | 45 | 36.6 |
| Other/Unknown/ Not genotyped | 11 | 8.9 |
| Pancreatic insufficient[1] | 117 | 95.1 |
| Age Group | ||
| 10-17 yrs | 43 | 35.0 |
| ≥18 yrs | 80 | 65.0 |
| FEV1 % Predicted[2] | ||
| <50% | 49 | 39.8 |
| 50%-69% | 34 | 27.6 |
| 70%-89% | 26 | 21.1 |
| ≥90% | 7 | 5.7 |
| FEV1 drop ≥10% predicted presenting for PE[3] | 66 | 53.7 |
| IV abx for PE in 6 months prior[4] | 51 | 41.5 |
| Microbiology[5] | ||
| Pa | 79 | 64.2 |
| MSSA | 34 | 27.6 |
| MRSA | 40 | 32.5 |
| B. cepacia | 4 | 3.3 |
| S. maltophilia | 15 | 12.2 |
| A. xylosoxidans | 11 | 8.9 |
| H. influenza | 4 | 3.3 |
| Aspergillus | 9 | 7.3 |
| CF Related Diabetes | 37 | 30.1 |
| Mean | SD | |
| Age (years) | 23.1 | 10.2 |
| Body Mass Index (kg/m2) | 19.9 | 3.4 |
| FEV1 % Predicted at enrollment[2] | 55.8 | 20.9 |
| FEV1 % Best in 6 months prior to visit 1[6] | 63.7 | 23.9 |
Evidence of pancreatic enzyme use
Spirometry % predicted is calculated using the Wang equations24 for females less than 16 years of age and males less than 18 years of age. The Hankinson equations23 are used for females 16 years and older, males 18 years and older
As reported as criterion on pulmonary exacerbation diagnostic form
One or more episodes of IV antibiotic for reason “pulmonary exacerbation” recorded in the CFF Registry in 6 months prior to study PEx
Among respiratory cultures isolated at time of enrollment (n=107) or in CFF registry in 6 months prior to enrollment (n= 16). Pseudomonas aeruginosa (Pa), Methicillin-sensitive Staphylococcus aureus (MSSA), Methicillin-resistant Staphylococcus aureus (MRSA), Burkolderia (B.), Stenotrophomonas (S.), Achromobacter (A.), Haemophilus (H.)
Best of all spirometry in CFF Registry in 6 months prior to study PEx
PEx Treatment
PEx treatment features are in Table 2 by age. None of the pediatric patients were treated exclusively at home and adults were treated on average 4.2 days longer than those under 18 years (95% CI=(1.5, 6.9), p=0.003). IV tobramycin was the most commonly prescribed antibiotic in combination; pediatric patients were more likely to get a MRSA active antibiotic (65.1% versus 45.0%, p=0.039), and 16.3% overall received systemic corticosteroids.
Table 2.
Pulmonary exacerbation treatment by age group
| Age Group |
|||
|---|---|---|---|
| < 18 years N=43 | ≥18years N=80 | All N=123 | |
|
Duration of IV antibiotics mean (SD) n (%) |
14.0 (5.6) | 18.2 (9.8) | 16.7 (8.8) |
| 0-10 days | 11 (25.6) | 9 (11.3) | 20 (16.3) |
| >10-14 days | 21 (48.8) | 26 (32.5) | 47 (38.2) |
| >14-21 days | 8 (18.6) | 31 (38.8) | 39 (31.7) |
| >21 days | 3 (7.0) | 14 (17.5) | 17 (13.8) |
| Location of Treatment n(%) | |||
| Exclusively hospital | 28 (65.1) | 35 (43.8) | 63 (51.2) |
| Exclusively home | 0 (0) | 21 (26.3) | 21 (17.1) |
| Both hospital and home | 13 (30.2) | 23 (28.8) | 36 (29.3) |
| Unknown | 2 (4.7) | 1 (1.3) | 3 (2.4) |
| Number of IV Antibiotics n(%) | |||
| 1 | 1 (2.3) | 1 (1.3) | 2 (1.6) |
| 2 | 23 (53.5) | 46 (57.5) | 69 (56.1) |
| 3 | 16 (37.2) | 25 (31.3) | 41 (33.3) |
| 4+ | 3 (7.0) | 8 (10.0) | 11 (8.9) |
| Most common IV antibitiotics n(%) | |||
| Tobramycin | 22 (51.2) | 54 (67.5) | 76 (61.8) |
| Meropenem | 12 (27.9) | 45 (56.3) | 57 (46.3) |
| Ceftazidime | 7 (16.3) | 27 (33.8) | 34 (27.6) |
| Vancomycin | 18 (41.9) | 11 (13.8) | 29 (23.6) |
| Cefepime | 11 (25.6) | 12 (15.0) | 23 (18.7) |
| MRSA[1] active drug n(%) | 28 (65.1) | 36 (45.0) | 64 (53.0) |
| Inhaled Antibiotics n(%) | 5 (11.6) | 7 (8.8) | 12 (9.8) |
| Oral Antibiotics n(%) | 5 (11.6) | 22 (27.5) | 27 (22.0) |
| Steroids n(%) | 7 (16.3) | 13 (16.3) | 20 (16.3) |
Received any of: doxycycline, linezolid, minocycline, rifampin, tigecycline, trimethoprim/sulfamethoxazole, or vancomycin
PEx Outcomes
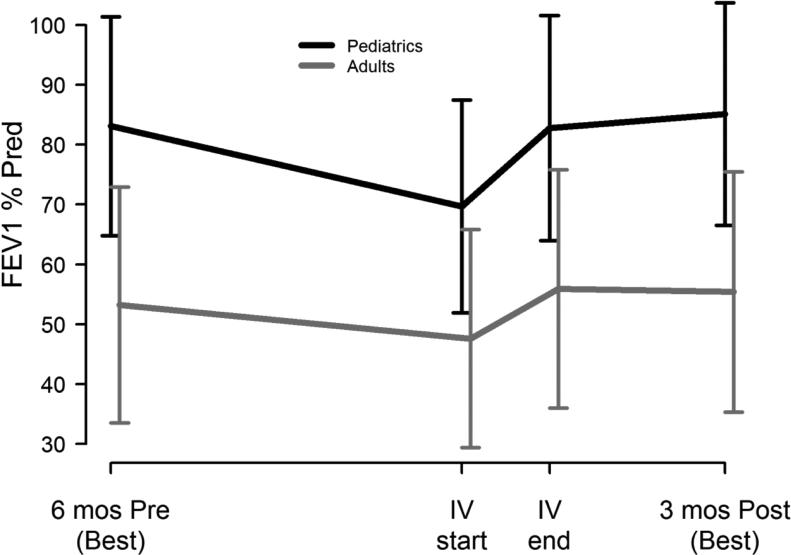
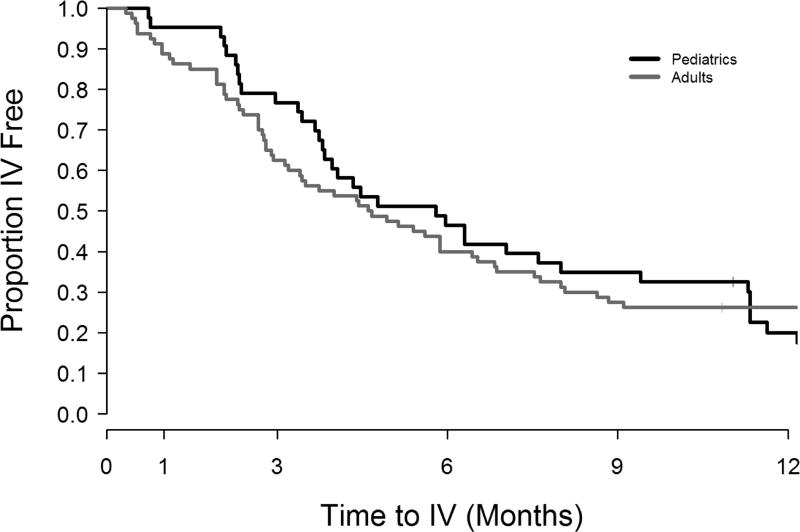
Supplementary Figure 1 shows enrollment and follow-up for each of the outcomes. Table 3 summarizes short and long term clinical outcomes by age group. Among the 102 participants with spirometry at enrollment and at or near the end of IV antibiotic treatment, 73% had lung function measured within ±2 days of IV end, 93% were within a week; 2 had PFTs not within 10 days of IV end and were removed from the calculations. There was marked improvement in immediate FEV1 response in the younger patients (5.9% predicted more than adults, 95% CI=(2.3, 9.5), p=0.002) (Figure 1), while both age groups had similar response in symptom severity, baseline FEV1 recovery in subsequent 3 months, and IV antibiotic need (by 30, 90 and 180 days) after treatment for study PEx. The Kaplan-Meier curves (Figure 2) for time to next IV antibiotic did not significantly differ by age group (p-value=0.74).
Table 3.
Clinical Response by age group and overall
| Age Group |
Total N=123 | |||
|---|---|---|---|---|
| < 18 N=43 | ≥ 18 N=80 | |||
| Short Term Treatment Response | ||||
| Improvement in symptom score[1] | n | 36 | 57 | 93 |
| mean (SD) | −21.7 (13.9) | −22.2 (14.9) | −22.0 (14.4) | |
| Change in FEV1 (% predicted)[2] | n | 35 | 65 | 100 |
| mean (SD) | 13.9 (9.2) | 8.0 (8.3) | 10.0 (9.1) | |
| < 10% improvement FEV1 (L)[3] | n (%) | 10 (28.6) | 23 (35.4) | 33 (33.0) |
| IV antibiotic within 30 days of completing IV for study PE[4] | n (%) | 2 (4.7) | 9 (11.3) | 11 (8.9) |
| Interim Response: 3 months post study PE | ||||
| Change in best FEV1 (% predicted) | n | 37 | 71 | 108 |
| from 6 mos prior to 3 mos post PE | mean (SD) | 1.6 (12.4) | 2.5 (7.9) | 2.2 (9.6) |
| < 90% FEV1 baseline recovery[5] | n (%) | 5 (13.5) | 8 (11.3) | 13 (12.0) |
| IV antibiotic within 90 days of completing IV for study PE[4] | n (%) | 10 (23.3) | 30 (37.5) | 40 (32.5) |
| Long Term Response: | ||||
| IV antibiotic within 180 days of completing IV for study PE | n (%) | 23 (53.5) | 48 (60.0) | 71 (57.7) |
| Time to next IV antibiotic[4](days) | mean (SD) | 236.5 (190.3) | 234.7 (235.9) | 235.3 (220.2) |
| median | 174.0 | 139.0 | 143.0 | |
| min, Max | 22.0, 740.0 | 10.0, 984.0 | 10.0, 984.0 | |
Improvement in CF Respiratory Infection Symptom Score (CRISS) during treatment for study PE
Absolute change in percent predicted during study PE treatment from visit 1 to visit 2
<10% relative change in FEV1 (L) during study PE treatment (N=100, n<18=35, n≥18=65)
Only IV antibiotic episodes occurring 7 or more days after end of treatment for study PE are considered
Best FEV1 (% predicted) 3 months post study PE < 0.90 of best FEV1 (% predicted) 6 months prior to study PE4 (N=108, n<18=37, n≥18=71)
Figure 1. Lung function by age group.
FEV1 % of predicted mean ± 1SD for pediatric participants (black lines, n=37) and adult participants (gray lines, n=71): best during 6 months before study PEx, at time of IV initiation for PEx, at end of IV for PEx, and best during 3 months after study PEx.
Figure 2. Kaplan-Meier survival curves for time to next IV antibiotic episode by age group.
Proportion IV antibiotic free by time (after study PEx) in pediatric (black lines, n=43) and adult participants (gray lines, n=80).
Association of demographic, clinical, and treatment factors with outcomes
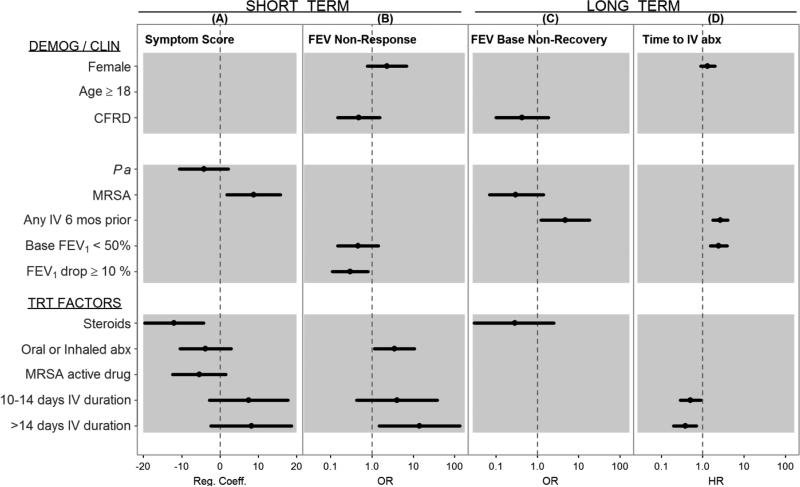
Figure 3 shows the association of clinical and treatment factors with each of the clinical responses (p<0.30). MRSA was associated with a diminished symptom severity reduction (average 8.8 point increase, 95%CI=(1.8, 15.8), p-value= 0.015), while treatment choices such as steroids (−12.0 points, 95%CI=(−19.7, −4.3), p-value= 0.003), oral/inhaled antibiotics, and MRSA active drugs were associated with larger reductions in symptom severity. Longer treatment durations (10-14 days and >14 days as compared to <10 days) were associated with less symptom improvement but not statistically significant.
Figure 3. Factors associated with short and long term outcomes of PEx treatment.
(A) Multivariate regression coefficients (Reg. Coeff) and 95% CIs for demographic (DEMOG), clinical (CLIN) and treatment (TRT) factors associated with CRISS symptom score (- is an improvement). (B) Multivariate odds ratios (OR) and 95% CIs for factors associated with <10% immediate response in FEV1 (L) during treatment. (C) Multivariate ORs and 95% CIs for factors associated with <90% baseline (Base.) FEV1 recovery. (D) Multivariate hazard ratios (HR) and 95% CIs for factors associated with time to next IV antibiotic (abx). Factors with p-value <0.30 were dropped from model and not estimated or plotted in figure. CFRD=cystic fibrosis related diabetes; Pa=P. aeruginosa colonized at time of PEx; MRSA=colonized at time of PEx; Base FEV (<50%)=FEV1 percent predicted at time of PEx; FEV1 Drop= FEV1 drop ≥ 10% presenting for PEx; Oral or Inh=Use of oral or inhaled antibiotics during treatment for PEx; MRSA active drug= use of doxycycline, linezolid, minocycline, rifampin, tigecycline, vancomycin or trimethoprim/sulfamethoxazole; 10-14 Days= total duration of IV antibiotic therapy compared to reference <10 Days; >14 Days=total duration of IV antibiotic therapy compared to reference <10 Days.
Immediate FEV1 non-response was associated with use of oral/inhaled antibiotics (OR=3.5, 95%CI=(1.2,10.6), p-value=0.027) and longer treatment duration (>14 days compared to <10 days, p-value=0.021), whereas a drop of 10% FEV1 at admission had a protective effect and was associated with a reduced odds of failure to recover (OR=0.30, 95%CI=(0.11, 0.81), p-value=0.017). CFRD was the only covariate with a common, though weak, association with both immediate FEV1 response (p-value=0.221) and recovery of baseline FEV1 (p-value=0.257). History of IV antibiotics was the only factor significantly associated (OR=4.8, 95%CI=(1.2, 18.4), p=0.023) with non-recovery of baseline FEV1 post treatment.
The hazard of subsequent IV antibiotic was significantly increased by history of IV episodes (HR=2.7, 95%CI=(1.8, 4.1), p-value<0.001) and low FEV1 at admission (HR=2.4, 95%CI=(1.5, 3.9), p-value<0.001), whereas longer treatment duration was associated with a reduced time to next exacerbation (10-14 days: HR=0.50, 95%CI=(0.29, 0.889), p-value=0.018; >14 days: HR=0.38, 95%CI=(0.20, 0.70), p-value=0.002).
Short term response and subsequent outcomes
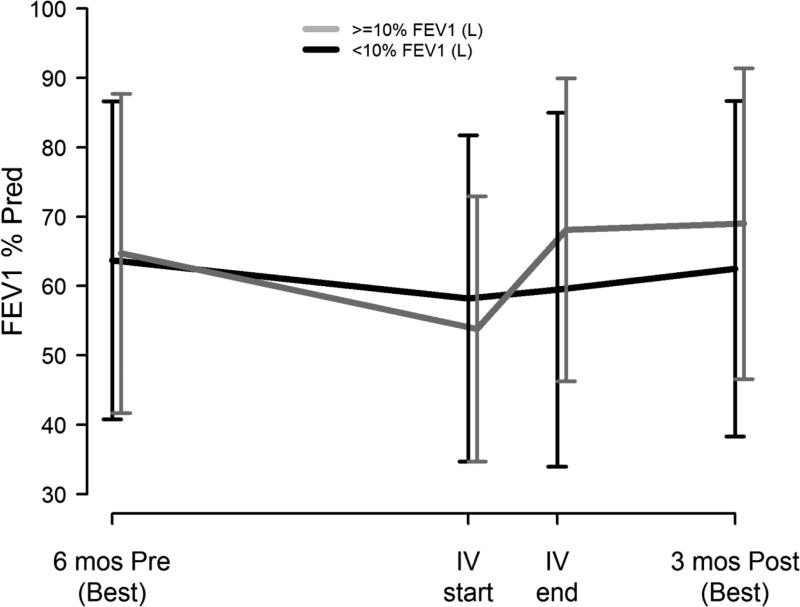
Change in symptom score was not associated with recovery of baseline lung function (p-value =0.49) or time to next IV antibiotic (p-value= 0.69) when added to the multivariate logistic and Cox regression models presented in Figure 3 and Supplementary Table 1, respectively. Immediate non-response in FEV1 (<10% during treatment) significantly increased the odds of non-recovery of baseline (OR=7.8, 95% CI=(1.9, 31.6), p-value =0.004); no other covariates were significantly associated with post-PEx recovery when adjusted for short term FEV1 response. Among those with immediate FEV1 response, 95% recovered baseline by 3 months, whereas among those who had <10% improvement during treatment, 73% recovered baseline. Figure 4 shows lung function by short term FEV1 responder status.
Figure 4. Lung function by immediate FEV1 response with treatment.
FEV1 % of predicted mean ± 1SD for participants with <10% relative FEV1 (L) response during PEx treatment (black lines, n=35) and participants ≥10% relative FEV1 (L) response during treatment (gray lines, n=67): best during 6 months before study PEx, at time of IV initiation for PEx, at end of IV for PEx, and best during 3 months after study PEx.
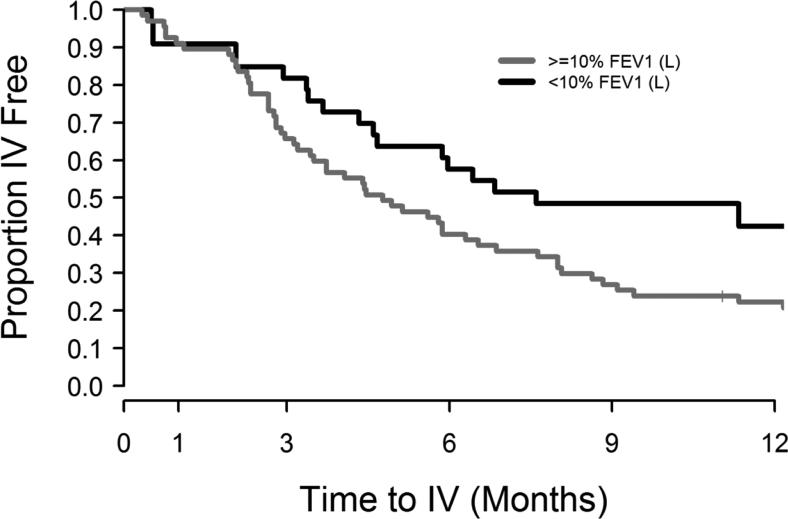
In contradiction, short term FEV1 non-response significantly decreased the risk of subsequent IV antibiotic event (HR=0.48, 95% CI=(0.27, 0.85), p-value=0.011) along with other significant factors that increased the risk: being female (HR=1.8, 95%CI=(1.1, 3.1), p-value=0.032), history of IV antibiotic event (HR=2.8, 95%CI=(1.6, 4.8), p-value<0.001), and low FEV1 at start of study PEx (HR=2.0, 95%CI=(1.2, 3.4), p-value=0.01). Figure 5 shows the Kaplan-Meier curves to next IV antibiotic episode by short term FEV1 responder status (p-value= 0.07).
Figure 5. Kaplan-Meier survival curves for time to next IV antibiotic episode by immediate FEV1 response with treatment.
Proportion IV antibiotic free by time (after study PEx) in participants with <10% relative FEV1 (L) response during PEx treatment (black lines, n=35) and those with ≥10% relative FEV1 (L) response during treatment (gray lines, n=67).
DISCUSSION
CF pulmonary exacerbations are managed with a wide array of treatment regimens that may be based on patient or clinician preference, pathogen isolation, historical response, or trajectory of response to treatment for the current PEx. In general, the short term goals of treatment are to resolve symptoms and recover lost lung function, but there may also be long term goals, such as preservation of lung function and prevention of subsequent acute events. We show, across multiple centers, that patient clinical characteristics and treatment factors that were associated with short term clinical improvement differed from those associated with subsequent lung function recovery and time to next acute event treated with IV antibiotics. In addition, immediate symptom resolution and lung function response did not have a clear relationship with longer term clinical outcomes.
To our knowledge, this is the first study to examine the multi-factorial association between clinical and treatment features with patient reported symptoms during a pulmonary exacerbation. Improvement (i.e. reduction in symptom score) was seen in nearly all patients; however presence of MRSA was related to a diminished effect while steroids were significantly associated with a greater effect. This is contrary to a small randomized trial of steroids in CF children that failed to see an effect of steroids on symptoms.29 Treating to improve patient signs and symptoms is a hallmark component of PEx management; however it may not be indicative of downstream clinical outcomes. A CF symptom score during PEx30 has been associated with extending IV antibiotic duration17 and time to next PEx,31 but not when adjusting for other factors –similar to the findings of this study. The antibiotic course may be extended because of seeming lack of symptom improvement but it is unclear if there are advantages to longer treatment that outweigh cost, time, and potential toxicities.
The other common goal of PEx treatment is recovery of lost lung function. Nearly 90% of this sample recovered baseline lung function –higher than the 75% observed in the Registry from 2003-2006.4 We found that failure to improve FEV1 during treatment was associated with failure to recover pre-PEx lung function in the subsequent three months; however, in contradiction short term FEV1 non-response was associated with longer time to next IV antibiotic episode. Perhaps the patients with the highly variable, oscillating swings in lung function –large drops and big gains with treatment are easier to identify as requiring treatment for exacerbation,32 while those who are less responsive to IV antibiotics may be less likely to be admitted again for IV treatment when it didn't appear to improve the patient's lung function. This analysis specifically examined the association of relative FEV1 improvement during treatment for PEx and subsequent ‘baseline recovery’ while adjusting for ‘sick decline’ and lung function at admission. An alternative short term metric would be recovery of baseline lung function (best 6 months prior) at the end of treatment; however such a measure would confound ‘sick decline’ rather than letting the analysis tease out any effect. Also, the calculation of baseline recovery includes the FEV1 measured at end of treatment (which is often the highest in the 3 months post IV therapy); these measures of ‘immediate response’ and ‘recovery of baseline’ guaranteed mutually exclusive endpoints.
Extending the PEx free interval is an important feature of CF disease management because these acute events have been shown to have long term detrimental effects.1,18,33 For this reason, time to next IV antibiotic course or PEx is a common and useful endpoint in clinical trials of chronic therapies;34,35 however it could be argued that because the majority of these events occur 4-6 months after treatment, they are too far downstream and confounded by factors not related to the acute management of the preceding PEx to serve as useful outcomes when studying success or failure PEx treatment regimens. Similarly, long term lung function decline has been explored within the context of PEx; however for studying PEx treatment regimens, an immediate outcome is necessary for both practicality and to reduce the influence of confounding factors. Several have shown the detrimental effect of exacerbation on long term lung function in CF.4,18 These study data support those findings (history of IV antibiotics significantly increased the risk of failing to recover baseline lung function) and likely demonstrate that recurrent PEx events and underlying phenotype such as CFRD and MRSA colonization are responsible for persistent respiratory decline more than a particular treatment regimen or response.
The inclusion criteria of this study required dual systemic, intravenous antibiotic therapy and thus likely excluded patients treated solely for MRSA infection (either because of exclusively oral or mono-therapeutic choices). Thereby; inference here applies only to CF exacerbations that required systemic dual therapy. The PEx guidelines found insufficient evidence to necessitate two antibiotics when Pa is suspected; but neither was there support for less than two, and thus yielded to standard practice of two. 13 Pa prevalence in this study is consistent with the general CF population. 6 PEx treatment guidelines also cited insufficient evidence of either benefit or harm from acute steroid use to treat CF exacerbations. 13 While this study found some moderate benefit from steroid use, interpretations should be exercised with caution because of the potential for indication bias in a non-randomized study. The question of acute corticosteroids for treating CF PEx is of tremendous interest and would benefit from carefully controlled, randomized research.
There were limitations to our analysis of this observational study, one being that treatments may have been proxy for baseline clinical features or confounded with response. For example, duration was a treatment factor in this analysis; however antibiotic regimens often changed and extended mid-course presumably for reasons of clinical non-response, therefore treatment was confounded with outcomes and has been treated as such in other studies.15,17 Additions or changes to the treatment may have been related to microbial susceptibility testing, or convenience/preference. For these reasons it is challenging to interpret the relationship between treatment factors and outcomes. This multi-site pediatric and adult study augmented with CF NPR data yields generalizable findings for short and long term PEx outcomes –perhaps at the expense of increased heterogeneity resulting from site variability, irregular spirometry timing, and unmonitored supplemental data. Lastly, all our statistical models were estimable and coefficients were stable; however the sample size is moderately small and no adjustments were made for multiple comparisons, therefore associations and p-values should be interpreted with caution.
Symptom resolution and immediate improvement in FEV1 are meaningful measures of acute PEx therapy, and we show them to be more associated with treatment features than long term measures. Short term non-response in symptoms or lung function during treatment was not clearly related with subsequent PEx; however immediate FEV1 response does appear to be associated with recovery of baseline lung function. Also, some of the contradictory results we observed support the use of a composite response15 of short term clinical features to serve as a trial outcome (e.g. lung function or symptom improvement without additional IV antibiotics within a month of the initial treatment). A larger cohort may support or refute our results, while randomized controlled studies of PEx treatment will ultimately produce the evidence necessary to improve the overall wellbeing of patients with CF.
Supplementary Material
What is the key question?
How do varying treatments for pulmonary exacerbation in CF correspond with clinical response, including symptom severity, short and long term lung function, and time to next PEx?
What is the bottom line? Across multiple centers, the patient clinical characteristics and treatment factors that are associated with short term clinical improvement differ from those associated with subsequent lung function recovery and time to next acute event treated with IV antibiotics.
Why read on? This is the only multi-center evaluation of clinically-defined pulmonary exacerbations in both pediatric and adult CF patients to examine a comprehensive set of clinical responses for determining an outcome measure for future studies of exacerbation therapies.
ACKNOWLEDGEMENTS
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions. We also recognize the three anonymous peer reviewers whose suggestions greatly improved this manuscript.
Funding: This research was supported by Cystic Fibrosis Foundation Therapeutics (SAGEL07B0, SAGEL11CS0, HAMBLE10A0, HELTSH13A0), the National Institutes of Health (NIDDK P30 DK089507, KL2 TR000428), the SCTR Institute at the Medical University of South Carolina (NIH UL1 TR000062), NCATS Colorado CTSI (NIH UL1 TR000154), and the University of Wisconsin-Madison ICTR (NIH UL1 TR000427).
Footnotes
Author Contributions: SLH, SDS, and PAF designed the study, SLH, VT, SDS, CHG, DBS, BCM, and PAF contributed substantially to the data analysis and interpretation, and the writing of the manuscript.
Disclosures: The authors have no competing interests or disclosures relevant to the work presented here.
REFERENCES
- 1.Liou TG, Adler FR, Fitzsimmons SC, et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer K, Vandemheen KL, Tullis E, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66:680–685. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 3.Sawicki GS, Rasouliyan L, McMullen AH, et al. Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol. 2011;46:36–44. doi: 10.1002/ppul.21325. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Bittner RC, Rosenfeld M, et al. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briesacher BA, Quittner AL, Fouayzi H, et al. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001-2007. Pediatr Pulmonol. 2011;46:770–776. doi: 10.1002/ppul.21441. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation Patient Registry . Annual Data Report. Cystic Fibrosis Foundation; Bethesda, MD: 2012. 2012. [Google Scholar]
- 7.Kraynack NC, Gothard MD, Falletta LM, et al. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011;46:870–881. doi: 10.1002/ppul.21442. [DOI] [PubMed] [Google Scholar]
- 8.Wagener JS, Rasouliyan L, VanDevanter DR, et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48:666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proesmans M, Heyns L, Moons P, et al. Real life evaluation of intravenous antibiotic treatment in a paediatric cystic fibrosis centre: outcome of home therapy is not inferior. Respir Med. 2009;103:244–250. doi: 10.1016/j.rmed.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Thornton J, Elliott R, Tully MP, et al. Long term clinical outcome of home and hospital intravenous antibiotic treatment in adults with cystic fibrosis. Thorax. 2004;59:242–246. doi: 10.1136/thx.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collaco JM, Green DM, Cutting GR, et al. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med. 2010;182:1137–1143. doi: 10.1164/rccm.201001-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolter JM, Bowler SD, Nolan PJ, et al. Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. Eur Respir J. 1997;10:896–900. [PubMed] [Google Scholar]
- 13.Flume PA, Mogayzel PJ, Jr., Robinson KA, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 14.Blumer JL, Saiman L, Konstan MW, et al. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest. 2005;128:2336–2346. doi: 10.1378/chest.128.4.2336. [DOI] [PubMed] [Google Scholar]
- 15.Parkins MD, Rendall JC, Elborn JS. Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest. 2012;141:485–493. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]
- 16.VanDevanter DR, O'Riordan MA, Blumer JL, et al. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010;11:137. doi: 10.1186/1465-9921-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequeiros IM, Jarad NA. Extending the course of intravenous antibiotics in adult patients with cystic fibrosis with acute pulmonary exacerbations. Chron Respir Dis. 2012;9:213–220. doi: 10.1177/1479972312445903. [DOI] [PubMed] [Google Scholar]
- 18.Waters V, Stanojevic S, Atenafu EG, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 19.Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med. 2011;17:442–447. doi: 10.1097/MCP.0b013e32834b8c04. [DOI] [PubMed] [Google Scholar]
- 20.Clinical practice guidelines for cystic fibrosis. Cystic Fibrosis Foundation; Bethesda, MD: 1997. Appendix VIII, Table 7. [Google Scholar]
- 21.Sagel SD, Thompson V, Chmiel JF, et al. Effect of Treatment of Cystic Fibrosis Pulmonary Exacerbations on Systemic Inflammation. Ann Am Thorac Soc. 2015 Feb 25; doi: 10.1513/AnnalsATS.201410-493OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Am J Respir Crit Care Med. Vol. 152. American Thoracic Society; 1995. Standardization of Spirometry, 1994 Update. pp. 1107–1136. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 25.Goss CH, Edwards TC, Ramsey BW, et al. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8:245–252. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld M, Emerson J, Williams-Warren J, et al. Defining a pulmonary exacerbation in cystic fibrosis. J Pediatr. 2001;139:359–365. doi: 10.1067/mpd.2001.117288. [DOI] [PubMed] [Google Scholar]
- 27.Goss CH, Caldwell E, Gries KS, et al. Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis: CFRSD-CRISS. Pediatr Pulmonol. 2013;S36:1. [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. Wiley; New York, NY: 2000. pp. 91–142. [Google Scholar]
- 29.Dovey M, Aitken ML, Emerson J, et al. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest. 2007;132:1212–1218. doi: 10.1378/chest.07-0843. [DOI] [PubMed] [Google Scholar]
- 30.Jarad NA, Sequeiros IM. A novel respiratory symptom scoring system for CF pulmonary exacerbations. QJM. 2012;105:137–143. doi: 10.1093/qjmed/hcr149. [DOI] [PubMed] [Google Scholar]
- 31.Sequeiros IM, Jarad N. Factors associated with a shorter time until the next pulmonary exacerbation in adult patients with cystic fibrosis. Chron Respir Dis. 2012;9:9–16. doi: 10.1177/1479972311433575. [DOI] [PubMed] [Google Scholar]
- 32.Morgan WJ, Wagener JS, Yegin A, et al. Probability of treatment following acute decline in lung function in children with cystic fibrosis is related to baseline pulmonary function. J Pediatr. 2013;163:1152–1157. e1152. doi: 10.1016/j.jpeds.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCoy KS, Quittner AL, Oermann CM, et al. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.