Abstract
Surgery is the most efficacious treatment for postprostatectomy incontinence. The ideal surgical approach depends on a variety of patient factors including history of prior incontinence surgery or radiation treatment, bladder contractility, severity of leakage, and patient expectations. Most patients choose to avoid a mechanical device, opting for the male sling over the artificial urinary sphincter. The modern male sling has continued to evolve with respect to device design and surgical technique. Various types of slings address sphincteric incompetence via different mechanisms of action. The recommended surgery, however, must be individualized to the patient based on degree of incontinence, detrusor contractility, and urethral compliance. A thorough urodynamic evaluation is indicated for the majority of patients, and the recommendation for an artificial urinary sphincter, a transobturator sling, or a quadratic sling will depend on urodynamic findings and the patient's particular preference. As advancements in this field evolve, and our understanding of the pathophysiology of incontinence and mechanisms of various devices improves, we expect to see continued evolution in device design.
Keywords: Artificial urinary sphincter, Prostatectomy, Stress urinary incontinence, Suburethral slings, Urodynamics
INTRODUCTION
Approximately 4% of radical prostatectomy (RP) patients will have incontinence of sufficient bother to elect surgery to treat their postprostatectomy incontinence (PPI) [1]. In the 20th century, transurethral injection of periurethral bulking agents and placement of an artificial urinary sphincter (AUS) were the two most common surgical treatments for PPI [2]. With periurethral bulking injection, the majority of patients fail to achieve meaningful improvements in continence [3]. In contrast, the AUS has enjoyed a 30-year reign as the most predictably efficacious treatment for PPI. There are, however, well-known surgical complications, including urethral erosion (6%), device infection (5.5%), and mechanical failure (6%) [4] necessitating a revision rate of 21% at 5 years and 50% at 10 years postoperatively [5]. The male sling arose out of necessity for a less invasive and less risky alternative to AUS, and a patient-driven desire to avoid a mechanical device [6].
The modern male sling has evolved over the past 40 years. The Kaufman device, comprised of a hemispherical silicone-gel filled prosthesis and polyurethane straps compressing the urethra, was replaced by the pubourethral sling [7], which was in turn supplanted by the bone-anchored male sling (BAMS, InVance, American Medical Systems, Minnetonka, MN, USA) and the transobturator (TO) sling (AdVance, American Medical Systems). The BAMS is based upon a 4 × 7-cm silicone-coated polyester mesh, secured to the descending pubic rami via 6 titanium bone screws, compressing the perineal urethra [8]. The TO sling, in contrast, improves urinary continence in a noncompressive manner, by relocating the proximal urethra more proximally [9]. Each device has an established track record of success in men with mild to moderate PPI. More recently, the quadratic sling (VIRTUE, Coloplast, Humlebaek, Denmark), a four-armed polypropylene mesh that provides both proximal urethral relocation via a TO component and perineal urethral compression via a prepubic (PP) component, [10] has gained popularity among urologists and patients. Several adjustable slings have been introduced (Argus, Promedon, Cordoba, Argentina and Remeex, Neomedic, Barcelona, Spain) with the potential advantage of postoperative tightening or loosening [11,12]. As a testament to improvements in design and efficacy, male slings recently superseded the AUS and urethral bulking as the most common surgical procedures for PPI [2].
Despite different mechanisms of action, the primary goals of the various male slings remain: (1) tensioning to adequately compress the bulbous urethra and/or sufficiently relocate the proximal urethra; (2) maintaining tension to prevent recurrent leakage; and (3) balancing sling tension and detrusor contractility in order to avoid urinary retention.
URODYNAMIC EVALUATION
Prior to offering invasive surgery, the urologist must determine the pathophysiology driving the patient's incontinence. PPI may result from bladder dysfunction and/or intrinsic sphincter deficiency (ISD). Additionally, there may be an obstructive anastomotic stricture in 3% to 21% of men [13,14]. Furthermore, patients with detrusor underactivity may be at increased risk of urinary retention following surgery, as adequate detrusor contractility is necessary to overcome the fixed resistance of a compressive device [5]. Just as the compressive sling is designed to prevent leakage with straining, it is likely to interfere with valsalva voiding in those with inadequate contractility. Measurement of leak point pressure (LPP) is not absolutely necessary, as LPP neither correlates with pad weight, nor does it typically alter the plan of treatment [15]. On the other hand, pad use does correlate with actual urine loss, thereby justifying daily pad use as a measure of incontinence severity [16]. Based on these findings, the degree of incontinence is therefore best quantified by pad use or the pad test.
Urodynamic evidence of bladder dysfunction must be interpreted cautiously in men with PPI. While de novo reduced bladder compliance may affect greater than 25% of patients up to three years post-operatively [17], this finding does not usually impact surgical outcome. In severely incontinent patients, urodynamic diminished compliance and low volume detrusor overactivity are the most likely artifacts identified during supra-physiologic filling of bladders that have been chronically underfilled, and do not typically affect AUS surgical outcome [18,19].
Detrusor hypocontractility may be seen in 25% to 40% of patients greater than 1 year postoperatively, and can occur de novo in up to 10% [20,21]. Since the cuff is deflated during voiding, allowing efficient bladder evacuation via bladder contraction or valsalva abdominal straining, AUS success does not differ in patients with normal versus underactive bladders [4,22]. In contrast, adequate detrusor contractility is necessary to expel urine past a compressive sling [5]. AUS or a TO sling is preferred in those with an underactive bladder, whereas a compressive quadratic sling should be considered only in those with adequate detrusor contractility.
While there is no universally agreed upon measure of normal detrusor contractility following prostate removal, bladder contractility index and measurement of isovolumetric detrusor contraction pressure [17,23] are commonly used. Voiding pressure is an unreliable determinant of detrusor strength in patients with low urethral resistance, as the contractile pressure required to maintain axial flow can approach zero. Therefore, using nomograms based upon a population of men with prostate enlargement is inaccurate in men status post RP [23]. A direct measure of contractility is the isometric detrusor contraction pressure (Piso), correlating highly with the Watts factor [24], which is generally recognized as the most reliable approximation of bladder contraction strength. Piso can be determined via the mechanical stop test [25,26], whereby the examiner manually compresses the penile urethra during voiding, preventing urinary flow, but gently enough to prevent causing a pelvic floor contraction, which may otherwise abort a detrusor contraction. A minimum Piso of 60-cm water is necessary to overcome the fixed resistance of a compressive urethral sling, which intraoperatively is typically tensioned to 60-cm retrograde leak point pressure (RLPP).
1. Video fluoroscopy
The addition of video fluoroscopy to urodynamic testing is useful for determining the degree of bladder neck and proximal urethral mobility. As a mechanism of action, the TO sling relies on repositioning of the descended sphincter unit rather than on direct compression of the bulbar urethra [27], therefore it is important to evaluate the degree to which the posterior urethra may ultimately be repositioned. The "repositioning test" is based on the finding that abdominal LPP increases upon gently pushing the perianal perineum in a cephalad direction (while avoiding direct compression of the urethral bulb) [28]. Cystoscopically visible sphincter closure occurs upon perineal elevation in men with sufficient residual sphincter function (i.e., mild ISD) [29]-a finding that has been shown to predict surgical success [9,27,30]. Fluoroscopic demonstration of bladder neck and proximal urethral descent is an alternative to the repositioning test. Men with stress urinary incontinence (SUI) following RP alone had significantly more proximal urethral descent with straining than men status post adjuvant radiation or primary radiotherapy [31]. Therefore, those with adequate mobility are the most appropriate candidates for TO sling placement, whereas men with an immobile proximal urethra are more appropriately treated with a compressive device such as the quadratic sling or AUS.
SURGICAL TREATMENT OF PPI
For more than 30 years, the AUS has been regarded as the most reliable surgical option for treating PPI, with success rates typically above 80%. However, with infection rates of 5% to 6%, erosion rates of 6% to 8%, and mechanical failure in 6% to 23% of cases over 7 to 13 years [4,32], there has been an incentive to search for an alternative surgical approach.
1. Bone-anchored male sling
The BAMS improves continence via direct compression of the distal bulbar and perineal urethra against the genitourinary diaphragm. The fixation of a permanent synthetic sling material, using titanium bone screws and polypropylene sutures, contributed to a high level of surgical success and durability. Several large prospective cohort studies have demonstrated sustained efficacy of the BAMS over 3 to 5 years of follow-up-with success (less than or equal to 1 pad per day) rates of 65% to 80% and pad-free rates generally ranging from 50% to 65% [33,34]. In these series complication rates were low, with an overall infection rate of 3%, an erosion rate of less than 2%, and perineal pain (typically resolved by 3 months) only occurring in 16% to 19% of patients. However, the concern for osseous complications combined with the high cost of bone screws inspired development of an anchorless perineal sling.
2. TO sling
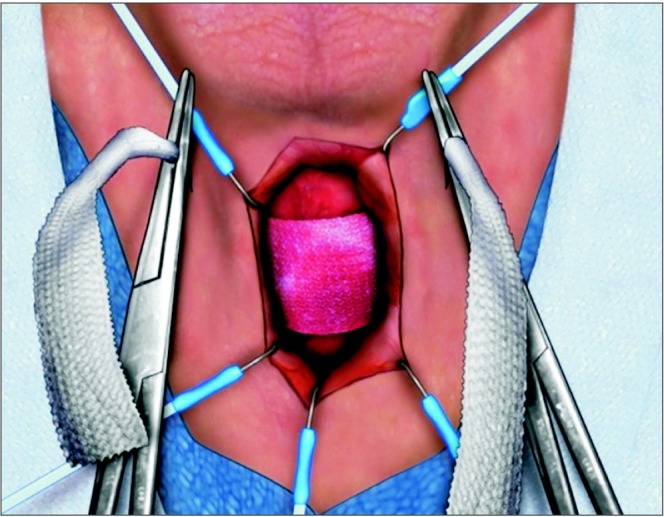
The TO sling was introduced in 2005, based upon a minimally invasive approach similar to the female midurethral TO sling (Fig. 1). Whereas this sling only nominally compresses the bulbar urethra, its main mechanism of action relies upon proximal urethral relocation. It is hypothesized that inefficacious coaptation of the urethral sphincter complex results from laxity of posterior urethral support and relative misalignment of the proximal urethra [28]. Therefore a TO sling that restores the pre-prostatectomy configuration by realigning the mobile sphincter complex will ultimately prevent proximal urethral descent and improve intrinsic continence. Following appropriate sling tensioning, the bulbar urethra is relocated proximally by a distance of 2 to 3 cm, into the higher pressure pelvic outlet, thus functioning as a physiologic "backstop" during straining [35].
Fig. 1. Transobturator sling. Upon tensioning, the bulbar urethra will move proximally.
Success rates from prospective series range from 54% to 80%, with cure rates generally around 50% [36,37,38]. Transient urinary retention has been reported in 3% to 23%, typically resolving by 12 weeks [6]. Perineal pain rates vary widely from 0% to greater than 20%, depending on the definition of postoperative pain [39,40,41] but most reports show significant pain in less than 10% which resolves by 3 months postoperatively. Serious complications requiring sling explantation are generally less than 1% [8].
3. Quadratic sling
The quadratic sling can increase urethral resistance to a greater degree than either a purely perineal or TO device, by combining the mechanisms of action of the BAMS and TO sling. This additive effect has been mechanistically proven by demonstrating that sequential tightening of the TO and then the PP extensions caused a cumulative increase in RLPP [10,42].
In a multinational clinical trial, the quadratic sling with fixation achieved an objective success rate (greater than 50% improvement in 24-hour pad weight) of 79%, subjective success rate (Patient Global Impression of Improvement score of much or very much improved) of 71%, and a median 24-hour pad weight reduction of 88% at 1 year. Cure (less than 1.3 g of leakage on 24-hour pad weight) was achieved in 46% at 1 year. In this cohort, the majority of patients had moderate (100 to 400 g/d) or severe (greater than 400 g/d) incontinence and complications were only Clavien grade 1. Overall, only 19% had mild genital paresthesias, and 12% experienced mild perineal pain, which is similar to the rates of pain of the aforementioned slings. By the 6-month follow-up visit, all paresthesias had resolved, and only 2 cases of mild perineal discomfort persisted, with neither requiring further treatment [43]. The results of the various male sling procedures have been summarized (Table 1).
Table 1. Results of postprostatectomy incontinence surgical procedures.
| Surgery | Success (cure/improved) | Most common complications (typical range) | |
|---|---|---|---|
| Bone anchored male sling | 65%-80% | Infection/erosion | 2%-3% |
| Urinary retention | 1%-2% | ||
| Pelvic pain | 16%-19% | ||
| Retroluminal sling | 63%-80% | Infection/erosion | <1% |
| Urinary retention | 3%-23% | ||
| Pelvic pain | 0%-10% | ||
| Quadratic sling with fixation | 70%-79% | Infection/erosion | 0% |
| Urinary retention | 0% | ||
| Pelvic pain | 12%-19% | ||
| Artificial urinary sphincter | >80% | Infection/erosion | 5%-8% |
| Urinary retention | 0% | ||
| Mechanical failure | 6%-23% | ||
4. Adjustable sling
The Argus device consists of a silicone foam pad placed under the bulbar urethra, attached to silicone columns that may be passed through a retropubic or TO approach, fixed over the rectus or obturator fascia using silicone washers [11,44]. The Remeex device consist of a short polypropylene mesh placed over the bulbar urethra, with monofilament threads passed retropubically and a permanent implanted mechanical tension device placed over the rectus fascia. In the event of suboptimal intraoperative tension, sling tightening may be accomplished through a minimally invasive "re-adjustment" technique for each of these devices.
In a systematic review by Trost and Elliott [45] in 2012, out of 4 studies with a total of 273 patients, excluding salvage procedures, the Argus retropubic sling was associated with a 17% to 79% success rate. In the largest study by Hubner et al. [11] of 101 patients, Argus sling adjustment was required in 39% of men. The moderate silicone burden, however, is prone to complications with a 5% to 7% infection, 3% to 13% erosion and 12% to 35% rate of explantation [45]. These findings are similar to a recent unblinded nonrandomized study in 44 patients comparing the TO approach Argus to the AdVance sling by Chung et al. [46], with Argus revision required in 24% of men and subsequent social continence (0 to 1 pad per day) achieved in 92% with the Argus and 84% with AdVance at a mean follow-up of 36 and 33 months, respectively.
With regards to the Remeex system, Sousa-Escandon et al. [12] reported their series of 51 patients from 2002 to 2005 and found a self reported 65% cure rate and 85% of men improved at 32 months. The retropubic permanent suture passage was associated with a 10% bladder perforation rate, 6% infection/erosion, and 86% required at least 2 adjustment revisions. Findings were similar to Jimenez Parra et al. [47], in their small series of 14 patients published in 2010, they reported a 21% explantation rate, 29% bladder perforation, and 36% urinary retention. At a mean follow-up of 18.6 months 42% of patients were totally continent and 33% reported light incontinence.
The nonadjustable and adjustable slings appear to be equally efficacious, but the latter have a higher explantation rate [45]. A properly tensioned sling with appropriate fixation that does not lose tension over time is just as efficacious as an undertensioned adjustable sling where the surgeon has the ability to increase tension postoperatively.
SLING TENSIONING
The observation of either proximal relocation of the bulbar urethra or luminal coaptation on cystoscopy suffices for appropriate tensioning of the TO sling. On the other hand, for a compressive perineal sling, a more precise quantification of sling tensioning is necessary-such as RLPP, a standardized measure of urethral sphincteric competence in men with ISD [48,49,50]. While abdominal LPP cannot be measured during surgery under general anesthesia, RLPP can be measured via perfusion sphincterometry, and has been validated as a useful quantification of urethral resistance during male anti-incontinence surgery [51,52].
Sufficient tension must be maintained to achieve long-term continence, whereas loss of tension equates to loss of sling efficacy. Hence it is not surprising that the use of a resorbable allograft or xenograft BAMS has a failure rate of greater than 90% at 6 months [53]. In contrast, 3- to 5-year efficacy does not diminish with a non-absorbable polypropylene BAMS [33,34,54]. Objective urodynamic evidence of sustained sling tension has also been demonstrated at 2 years postoperatively [52], thus demonstrating that tension and continence can be maintained with the use of a reliable fixation method and permanent sling material.
Similarly, early reports of the TO sling were associated with a loss of efficacy over time. The Cleveland Clinic group found that patient-determined success decreased from 87% to 63% with the retroluminal sling, with average daily pad use more than doubling over 2 years postoperatively [55]. Specific risk factors predisposing to TO sling failure include the use of absorbable fixation sutures, fewer than 4 sutures, and the absence of subcutaneous sling tunneling [29]. Further experience, however, and improved sling fixation has led to substantially better medium-term results, with 77% success at 3 years attributed to more effective fixation [39]. With the goal of facilitating sling fixation by incorporating a chevron-type tissue anchor, the AdVance XP (American Medical Systems) has recently been introduced, [56] but has not received approval in the United States.
The unfixed quadratic sling, similar to other slings, demonstrated progressive reduction in efficacy over 12 months, prior to the incorporation of an effective method of mesh fixation. Following initial objective and subjective success rates of 61% and 56%, respectively, only 42% of patients realized subjective and objective success at the end of 1 year. The original surgical technique has since been revised with a straight-forward fixation of the TO and PP arms and subsequent improvement in efficacy through the elimination of early sling slippage [43]. Objective success was achieved in 79% and subjective success in over 70% at 1 year, despite patients with moderate to severe incontinence comprising the majority of the cohort-indicative of efficacious sling fixation. In fact, for the fixation cohort, 24-hour pad weight was reduced by 88% at 12 months.
WHICH OPERATION FOR WHICH PATIENT
1. What patients choose
When given a choice, patients overwhelming prefer the male sling over the AUS for treating PPI. Kumar et al. [6] reported that when given a choice between AUS placement and sling surgery, 22 out of 24 men (92%) indeed chose the male sling, while in 63 men for whom AUS was recommended, a remarkable 25% still opted for sling surgery. Consistent with this preference has been the change in prevalence of sling surgery for the treatment of PPI, which between 2001 and 2011 has increased dramatically, from only 15% of PPI surgeries in 2001, up to 51% in 2011 [2].
2. Timing of anti-incontinence surgery
A period of watching and waiting along with Kegel exercises is advocated for 6 to 12 months after RP. However according to a recent Cochrane systematic review of pelvic floor muscle training after RP, there was no statistical improvement in urinary incontinence in the treatment group at 12 months (risk ratio, 0.85; 95% confidence interval. 0.60-1.22) with 57% of men reporting urinary incontinence compared to 62% in the control group [57]. In a prospective cohort of 500 men by Lepor and Kaci [58], following open RP 80.6%, 95.2%, and 98.5% self reported continence at 3, 12, and 24 months, respectively. Since improvements in continence are generally realized by 12 months, it is suggested that conservative management be utilized the first year after surgery.
3. Specific patient factors
1) Degree of incontinence
Severity of incontinence (quantified by pad weight) affects sling outcome. In men with greater than 200 g/d urine loss, the TO sling has been associated with lower success rates [59,60]. For each 1 g increase on the 24-hour pad weight test, cure rate decreases by 0.4%, such that with a pad weight of greater than 400 g/d, success was 80% lower than those patients with just 200 g/d leakage [60]. Similarly, for the BAMS, increased pad weight was associated with a lower efficacy, such that AUS should be recommended over sling when pad weight exceeds 450 g/d [61]. In contrast, the quadratic sling with fixation has shown no difference in the success rates for mild (less than 100 g/d), moderate (100 to 400 g/d), or severe (greater than 400 g/d) leakage [43].
2) Prior radiation
Prior radiotherapy has been reported as a risk factor for sling failure [60,62,63]. Adequate urethral tissue compliance is necessary for successful urethral compression. Radiation was the most significant factor predicting failure for the Northwestern pubourethral sling [63] and has also been shown to adversely affect the BAMS [62]. Radiation also limits urethral mobility [31], and hinders the ability of the TO sling to achieve adequate proximal urethral relocation. In a retrospective cohort study, Torrey et al. [64] reported that no patients were cured and only 29% were improved if men had undergone radiation therapy prior to sling placement, versus a 63% cure rate and 27% improvement rate in men undergoing TO sling who were not radiated. In another cohort, TO sling patients who were radiated realized only a 25% cure and 25% improvement rate, compared to a success rate of almost 80% in nonradiated patients [60]. Based on the poor outcomes of slings in men who have undergone prior radiation, the AUS remains the treatment of choice in this select patient population.
3) Prior AUS
Sling placement is generally ineffective following prior AUS explantation, which typically causes a poorly compliant and relatively noncompressible fibrotic urethra [33,54]. In contrast, AUS reimplantation has a predictably high success rate. Compared to men undergoing salvage sling implantation, those undergoing repeat AUS surgery were three times more likely to achieve adequate continence [65]. Urethral atrophy may be addressed by replacing or up-pressurizing the balloon reservoir, revising the urethral cuff (repositioning, downsizing, tandem cuff, or transcorporal cuff), or urethral buttressing with a collagen matrix [66,67,68,69].
4) Prior sling
Following sling surgery, recurrence of incontinence ranges from 20% to 35%. After a failed sling, conservative measures such as pelvic floor exercises are unlikely to provide sufficient relief. It is important to evaluate whether recurrent or persistent incontinence represents detrusor or sphincter dysfunction. If the patient indeed has SUI due to sphincteric insufficiency, then reoperation is indicated. There is a paucity of data to suggest that periurethral bulking agent would be any more effective following a failed sling than it would be when used as a primary treatment for postprostatectomy SUI. However, there is a growing body of literature regarding the efficacy of repeat sling surgery versus AUS following sling failure.
AUS surgery is equally efficacious in the patient who has failed a prior sling as it is in the patient who has not had prior incontinence surgery. In the setting of prior TO sling, the AUS can be implanted in a straightforward manner, via a perineal approach. It is neither simple nor necessary to release the retroluminal sling. Rather the AUS can be placed distal to the membranous urethra and proximal bulb in routine fashion. Similarly, following failed BAMS, the AUS may be placed via a trans-scrotal approach, somewhat more distal than would be achieved during the perineal approach. In these select cases, the prior sling can thereby act as a "pseudo double cuff", while insufficient on its own, may actually provide some degree of sphincteric coaptation and improve AUS efficacy [13]. If the surgeon prefers the perineal approach and a more proximally placed AUS, then the bone anchored sling may be divided in the midline and dissected off the bulbospongiosus muscle, thus leaving an unscarred urethra for AUS cuff placement [5].
The efficacy of a repeat sling appears to depend on the time to sling failure, and the residual sphincteric function. In those patients who either never achieved sling efficacy or who had early sling failure (less than 6 months postoperatively), the efficacy of repeat sling surgery was lower (20% cure, 20% improved) compared to those who failed initial sling surgery later (greater than 6 months postoperatively). This latter group realized a 63% cure and 13% improvement rate at 1-year following repeat sling surgery [70]. Soljanik et al. [71] showed similar efficacy rates for repeat TO sling surgery. In their cohort, 46% of patients were dry 6 months following repeat sling surgery. However in this group, it must be noted that patients were carefully selected such that only those with a positive repositioning test were offered revision sling surgery, whereas those with more substantial ISD were not. Similarly, Webster's group from Duke found a low success rate with repeat sling, noting a 55% failure rate, and a seven times higher likelihood of failure than AUS placement [72].
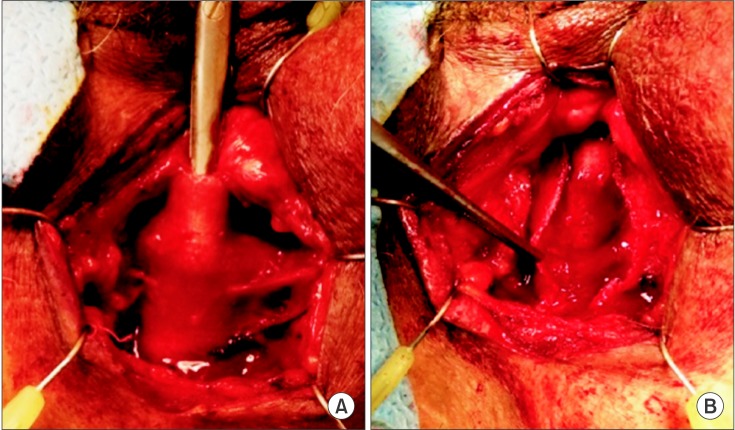
Given the relatively high failure rate in repeat sling surgery, and the relatively high success rate of salvage AUS surgery, implantation of an AUS remains the procedure of choice in men who have failed primary sling. Overall, up to 13% of men who undergo male sling surgery will ultimately have implantation of an AUS [73]. A number of small case series have shown that AUS placement after failed male sling has a high success rate of 80% to 90% without an increase in the expected complication rate [74,75,76,77,78]. Whereas the TO sling may be left in situ without affecting AUS placement, the quadratic sling must be partially excised to permit access to the bulbar and perineal urethra for proper AUS cuff placement. Fortunately, the identification of the quadratic sling is quite simple through a perineal incision, and dissection of the sling off the underlying bulbospongiosus muscle is also straightforward [79]. The preserved muscle can then be divided, leaving an unscarred urethra, which is readily amenable to circumferential dissection and AUS cuff placement (Fig. 2). ProACT (not available in the United States) has been examined as a salvage procedure following failed TO sling surgery. In a recent cohort study, 15 of 40 men who failed a TO sling (all of whom had severe leakage preoperatively), were successfully salvaged with either AUS or ProACT [80], with complication rates similar to that experienced with primary AUS or ProACT insertion.
Fig. 2. (A) The quadratic sling is identified and readily dissected off the urethra. (B) After incising the sling, the bulbospongiosus is exposed, thus permitting a straightforward artificial urinary sphincter placement.
SUMMARY
The modern male sling has evolved with respect to both design and surgical technique, from the original Kaufman prosthesis, to the pubourethral sling, to the perineal BAMS, to the noncompressive TO sling, and most recently to the quadratic sling that combines the mechanisms of action of the predecessor devices. No single device should be considered exclusively as the gold-standard option for treating PPI. Rather, different devices are best suited for patients depending on history of radiation, previous incontinence surgery, degree of leakage, and bladder contractility (Table 2).
Table 2. Indications and contraindications for the surgical management of postprostatectomy incontinence.
| Surgery | Indication | Contraindication |
|---|---|---|
| Retroluminal sling | SUI | History of radiation |
| Mild-moderate leakage | Poor residual sphincter function | |
| Prior AUS | ||
| Quadratic sling with fixation | SUI | Detrusor hypocontractility |
| Moderate-severe leakage | Prior AUS | |
| [OK in radiated patient if >6 months prior] | ||
| Artificial urinary sphincter | SUI | None |
| Any degree of leakage | ||
| [OK in radiated patient] | ||
| [OK after prior AUS] | ||
| [OK after sling] |
SUI, stress urinary incontinence; AUS, artificial urinary sphincter.
In a man demonstrating leakage with straining that stops at the cessation of the straining maneuver, the diagnosis of ISD can made without further testing. A voiding diary is sufficient to demonstrate adequate bladder capacity, and a bladder scan can evaluate the patient's ability to empty his bladder. However with regards to bladder contractility, this can only be ascertained by detailed urodynamic evaluation. Given the recent expansion of therapeutic options for treating SUI, a thorough evaluation should also include pad weight and urodynamic studies-to best direct specific surgical therapy.
Adequate urethral tissue compliance is necessary for successful urethral compression and/or proximal repositioning with a sling. Radiation and previous AUS explantation, both of which may result in a relatively noncompressible urethra, are associated with diminished sling efficacy. With the exception of the occasional patient with persistent mild to moderate SUI following prior sling, for persistent PPI and a positive repositioning test, AUS implantation is the treatment of choice because it can provide the circumferential urethral compression necessary for adequate coaptation even in the setting of diminished urethral compliance.
In men who have not been radiated and have not had prior incontinence surgery, factors such as degree of leakage, proximal urethral mobility, and detrusor contractility can help determine the preferred surgical approach. In those with less than 200 g/d leakage and a positive repositioning test as well as adequate urethral mobility on video urodynamics, retroluminal or quadratic sling is the preferred approach, because of the lower complication rates compared to AUS placement and the fact that neither sling complicates future AUS placement. With detrusor underactivity, the TO sling may be preferred, given its noncompressive mechanism of action. For those with 200 to 400 g/d leakage, the quadratic sling may be preferred, given the compressive nature of this surgical device, providing superior resistance compared to a purely TO approach that relies on adequate residual sphincter function. However, adequate detrusor contractility is necessary to overcome the resistance of this compressive device. In the setting of detrusor underactivity and moderate incontinence, AUS is preferred. With leakage greater than 400 g/d, AUS is the recommended option unless the patient refuses a mechanical device, in which case a compressive sling would be recommended over a noncompressive sling.
The evaluation and management of PPI has changed and improved dramatically over the past 2 decades. Further innovations and developments in sling design are likely, as this field continues to evolve.
Footnotes
CONFLICTS OF INTEREST: Craig V. Comiter (Coloplast, Consultant and Clinical Investigator). Except for that, the authors have nothing to disclose.
References
- 1.Nam RK, Herschorn S, Loblaw DA, Liu Y, Klotz LH, Carr LK, et al. Population based study of long-term rates of surgery for urinary incontinence after radical prostatectomy for prostate cancer. J Urol. 2012;188:502–506. doi: 10.1016/j.juro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Chughtai B, Sedrakyan A, Isaacs AJ, Mao J, Lee R, Te A, et al. National study of utilization of male incontinence procedures. Neurourol Urodyn. 2014 Oct 18; doi: 10.1002/nau.22683. [Epub] [DOI] [PubMed] [Google Scholar]
- 3.Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner-Hermanns R, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29:179–190. doi: 10.1002/nau.20844. [DOI] [PubMed] [Google Scholar]
- 4.Lai HH, Hsu EI, Teh BS, Butler EB, Boone TB. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol. 2007;177:1021–1025. doi: 10.1016/j.juro.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 5.Comiter CV. Surgery Insight: surgical management of postprostatectomy incontinence: the artificial urinary sphincter and male sling. Nat Clin Pract Urol. 2007;4:615–624. doi: 10.1038/ncpuro0935. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Litt ER, Ballert KN, Nitti VW. Artificial urinary sphincter versus male sling for post-prostatectomy incontinence: what do patients choose? J Urol. 2009;181:1231–1235. doi: 10.1016/j.juro.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Stern JA, Clemens JQ, Tiplitsky SI, Matschke HM, Jain PM, Schaeffer AJ. Long-term results of the bulbourethral sling procedure. J Urol. 2005;173:1654–1656. doi: 10.1097/01.ju.0000157972.11229.4e. [DOI] [PubMed] [Google Scholar]
- 8.Welk BK, Herschorn S. The male sling for post-prostatectomy urinary incontinence: a review of contemporary sling designs and outcomes. BJU Int. 2012;109:328–344. doi: 10.1111/j.1464-410X.2010.10502.x. [DOI] [PubMed] [Google Scholar]
- 9.Rehder P, Berger T, Kiss G, Madersbacher H, Gozzi C. Advance male sling: anatomic evidence of retrourethral position after tensioning without direct urethral compression. Eur Urol Suppl. 2008;7:87. [Google Scholar]
- 10.Comiter CV, Nitti V, Elliot C, Rhee E. A new quadratic sling for male stress incontinence: retrograde leak point pressure as a measure of urethral resistance. J Urol. 2012;187:563–568. doi: 10.1016/j.juro.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 11.Hubner WA, Gallistl H, Rutkowski M, Huber ER. Adjustable bulbourethral male sling: experience after 101 cases of moderate-to-severe male stress urinary incontinence. BJU Int. 2011;107:777–782. doi: 10.1111/j.1464-410X.2010.09619.x. [DOI] [PubMed] [Google Scholar]
- 12.Sousa-Escandon A, Cabrera J, Mantovani F, Moretti M, Ioanidis E, Kondelidis N, et al. Adjustable suburethral sling (male remeex system) in the treatment of male stress urinary incontinence: a multicentric European study. Eur Urol. 2007;52:1473–1479. doi: 10.1016/j.eururo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Kao TC, Cruess DF, Garner D, Foley J, Seay T, Friedrichs P, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol. 2000;163:858–864. [PubMed] [Google Scholar]
- 14.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172(6 Pt 1):2227–2231. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 15.Twiss C, Fleischmann N, Nitti VW. Correlation of abdominal leak point pressure with objective incontinence severity in men with post-radical prostatectomy stress incontinence. Neurourol Urodyn. 2005;24:207–210. doi: 10.1002/nau.20120. [DOI] [PubMed] [Google Scholar]
- 16.Nitti VW, Mourtzinos A, Brucker BM SUFU Pad Test Study Group. Correlation of patient perception of pad use with objective degree of incontinence measured by pad test in men with post-prostatectomy incontinence: the SUFU Pad Test Study. J Urol. 2014;192:836–842. doi: 10.1016/j.juro.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Giannantoni A, Mearini E, Zucchi A, Costantini E, Mearini L, Bini V, et al. Bladder and urethral sphincter function after radical retropubic prostatectomy: a prospective long-term study. Eur Urol. 2008;54:657–664. doi: 10.1016/j.eururo.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Lai HH, Hsu EI, Boone TB. Urodynamic testing in evaluation of postradical prostatectomy incontinence before artificial urinary sphincter implantation. Urology. 2009;73:1264–1269. doi: 10.1016/j.urology.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Afraa TA, Campeau L, Mahfouz W, Corcos J. Urodynamic parameters evolution after artificial urinary sphincter implantation for post-radical prostatectomy incontinence with concomitant bladder dysfunction. Can J Urol. 2011;18:5695–5698. [PubMed] [Google Scholar]
- 20.Giannantoni A, Mearini E, Di Stasi SM, Mearini L, Bini V, Pizzirusso G, et al. Assessment of bladder and urethral sphincter function before and after radical retropubic prostatectomy. J Urol. 2004;171:1563–1566. doi: 10.1097/01.ju.0000118957.24390.66. [DOI] [PubMed] [Google Scholar]
- 21.Matsukawa Y, Hattori R, Komatsu T, Funahashi Y, Sassa N, Gotoh M. De novo detrusor underactivity after laparoscopic radical prostatectomy. Int J Urol. 2010;17:643–648. doi: 10.1111/j.1442-2042.2010.02529.x. [DOI] [PubMed] [Google Scholar]
- 22.Trigo Rocha F, Gomes CM, Mitre AI, Arap S, Srougi M. A prospective study evaluating the efficacy of the artificial sphincter AMS 800 for the treatment of postradical prostatectomy urinary incontinence and the correlation between preoperative urodynamic and surgical outcomes. Urology. 2008;71:85–89. doi: 10.1016/j.urology.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Elliott CS, Comiter CV. Maximum isometric detrusor pressure to measure bladder strength in men with postprostatectomy incontinence. Urology. 2012;80:1111–1115. doi: 10.1016/j.urology.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths D. Detrusor contractility: order out of chaos. Scand J Urol Nephrol Suppl. 2004;(215):93–100. doi: 10.1080/03008880410015426. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MP, DuBeau CE, Resnick NM, Cravalho EG, Yalla SV. Continuous occlusion test to determine detrusor contractile performance. J Urol. 1995;154:1834–1840. [PubMed] [Google Scholar]
- 26.McIntosh SL, Griffiths CJ, Drinnan MJ, Robson WA, Ramsden PD, Pickard RS. Noninvasive measurement of bladder pressure. Does mechanical interruption of the urinary stream inhibit detrusor contraction? J Urol. 2003;169:1003–1006. doi: 10.1097/01.ju.0000049031.40088.45. [DOI] [PubMed] [Google Scholar]
- 27.Bauer RM, Mayer ME, Gratzke C, Soljanik I, Buchner A, Bastian PJ, et al. Prospective evaluation of the functional sling suspension for male postprostatectomy stress urinary incontinence: results after 1 year. Eur Urol. 2009;56:928–933. doi: 10.1016/j.eururo.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 28.Rehder P, Freiin von Gleissenthall G, Pichler R, Glodny B. The treatment of postprostatectomy incontinence with the retroluminal transobturator repositioning sling (Advance): lessons learnt from accumulative experience. Arch Esp Urol. 2009;62:860–870. doi: 10.4321/s0004-06142009001000011. [DOI] [PubMed] [Google Scholar]
- 29.Soljanik I, Gozzi C, Becker AJ, Stief CG, Bauer RM. Risk factors of treatment failure after retrourethral transobturator male sling. World J Urol. 2012;30:201–206. doi: 10.1007/s00345-011-0671-6. [DOI] [PubMed] [Google Scholar]
- 30.Bauer RM, Gozzi C, Roosen A, Khoder W, Trottmann M, Waidelich R, et al. Impact of the repositioning test on postoperative outcome of retroluminar transobturator male sling implantation. Urol Int. 2013;90:334–338. doi: 10.1159/000347123. [DOI] [PubMed] [Google Scholar]
- 31.Comiter C, Payne C, Vecchiotti R. Abstract 2160: A prospective analysis of video-urodynamic data to measure urethral mobility in men with post-prostatectomy incontinence. J Urol. 2011;185(4 Suppl):e864–e865. Abstract No. 2160. [Google Scholar]
- 32.Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective review from the University of Michigan. J Urol. 2008;179:1912–1916. doi: 10.1016/j.juro.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Comiter CV. The male perineal sling: intermediate-term results. Neurourol Urodyn. 2005;24:648–653. doi: 10.1002/nau.20166. [DOI] [PubMed] [Google Scholar]
- 34.Carmel M, Hage B, Hanna S, Schmutz G, Tu le M. Long-term efficacy of the bone-anchored male sling for moderate and severe stress urinary incontinence. BJU Int. 2010;106:1012–1016. doi: 10.1111/j.1464-410X.2010.09207.x. [DOI] [PubMed] [Google Scholar]
- 35.De Ridder D, Rehder P. The advance male sling: anatomic features in relation to mode of action. Eur Urol Suppl. 2011;10:383–389. [Google Scholar]
- 36.Rehder P, Haab F, Cornu JN, Gozzi C, Bauer RM. Treatment of postprostatectomy male urinary incontinence with the transobturator retroluminal repositioning sling suspension: 3-year follow-up. Eur Urol. 2012;62:140–145. doi: 10.1016/j.eururo.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Zuckerman JM, Edwards B, Henderson K, Beydoun HA, Mc-Cammon KA. Extended outcomes in the treatment of male stress urinary incontinence with a transobturator sling. Urology. 2014;83:939–945. doi: 10.1016/j.urology.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 38.Osman NI. Slings in the management of male stress urinary incontinence. Curr Opin Urol. 2013;23:528–535. doi: 10.1097/MOU.0b013e328364fae1. [DOI] [PubMed] [Google Scholar]
- 39.Rehder P, Mitterberger MJ, Pichler R, Kerschbaumer A, Glodny B. The 1 year outcome of the transobturator retroluminal repositioning sling in the treatment of male stress urinary incontinence. BJU Int. 2010;106:1668–1672. doi: 10.1111/j.1464-410X.2010.09400.x. [DOI] [PubMed] [Google Scholar]
- 40.Cornu JN, Sebe P, Ciofu C, Peyrat L, Cussenot O, Haab F. Mid-term evaluation of the transobturator male sling for post-prostatectomy incontinence: focus on prognostic factors. BJU Int. 2011;108:236–240. doi: 10.1111/j.1464-410X.2010.09765.x. [DOI] [PubMed] [Google Scholar]
- 41.Bauer RM, Mayer ME, May F, Gratzke C, Buchner A, Soljanik I, et al. Complications of the AdVance transobturator male sling in the treatment of male stress urinary incontinence. Urology. 2010;75:1494–1498. doi: 10.1016/j.urology.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Comiter CV, Rhee EY. The 'ventral urethral elevation plus' sling: a novel approach to treating stress urinary incontinence in men. BJU Int. 2008;101:187–191. doi: 10.1111/j.1464-410X.2007.07277.x. [DOI] [PubMed] [Google Scholar]
- 43.Comiter CV, Rhee EY, Tu LM, Herschorn S, Nitti VW. The virtue sling--a new quadratic sling for postprostatectomy incontinence--results of a multinational clinical trial. Urology. 2014;84:433–438. doi: 10.1016/j.urology.2014.02.062. [DOI] [PubMed] [Google Scholar]
- 44.Bauer RM, Rutkowski M, Kretschmer A, Casuscelli J, Stief CG, Huebner W, et al. Efficacy and complications of the adjustable sling system ArgusT for male incontinence: results of a prospective 2-center study. Urology. 2015;85:316–320. doi: 10.1016/j.urology.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Trost L, Elliott DS. Male stress urinary incontinence: a review of surgical treatment options and outcomes. Adv Urol. 2012;2012:287489. doi: 10.1155/2012/287489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung E, Smith P, Malone G, Cartmill R. Adjustable versus non-adjustable male sling for post-prostatectomy urinary incontinence: a prospective clinical trial comparing patient choice, clinical outcomes and satisfaction rate with a minimum follow up of 24 months. Neurourol Urodyn. 2015 Feb 14; doi: 10.1002/nau.22731. [Epub] [DOI] [PubMed] [Google Scholar]
- 47.Jimenez Parra JD, Cebrian Lostal JL, Hualde Alfaro A, Alvarez Bandres S, Garcia García D, Torres Varas L, et al. REMEEX® system for the treatment of male urinary stress incontinence: our experience. Actas Urol Esp. 2010;34:802–805. [PubMed] [Google Scholar]
- 48.Comiter CV, Sullivan MP, Yalla SV. Retrograde leak point pressure for evaluating postradical prostatectomy incontinence. Urology. 1997;49:231–236. doi: 10.1016/S0090-4295(96)00427-X. [DOI] [PubMed] [Google Scholar]
- 49.Bamshad BR, Hadley HR, Ruckle HC, Lui PD. Perfusion sphincterometry for objective evaluation of postprostatectomy intrinsic sphincter deficiency. Urology. 1999;53:968–973. doi: 10.1016/s0090-4295(98)00630-x. [DOI] [PubMed] [Google Scholar]
- 50.Comiter CV, Sullivan MP, Yalla SV. Correlation among maximal urethral closure pressure, retrograde leak point pressure, and abdominal leak point pressure in men with postprostatectomy stress incontinence. Urology. 2003;62:75–78. doi: 10.1016/s0090-4295(03)00123-7. [DOI] [PubMed] [Google Scholar]
- 51.Choe JM, Battino BS, Bell TE. Retrograde perfusion sphincterometry with a flexible cystoscope: method of troubleshooting the AMS 800. Urology. 2000;56:317–319. doi: 10.1016/s0090-4295(00)00570-7. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich NF, Comiter CV. The male sling for stress urinary incontinence: urodynamic and subjective assessment. J Urol. 2004;172:204–206. doi: 10.1097/01.ju.0000132146.79081.da. [DOI] [PubMed] [Google Scholar]
- 53.Samli M, Singla AK. Absorbable versus nonabsorbable graft: outcome of bone anchored male sling for post-radical prostatectomy incontinence. J Urol. 2005;173:499–502. doi: 10.1097/01.ju.0000150106.65523.16. [DOI] [PubMed] [Google Scholar]
- 54.Giberti C, Gallo F, Schenone M, Cortese P, Ninotta G. The bone anchor suburethral synthetic sling for iatrogenic male incontinence: critical evaluation at a mean 3-year followup. J Urol. 2009;181:2204–2208. doi: 10.1016/j.juro.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Gill BC, Nowacki AS, Montague DK, Angermeier KW, Wood HM, et al. Therapeutic durability of the male transobturator sling: midterm patient reported outcomes. J Urol. 2012;187:1331–1335. doi: 10.1016/j.juro.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 56.Collado Serra A, Resel Folkersma L, Domínguez-Escrig JL, Gomez-Ferrer A, Rubio-Briones J, Solsona Narbon E. AdVance/AdVance XP transobturator male slings: preoperative degree of incontinence as predictor of surgical outcome. Urology. 2013;81:1034–1039. doi: 10.1016/j.urology.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Anderson CA, Omar MI, Campbell SE, Hunter KF, Cody JD, Glazener CM. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2015;1:CD001843. doi: 10.1002/14651858.CD001843.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lepor H, Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: a prospective assessment using validated self-administered outcome instruments. J Urol. 2004;171:1216–1219. doi: 10.1097/01.ju.0000113964.68020.a7. [DOI] [PubMed] [Google Scholar]
- 59.Cornu JN, Sebe P, Ciofu C, Peyrat L, Beley S, Tligui M, et al. The AdVance transobturator male sling for postprostatectomy incontinence: clinical results of a prospective evaluation after a minimum follow-up of 6 months. Eur Urol. 2009;56:923–927. doi: 10.1016/j.eururo.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Bauer RM, Soljanik I, Fullhase C, Karl A, Becker A, Stief CG, et al. Mid-term results for the retroluminar transobturator sling suspension for stress urinary incontinence after prostatectomy. BJU Int. 2011;108:94–98. doi: 10.1111/j.1464-410X.2010.09729.x. [DOI] [PubMed] [Google Scholar]
- 61.Fischer MC, Huckabay C, Nitti VW. The male perineal sling: assessment and prediction of outcome. J Urol. 2007;177:1414–1418. doi: 10.1016/j.juro.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 62.Castle EP, Andrews PE, Itano N, Novicki DE, Swanson SK, Ferrigni RG. The male sling for post-prostatectomy incontinence: mean followup of 18 months. J Urol. 2005;173:1657–1660. doi: 10.1097/01.ju.0000154782.86431.41. [DOI] [PubMed] [Google Scholar]
- 63.Schaeffer AJ, Clemens JQ, Ferrari M, Stamey TA. The male bulbourethral sling procedure for post-radical prostatectomy incontinence. J Urol. 1998;159:1510–1515. doi: 10.1097/00005392-199805000-00026. [DOI] [PubMed] [Google Scholar]
- 64.Torrey R, Rajeshuni N, Ruel N, Muldrew S, Chan K. Radiation history affects continence outcomes after advance transobturator sling placement in patients with post-prostatectomy incontinence. Urology. 2013;82:713–717. doi: 10.1016/j.urology.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 65.Tuygun C, Imamoglu A, Gucuk A, Goktug G, Demirel F. Comparison of outcomes for adjustable bulbourethral male sling and artificial urinary sphincter after previous artificial urinary sphincter erosion. Urology. 2009;73:1363–1367. doi: 10.1016/j.urology.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 66.Margreiter M, Farr A, Sharma V, Schauer I, Klingler HC. Urethral buttressing in patients undergoing artificial urinary sphincter surgery. J Urol. 2013;189:1777–1781. doi: 10.1016/j.juro.2012.11.152. [DOI] [PubMed] [Google Scholar]
- 67.Guralnick ML, Miller E, Toh KL, Webster GD. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075–2078. [PubMed] [Google Scholar]
- 68.Trost L, Elliott D. Small intestinal submucosa urethral wrap at the time of artificial urinary sphincter placement as a salvage treatment option for patients with persistent/recurrent incontinence following multiple prior sphincter failures and erosions. Urology. 2012;79:933–938. doi: 10.1016/j.urology.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Wang R, McGuire EJ, He C, Faerber GJ, Latini JM. Long-term outcomes after primary failures of artificial urinary sphincter implantation. Urology. 2012;79:922–928. doi: 10.1016/j.urology.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 70.Martinez EJ, Zuckerman JM, Henderson K, Edwards B, Mc-Cammon K. Evaluation of salvage male transobturator sling placement following recurrent stress urinary incontinence after failed transobturator sling. Urology. 2015;85:478–482. doi: 10.1016/j.urology.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Soljanik I, Becker AJ, Stief CG, Gozzi C, Bauer RM. Repeat retrourethral transobturator sling in the management of recurrent postprostatectomy stress urinary incontinence after failed first male sling. Eur Urol. 2010;58:767–772. doi: 10.1016/j.eururo.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 72.Ajay D, Zhang H, Gupta S, Selph JP, Belsante MJ, Lentz AC, et al. The artificial urinary sphincter is superior to a secondary transobturator male sling in cases of a primary sling failure. J Urol. 2015;194:1038–1042. doi: 10.1016/j.juro.2015.04.106. [DOI] [PubMed] [Google Scholar]
- 73.Kim PH, Pinheiro LC, Atoria CL, Eastham JA, Sandhu JS, Elkin EB. Trends in the use of incontinence procedures after radical prostatectomy: a population based analysis. J Urol. 2013;189:602–608. doi: 10.1016/j.juro.2012.08.246. [DOI] [PubMed] [Google Scholar]
- 74.Belot PY, Fassi-Fehri H, Crouzet S, Codas R, Badet L, Gelet A, et al. Treatment of stress urinary incontinence after prostate surgery: results of the artificial urinary sphincter after suburethral sling failure. Prog Urol. 2012;22:644–649. doi: 10.1016/j.purol.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Romano SV, Metrebian SE, Vaz F, Muller V, D'Ancona CA, de Souza EA, et al. Long-term results of a phase III multicentre trial of the adjustable male sling for treating urinary incontinence after prostatectomy: minimum 3 years. Actas Urol Esp. 2009;33:309–314. doi: 10.1016/s0210-4806(09)74146-4. [DOI] [PubMed] [Google Scholar]
- 76.Abdou A, Cornu JN, Sebe P, Ciofu C, Peyrat L, Cussenot O, et al. Salvage therapy with artificial urinary sphincter after Advance™ male sling failure for post-prostatectomy incontinence: a first clinical experience. Prog Urol. 2012;22:650–656. doi: 10.1016/j.purol.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Lentz AC, Peterson AC, Webster GD. Outcomes following artificial sphincter implantation after prior unsuccessful male sling. J Urol. 2012;187:2149–2153. doi: 10.1016/j.juro.2012.01.119. [DOI] [PubMed] [Google Scholar]
- 78.Staskin DR, Comiter CV. Surgical treatment of male sphincteric urinary incontinence: the male perineal sling and artificial urinary sphincter. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders; 2007. pp. 2391–2404. [Google Scholar]
- 79.Comiter C. Surgery for postprostatectomy incontinence: which procedure for which patient? Nat Rev Urol. 2015;12:91–99. doi: 10.1038/nrurol.2014.346. [DOI] [PubMed] [Google Scholar]
- 80.Al-Najar A, Kaufmann S, Boy S, Naumann CM, Junemann PK, Van Der Horst C. Management of recurrent post-prostatectomy incontinence after previous failed retrourethral male slings. Can Urol Assoc J. 2011;5:107–111. doi: 10.5489/cuaj.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]